Abstract
Most people are exposed to at least one traumatic event during the course of their lives, but large numbers of people do not develop posttraumatic stress disorders. Although previous studies have shown that repeated and chronic stress change the brain’s structure and function, few studies have focused on the long-term effects of acute stressful exposure in a nonclinical sample, especially the morphology and functional connectivity changes in brain regions implicated in emotional reactivity and emotion regulation. Forty-one months after the 5/12 Wenchuan earthquake, we investigated the effects of trauma exposure on the structure and functional connectivity of the brains of trauma-exposed healthy individuals compared with healthy controls matched for age, sex, and education. We then used machine-learning algorithms with the brain structural features to distinguish between the two groups at an individual level. In the trauma-exposed healthy individuals, our results showed greater gray matter density in prefrontal-limbic brain systems, including the dorsal anterior cingulate cortex, medial prefrontal cortex, amygdala and hippocampus, than in the controls. Further analysis showed stronger amygdala-hippocampus functional connectivity in the trauma-exposed healthy compared to the controls. Our findings revealed that survival of traumatic experiences, without developing PTSD, was associated with greater gray matter density in the prefrontal-limbic systems related to emotional regulation.
Introduction
Trauma exposure is common and could increase lifetime vulnerability to mental health problems when individuals encounter stress or adversity, especially the most traumatic events, such as a massive earthquake, terrorism or war [1–3]. Although only a minority of humans develop posttraumatic stress disorders (PTSD) [4, 5], traumatic experiences predict an increased risk for psychopathology later in life in the general population [6–9]. Although previous studies have indicated that trauma exposure could impact brain function and structure in nonclinical individuals [10–12], few studies have focused on the long-term effects of trauma exposure in a nonclinical sample. Moreover, it is not yet clear how the long-term effects of trauma exposure cause the change of brain structure and function associated with emotion and memory processing in trauma-exposed individuals. Thus, the changes in brain structure and functions might help us understand the neural mechanisms underlying both vulnerable and resilient individuals in this nonclinical sample.
Extensive neuroimaging studies of patients with mood disorders have shown significant alternations in brain structure and function [13–16], which have also been found in healthy adults after stressful life events [10, 17, 18]. Other researches have indicated that the amygdala, hippocampus and ventral medial prefrontal cortex (vmPFC) play an important role in emotion regulation, memory and coping with stress responsively [19–24]. Animal studies also indicate that these brain regions are associated with emotion processing and regulation of the hypothalamic pituitary adrenal axis under stressful situations [25–28]. The amygdala is hyper-responsive in PTSD, which is associated with exaggerated fear responses and emotional arousal [29]. Exposure to stress could induce increased activity within the amygdala related to traumatic events [30, 31], and a hypo-responsive vmPFC and hyper-responsive amygdala are exhibited in anxiety-related disorders [32, 33]. Previous animal and human studies have shown that exposure to stress induces neural structural and functional abnormalities of the hippocampus associated with dysfunctional episodic and autobiographical memories [34–40]. Moreover, recent studies with nonclinical samples have also found decreased volume in the frontal-limbic regions, such as the hippocampus, anterior cingulate and medial prefrontal cortex, which were related to serious chronic life stress [41], closer proximity to the disaster on 9/11 [10] and more cumulative adverse events [17]. Additionally, some studies have indicated that the experience of acute events has a short-term effect on structure and function [11, 42] in the prefrontal-limbic, parietal and striatal brain systems. As a consequence, it is important to explore the long-term effects of experiences of acute events on brain structure and function and to better understand the neural circuits underlying resilience in these trauma-exposed individuals with no psychiatric disorders.
Rather than mass univariate analyses, multivariate-pattern-analysis (MVPA) is sensitive to spatially distributed effects and can be used to separate patients from the healthy controls using structural MRI (magnetic resonance imaging) data or the functional MRI data [43, 44]. Previous mass univariate analyses studies have reported differences between patients and controls at the group level. Unlike univariate analyses, MVPA neuroimaging studies generally trend to make inferences at the level of the individual rather than the group [45]. Apparently, mass-univariate and multivariate methods are complementary approaches. Thus, we combined the two types of analyses to reveal the neuroanatomical correlates of trauma-exposed individuals with no psychiatric disorders.
In the present study, more than 3 years after the 5/12 Wenchuan earthquakes, we used voxel-based morphometry (VBM) and resting state fMRI (rs-fMRI) to investigate the changes in brain structure and functional connectivity between the stress-related brain areas in trauma survivors compared to healthy controls.
Methods
Subjects
A total of forty-two healthy undergraduate students participated in this study about forty-one months after the 5/12 Wenchuan earthquake. Twenty-one individuals (14 female, ages 20.5 ± 1.3) from the Wenchuan earthquake disaster area were identified by the post-traumatic stress disorder self-rating scale (PTSD-SS) (with score < 60) as resilient trauma survivors [46]. The PTSD-SS was constructed based on the definition and diagnostic criteria of PTSD described in the Diagnostic and Statistical Manual of Mental Disorders: Fourth Edition (DSM-IV). And participants who have got the total score below 60 in PTSD-SS are thought of no serious PTSD symptoms [47]. PTSD-SS serve as a screening tool for PTSD. Subjects were excluded if they (1) had clinically significant PTSD symptoms (PTSD-SS total score over 60); (2) had undergone any form of psychotherapy or taken psychotropic medications after the Wenchuan earthquake; (3) or had only experienced psychotic illness through the files of mental health education. Moreover, twenty-one healthy controls (12 female, ages 21 ± 1.1) who were not exposed to the earthquake were recruited from the local campus by advertisements. The two groups were well matched for age (p = 0.24), sex (p = 0.58) and length of education (p = 0.62) (two sample t-test using SPSS, Inc., Chicago, IL, USA). Two participants were tested but excluded from the VBM analysis due to problems in the image registration.
The Impact of Event Scale Revised (IES-R) is a self-administered, 22-item questionnaire as indicators of PTSD. It should be administered due to trauma and have no other medical basis, and perhaps it is the most widely used self-administered assessment in the field of traumatic stress. The IES-R was administered after scanning for all subjects to record their current subjective distress. They were told: “Please read each item, and then indicate how distressing each difficulty has been for you during the past 7 days with respect to wenchuan earthquake [48].” The IES-R is not a diagnostic or screening tool for PTSD. We aimed to observe the degree of distress especially in resilient trauma survivors that they respond to the traumatic event. There are no specific cut-off scores for the IES-R and the higher scores are representative of greater distress.
The Spielberger State-Trait Anxiety Inventory (STAI) [49] was used to measure the difference in the trait component and current state of stress response between the two groups, a long time after the earthquake. All subjects had normal or corrected-to-normal vision and none had a history of neurological or psychiatric disease. The Southwest University Brain Imaging Center Institutional Review Board approved this study. Written informed consent was obtained from all subjects.
Functional magnetic resonance image acquisition
All MRI data were performed on a 3T scanner (Siemens Trio, Erlangen, Germany) at the Brain Imaging Research Central at Southwest University. First, resting-state functional MR images were obtained using an Echo Planar Imaging (EPI) sequence with the following parameters: time repetition [TR] = 2000 ms; time echo [TE] = 40 ms; flip angle [FA] = 90, slices = 28, matrix = 64×64; field of view [FOV] = 192 mm; acquisition voxel size = 3.4 × 3.4 × 4 mm. A total of 242 volumes were collected for each subject. During fMRI scanning, subjects were instructed to close their eyes, not to move, think about anything particular, or fall asleep [50, 51]). Each subject reported not having fallen asleep using a simple questionnaire after the scan. Second, a high-resolution 3D Magnetization Prepared Rapid Gradient Echo structural image was acquired with the following parameters (TR/TE/FA = 1900 ms/2.2 ms/9°, resolution = 256×256 matrix, slices = 176, thickness = 1.0 mm).
Functional magnetic resonance image processing
The resting-state data preprocessing was performed using a Matlab (Math works Inc., Natick, MA) toolbox Data Processing Assistant for Resting-State fMRI (DPARSF, http://resting-fmri.sourceforge.net/). This included the following steps: the first 10 functional images were discarded due to signal instability and the subject’s adaptation to the scanning noise. The slices of the remaining 232 volumes for each subject were corrected for different collection times of signals by slice time correction. Subsequently, the functional image time series were motion-corrected by realigning all images to the middle image volume. The individual structural image of each subject was co-registered to the mean functional image generated after motion correction. Third, the functional images were spatially normalized into the Montreal Neurological Institute (MNI) space using the transformation information generated by segmentation and resampled into 3 mm cubic voxels. The normalized images were smoothed with an isotropic Gaussian kernel (FWHM = 4 mm).
Structural data preprocessing
The structural MRI images were processed using SPM8 software (Welcome Department of Cognitive Neurology, London, UK; www.fil.ion.ucl.ac/spm). For better image registration, the reorientation of the images was manually set to the anterior commissure. The structural image was then segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CF). Subsequently, we performed diffeomorphic anatomical registration through exponentiated lie (DARTEL) algebra in SPM8 for registration, normalization and modulation. Then, the registered images were transformed to Montreal Neurological Institute (MNI) space. Finally, the normalized, non-modulated images (gray matter and white matter density images) were smoothed with a 10-mm full-width at half-maximum Gaussian kernel to increase the signal to noise ratio.
Traditional univariate VBM analysis
The general linear model (GLM) approach was used to test the gray matter density (GMD) differences between the trauma-exposed group and the healthy controls. To control for the effects of age, gender, and whole brain gray matter density, these variables were added as additional covariates. We also applied absolute threshold masking (all voxels with GM values of < 0.2 were excluded) to avoid edge effects between gray and white matter. To explore whether there ware structural differences in the amygdala, parahippocampal and dACC, which have been considered to be involved in emotion regulation and stress responsively [19–24], small volume corrections (SVC) were applied using masks created using the WFU PickAtlas toobox [52–54]. Finally, to further investigate the predictive ability of regional gray matter density, the significant differences map (Height threshold: p < 0.005; Extent cluster threshold: p < 0.05, with a whole brain FWE-corrected) and separate clusters obtained from the univariate analysis were used as a region of interest mask, and we used Support Vector Machine (SVM) [55] to discriminative between trauma-exposed group and healthy controls.
Multivariate pattern analysis based on VBM
The PRONTO toolbox based on pattern classification techniques was used for the analysis of neuroimaging data [56, 57]. The dataset is usually divided into two sets: training and testing. During the training phase, an algorithm learns some mapping between patterns and the labels [56]; during the test phase, the learned function is used to predict the group membership of the test individuals. To make the test data set independent from the training set, a mean centering and leave one out cross-validation (LOOCV) procedure [58] was performed for each subject. Because the variance of the importance weights can be large or even infinite, leave-one-out may lead to an unreliable estimate [59–61]. However, it provides an almost unbiased estimate of the generalization ability of a classifier [61]. Most of statistical theory and machine learning theory are based on the assumption that the data is independently and identically distributed. However, in neuroimaging research, this assumption is often not met [62]. Therefore, classical estimates of confidence intervals may not always be appropriate. Permutation testing is a non-parametric procedure that allows to obtain meaningful confidence intervals and p-values in this case [62]. In order to obtain more meaningful confidence intervals and p-values of each cluster, a random permutation test (10,000 times) was used to examine the statistical significance of the classification models [63, 64].
Functional connectivity: Region of interest selection and data analysis
Functional connectivity was performed by applying a seed region correlation approach [65] using the Resting-State fMRI Data Analysis Toolkit (REST) software package [66]. Previous studies have shown that acute stress was closely interrelated with structural or functional alternation of the amygdale, medial prefrontal cortex and hippocampus [19–24]. Therefore, both sides of the three brain areas were chosen as the regions of interest (ROI) using a previously validated, anatomically labeled (AAL) template image [53]. Subsequently, the band pass filtering (0.01–0.08 Hz) and linear detrending were performed. The time courses for the various covariates (white matter, cerebrospinal fluid, and 24 motion parameters for head movement) were extracted and regressed out to cancel out the potential impact of physiological artifacts. Here, the Friston 24-parameter model, which includes 6 current head motion parameters, 6 head motion parameters from the previous imaging volume, and the 12 corresponding squared items, was utilized to regress out head motion effects from the realigned data based on previous reports demonstrating benefits of higher-order models in reducing head micromovements [67, 68]. Then, we implemented a “scrubbing” procedure to censor high motion volumes [69, 70]. The time course for each ROI was extracted by averaging the time series of all voxels within each ROI. Finally, the correlation coefficients were transformed to z-values using Fisher's r-to-z transformation to improve the normality of the partial correlation coefficients [71, 72]and to enable group comparisons. In the group comparison analysis, we tested for strength connection (z-transformed r value) between the trauma survivors and the healthy controls. To investigated whether the functional connectivity (amygdala-medial prefrontal cortex, amygdala-hippocampus and medial prefrontal cortex-hippocampus) would be affected by the IES-R scores between the earthquake exposed group and the control group, we calculated the Pearson correlation coefficients between the z-scores of each ROI-to-ROI and the IES-R scores with controlling the sex and age as regressors of no interest.
Results
5/12 earthquake-exposed versus comparison group
The levels of anxiety were within the normal range across all groups, and the STAI scores were not significantly different between the trauma survivors and healthy controls (Table 1). Age, sex and length of education were not significantly different between the two groups. The PTSD-SS scores of the trauma survivors were significantly higher than the healthy controls’ (t = 4.3; p< 0.001). The IES-R scores for the healthy control group fell well within the normal range, and were significantly lower than trauma survivors’ (t = -3.5; p< 0.001).
Table 1. Demographic Characteristics of trauma Survivors and healthy controls.
| Characteristics | Survivors ± SD (n = 21) | Controls ± SD (n = 21) | P |
|---|---|---|---|
| Female to male, no | 14:7 | 12:9 | p = 0.58 |
| Mean age, y | 20.5 ± 1.3 | 20.9 ± 1.1 | p = 0.24 |
| Years of education | 13.7 ± 1.4 | 13.9 ± 1.1 | p = 0.62 |
| PTSD-SS Scores | 46.5 ± 11.3 | 32.9 ± 7.7 | p < 0.001** |
| STAI(S-AI) Scores | 39.7 ± 6.8 | 43.7 ± 9.9 | p = 0.14 |
| STAI(T-AI) Scores | 41.7 ± 7.5 | 46 ± 11.2 | p = 0.15 |
| IES-R Scores | 24.3 ± 15.1 | 11.5±5.8 | p < 0.001** |
The VBM results of univariate analysis and MVPA
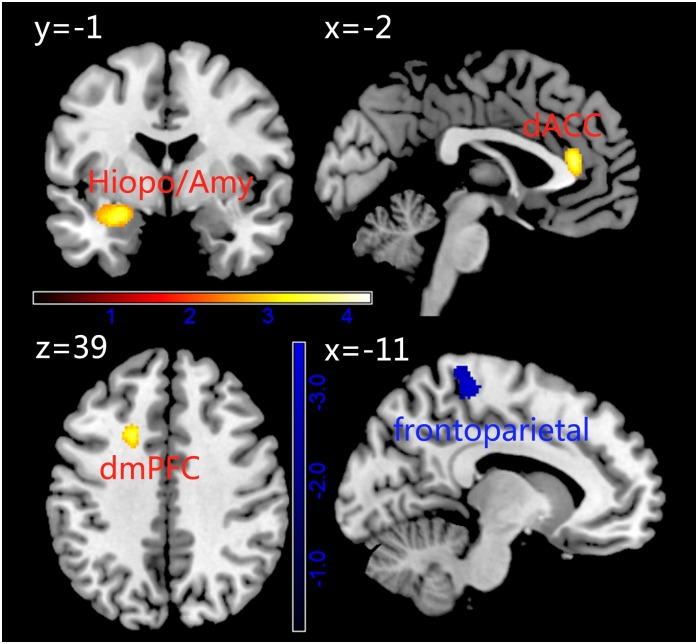
Compared with the healthy controls, the trauma survivors showed that greater GMD in the dorsal medial frontal cortex (dmPFC), dorsal anterior cingulate cortex (dACC) extended to the rostral anterior cingulated cortex (rACC) and bilaterally in the hippocampus/amygdala and lower GMD of the frontoparietal association cortex (Fig 1 and Table 2).
Fig 1. Differences in gray matter density (GMD) between Trauma Survivors and Healthy Controls using univariate analysis based on voxel-based morphometry.
The hot in the map represent represents the results of GMD Trauma > Control. While, the blue represents the result Control > Trauma.
Table 2. The differences in gray matter density between trauma survivors and controls.
| Brain structure | cluster | t | MNI | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Trauma survivors > Controls | |||||
| L dMPFC* | 528 | 3.69 | -20 | 14 | 38 |
| dACC*** | 1005 | 4.29 | 19 | 26 | 17 |
| Parahippocampal/Amygdala** | 498 | 3.56 | -27 | -3 | -15 |
| Trauma survivors < Controls | |||||
| Frontoparietal cortex * | 612 | 3.88 | -23 | -38 | 75 |
* Height threshold p< 0.005, uncorrected.
** p < 0.01, small volume corrected.
*** p < 0.005, small volume corrected.
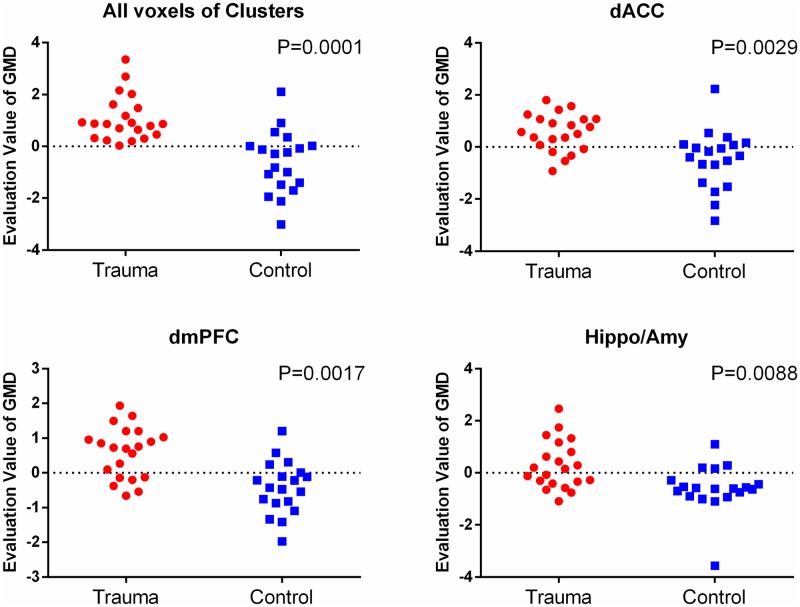
We also used MVPA to further investigate the predictive ability of regional gray matter density at the individual level, based on the GMD differences map (GMD map) and regions of interest obtained from the univariate analysis. The applications of SVM to dmPFC clusters classified the trauma-exposed and healthy control groups with a sensitivity of 71.4% and 73.7%, respectively, leading to an overall accuracy of 72.5%; dACC (76.2%, 68.4%, 72.5%); hippocampus/amygdala (52.4%, 78.9%, 65%). When all these clusters were considered simultaneously, it classified the trauma-exposed and healthy control groups with a sensitivity of 100% and 68.4%, respectively, leading to an overall accuracy of 85%. Permutation testing indicated that these predictive accuracies were all statistically significant (see Fig 2).
Fig 2. The classfication plot of individual diagnosis results.
A support vector machine was used to construct multivariate models and to classify participants as trauma survivors or controls. Evaluation value of GMD: the output value of the machine’s decision function for each test sample. The decision threshold is displayed by a horizontal line at the centre of the plot. Statistical significance P-values were generated by using permutation test (n = 10,000).
The Strength of connections between ROIs and impact of event scale revised
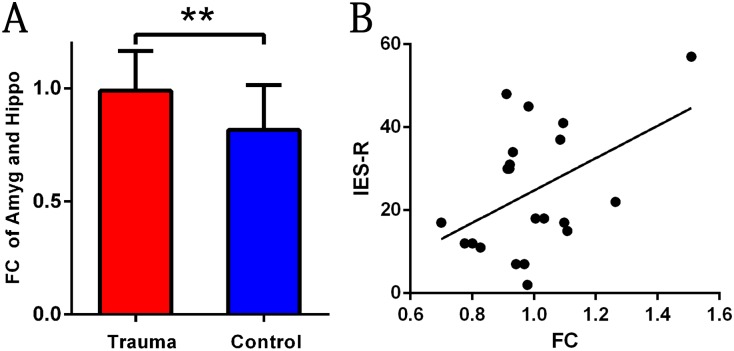
A group comparison analysis revealed that there were no significant differences in the functional connection of the amygdala with the mPFC between the two groups (t = -1.56; p = 0.126). However, an increased strength of the connection of the amygdala with the hippocampus was found in the trauma survivors compared to the healthy controls (t = 2.86; p = 0.007, Fig 3A). Additionally, there was a significant positive correlation between the strength of the connection and IES-R scores in the trauma survivors (r = 0.45; p = 0.04, Fig 3B).
Fig 3. Differences in Functional Connectivity Maps for Trauma Survivors compared with Healthy Controls.
(A) Trauma survivors showed significant stronger functional connectivity of the amygdala-hippocampus. (B) The correlation between the strength of the connection and IES-R scores in the trauma survivors.
Discussion
In the current study, we investigated the long-term effect of the traumatic event (the Wenchuan Earthquake) on human brain structure and functional connectivity. We found that resilient trauma-exposed survivors showed greater gray matter density in prefrontal-limbic brain systems and lower gray matter density in the frontoparietal association cortex than controls. Resting-state functional connectivtiy analysis found that resilient trauma-exposed survivors showed strengthened functional connectivity between the amygdala and hippocampus compared to controls.
The prefrontal cortex plays an important role in top-down emotion regulation and it can regulate the neural activity of the limbic systems, especially the amygdala and hippocampus [20, 21, 73–75]. The previous review which focused on the neuroimaging findings in PTSD patients has suggested that volume reductions in the prefrontal cortex might be related to a reduced capacity to inhibit fear and modulate affective responses [76]. Similar studies have revealed that resilient trauma survivors showed an increased gray matter volume in the right middle prefrontal gyrus compared with PTSD patients [77] and that healthy subjects who experienced the traumatic event showed increased resting-state activity in the left lateral prefrontal cortex compared to healthy controls [11]. Moreover, the structural abnormalities in the dACC and amygdala may reflect predisposed neural abnormalities that increased the likelihood of developing PTSD following exposure to trauma [78]. Sekiguchi et al. revealed that subjects with lower gray matter volume in the ACC before the earthquake were likely to have PTSD symptoms [79]. Thus, combine our results, greater gray matter density in the prefrontal-limbic systems might be associated with the better ability to control the sustained hyper-activation in limbic systems due to the traumatic experience [42].
It has recently been highlighted that periods of traumatic stress might alter the structural and activity in the human parietal lobule [77, 80–83]. An MRI study found that resilient trauma survivors showed less gray matter volume in the parietal cortex compared to healthy controls [81]. Interestingly, there was a pattern of increases cerebral blood flow in PTSD patients and decreases in resilient trauma survivors in the parietal cortex with exposure to traumatic pictures and sounds [82]. These findings suggested that the frontoparietal association cortex is sensitive to traumatic stress. The frontoparietal association cortex was implicated in the visual imagery representing, an important component of the visuospatial processing of preparation for responding to a physical threat, as well as a critical component of flashbacks and similar PTSD symptoms. Therefore, combine our results with previous studies, the density reduction in the frontoparietal association cortex might be related to reduced flashbacks and non-excessive reaction to a threat.
Furthermore, trauma not only induces an anxious state and emotional arousal but can also impair memory through the amygdala’s interactions with other brain regions [27, 84, 85]. The hippocampus is widely implicated in memory encoding and maintenance, forming and storing memories associated with emotional events [86, 87] and autobiographical memory [88, 89]. Many studies have shown abnormal hippocampus activity [19, 90] and decreased hippocampal volume [37, 91] in stress-related pathologies, such as major depressive disorder and PTSD. Resilient early life stress subjects yet showed increased degree of the hippocampus in graph network relative to healthy controls [92]. In addition, decreased hippocampal volume is also associated with serious and long-lasting traumatic stress [93]. In this study, we have observed increased functional connectivity between the amygdala and hippocampus in the resilient trauma survivors. Previous studies suggested that increased amygdala-hippocampus correlations during recollection of negative autobiographical memories [94, 95] or emotional events [94, 96]. For example, Smith et al. (2004) showed that retrieval of emotionally valenced contextual information is associated with increased connectivity between the hippocampus and amygdala [97]. Greenberg et al. observed co-activation of the amygdala and hippocampus during autobiographical memory retrieval task (recollection of episodes from the personal past) [98]. This hippocampal-amygdala effects in autobiographical memory also were found in other studies [99, 100]. Admon et al. (2009) showed that amygdala activity could predict the subsequent reactivity of the hippocampus in resilient trauma-exposed individuals [38]. Several studies indicated that the connectivity between the amygdala and hippocampus is associated with the modulation of stress effects on memory consolidation, memory retrieval [24, 101–103]. Thus, increased connectivity between the amygdala and hippocampus may store and maintain negative autobiographical memory. Moreover, resilient trauma survivors can retrieve autobiographical memories more specifically than PTSD patients [104]. The connectivity strengths might imply one potential mechanism by which increased functional connectivity between the amygdala and hippocampus indexes lower risk for PTSD.
However, we observed a significant positive correlation between the connectivity strength and distress. It is not consistent with the notion that greater connectivity serves as a protective role in developing PTSD. Thus, this correlation should generally be interpreted with caution. To begin with, the range of the symptom severity might not large enough to explore the brain-behavioral relationship. Perhaps there would be an inverse association between symptoms and hippocampus-amygdala connectivity in subjects with high-level symptoms. Besides, the trauma-exposed condition is characterized by other three typical symptom clusters: re-experiencing, avoidance and hyperarousal symptoms [105, 106]. It is possible to observe the significant negative correlations between other specific symptom clusters and the connectivity strength. Last but not least, the correlation between distress and connectivity might not be explained by linear regression. Perhaps, performing the nonlinear correlation analysis would be more acceptable. These interpretations remain largely speculative and need further investigation.
Our study revealed that resilient trauma survivors showed greater gray matter density in the prefrontal-limbic systems that were implicated in emotional regulation. The emotional regulation ability plays a critical role in preventing the onset of PTSD in those trauma-exposed nonclinical adults. However, there are two possible explanations for the current findings. One possibility is that these structural differences might be a pre-existing factor and those participants did not develop PTSD due to these biological protective factor. Nevertheless, we cannot rule out the possibility that the structural differences are the brain “scar” after the traumatic event. Our study is quite preliminary in nature and only longitudinal studies that examine the changes in behaviors and neuroimaging measures in individuals before and after the traumatic stress could fully rule out one of the possibilities.
Of note, there are other limitations in our study. First at all, we did not include the participants who experienced the Wenchuan earthquake and then developed the PTSD in our study. This limitation restricted our ability to differentiate the resilience and vulnerability factor at the neural level. We expect to recruit an additional PTSD patients group and carry out the longitudinal project in the future. Besides, the sample size of our study was modest and the range of symptoms severity is limited. This point largely affects the reliability of our brain-behavior correlational analysis. Finally, the assessments of symptom in this study are not comprehensive enough. The assessments should be multiplex and include different symptoms clusters, such as re-experiencing, avoidance and hyperarousal symptoms along with a high rate of dissociative symptoms [107, 108].
Our results are largely consistent with findings from studies on other stressors (and stress in general) in the literature [11, 19–24, 77]. Although the sample size of the study was modest, we have provided a reliable and valid stress model especially for gray matter density effects through MVPA analysis. Previous studies mainly focused on the short-term effect of trauma exposure or patients with PTSD, while our study investigated the long-term effects of trauma exposure in a nonclinical sample. Furthermore, our findings revealed the structural and functional differences in brain regions that are usually implicated in emotional regulation. In conclusion, our study revealed that survival of traumatic experiences, without developing PTSD, was associated with greater gray matter density in the prefrontal-limbic systems related to emotional regulation.
Supporting Information
(XLSX)
Acknowledgments
Additional members of the Qiu Lab who provided valuable assistance include: Meng Zhang, who assisted with data acquisition and Xin Wu, who assisted with data analysis. We also extend our gratitude to the subjects, especially the Wenchuan earthquake survivors.
Data Availability
Data are available within the paper and its Supporting Information files and at https://dx.doi.org/10.6084/m9.figshare.4299389.
Funding Statement
This research was supported by the National Natural Science Foundation of China (31271087, Jiang Qiu) and the National High-level personnel of special support program (31571137, Jiang Qiu).
References
- 1.Steel Z, Silove D, Phan T, Bauman A. Long-term effect of psychological trauma on the mental health of Vietnamese refugees resettled in Australia: a population-based study. Lancet. 2002;360(9339):1056 10.1016/S0140-6736(02)11142-1 [DOI] [PubMed] [Google Scholar]
- 2.Turner RJ, Lloyd DA. Lifetime traumas and mental health: The significance of cumulative adversity. Journal of Health and Social Behavior. 1995:360–76. [PubMed] [Google Scholar]
- 3.Goenjian AK, Steinberg AM, Najarian LM, Fairbanks LA, Tashjian M, Pynoos RS. Prospective study of posttraumatic stress, anxiety, and depressive reactions after earthquake and political violence. American Journal of Psychiatry. 2000;157(6):911–895. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of general psychiatry. 1995;52(12):1048 [DOI] [PubMed] [Google Scholar]
- 5.Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma, Violence, & Abuse. 2009;10(3):198–210. [DOI] [PubMed] [Google Scholar]
- 6.Harley M, Kelleher I, Clarke M, Lynch F, Arseneault L, Connor D, et al. Cannabis use and childhood trauma interact additively to increase the risk of psychotic symptoms in adolescence. Psychological medicine. 2010;40(10):1627–34. 10.1017/S0033291709991966 [DOI] [PubMed] [Google Scholar]
- 7.Heim C, Wagner D, Maloney E, Papanicolaou DA, Solomon L, Jones JF, et al. Early adverse experience and risk for chronic fatigue syndrome: results from a population-based study. Archives of general psychiatry. 2006;63(11):1258–66. 10.1001/archpsyc.63.11.1258 [DOI] [PubMed] [Google Scholar]
- 8.Pfeifer S, van Os J, Hanssen M, Delespaul P, Krabbendam L. Subjective experience of cognitive failures as possible risk factor for negative symptoms of psychosis in the general population. Schizophrenia bulletin. 2009;35(4):766–74. 10.1093/schbul/sbn004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bechdolf A, Thompson A, Nelson B, Cotton S, Simmons M, Amminger G, et al. Experience of trauma and conversion to psychosis in an ultra‐high‐risk (prodromal) group. Acta Psychiatrica Scandinavica. 2010;121(5):377–84. 10.1111/j.1600-0447.2010.01542.x [DOI] [PubMed] [Google Scholar]
- 10.Ganzel BL, Kim P, Glover GH, Temple E. Resilience after 9/11: Multimodal neuroimaging evidence for stress-related change in the healthy adult brain. Neuroimage. 2008;40(2):788–95. 10.1016/j.neuroimage.2007.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lui S, Huang X, Chen L, Tang H, Zhang T, Li X, et al. High-field MRI reveals an acute impact on brain function in survivors of the magnitude 8.0 earthquake in China. Proceedings of the National Academy of Sciences. 2009;106(36):15412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold A, Shin L, Orr S, Carson M, Rauch S, Macklin M, et al. Decreased regional cerebral blood flow in medial prefrontal cortex during trauma-unrelated stressful imagery in Vietnam veterans with post-traumatic stress disorder. Psychological medicine. 2011;41(12):2563–72. 10.1017/S0033291711000730 [DOI] [PubMed] [Google Scholar]
- 13.McEwen BS. Effects of adverse experiences for brain structure and function. Biological psychiatry. 2000;48(8):721–31. [DOI] [PubMed] [Google Scholar]
- 14.Frodl T, Reinhold E, Koutsouleris N, Donohoe G, Bondy B, Reiser M, et al. Childhood stress, serotonin transporter gene and brain structures in major depression. Neuropsychopharmacology. 2010;35(6):1383–90. 10.1038/npp.2010.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papagni SA, Benetti S, Arulanantham S, McCrory E, McGuire P, Mechelli A. Effects of stressful life events on human brain structure: A longitudinal voxel-based morphometry study. Stress. 2011;14(02):227–32. [DOI] [PubMed] [Google Scholar]
- 16.Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10(6):410–22. 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative Adversity and Smaller Gray Matter Volume in Medial Prefrontal, Anterior Cingulate, and Insula Regions. Biological psychiatry. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veer IM, Oei NYL, Spinhoven P, van Buchem MA, Elzinga BM, Rombouts SARB. Beyond acute social stress: increased functional connectivity between amygdala and cortical midline structures. Neuroimage. 2011;57(4):1534–41. 10.1016/j.neuroimage.2011.05.074 [DOI] [PubMed] [Google Scholar]
- 19.Shin LM, Rauch SL, PITMAN RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences. 2006;1071(1):67–79. [DOI] [PubMed] [Google Scholar]
- 20.Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioural brain research. 2011;223(2):403–10. 10.1016/j.bbr.2011.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim MJ, Whalen PJ. The structural integrity of an amygdala—prefrontal pathway predicts trait anxiety. The Journal of Neuroscience. 2009;29(37):11614–8. 10.1523/JNEUROSCI.2335-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ressler KJ. Amygdala activity, fear, and anxiety: modulation by stress. Biological psychiatry. 2010;67(12):1117 10.1016/j.biopsych.2010.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nutt DJ, Malizia AL. Structural and functional brain changes in posttraumatic stress disorder. Journal of Clinical Psychiatry; Journal of Clinical Psychiatry. 2004. [PubMed] [Google Scholar]
- 24.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature Reviews Neuroscience. 2009;10(6):423–33. 10.1038/nrn2651 [DOI] [PubMed] [Google Scholar]
- 25.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29(8):1201–13. 10.1016/j.pnpbp.2005.08.006 [DOI] [PubMed] [Google Scholar]
- 26.Urry HL, Van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience. 2006;26(16):4415–25. 10.1523/JNEUROSCI.3215-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7(1):54–64. 10.1038/nrn1825 [DOI] [PubMed] [Google Scholar]
- 28.Cunningham-Bussel AC, Root JC, Butler T, Tuescher O, Pan H, Epstein J, et al. Diurnal cortisol amplitude and fronto-limbic activity in response to stressful stimuli. Psychoneuroendocrinology. 2009;34(5):694–704. 10.1016/j.psyneuen.2008.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNally RJ. Cognitive abnormalities in post-traumatic stress disorder. Trends in cognitive sciences. 2006;10(6):271–7. 10.1016/j.tics.2006.04.007 [DOI] [PubMed] [Google Scholar]
- 30.van Marle HJF, Hermans EJ, Qin S, Fernández G. From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biological psychiatry. 2009;66(7):649–55. 10.1016/j.biopsych.2009.05.014 [DOI] [PubMed] [Google Scholar]
- 31.Van Marle HJF, Hermans EJ, Qin S, Fernández G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage. 2010;53(1):348–54. 10.1016/j.neuroimage.2010.05.070 [DOI] [PubMed] [Google Scholar]
- 32.Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological psychiatry. 2007;62(2):168–78. 10.1016/j.biopsych.2006.08.024 [DOI] [PubMed] [Google Scholar]
- 33.Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, et al. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage. 2006;29(2):347–57. 10.1016/j.neuroimage.2005.03.047 [DOI] [PubMed] [Google Scholar]
- 34.Pavlides C, Nivón LG, McEwen BS. Effects of chronic stress on hippocampal long‐term potentiation. Hippocampus. 2002;12(2):245–57. 10.1002/hipo.1116 [DOI] [PubMed] [Google Scholar]
- 35.Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work? Trends in cognitive sciences. 2006;10(4):152–8. 10.1016/j.tics.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 36.Brambilla P, Nicoletti MA, Harenski K, Sassi RB, Mallinger AG, Frank E, et al. Anatomical MRI study of subgenual prefrontal cortex in bipolar and unipolar subjects. Neuropsychopharmacology. 2002;27(5):792–9. 10.1016/S0893-133X(02)00352-4 [DOI] [PubMed] [Google Scholar]
- 37.Smith ME. Bilateral hippocampal volume reduction in adults with post‐traumatic stress disorder: A meta‐analysis of structural MRI studies. Hippocampus. 2005;15(6):798–807. 10.1002/hipo.20102 [DOI] [PubMed] [Google Scholar]
- 38.Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, et al. Human vulnerability to stress depends on amygdala's predisposition and hippocampal plasticity. Proceedings of the National Academy of Sciences. 2009;106(33):14120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Admon R, Lubin G, Rosenblatt JD, Stern O, Kahn I, Assaf M, et al. Imbalanced neural responsivity to risk and reward indicates stress vulnerability in humans. Cerebral Cortex. 2012. [DOI] [PubMed] [Google Scholar]
- 40.Cabeza R, Nyberg L. Neural bases of learning and memory: functional neuroimaging evidence. Current opinion in neurology. 2000;13(4):415–21. [DOI] [PubMed] [Google Scholar]
- 41.Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 2007;35(2):795–803. 10.1016/j.neuroimage.2006.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lui S, Chen L, Yao L, Xiao Y, Wu Q-Z, Zhang J-R, et al. Brain structural plasticity in survivors of a major earthquake. Journal of psychiatry & neuroscience: JPN. 2013;38(6):381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu CH, Mourao-Miranda J, Costafreda SG, Khanna A, Marquand AF, Williams SC, et al. Pattern classification of sad facial processing: toward the development of neurobiological markers in depression. Biological psychiatry. 2008;63(7):656–62. 10.1016/j.biopsych.2007.08.020 [DOI] [PubMed] [Google Scholar]
- 44.Modinos G, Pettersson-Yeo W, Allen P, McGuire PK, Aleman A, Mechelli A. Multivariate pattern classification reveals differential brain activation during emotional processing in individuals with psychosis proneness. Neuroimage. 2012;59(3):3033–41. 10.1016/j.neuroimage.2011.10.048 [DOI] [PubMed] [Google Scholar]
- 45.Orrù G, Pettersson-Yeo W, Marquand AF, Sartori G, Mechelli A. Using support vector machine to identify imaging biomarkers of neurological and psychiatric disease: a critical review. Neuroscience & Biobehavioral Reviews. 2012;36(4):1140–52. [DOI] [PubMed] [Google Scholar]
- 46.Liu X, Ma D, Liu L, Zhao G, Li C, Yang J, et al. Development of the post-traumatic stress disorder self-rating scale and its reliability and validity. Chinese Journal of Behavioral Medical Science. 1998;7:93–6. [Google Scholar]
- 47.Liu X, Ma D, Liu L, Zhao G, Li C, Yang J, et al. Development of the post-traumatic stress disorder self-rating scale and its reliability and validity. Chin J Behav Med Sci. 1998;7:93–6. [Google Scholar]
- 48.Weiss DS, Marmar CR. The impact of event scale-revised. Assessing psychological trauma and PTSD. 1997;2:168–89. [Google Scholar]
- 49.Spielberger CD. State‐Trait anxiety inventory: Wiley Online Library; 2010.
- 50.Wang Z, Yan C, Zhao C, Qi Z, Zhou W, Lu J, et al. Spatial patterns of intrinsic brain activity in mild cognitive impairment and alzheimer's disease: A resting‐state functional MRI study. Human brain mapping. 2011;32(10):1720–40. 10.1002/hbm.21140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, et al. The Association between Resting Functional Connectivity and Creativity. Cerebral Cortex. 2012. [DOI] [PubMed] [Google Scholar]
- 52.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human brain mapping. 1996;4(1):58–73. [DOI] [PubMed] [Google Scholar]
- 53.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- 54.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–9. [DOI] [PubMed] [Google Scholar]
- 55.Burges CJ. A tutorial on support vector machines for pattern recognition. Data mining and knowledge discovery. 1998;2(2):121–67. [Google Scholar]
- 56.Schrouff J, Rosa MJ, Rondina JM, Marquand AF, Chu C, Ashburner J, et al. PRoNTo: pattern recognition for neuroimaging toolbox. Neuroinformatics. 2013;11(3):319–37. 10.1007/s12021-013-9178-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Almeida J, Mourao-Miranda J, Aizenstein H, Versace A, Kozel F, Lu H, et al. Pattern recognition analysis of anterior cingulate cortex blood flow to classify depression polarity. The British Journal of Psychiatry. 2013;203(4):310–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stone M. Cross-validatory choice and assessment of statistical predictions. Journal of the Royal Statistical Society Series B (Methodological). 1974:111–47. [Google Scholar]
- 59.Peruggia M. On the variability of case-deletion Importance sampling Weights in the Bayesian linear model. Journal of the American Statistical Association. 1997;92(437):199–207. [Google Scholar]
- 60.Epifani I, MacEachern SN, Peruggia M. Case-deletion importance sampling estimators: Central limit theorems and related results. Electronic Journal of Statistics. 2008;2:774–806. [Google Scholar]
- 61.Efron B. Nonparametric estimates of standard error: the jackknife, the bootstrap and other methods. Biometrika. 1981;68(3):589–99. [Google Scholar]
- 62.Golland P, Fischl B, editors. Permutation tests for classification: towards statistical significance in image-based studies IPMI; 2003: Springer. [DOI] [PubMed] [Google Scholar]
- 63.Stelzer J, Chen Y, Turner R. Statistical inference and multiple testing correction in classification-based multi-voxel pattern analysis (MVPA): random permutations and cluster size control. Neuroimage. 2013;65:69–82. 10.1016/j.neuroimage.2012.09.063 [DOI] [PubMed] [Google Scholar]
- 64.Etzel JA, editor MVPA Permutation Schemes: Permutation Testing for the Group Level. Pattern Recognition in NeuroImaging (PRNI), 2015 International Workshop on; 2015: IEEE.
- 65.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine. 1995;34(4):537–41. [DOI] [PubMed] [Google Scholar]
- 66.Song X-W, Dong Z-Y, Long X-Y, Li S-F, Zuo X-N, Zhu C-Z, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PloS one. 2011;6(9):e25031 10.1371/journal.pone.0025031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–56. 10.1016/j.neuroimage.2012.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement‐related effects in fMRI time‐series. Magnetic resonance in medicine. 1996;35(3):346–55. [DOI] [PubMed] [Google Scholar]
- 69.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage. 2013;76:439–41. 10.1016/j.neuroimage.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carp J. Optimizing the order of operations for movement scrubbing: Comment on Power et al. Neuroimage. 2013;76:436–8. 10.1016/j.neuroimage.2011.12.061 [DOI] [PubMed] [Google Scholar]
- 71.Salvador R, Suckling J, Coleman MR, Pickard JD, Menon D, Bullmore E. Neurophysiological architecture of functional magnetic resonance images of human brain. Cerebral Cortex. 2005;15(9):1332–42. 10.1093/cercor/bhi016 [DOI] [PubMed] [Google Scholar]
- 72.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8(9):700–11. 10.1038/nrn2201 [DOI] [PubMed] [Google Scholar]
- 73.Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011;56(3):881–9. 10.1016/j.neuroimage.2011.02.064 [DOI] [PubMed] [Google Scholar]
- 74.Chepenik LG, Raffo M, Hampson M, Lacadie C, Wang F, Jones MM, et al. Functional connectivity between ventral prefrontal cortex and amygdala at low frequency in the resting state in bipolar disorder. Psychiatry research. 2010;182(3):207 10.1016/j.pscychresns.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strakowski SM, Eliassen JC, Lamy M, Cerullo MA, Allendorfer JB, Madore M, et al. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biological psychiatry. 2011;69(4):381–8. 10.1016/j.biopsych.2010.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience. 2012;13(11):769–87. 10.1038/nrn3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herringa R, Phillips M, Almeida J, Insana S, Germain A. Post-traumatic stress symptoms correlate with smaller subgenual cingulate, caudate, and insula volumes in unmedicated combat veterans. Psychiatry Research: Neuroimaging. 2012;203(2):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen JE, Shalev H, Admon R, Hefetz S, Gasho CJ, Shachar LJ, et al. Emotional brain rhythms and their impairment in post‐traumatic patients. Human brain mapping. 2013;34(6):1344–56. 10.1002/hbm.21516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sekiguchi A, Sugiura M, Taki Y, Kotozaki Y, Nouchi R, Takeuchi H, et al. Brain structural changes as vulnerability factors and acquired signs of post-earthquake stress. Molecular psychiatry. 2013;18(5):618–23. 10.1038/mp.2012.51 [DOI] [PubMed] [Google Scholar]
- 80.Bremner JD. Neuroimaging studies in post-traumatic stress disorder. Current psychiatry reports. 2002;4(4):254–63. [DOI] [PubMed] [Google Scholar]
- 81.Stoppel C, Heinze H-J. Structural alterations in lateral prefrontal, parietal and posterior midline regions of men with chronic posttraumatic stress disorder. Journal of psychiatry & neuroscience: JPN. 2011;36(3):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biological psychiatry. 1999;45(7):806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weber DL, Clark CR, McFarlane AC, Moores KA, Morris P, Egan GF. Abnormal frontal and parietal activity during working memory updating in post-traumatic stress disorder. Psychiatry Research: Neuroimaging. 2005;140(1):27–44. 10.1016/j.pscychresns.2005.07.003 [DOI] [PubMed] [Google Scholar]
- 84.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala—frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2(4):303–12. 10.1093/scan/nsm029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsoory M, Vouimba R, Akirav I, Kavushansky A, Avital A, Richter-Levin G. Amygdala modulation of memory-related processes in the hippocampus: potential relevance to PTSD. Progress in brain research. 2008:167. [DOI] [PubMed] [Google Scholar]
- 86.Shapiro ML, Eichenbaum H. Hippocampus as a memory map: synaptic plasticity and memory encoding by hippocampal neurons. Hippocampus. 1999;9(4):365–84. [DOI] [PubMed] [Google Scholar]
- 87.Hamann S. Cognitive and neural mechanisms of emotional memory. Trends in cognitive sciences. 2001;5(9):394–400. [DOI] [PubMed] [Google Scholar]
- 88.Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss W-D. Cerebral representation of one’s own past: neural networks involved in autobiographical memory. The Journal of Neuroscience. 1996;16(13):4275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. Trends in cognitive sciences. 2007;11(5):219–27. 10.1016/j.tics.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 90.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American journal of psychiatry. 2007;164(10):1476 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Campbell S, MacQueen G. The role of the hippocampus in the pathophysiology of major depression. Journal of Psychiatry and Neuroscience. 2004;29(6):417 [PMC free article] [PubMed] [Google Scholar]
- 92.Cisler J, James G, Tripathi S, Mletzko T, Heim C, Hu X, et al. Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychological medicine. 2013;43(03):507–18. [DOI] [PubMed] [Google Scholar]
- 93.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–45. 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- 94.St Jacques PL, Botzung A, Miles A, Rubin DC. Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. Journal of psychiatric research. 2011;45(5):630–7. 10.1016/j.jpsychires.2010.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, et al. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. Journal of Psychiatry & Neuroscience: JPN. 2012;37(4):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brohawn KH, Offringa R, Pfaff DL, Hughes KC, Shin LM. The neural correlates of emotional memory in posttraumatic stress disorder. Biological psychiatry. 2010;68(11):1023–30. 10.1016/j.biopsych.2010.07.018 [DOI] [PubMed] [Google Scholar]
- 97.Smith A, Henson R, Dolan R, Rugg M. fMRI correlates of the episodic retrieval of emotional contexts. Neuroimage. 2004;22(2):868–78. 10.1016/j.neuroimage.2004.01.049 [DOI] [PubMed] [Google Scholar]
- 98.Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, LaBar KS. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia. 2005;43(5):659–74. 10.1016/j.neuropsychologia.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 99.Maguire EA. Neuroimaging studies of autobiographical event memory. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2001;356(1413):1441–51. 10.1098/rstb.2001.0944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith AP, Stephan KE, Rugg MD, Dolan RJ. Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron. 2006;49(4):631–8. 10.1016/j.neuron.2005.12.025 [DOI] [PubMed] [Google Scholar]
- 101.Roozendaal B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiology of learning and memory. 2002;78(3):578–95. [DOI] [PubMed] [Google Scholar]
- 102.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. 10.1146/annurev.neuro.27.070203.144157 [DOI] [PubMed] [Google Scholar]
- 103.McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, et al. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(30):10718–23. 10.1073/pnas.0504436102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McNally RJ, Lasko NB, Macklin ML, Pitman RK. Autobiographical memory disturbance in combat-related posttraumatic stress disorder. Behaviour research and therapy. 1995;33(6):619–30. [DOI] [PubMed] [Google Scholar]
- 105.Boehnlein JK. The process of research in posttraumatic stress disorder. Perspectives in biology and medicine. 1989;32(3):455–65. [DOI] [PubMed] [Google Scholar]
- 106.Paige SR, Reid GM, Allen MG, Newton JE. Psychophysiological correlates of posttraumatic stress disorder in Vietnam veterans. Biological psychiatry. 1990;27(4):419–30. [DOI] [PubMed] [Google Scholar]
- 107.Yin Y, Jin C, Hu X, Duan L, Li Z, Song M, et al. Altered resting-state functional connectivity of thalamus in earthquake-induced posttraumatic stress disorder: a functional magnetic resonance imaging study. Brain research. 2011;1411:98–107. 10.1016/j.brainres.2011.07.016 [DOI] [PubMed] [Google Scholar]
- 108.Douglas J. Dissociation and posttraumatic stress disorder in Vietnam combat veterans. The American journal of psychiatry. 1992;149:328–32. 10.1176/ajp.149.3.328 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
Data are available within the paper and its Supporting Information files and at https://dx.doi.org/10.6084/m9.figshare.4299389.