Abstract
Glycan biosynthesis occurs mainly in Golgi. Molecular organization and functional regulation of this process are not well understood. We evaluated the extrinsic effect of lectin domains (β-trefoil fold) of polypeptide GalNAc-transferases (ppGalNAc-Ts) on catalytic activity of glycosyltransferases during O-GalNAc glycan biosynthesis. The presence of lectin domain T3lec or T4lec during ppGalNAc-T2 and ppGalNAc-T3 catalytic reaction had a clear inhibitory effect on GalNAc-T activity. Interaction of T3lec or T4lec with ppGalNAc-T2 catalytic domain was not mediated by carbohydrate. T3lec, but not T2lec and T4lec, had a clear activating effect on Drosophila melanogaster core 1 galactosyltransferase enzyme activity and a predominant inhibitory effect on in vivo human core 1 glycan biosynthesis. The regulatory role of the β-trefoil fold of ppGalNAc-Ts in enzymatic activity of glycosyltransferases involved in the O-glycan biosynthesis pathway, described here for the first time, helps clarify the mechanism of biosynthesis of complex biopolymers (such as glycans) that is not template-driven.
Keywords: glycobiology, glycoprotein biosynthesis, glycosylation inhibitor, lectin, mucin
Introduction
Glycobiology is the study of biosynthesis, structure, function, and evolution of naturally occurring glycans and the proteins that recognize them. Glycan biosynthesis consists of the polymerization of sugars in an “assembly line” in which enzymes (glycosyltransferases (glycosyl-Ts)2) catalyze the synthesis of glycosidic linkages by saccharide transfer from sugar donor to an acceptor. In contrast to synthesis of DNA, RNA, or protein molecules, glycosylation is not template-driven; rather the monosaccharide sequence is determined by the action of specific glycosyl-Ts (1). Glycan biosynthesis occurs mainly in Golgi. Molecular organization and functional regulation of this process are not well understood. A self-assembled enzyme complex has been proposed as a mechanism responsible for correct positioning of glycosyl-Ts in Golgi (2, 3). According to this model, glycosyl-T domains facilitate protein/protein interactions related to formation of a “low affinity/high specificity” complex that controls enzyme ratios and optimizes flow in the sugar assembly line.
In O-GalNAc glycan (mucin) biosynthesis, the first step is the transfer of N-acetylgalactosamine (GalNAc) from the sugar donor, UDP-GalNAc, to selected Ser/Thr residues of the acceptor protein to yield GalNAcα1-O-Ser/Thr (Tn antigen) (4). This key initial step is catalyzed by a multigene family of enzyme polypeptide GalNAc-transferases (ppGalNAc-Ts). Various ppGalNAc-T isoforms are differentially expressed in mammalian cells and tissues during development and differentiation. The second monosaccharide linked to GalNAcα1-O-Ser/Thr may be galactose (Gal) or N-acetylglucosamine (GlcNAc) to yield core 1 (Galβ3GalNAcα1-O-Ser/Thr) or core 3 (GlcNAcβ3GalNAcα1-O-Ser/Thr) glycan, respectively. Core 1 glycan biosynthesis involves core 1 β3Gal-T (C1GalT), a ubiquitous enzyme found in most mammalian cells. Core 3 β3GlcNAc-T catalyzes biosynthesis of core 3 glycan, the predominant glycan in colonic and salivary mucins (5). Core 2 and core 4 glycans are the products of β6-GlcNAc-T action on GalNAcα of core 1 and core 3, respectively. Addition of repeating Galβ3/4GlcNAc units gives rise to the backbone region of glycans. The peripheral regions of saccharide chains display high structural diversity. Fucose and N-acetylneuraminic acid are frequent capping residues in these regions.
In studies of structure-function relationships, ppGalNAc-Ts were shown to be type II transmembrane proteins containing a short N-terminal cytoplasmic tail, a small transmembrane anchor, and a Golgi lumenal region. The lumenal region consists of a stem section, catalytic domain, and C terminus. This C-terminal domain has sequence and predicted structural homology to a lectin. Lectins are glycan-binding proteins that play important roles in mammalian cellular homeostasis (6). Some lectins have bifunctional properties; they have both a glycan-binding site and a pocket involved in protein/protein interaction (7). R-type lectins, an intracellular lectin family, have a β-trefoil fold motif similar to that of the Gal-binding B chain of ricin, i.e. three lobes arranged as a β-trefoil around a 3-fold axis (8). This motif, essentially a flexible lectin scaffold, is present in the C-terminal domain of all ppGalNAc-T isoforms (9, 10).
The lectin domains of the isoforms ppGalNAc-T4 (T4lec), -T2 (T2lec), and -T3 (T3lec) show carbohydrate binding specificity for GalNAc (11–13). The intrinsic function of these lectin domains is to modulate GalNAc-T activity resulting in dense O-GalNAc glycosylation without affecting the GalNAc-T activity on naked peptides. These studies demonstrated the intrinsic role of ppGalNAc-T lectin domains in activity of the enzyme itself.
We examined the extrinsic functions of ppGalNAc-T lectin domains on catalytic activity of neighboring glycosyl-Ts to clarify the intermolecular interactions that regulate activity of enzymes involved in O-GalNAc glycan biosynthesis. Our focus was on effects of T3lec and T4lec on glycosyl-T activity of ppGalNAc-T2, ppGalNAc-T3, and C1GalT, enzymes that play critical roles in the sugar assembly line of such biosynthesis.
Results
Effect of Lectin Domains on Catalytic Activity of ppGalNAc-Ts
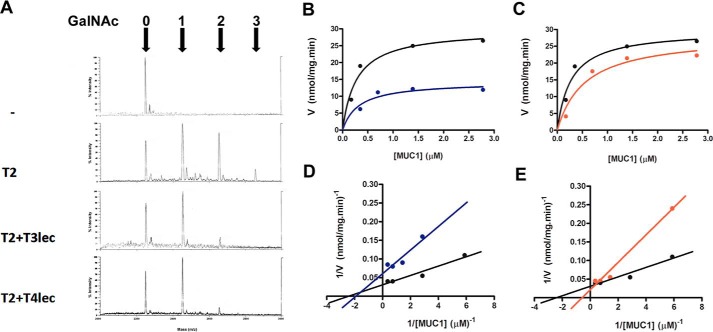
Enzymes and lectin domains were expressed in insect cells and purified by affinity chromatography (supplemental Fig. S-1). To assess the effect of lectin domains on catalytic activity of neighboring glycosyl-Ts, we measured GalNAc-T activity in the presence of purified lectin domains. The effects of T3lec and T4lec on catalytic activity of ppGalNAc-T2 and -T3 were determined using UDP-GalNAc as sugar donor and a mucin peptide as acceptor. Enzymatic reaction products were analyzed by mass spectrometry, and the number of O-GalNAc groups linked to the peptide was determined. Mass spectrometry of mucin-derived glycopeptides revealed the number of O-GalNAc sites following catalysis by ppGalNAc-T2 (Fig. 1A). The presence of T3lec and T4lec during the catalytic reaction caused a notable reduction in the number of O-glycosylated MUC1 sites, indicating that both lectin domains have an inhibitory effect on GalNAc-T activity of ppGalNAc-T2. Enzyme kinetics of glycosyl-Ts was evaluated by colorimetric assay, and the data were fitted using a non-linear regression algorithm. This method revealed a significant inhibitory effect of T3lec and T4lec on GalNAc-T activity with MUC1 as acceptor substrate (Fig. 1, B and C) as evidenced by altered kinetic parameters Vmax (maximal velocity) and Km (Michaelis constant) of ppGalNAc-T2 (supplemental Table S-1). These two lectins also had an inhibitory effect on ppGalNAc-T2 catalytic activity with MUC2 as acceptor substrate (supplemental Fig. S-2 and Table S-1).
FIGURE 1.
Effects of lectin domains on enzyme activity of ppGalNAc-T2 (T2). A, MUC1 glycosylation product was analyzed by MALDI-TOF. Numbers of α-GalNAc incorporated were determined on acceptor peptide without ppGalNAc-T2 enzyme (control), with ppGalNAc-T2 (8 nm), and with ppGalNAc-T2 (8 nm) in the presence of 1.6 μm T3lec or T4lec domain. B–E, kinetics plots of MUC1 peptide glycosylation assay under initial velocity condition of ppGalNAc-T2 (1.6 nm) enzymatic reaction (black). Effects of 0.16 μm T3lec (B, blue) and T4lec (C, red) on ppGalNAc-T2 activity were assayed. Plots were fitted to the Michaelis-Menten equation using the GraphPad software program, yielding R2 values of 0.94 (black), 0.81 (blue), and 0.94 (red). Double reciprocal plots of enzyme activity without lectin domain (black) or in the presence of 0.16 μm T3lec (D, blue) or T4lec (E, red) indicated the type of inhibition of ppGalNAc-T2 activity. Plots were fitted to linear regression using the GraphPad software program, yielding R2 values of 0.94 (black), 0.88 (blue), and 0.98 (red). Results are representative of three independent experiments.
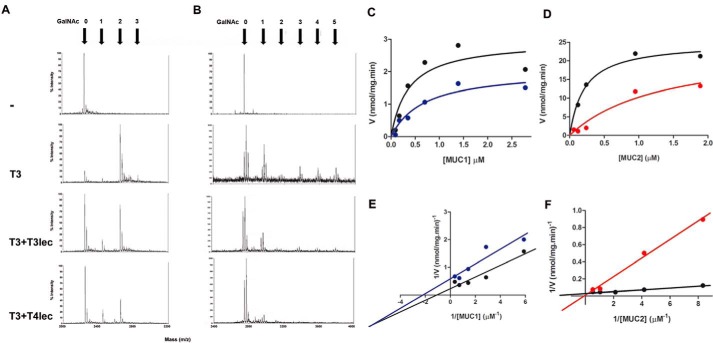
Similar results were obtained when the GalNAc-T activity of ppGalNAc-T3 was studied in presence of T3lec and T4lec. Both lectin domains caused a notable reduction in the number of O-glycosylated MUC1 and MUC5B sites by mass spectrometry (Fig. 2, A and B). Enzyme kinetics of ppGalNAc-T3 revealed a significant inhibitory effect of T3lec on GalNAc-T activity with MUC1 as acceptor substrate (Fig. 2C) as evidenced by altered kinetic parameters Vmax and Km of ppGalNAc-T3 (supplemental Table S-2). This lectin also had an inhibitory effect on ppGalNAc-T3 catalytic activity with MUC2 as acceptor substrate (Fig. 2D and supplemental Table S-2).
FIGURE 2.
Effects of lectin domains on enzyme activity of ppGalNAc-T3 (T3). MUC1 (A) and MUC5B (B) glycosylation products were analyzed by MALDI-TOF. Numbers of α-GalNAc incorporated were determined on acceptor peptide without ppGalNAc-T3 enzyme (control), with ppGalNAc-T3 (8 nm), and with ppGalNAc-T3 (8 nm) in the presence of 0.8 μm T3lec or T4lec domain. Kinetics plots of peptide glycosylation assay under initial velocity condition of ppGalNAc-T3 (1.6 nm) enzymatic reaction (black) are shown. Effects of 0.08 μm T3lec on ppGalNAc-T3 activity with MUC1 (C) or MUC2 (D) as acceptor peptide were assayed. Plots were fitted to the Michaelis-Menten equation using the GraphPad software program, yielding R2 values of 0.83 (C, black), 0.92 (C, blue), 0.96 (D, black), and 0.95 (D, red). Double reciprocal plots of enzyme activity without lectin domain (black) or in the presence of 0.08 μm T3lec (E, blue and F, red) indicated the type of inhibition on ppGalNAc-T3 activity. Plots were fitted to linear regression using the GraphPad software program, yielding R2 values 0.92 (E, black), 0.88 (E, blue) 0.98 (F, black), and 0.99 (F, red). Results are representative of three independent experiments.
Double reciprocal plots of enzyme activity were constructed to evaluate the type of inhibition on ppGalNAc-Ts. With ppGalNAc-T2 and MUC1 as acceptor substrate, T3lec had a plot characteristic of a non-competitive inhibitor (Fig. 1D), whereas T4lec had a plot characteristic of a competitive inhibitor (Fig. 1E). Using MUC2 as acceptor substrate, T3lec appeared as a competitive inhibitor in the double reciprocal plot (supplemental Fig. S-2), demonstrating the important effect of acceptor substrate on inhibition type of lectin domains. Inhibition constants (Ki values) were determined for T3lec and T4lec using MUC1 and MUC2 as acceptors (supplemental Table S-3). Meanwhile, a double reciprocal plot of ppGalNAc-T3 activity showed that T3lec had a plot characteristic of a mixed inhibitor when MUC1 is acceptor and a plot of a competitive inhibitor with MUC2 as acceptor (Fig. 2, E and F). Ki values were determined for T3lec using MUC1 and MUC2 as acceptor substrates of ppGalNAc-T3 activity (supplemental Table S-4).
In Vivo Study of Catalytically Inactive ppGalNAc-T3 in O-GalNAc Glycan Biosynthesis
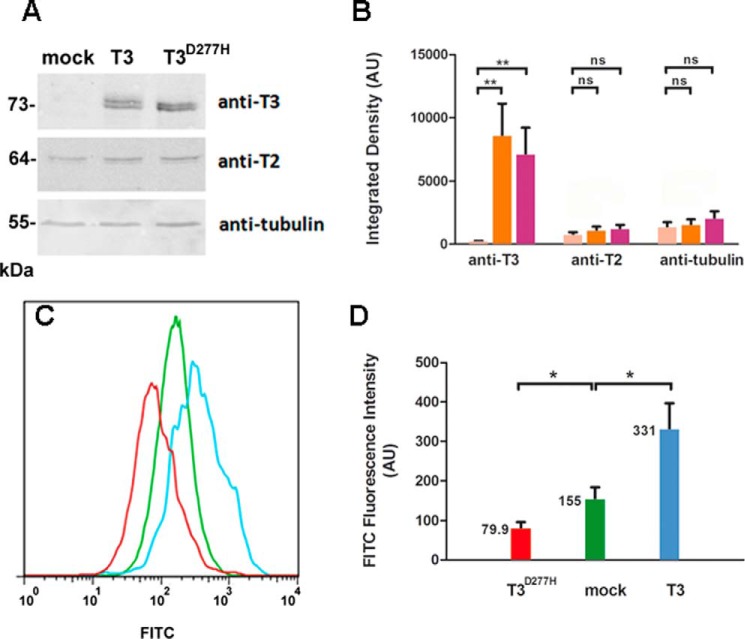
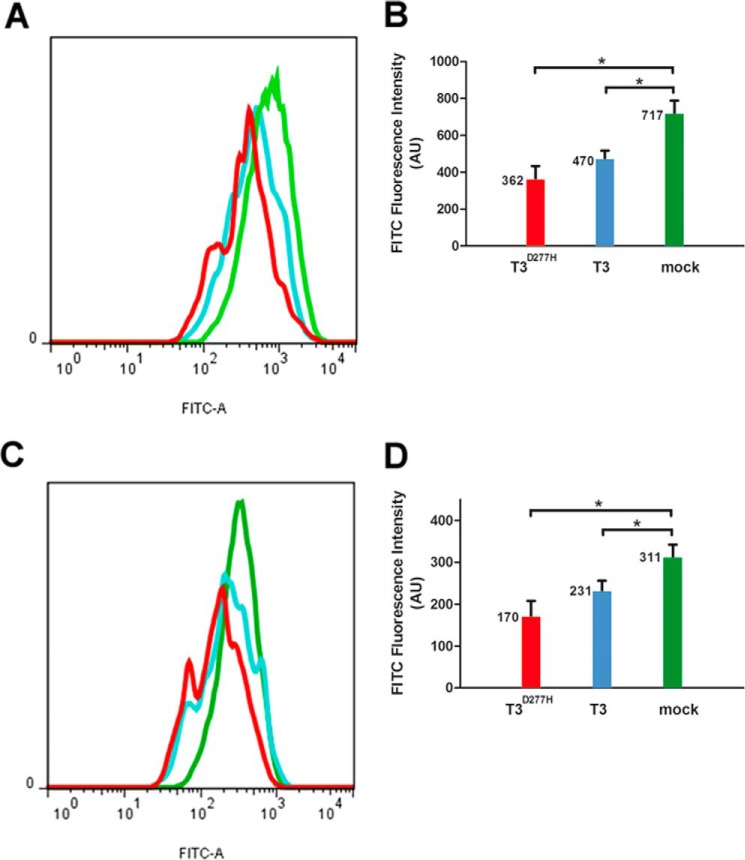
We used Chinese Hamster ovary (CHO) mutant cell line ldlD to study the initiation of O-GalNAc glycosylation in vivo. CHO ldlD cells are deficient in UDP-Gal and UDP-GalNAc 4-epimerase and are therefore unable to synthesize UDP-Gal or UDP-GalNAc. External addition of GalNAc and Gal restores normal levels of the two sugar nucleotides (14). When GalNAc is added to cell culture, O-GalNAc glycosylation is truncated following incorporation of the first monosaccharide, and Tn antigen can be detected using Vicia villosa lectin (VVL), which recognizes terminal α-GalNAc. We transiently transfected CHO ldlD cells with several vectors and evaluated ppGalNAc-T3 expression by Western blotting using a specific antibody (Fig. 3, A and B). Overexpression of ppGalNAc-T3 and ppGalNAc-T3D277H (a mutant enzyme that has a normal lectin domain but is catalytically inactive) was clearly observed, whereas endogenous ppGalNAc-T3 was not detected when mock vector was used. Overexpression of ppGalNAc-T3 or ppGalNAc-T3D277H had no effect on the endogenous expression level of ppGalNAc-T2. Flow cytometric analysis showed that overexpression of ppGalNAc-T3 caused a 2.14-fold increase (from 155 to 331) of Tn antigen expression level in ldlD cells relative to mock vector treatment (Fig. 3, C and D). The O-GalNAc glycan level was 49% lower (79 compared with 155) in cells expressing ppGalNAc-T3D277H than in mock vector-treated cells. Thus, this protein with lectin activity affects O-GalNAc glycosylation in vivo, consistent with results of in vitro assays.
FIGURE 3.
Effect of T3lec on O-GalNAc glycosylation in vivo. CHO ldlD cells were transfected with mock vector, ppGalNAc-T3 (T3), or catalytically inactive mutant ppGalNAc-T3D277H (T3D277H). A, overexpression of recombinant proteins was assayed by Western blotting using anti-ppGalNAc-T3 antibody with anti-ppGalNAc-T2 (T2) and anti-tubulin antibodies as internal control and loading control, respectively. B, bands of Western blotting by using anti-ppGalNAc-T3, anti-ppGalNAc-T2, and anti-tubulin antibodies on CHO ldlD cells transfected with mock vector (pink), ppGalNAc-T3 (orange), or ppGalNAc-T3D277H (fuchsia) were quantified by using ImageJ software. Error bars represent S.D. of three independent experiments. C, effects of mock vector (green), ppGalNAc-T3 (light blue), and ppGalNAc-T3D277H (red) overexpression on O-GalNAc glycosylation were evaluated by flow cytometry of cells stained with biotinylated VVL. D, fluorescence intensity values of FITC (expressed in arbitrary units (AU)) corresponding to means and S.D. of three independent experiments. **, p < 0.01; *, p < 0.05; ns, not significant.
Interactions of Lectin Domains with ppGalNAc-T2
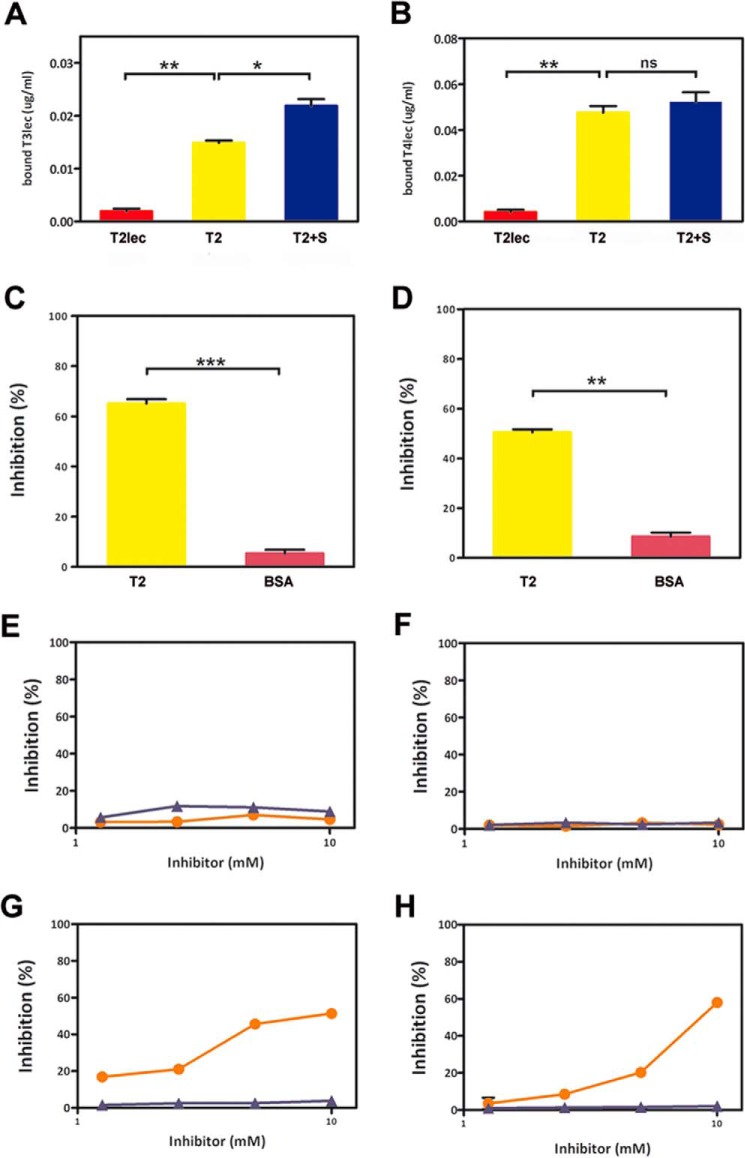
In view of the effect of lectin domains on catalytic activity of ppGalNAc-T2, we studied interactions between them. T3lec showed significant binding to ppGalNAc-T2, whereas the interaction was not significant with T2lec (Fig. 4A). Preincubation of ppGalNAc-T2 with substrates (UDP-GalNAc and MUC1 without Mn2+) greatly enhanced its interaction with T3lec. These findings indicate an important role of the ppGalNAc-T2 catalytic domain in interaction with T3lec.
FIGURE 4.
Interactions between lectin domains and ppGalNAc-T2. Binding assays were developed to assess the type of interaction of T3lec (A) and T4lec (B) with T2lec (red), ppGalNAc-T2 (T2) (yellow), and ppGalNAc-T2 plus substrates (T2+S) (blue). Specificities of interactions of T3lec (C) and T4lec (D) with ppGalNAc-T2 were analyzed by competitive assays using ppGalNAc-T2 (yellow) or BSA (pink) as inhibitor. Error bars represent S.D. of triplicates. Carbohydrates were evaluated as potential inhibitors to study glycan mediation in lectin domain/ppGalNAc-T2 interaction. Effects of BzlαGalNAc (orange) and MeαGlcNAc (purple) on interaction of T3lec (E) and T4lec (F) with ppGalNAc-T2 were assayed. Competitive assays were developed to evaluate glycan binding properties of lectin domains during interaction of T3lec (G) or T4lec (H) with dOSM using BzlαGalNAc (orange) and MeαGlcNAc (purple) as potential inhibitors. Measures are expressed as percentage of inhibition of triplicates. ***, p < 0.001; **, p < 0.01; *, p < 0.05; ns, not significant.
Similar results were obtained for T4lec (Fig. 4B). T4lec bound strongly to ppGalNAc-T2, but interaction with T2lec was not relevant. In conclusion, the two lectin domains T3lec and T4lec interacted with the catalytic domain of ppGalNAc-T2.
Interactions of T3lec and T4lec with ppGalNAc-T2 Are Not Mediated by Glycans
A competitive assay is a useful tool for evaluating lectin interaction specificity and glycan recognition. Soluble ppGalNAc-T2, used as competitor, clearly inhibited interaction of T3lec or T4lec with adsorbed ppGalNAc-T2 (Fig. 4, C and D). Bovine serum albumin, used as competitor, had no such inhibitory effect, demonstrating the specificity of lectin/ppGalNAc-T2 interaction. Similar competitive assays were performed using various carbohydrates as potential inhibitors. BzlαGalNAc and MeαGlcNAc had no inhibitory effect on binding of T3lec or T4lec to ppGalNAc-T2 (Fig. 4, E and F), indicating that glycans are not involved in this interaction. For comparison, BzlαGalNAc and MeαGlcNAc were also assayed as potential inhibitors of the interaction of T3lec and T4lec with desialylated ovine submaxillary mucin (dOSM), a glycoprotein having terminal GalNAcα-Ser/Thr. BzlαGalNAc significantly inhibited interaction of both T3lec (IC50 = 5 mm) and T4lec (IC50 = 8 mm) with dOSM, whereas MeαGlcNAc had no such inhibitory effect (Fig. 4, G and H). These findings support a GalNAc recognition ability of lectin domains as reported previously (11, 13).
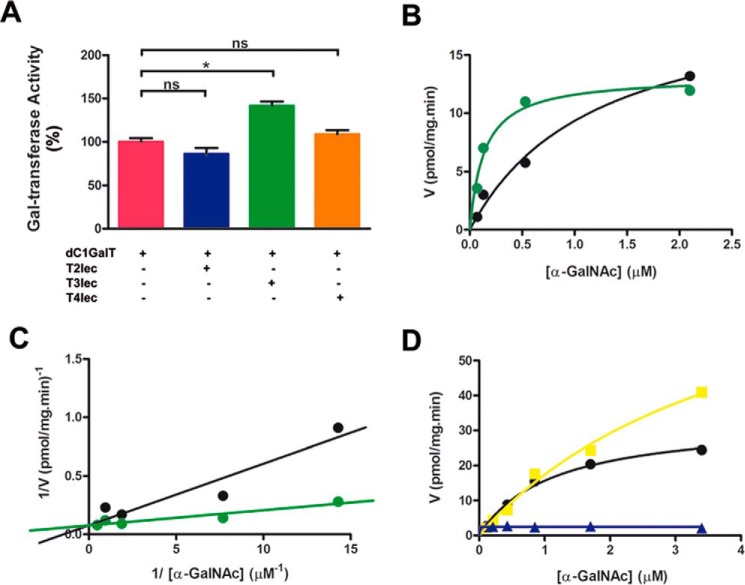
Effect of Lectin Domains on Gal-T Activity of Drosophila melanogaster C1GalT (dC1GalT)
Enzyme kinetics of dC1GalT was studied in the presence and absence of ppGalNAc-T lectin domains. The effects of T2lec, T3lec, and T4lec on Gal-transferase activity of dC1GalT are shown in Fig. 5A. T3lec had a clear enhancing effect on the catalytic activity, but T2lec and T4lec did not. Enzyme kinetics plots revealed the activation of dC1GalT by T3lec (Fig. 5, B and C) and a significant alteration of kinetic parameter Km (supplemental Table S-5). ppGalNAc-T3 had an enhancing effect on dC1GalT activity at high substrate concentration (Fig. 5D). Kinetic parameters Vmax and Km for dC1GalT in the presence and absence of ppGalNAc-T3 are shown in supplemental Table S-6. ppGalNAc-T3 alone (control) showed no Gal-transferase activity (Fig. 5D).
FIGURE 5.
Effects of lectin domains on dC1GalT enzyme activity. A, dC1GalT activity was assayed without (pink) or with 0.08 μm T2lec (blue), T3lec (green), and T4lec (orange). Error bars represent S.D. of three independent experiments. B, dC1GalT activity was assessed in the absence (black) and presence (green) of 0.08 μm T3lec with dOSM as acceptor substrate using non-linear fitting that yielded R2 values of 0.98 (black) and 0.96 (green). Data are representative of three independent experiments. C, double reciprocal plot of dC1GalT activity, demonstrating the activation ability of T3lec, with R2 values of 0.91 (black) and 0.89 (green) from linear regression fitting. D, Gal-T activity of dC1GalT with OSM as acceptor substrate in the absence (black) and presence of 0.16 μm ppGalNAc-T3 (yellow), yielding R2 values of 0.99 (black) and 0.99 (yellow) by non-linear fitting software. Control, Gal-T activity of 0.16 μm ppGalNAc-T3 (blue) without dC1GalT. Results are representative of three independent experiments. *, p < 0.05; ns, not significant.
In Vivo Effect of Catalytically Inactive ppGalNAc-T3 in Human Core 1 Glycan Biosynthesis
We used HeLa cells to study human core 1 glycan biosynthesis in vivo. Epithelial tumor cells are frequently deficient in the expression of core 2 β6GlcNAc-T and core 3 β3GlcNAc-T, yielding truncated O-glycans with overexpression of terminal core 1 glycan (15). This terminal disaccharide was detected using peanut agglutinin (PNA) and Agaricus bisporus lectin (ABL). We transiently transfected HeLa cells with ppGalNAc-T3 or ppGalNAc-T3D277H vector to determine the effect on core 1 glycan expression. Flow cytometric analysis showed that overexpression of ppGalNAc-T3 and ppGalNAc-T3D277H caused a reduction of core 1 glycan expression level in HeLa cells relative to mock vector treatment (Fig. 6). Core 1 glycan level was 34% (470 compared with 717) or 26% (231 compared with 311) lower in cells overexpressing ppGalNAc-T3 than in mock vector-treated cells when detected with PNA or ABL, respectively. Overexpression of ppGalNAc-T3D277H caused a reduction of terminal core 1 glycan expression of 49% (362 compared with 717) or 45% (170 compared with 311) relative to mock vector treatment by using PNA or ABL, respectively. Thus, ppGalNAc-T3 and ppGalNAc-T3D277H affected human core 1 glycan biosynthesis in HeLa cells.
FIGURE 6.
Influence of T3lec on human core 1 glycan biosynthesis in vivo. HeLa cells were transfected with mock vector, ppGalNAc-T3 (T3), or catalytically inactive mutant ppGalNAc-T3D277H (T3D277H). Effects of mock vector (green), ppGalNAc-T3 (light blue), and ppGalNAc-T3D277H (red) overexpression on core 1 glycan expression were evaluated by flow cytometry of cells stained with biotinylated PNA (A) or ABL (C). Fluorescence intensity values of FITC (expressed in arbitrary units (AU)) correspond to experimental conditions with PNA (B) or ABL (D). Bars represent means of three independent experiments, and error bars represent S.D. *, p < 0.05.
Discussion
Extrinsic effects resulting from lectin/glycan interactions are crucial events in cellular homeostasis. There are several examples involving N-glycan biosynthesis during protein glycosylation. Calnexin is a lectin chaperone molecule that plays a key role in quality control of glycoprotein folding based on carbohydrate recognition of the Glc1Man9GlcNAc2 residue (16). ERGIC-53 and VIP36 are leguminous type (L-type) lectins that function as cargo receptors for trafficking of certain N-linked glycoproteins in secretory pathways of animal cells. The two lectins have structural similarities in their carbohydrate recognition domains but distinct sugar binding specificities and affinities (17). The two known members of the P-type lectin (mannose 6-phosphate receptor) family are distinguished from all other lectins by their ability to recognize phosphorylated mannose residues (Man-6-P). The best characterized function of mannose 6-phosphate receptors is their ability to direct delivery to the lysosome of soluble lysosomal enzymes bearing Man-6-P on their N-linked oligosaccharides (18). The present study was focused on extrinsic effects of lectin domains present in ppGalNAc-Ts that control catalytic activity of glycosyl-Ts on O-GalNAc glycan biosynthesis.
The presence of lectin domains (T3lec and T4lec) in the catalytic reaction of ppGalNAc-T2 and ppGalNAc-T3 had a clear inhibitory effect on GalNAc-T activity. Mass spectrometric results showed a reduced number of glycosylated sites on MUC1 and MUC5B acceptor peptides when lectins were present in the enzyme reaction. Such inhibitory effect of lectin domains was also evidenced by analysis of initial velocity of GalNAc-T reaction. Enzyme kinetics plots showed that T3lec and T4lec had differing characteristics as inhibitors of ppGalNAc-T2 with MUC1 as acceptor. T3lec was a non-competitive inhibitor, whereas T4lec was a competitive inhibitor as shown by double reciprocal plots. The inhibition type of lectin domains was also affected by the acceptor peptide. In ppGalNAc-T2 catalysis, T3lec functioned as a non-competitive inhibitor with MUC1 as acceptor but as a competitive inhibitor with MUC2 as acceptor, whereas in ppGalNAc-T3 catalysis T3lec acted as a mixed inhibitor with MUC1 acceptor and as a competitive inhibitor with MUC2 acceptor. The lectin domains frequently yielded competitive inhibition by binding to the active site of enzymes, such as T4lec behavior and T3lec with MUC2 acceptor substrate. Moreover, T3lec in the presence of MUC1 showed non-competitive and mixed inhibition ability, revealing a preferential or additional recognition for substrate-enzyme complex over binding to enzyme alone. The lectin domain is the region of major amino acid divergence among ppGalNAc-T members. Enzyme kinetic of GalNAc-T in the presence of T3lec suggested a binding ability of this lectin domain to ppGalNAc-Ts in an additional locus from acceptor substrate site that affects the catalytic activity if MUC1 peptide is the acceptor. Thus, the β-trefoil fold could act as a regulator of enzyme activity, having a differential influence according to the amino acid sequence of acceptor substrate. Although we did not observe a sigmoid response by T3lec presence, allosteric properties on ppGalNAc-T enzymes cannot be discarded. The magnitude of the inhibition constant (Ki) depended on lectin domain, enzyme, and acceptor peptide.
CHO ldlD cells are a robust model for studying the initiation of O-GalNAc glycosylation. We used this model to assay the inhibitory effects of lectin domains. These in vivo studies showed a clear reduction of O-GalNAc glycosylation when the catalytically inactive mutant enzyme ppGalNAc-T3D277H was overexpressed. The inhibitory effect of the ppGalNAc-T3 lectin domain on O-GalNAc glycosylation was revealed by both in vitro and in vivo assays. This extrinsic effect of lectin domains is opposite to the previously described intrinsic effect whereby the lectin domain promotes the catalytic domain of its own enzyme to complete glycosylation of available sites and enhance O-GalNAc incorporation (12). There have been previous reports of proteins as inhibitors of enzyme activity. The Kunitz-soybean trypsin inhibitor protein family, a member of the β-trefoil fold superfamily, is a paradigm of protease/inhibitor interaction in particular and protein-protein recognition in general (19).
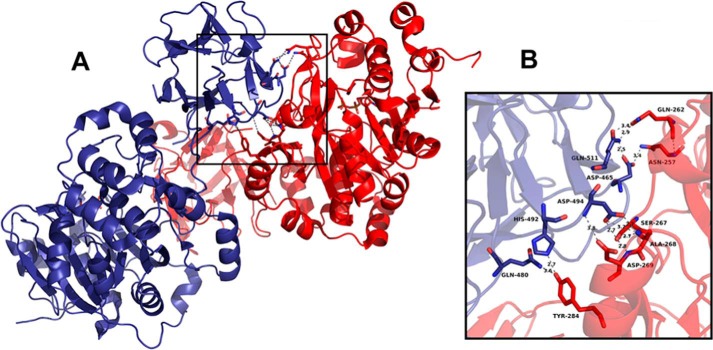
We developed binding assays to define the type of interaction between lectin domains and ppGalNAc-T2. The degree of interaction of T3lec or T4lec with T2lec was very low in comparison with interaction with ppGalNAc-T2 (catalytic and lectin domain). The interaction of ppGalNAc-T2 with T3lec was enhanced by the presence of enzyme substrates (UDP-GalNAc and MUC1). These findings and the observed effects of T3lec and T4lec on GalNAc-T activity indicate that the catalytic domain is the region of ppGalNAc-T2 involved in interaction with lectin domains. Moreover, the interaction of the catalytic domain of ppGalNAc-T2 with T2lec is observed in crystallographic studies (10). Crystal structure of ppGalNAc-T2 (Protein Data Bank code 2FFV) was analyzed using the PISA server (20). The analysis predicted an energetically favored interaction of the catalytic domain of a molecule with the lectin domain of neighboring ppGalNAc-T2, suggesting stable binding (supplemental Table S-7). In addition, the amino acids of domains involved in interaction were identified (Fig. 7). Crystals of ppGalNAc-T2 obtained in different experimental conditions (Protein Data Bank codes 4D0T, 4D0Z, and 4D11) exhibit similar interaction between catalytic and lectin domains, showing a conserved binding ability (21). Co-crystallization of ppGalNAc-Ts in the presence of different substrates should clarify allosteric properties of this enzyme family.
FIGURE 7.
Interaction between catalytic and lectin domains in crystal structure of ppGalNAc-T2 (Protein Data Bank code 2FFV). The two ppGalNAc-T2 molecules of the asymmetric unit exhibit conserved interaction regions (A). The enlarged view shows the amino acids corresponding to the lectin domain (blue) and catalytic domain (red) involved in the interaction (B). Hydrogen bonds are represented with gray dashed lines, and atomic distances are in Å. The figure was prepared using PyMOL.
Competitive assays helped elucidate the lectin domain/ppGalNAc-T2 interaction. Such interaction is not mediated by carbohydrate because GalNAc is the primary ligand in carbohydrate recognition of ppGalNAc-T lectin domains, and BzlαGalNAc had no inhibitory effect on the interaction. Galectin-3, a member of the galectin family, has intracellular functions (including effects on the nucleus) mediated by protein/protein interaction rather than protein/carbohydrate interaction (22). Another example of involvement of lectin domains in protein/protein interaction is the C-type lectin domain of lecticans, a family of aggregating chondroitin sulfate proteoglycans that bind tenascin-R independently of a carbohydrate moiety (23). In addition, in vivo studies showed that the Golgi lumenal region of glycosyltransferases from the N- and O-glycans biosynthesis pathway are involved in protein/protein interaction of self-assembled enzyme complex (24, 25).
We next studied the effects of ppGalNAc-T lectin domains on activity of dC1GalT, an enzyme that catalyzes transformation of Tn antigen in core 1 glycan (Galβ3GalNAcα1-O-Ser/Thr). In vertebrates, core 1 O-glycan disaccharide is the most common O-glycan core and is a precursor to more complex O-glycans (5). We found that T3lec played an important role as activator of dC1GalT activity, whereas T2lec and T4lec had no such role. Analysis of enzyme kinetic parameters of dC1GalT in the presence of T3lec revealed an 8.6-fold reduction of Kmvalue with a 36% reduction of Vmax. A double reciprocal plot of enzyme activity showed a significant change in slope of the line resulting from the presence of T3lec. ppGalNAc-T3 had an enhancing effect on Gal-T activity of dC1GalT at high concentration of substrate and an inhibitory effect at low substrate concentration that affected Vmax and Km. Consistent with the activator effect of the ppGalNAc-T3 lectin domain (β-trefoil fold) on dC1GalT activity, lectins from Macrolepiota procera and Clitocybe nebularis, which are fungal β-trefoil proteins, were found to enhance protease activity of trypsin and papain (26).
HeLa cells were assayed as a model for studying human core 1 glycan biosynthesis. These in vivo studies showed a clear reduction of core 1 glycan expression when ppGalNAc-T3 and the catalytically inactive mutant enzyme ppGalNAc-T3D277H were overexpressed. This extrinsic effect of ppGalNAc-T3s in human core 1 glycan biosynthesis is in agreement with that previously observed on dC1GalT whereby ppGalNAc-T3 reduces catalytic activity of Gal-T at low substrate concentration. The partial inconsistency between in vitro and in vivo results could be explained by the fact that in vitro assays were developed with Drosophila C1GalT, whereas human cells were used in in vivo assays. However, both models, in vitro and in vivo, showed that the lectin domain of ppGalNAc-T3 is able to modulate Gal-T activities in core 1 glycan biosynthesis.
The ability of the β-trefoil fold to support loops that vary greatly in length, sequence, and conformation and that comprise most of the protein surface suggests the possible creation of binding sites (27). The β-trefoil fold superfamily has very high plasticity in regard to interacting structures (proteins, DNA, and carbohydrates) (19, 28). Intrinsic functions of members of the superfamily are related to increased enzyme affinity for target glycans in hydrolytic activity of Streptomyces lividans endo-1–4xylanase (29, 30) and the role of GalNAc-T activity of ppGalNAc-Ts in promoting dense O-GalNAc glycosylation (12). Extrinsic functions of β-trefoil folds related to protein/protein interaction have been reported in protease-inhibitor Kunitz-soybean trypsin inhibitor proteins and in binding of interleukin 1 and fibroblast growth factor to their receptors (27).
O-GalNAc glycan biosynthesis is initiated in cis-Golgi, and ppGalNAc-T isoforms are localized in cis-, medial-, and trans-Golgi (5, 31), suggesting that additional functions of ppGalNAc-Ts are related to downstream glycosyl-Ts in the biosynthesis pathway. The β-trefoil fold of ppGalNAc-Ts appears to act as a scaffold structure. We propose that, in addition to the self-assembled enzymes in Golgi, lectin domains comprise part of a regulatory scaffold in O-GalNAc glycan biosynthesis that controls catalytic activity of glycosyl-Ts with a related role in sequence determination of O-glycans as shown schematically in Fig. 8.
FIGURE 8.
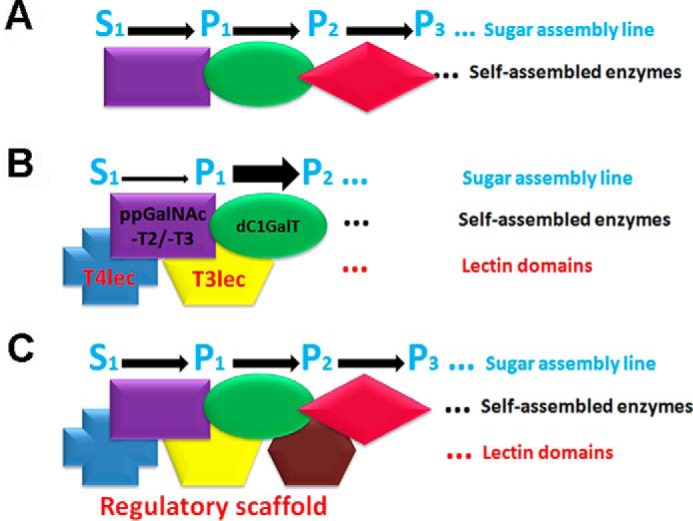
Proposed molecular organization (schematic) of glycosyl-Ts in Golgi and extrinsic functions of lectin domains. A, flow of the sugar assembly line (S0, initial substrate; P1, product 1; P2, product 2; P3, product 3) is optimized by self-assembled glycosyl-T complex in Golgi. B, T3lec and T4lec have extrinsic inhibitory effects on ppGalNAc-T2 and -T3 activity (thin arrow). T3lec has an enhancing effect of Gal-transferase on dC1GalT enzyme (wide arrow). C, lectin domains of various ppGalNAc-T isoforms act as a regulatory scaffold to control glycosyl-T activities involved in O-GalNAc glycan biosynthesis.
The present findings indicate a regulatory role of the β-trefoil fold of ppGalNAc-Ts in enzymatic activity of glycosyl-Ts involved in the O-glycan biosynthesis pathway. The lectin domains T3lec and T4lec reduced catalytic activity of ppGalNAc-T2 and ppGalNAc-T3. Core 1 glycan biosynthesis was modulated by T3lec but unaffected by T2lec or T4lec. This extrinsic function of lectin domains, described here for the first time, helps clarify the mechanism of biosynthesis of complex biopolymers (such as glycans) that is not template-driven.
Experimental Procedures
Expression and Purification of Enzymes and Lectin Domains
cDNAs of soluble enzymes and lectin domains (without the N-terminal cytoplasmic tail and transmembrane anchor) were cloned into baculovirus expression vector pAcGP67 as described previously (32). Constructs were inserted into pAcGP67-His downstream containing a His6 tag, generating pAcGP67-His-protein expression vectors. Sf9 insect cells were grown at 27 °C in Grace's insect medium containing 10% fetal bovine serum (FBS). pAcGP67-His-protein plasmids were co-transfected with BaculoGold DNA (BD Biosciences). Recombinant baculoviruses were obtained after two successive amplifications in Sf9 insect cells grown in serum-containing medium, and virus titers were estimated by titration in 24-well plates and monitoring of enzyme activity. All constructs correspond to human proteins except dC1GalT. dC1GalT has 50% amino acid identity with human C1GalT. The co-expression of human C1GalT and Cosmc (its specific chaperone) was attempted in insect cells; however, the expression of this human enzyme was not possible.
Secreted, soluble recombinant proteins were purified from supernatant of Sf9 insect cell culture medium after centrifugation at 1,500 rpm for 15 min at 4 °C. The supernatant was dialyzed (membrane molecular weight cutoff, <10,000) against PBS (10 mm sodium phosphate (pH 7.4) and 150 mm NaCl) and recentrifuged at 2,500 × g for 30 min at 4 °C. Proteins were purified using HisPurTM Cobalt Resin (Thermo Scientific) and eluted with 150 mm imidazole. Eluted proteins were dialyzed three times against PBS and concentrated by a centrifuge filter device (Millipore; membrane molecular weight cutoff, <10,000). A bicinchoninic acid assay was used to measure total proteins with BSA as the standard (Pierce, Thermo Scientific). Homogeneity of recombinant proteins was confirmed by SDS-PAGE and Coomassie Brilliant Blue staining.
ppGalNAc-T Assays
Mass Spectrometric Assay
A standard enzymatic assay was performed on a 50-μl total reaction mixture containing 25 mm cacodylate (pH 7.4), 10 mm MnCl2, 0.25% Triton X-100, 100 μm UDP-GalNAc, 8 nm ppGalNAc-T, and 20 μm acceptor peptide, incubating for 2 h at 37 °C. Glycosyl-T acceptor peptides assayed were MUC124 (TAPPAHGVTSAPDTRPAPGSTAPP), MUC233 (PTTTPITTTTTVTPTPTPTGTQTPTTTPISTTC), and MUC5B27 (SSTPGTTWILTELTTTATTTESTGSTA) synthesized by Neosystem (Strasbourg) or Cancer Research UK. Samples were applied directly to a probe for MALDI-TOF mass spectrometric analysis (12). The matrix used was 2,5-dihydroxybenzoic acid (25 mg/ml; Aldrich) dissolved in a mixture (2:1) of 0.1% trifluoroacetic acid in 30% aqueous acetonitrile (Rathburn Chemicals, Scotland, UK). Mass spectra were acquired on a Voyager-DE mass spectrometer equipped with delayed extraction (Perceptive Biosystems).
Colorimetric Assay
Polystyrene microtiter plates (Corning, Costar) were coated with 50 μl of peptides in carbonate buffer (pH 9.6) overnight at 4 °C, washed with PBS, and blocked with 0.1% Tween 20 in PBS for 1 h at room temperature. A catalytic reaction mixture containing 25 mm cacodylate (pH 7.4), 10 mm MnCl2, 0.05% Tween 20, 1.6 nm ppGalNAc-T, and 50 μm UDP-GalNAc was incubated for 15 min at 37 °C without or with lectin domains. Additional multiwells coated with several concentrations of MUC1-Tn (12) were used as a glycopeptide standard to determine GalNAc incorporation. Multiwells were washed again with PBS, incubated 60 min at room temperature with horseradish peroxidase (HRP)-Helix pomatia agglutinin lectin diluted 1:1,000 in PBS with 0.05% Tween 20, and washed again with PBS. Color was developed with 0.5 mg/ml o-phenylenediamine and 0.02% H2O2 in sodium citrate (pH 5.0) at room temperature, and the reaction was stopped by addition of 0.5 n H2SO4 (50 μl/well). Absorbance was read by microplate reader at 490 nm (33). Non-linear fitting of curves and enzyme kinetic parameters were obtained using the GraphPad Prism 5 software program.
In Vivo O-Glycan Biosynthesis Assay
HeLa cells and CHO mutant ldlD cells (14), deficient in UDP-Gal/UDP-GalNAc 4-epimerase, were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, 100 μg/ml streptomycin, and 200 units/ml penicillin. Cells were seeded into 35-mm plates and transfected for 4 h at 80% confluence using polyethylenimine (PEI) with pcDNA3.1-human ppGalNAc-T3, -T3D277H (a catalytically inactive mutant because DXH nucleotide-binding motif is critical for enzyme activity (34)), or mock vector. The transfection mixture was removed, and DMEM and 10% FBS were added to cells. CHO ldlD cells were supplemented with 1.5 mm GalNAc.
Western blotting of total lysates from transfected cells was performed by 10% SDS-PAGE. The antibodies used were rabbit anti-human ppGalNAc-T3 (1:500) (HPA007613, Sigma-Aldrich), rabbit anti-human ppGalNAc-T2 (1:500) (HPA011222, Sigma-Aldrich), and mouse anti-α-tubulin (1:2,500) (clone DM1A, T9026, Sigma-Aldrich). Goat anti-rabbit IRDye 800- and goat anti-mouse IRDye 680-conjugated secondary antibodies (LI-COR Biosciences) were used for infrared imaging with an Odyssey Infrared Imaging System (LI-COR Biosciences).
In other experiments, cells were cultured for 48 h; harvested; permeabilized with a Fixation/Permeabilization kit (eBioscience); treated with 0.1 unit/ml Clostridium perfringens neuraminidase (Sigma-Aldrich) for 45 min at 37 °C; stained with rabbit anti-human ppGalNAc-T3 (HPA007613, Sigma-Aldrich) and biotinylated VVL (Vector Laboratories), biotinylated PNA (Sigma-Aldrich), or biotinylated ABL for 1 h; washed; and incubated with anti-rabbit Alexa Fluor 633 (Life Technologies) or streptavidin-fluorescein isothiocyanate (FITC) (DakoCytomation) for 30 min. Permeabilization buffer (eBioscience) with 1% BSA was used for washing and antibody dilution. Cells were incubated with FITC-conjugated streptavidin and anti-rabbit Alexa Fluor 633 alone as control reactions. Flow cytometry was performed on a FACSCanto II instrument (BD Biosciences), and data were analyzed using the FlowJo software program (version 7.6.2).
Biotinylation of Lectin Domains
The pH of 1.5 ml of purified lectin domains (0.2 mg/ml) in PBS was adjusted to 9 using 1 m NaOH. The solution was combined with 60 μl of N-hydroxysuccinimidyl biotin dissolved in N,N-dimethylformamide (10 mg/ml), mixed end-over-end for 2 h at room temperature, and dialyzed three times against PBS (pH 7.4) after which an equal volume of glycerol (99.9%) was added. The biotinylated lectin domains were stored at −20 °C until use (35).
Lectin Binding Assay
Polystyrene microtiter plates (Corning, Costar) were coated with protein (ppGalNAc-T2, T2lec, or dOSM) in carbonate buffer (pH 9.6) overnight at 4 °C, washed with PBS, blocked with 0.1% Tween 20 in PBS for 1 h at room temperature, and washed again with PBS. Various concentrations of biotinylated lectin domains were added to the plates, incubated for 2 h at room temperature, washed with PBS, incubated for 60 min at room temperature with HRP-streptavidin in PBS with 0.05% Tween 20, and washed again with PBS (36). Color development and absorbance reading were as described under “Colorimetric Assay.”
Competitive Binding Assay
This procedure was the same as for the lectin binding assay as described above except that lectin domains (4 μg/ml) were preincubated with various concentrations of ppGalNAc-T2, BSA, or carbohydrate (BzlαGalNAc or MeαGlcNAc) for 1 h at room temperature before being added to wells.
Core 1 β3Gal-T Activity
Gal-T activity was developed using a colorimetric assay similar to that described above for GalNAc-T activity. Microtiter plates were coated with 50 μl of dOSM or OSM in carbonate buffer (pH 9.6) overnight at 4 °C, washed with PBS, and blocked with 0.1% Tween 20 in PBS for 1 h at room temperature. MUC1-Tn (12) was used as a glycopeptide standard to quantify terminal α-GalNAc, the sugar acceptor, on OSM and dOSM glycoproteins. GalNAc concentration was measured with HRP-H. pomatia agglutinin lectin (dilution, 1:100) (Sigma-Aldrich). 1 μm terminal α-GalNAc was measured in 10 μg/ml dOSM and 25 μg/ml OSM. A catalytic reaction mixture containing 50 mm MES (pH 7.0), 20 mm MnCl2, 0.05% Tween 20, 22 nm dC1GalT, and 400 μm UDP-Gal was incubated for 120 min at 37 °C without or with lectin domains of ppGalNAc-T or soluble ppGalNAc-T3; washed with PBS; incubated for 60 min at room temperature with HRP-PNA diluted 1:1,000 in PBS with 0.05% Tween 20; and washed again with PBS. Color development, absorbance reading, and non-linear curve fitting were as described under “Colorimetric Assay.” Different concentrations of asialoglycophorin (Sigma-Aldrich) were used as a core 1 glycan standard to determine galactose incorporation.
Statistical Analysis
Means were compared using an unpaired t test (GraphPad Prism software program). Error bars in figures represent standard deviation of three independent experiments. Statistical significances of differences between means are denoted as *** (p < 0.001), ** (p < 0.01), * (p < 0.05), or ns (not significant) in figures.
Author Contributions
V. L. and F. J. I. designed research. V. L., Y. D., and R. B. C. performed research. M. E. C., E. P. B., and H. C. contributed new reagents/analytic tools. V. L., G. A. N., and F. J. I. analyzed data. V. L. and F. J. I. wrote the paper.
Supplementary Material
Acknowledgments
We are grateful to S. Deza and G. Schachner for cell culture assistance, Dr. Y. Chiba for Core 1 Gal-T plasmid, Dr. H. Monaco for critical reading, and Dr. S. Anderson for English editing of the manuscript.
This work was supported by funding from Argentinean Institutions Consejo Nacional de Investigaciones Científicas y Técnicas; Secretaría de Ciencia y Tecnología (Universidad Nacional de Córdoba); Ministerio de Ciencia, Tecnología e Innovación Productiva (Córdoba); and Agencia Nacional de Promoción Científica y Tecnológica PICT 967 (to F. J. I.). The authors declare that they have no conflicts of interest with the contents of this article.
This work is dedicated to the memory of Enio J. A. Irazoqui.

This article contains supplemental Figs. S-1 and S-2 and Tables S-1–S-7.
- glycosyl-T
- glycosyltransferase
- ppGalNAc-T
- polypeptide GalNAc-transferase
- Tn antigen
- GalNAcα1-O-Ser/Thr
- GlcNAc
- N-acetylglucosamine
- C1GalT
- core 1 β3Gal-T
- lec
- lectin domain
- VVL
- V. villosa lectin
- Bzl
- benzyl
- OSM
- ovine submaxillary mucin
- dOSM
- desialylated ovine submaxillary mucin
- dC1GalT
- D. melanogaster C1GalT
- PNA
- peanut agglutinin
- ABL
- A. bisporus lectin
- Man-6-P
- mannose 6-phosphate
- Gal-T
- galactosyltransferase.
References
- 1. Xu D., and Esko J. D. (2009) A Golgi-on-a-chip for glycan synthesis. Nat. Chem. Biol. 5, 612–613 [DOI] [PubMed] [Google Scholar]
- 2. Young W. W. (2004) Organization of Golgi glycosyltransferases in membranes: complexity via complexes. J. Membr. Biol. 198, 1–13 [DOI] [PubMed] [Google Scholar]
- 3. Nilsson T., Au C. E., and Bergeron J. J. (2009) Sorting out glycosylation enzymes in the Golgi apparatus. FEBS Lett. 583, 3764–3769 [DOI] [PubMed] [Google Scholar]
- 4. Bennett E. P., Mandel U., Clausen H., Gerken T. A., Fritz T. A., and Tabak L. A. (2012) Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology 22, 736–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brockhausen I. (2006) Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 7, 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Varki A., Etzler M. E., Cummings R. D., and Esko J. D. (2009) Discovery and classification of glycan-binding proteins, in Essentials of Glycobiology (Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Bertozzi C. R., Hart G. W., and Etzler M. E., eds) 2nd Ed., pp. 375–386, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York: [PubMed] [Google Scholar]
- 7. Barondes S. H. (1988) Bifunctional properties of lectins: lectins redefined. Trends Biochem. Sci. 13, 480–482 [DOI] [PubMed] [Google Scholar]
- 8. Hazes B., and Read R. J. (1995) A mosquitocidal toxin with a ricin-like cell-binding domain. Nat. Struct. Biol. 2, 358–359 [DOI] [PubMed] [Google Scholar]
- 9. Imberty A., Piller V., Piller F., and Breton C. (1997) Fold recognition and molecular modeling of a lectin-like domain in UDP-GalNac:polypeptide N-acetylgalactosaminyltransferases. Protein Eng. 10, 1353–1356 [DOI] [PubMed] [Google Scholar]
- 10. Fritz T. A., Raman J., and Tabak L. A. (2006) Dynamic association between the catalytic and lectin domains of human UDP-GalNAc:polypeptide α-N-acetylgalactosaminyltransferase-2. J. Biol. Chem. 281, 8613–8619 [DOI] [PubMed] [Google Scholar]
- 11. Hassan H., Reis C. A., Bennett E. P., Mirgorodskaya E., Roepstorff P., Hollingsworth M. A., Burchell J., Taylor-Papadimitriou J., and Clausen H. (2000) The lectin domain of UDP-N-acetyl-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase-T4 directs its glycopeptide specificities. J. Biol. Chem. 275, 38197–38205 [DOI] [PubMed] [Google Scholar]
- 12. Wandall H. H., Irazoqui F., Tarp M. A., Bennett E. P., Mandel U., Takeuchi H., Kato K., Irimura T., Suryanarayanan G., Hollingsworth M. A., and Clausen H. (2007) The lectin domains of polypeptide GalNAc-transferases exhibit carbohydrate-binding specificity for GalNAc: lectin binding to GalNAc-glycopeptide substrates is required for high density GalNAc-O-glycosylation. Glycobiology 17, 374–387 [DOI] [PubMed] [Google Scholar]
- 13. Yoshimura Y., Nudelman A. S., Levery S. B., Wandall H. H., Bennett E. P., Hindsgaul O., Clausen H., and Nishimura S. (2012) Elucidation of the sugar recognition ability of the lectin domain of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase 3 by using unnatural glycopeptide substrates. Glycobiology 22, 429–438 [DOI] [PubMed] [Google Scholar]
- 14. Kingsley D. M., Kozarsky K. F., Hobbie L., and Krieger M. (1986) Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-GalUDP-GalNAc 4-epimerase deficient mutant. Cell 44, 749–759 [DOI] [PubMed] [Google Scholar]
- 15. Springer G. F. (1984) T and Tn, general carcinoma autoantigens. Science 224, 1198–1206 [DOI] [PubMed] [Google Scholar]
- 16. Sakono M., Seko A., Takeda Y., Aikawa J., Hachisu M., Koizumi A., Fujikawa K., and Ito Y. (2014) Glycan specificity of a testis-specific lectin chaperone calmegin and effects of hydrophobic interactions. Biochim. Biophys. Acta 1840, 2904–2913 [DOI] [PubMed] [Google Scholar]
- 17. Satoh T., Suzuki K., Yamaguchi T., and Kato K. (2014) Structural basis for disparate sugar-binding specificities in the homologous cargo receptors ERGIC-53 and VIP36. PLoS One 9, e87963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dahms N. M., Olson L. J., and Kim J.-J. (2008) Strategies for carbohydrate recognition by the mannose 6-phosphate receptors. Glycobiology 18, 664–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Azarkan M., Martinez-Rodriguez S., Buts L., Baeyens-Volant D., and Garcia-Pino A. (2011) The plasticity of the β-trefoil fold constitutes an evolutionary platform for protease inhibition. J. Biol. Chem. 286, 43726–43734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krissinel E., and Henrick K. (2007) Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 [DOI] [PubMed] [Google Scholar]
- 21. Lira-Navarrete E., Iglesias-Fernández J., Zandberg W. F., Compañón I., Kong Y., Corzana F., Pinto B. M., Clausen H., Peregrina J. M., Vocadlo D. J., Rovira C., and Hurtado-Guerrero R. (2014) Substrate-guided front-face reaction revealed by combined structural snapshots and metadynamics for the polypeptide N-acetylgalactosaminyltransferase-2. Angew. Chem. Int. Ed. Engl. 53, 8206–8210 [DOI] [PubMed] [Google Scholar]
- 22. Haudek K. C., Patterson R. J., and Wang J. L. (2010) SR proteins and galectins: what's in a name? Glycobiology 20, 1199–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aspberg A., Miura R., Bourdoulous S., Shimonaka M., Heinegârd D., Schachner M., Ruoslahti E., and Yamaguchi Y. (1997) The C-type lectin domains of lecticans, a family of aggregating chondroitin sulfate proteoglycans, bind tenascin-R by protein-protein interactions independent of carbohydrate moiety. Proc. Natl. Acad. Sci. U.S.A. 94, 10116–10121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hassinen A., Pujol F. M., Kokkonen N., Pieters C., Kihlström M., Korhonen K., and Kellokumpu S. (2011) Functional organization of Golgi N- and O-glycosylation pathways involves pH-dependent complex formation that is impaired in cancer cells. J. Biol. Chem. 286, 38329–38340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kellokumpu S., Hassinen A., and Glumoff T. (2016) Glycosyltransferase complexes in eukaryotes: long-known, prevalent but still unrecognized. Cell. Mol. Life Sci. 73, 305–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Žurga S., Pohleven J., Ko SJ., and Sabotič J. (2015) β-Trefoil structure enables interactions between lectins and protease inhibitors that regulate their biological functions. J. Biochem. 158, 83–90 [DOI] [PubMed] [Google Scholar]
- 27. Murzin A. G., Lesk A. M., and Chothia C. (1992) β-Trefoil fold: patterns of structure and sequence in the Kunitz inhibitors interleukins-1β and 1α and fibroblast growth factors. J. Mol. Biol. 223, 531–543 [DOI] [PubMed] [Google Scholar]
- 28. Wilson J. J., and Kovall R. A. (2006) Crystal structure of the CSL-Notch-Mastermind ternary complex bound to DNA. Cell 124, 985–996 [DOI] [PubMed] [Google Scholar]
- 29. Fujimoto Z. (2013) Structure and function of carbohydrate-binding module families 13 and 42 of glycoside hydrolases, comprising a β-trefoil fold. Biosci. Biotechnol. Biochem. 77, 1363–1371 [DOI] [PubMed] [Google Scholar]
- 30. Boraston A. B., Tomme P., Amandoron E. A., and Kilburn D. G. (2000) A novel mechanism of xylan binding by a lectin-like module from Streptomyces lividans xylanase 10A. Biochem. J. 350, 933–941 [PMC free article] [PubMed] [Google Scholar]
- 31. Röttger S., White J., Wandall H. H., Olivo J. C., Stark A., Bennett E. P., Whitehouse C., Berger E. G., Clausen H., and Nilsson T. (1998) Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus. J. Cell Sci. 111, 45–60 [DOI] [PubMed] [Google Scholar]
- 32. Bennett E. P., Hassan H., and Clausen H. (1996) cDNA cloning and expression of a novel human UDP-N-acetyl-α-d-galactosamine polypeptide N-acetylgalactosaminyltransferase, GalNAc-T3. J. Biol. Chem. 271, 17006–17012 [DOI] [PubMed] [Google Scholar]
- 33. Zlocowski N., Lorenz V., Bennett E. P., Clausen H., Nores G. A., and Irazoqui F. J. (2013) An acetylation site in lectin domain modulates the biological activity of polypeptide GalNAc-transferase-2. Biol. Chem. 394, 69–77 [DOI] [PubMed] [Google Scholar]
- 34. Hagen F. K., Hazes B., Raffo R., deSa D., and Tabak L. A. (1999) Structure-function analysis of the UDP-N-acetyl-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase. Essential residues lie in a predicted active site cleft resembling a lactose repressor fold. J. Biol. Chem. 274, 6797–6803 [DOI] [PubMed] [Google Scholar]
- 35. Jørgensen C. S., Heegaard N. H., Holm A., Højrup P., and Houen G. (2000) Polypeptide binding properties of the chaperone calreticulin. Eur. J. Biochem. 267, 2945–2954 [DOI] [PubMed] [Google Scholar]
- 36. Irazoqui F. J., Vozari-Hampe M. M., Lardone R. D., Villarreal M. A., Sendra V. G., Montich G. G., Trindade V. M., Clausen H., and Nores G. A. (2005) Fine carbohydrate recognition of Euphorbia milii lectin. Biochem. Biophys. Res. Commun. 336, 14–21 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.