Abstract
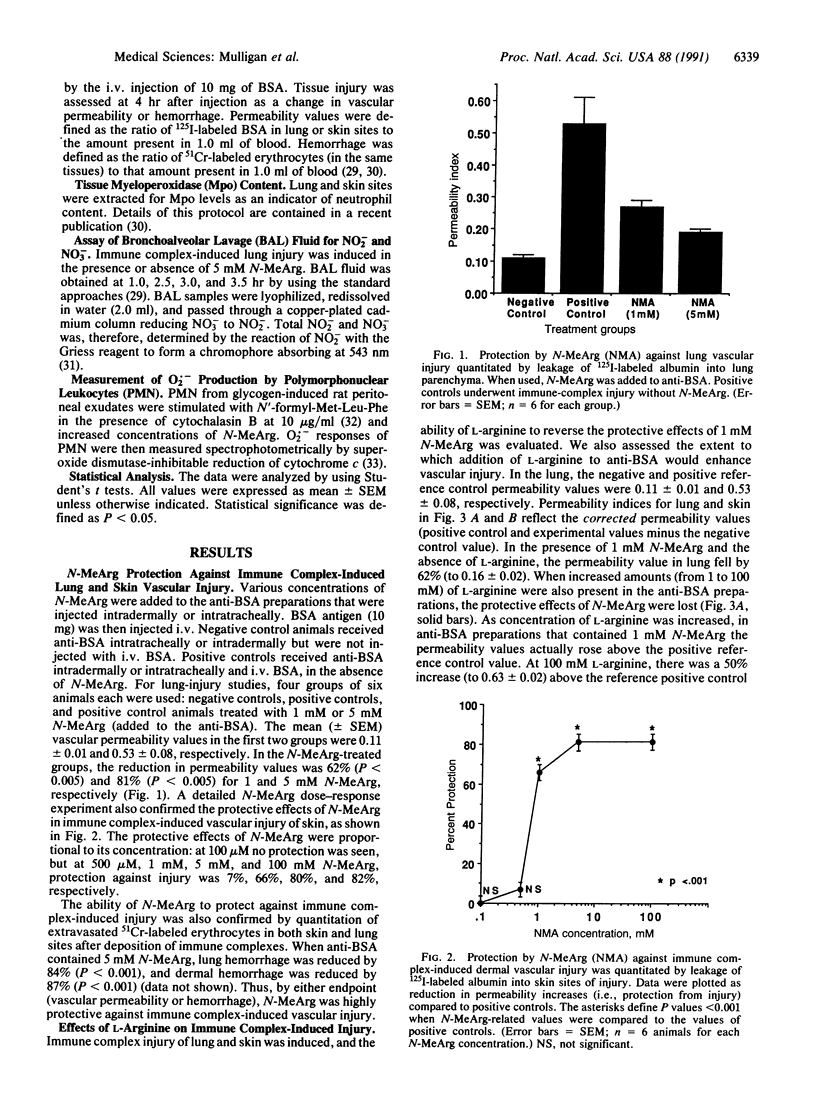
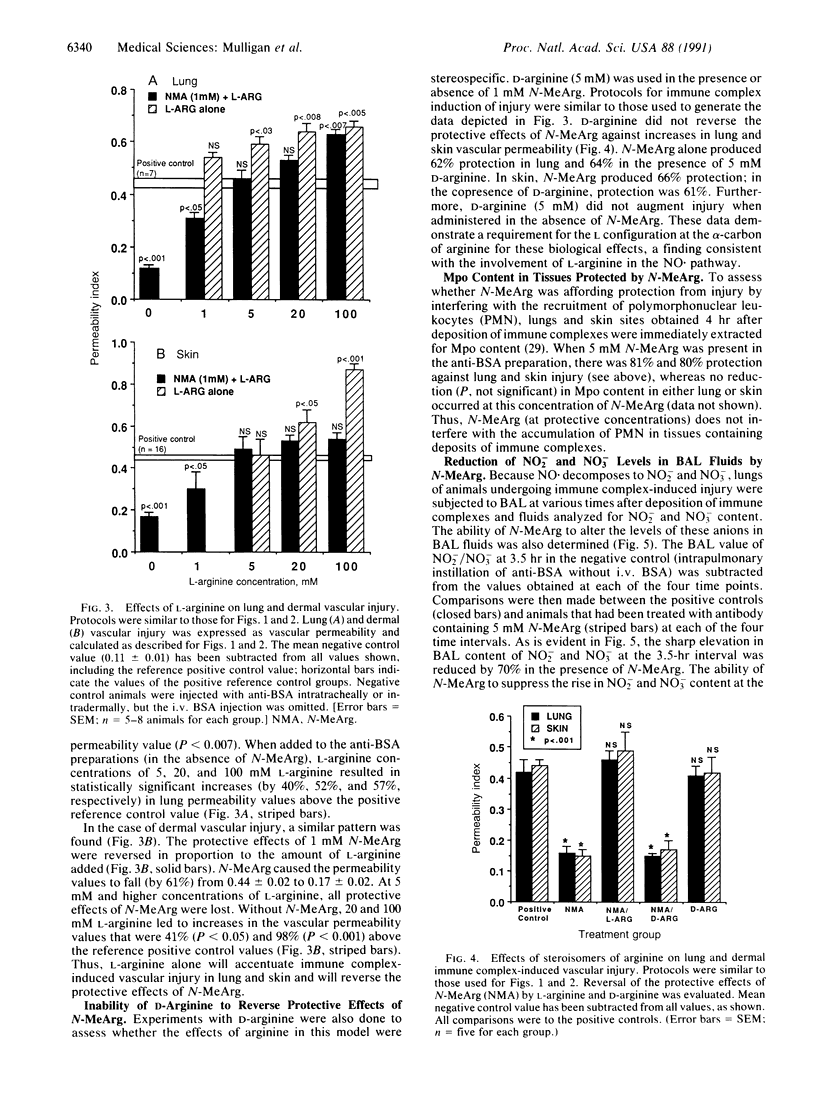
Nitric oxide (NO.), a free radical that is generated from L-arginine by stimulated endothelial cells, neutrophils, activated macrophages, and other cell types, reacts with superoxide anion (O2.-) to form peroxynitrite, which itself may be tissue toxic or can then react further to form the highly reactive and toxic hydroxyl radical (HO.). Because vascular injury produced by tissue deposition of immune complexes is linked to formation of toxic products derived from activated neutrophils, we have assessed whether immune complex-induced injury of rat lung and dermal vasculature is arginine dependent. The arginine analogue, NG-monomethyl-L-arginine (N-MeArg), which blocks NO. formation, protects against immune complex-induced vascular injury in rats. The protective effects of N-MeArg are reversed by the presence of L-arginine but not D-arginine. Additionally, in the absence of N-MeArg, injury is enhanced by the presence of L-arginine but not by D-arginine. Protection by N-MeArg is not associated with diminished recruitment of polymorphonuclear leukocytes. Bronchoalveolar lavage fluids from animals undergoing immune complex deposition in lung contain the decomposition products of NO.--namely, nitrite and nitrate. In the presence of N-MeArg these products are greatly diminished. These data suggest that immune complex-induced injury of rat lung and skin is L-arginine dependent. These data also suggest that in vivo metabolic products of L-arginine, such as NO(.), are directly or indirectly linked to immune complex-induced tissue injury.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Ferrari F. K., Williams D. L., Simmons R. L. Kupffer cell:hepatocyte cocultures release nitric oxide in response to bacterial endotoxin. J Surg Res. 1990 Apr;48(4):349–353. doi: 10.1016/0022-4804(90)90073-b. [DOI] [PubMed] [Google Scholar]
- Chester A. H., O'Neil G. S., Moncada S., Tadjkarimi S., Yacoub M. H. Low basal and stimulated release of nitric oxide in atherosclerotic epicardial coronary arteries. Lancet. 1990 Oct 13;336(8720):897–900. doi: 10.1016/0140-6736(90)92269-n. [DOI] [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Ochoa J. B., Harbrecht B. G., Flint S. G., Simmons R. L. Multiple cytokines are required to induce hepatocyte nitric oxide production and inhibit total protein synthesis. Ann Surg. 1990 Oct;212(4):462–471. doi: 10.1097/00000658-199010000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Fligiel S. E., Ward P. A., Johnson K. J., Till G. O. Evidence for a role of hydroxyl radical in immune-complex-induced vasculitis. Am J Pathol. 1984 Jun;115(3):375–382. [PMC free article] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Inhibition of rat mesangial cell mitogenesis by nitric oxide-generating vasodilators. Am J Physiol. 1989 Jul;257(1 Pt 2):F60–F66. doi: 10.1152/ajprenal.1989.257.1.F60. [DOI] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide-generating vasodilators inhibit mitogenesis and proliferation of BALB/C 3T3 fibroblasts by a cyclic GMP-independent mechanism. Biochem Biophys Res Commun. 1990 Aug 31;171(1):474–479. doi: 10.1016/0006-291x(90)91417-q. [DOI] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide-generating vasodilators inhibit mitogenesis and proliferation of BALB/C 3T3 fibroblasts by a cyclic GMP-independent mechanism. Biochem Biophys Res Commun. 1990 Aug 31;171(1):474–479. doi: 10.1016/0006-291x(90)91417-q. [DOI] [PubMed] [Google Scholar]
- Garlick P. B., Davies M. J., Hearse D. J., Slater T. F. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ Res. 1987 Nov;61(5):757–760. doi: 10.1161/01.res.61.5.757. [DOI] [PubMed] [Google Scholar]
- Granger D. N., McCord J. M., Parks D. A., Hollwarth M. E. Xanthine oxidase inhibitors attenuate ischemia-induced vascular permeability changes in the cat intestine. Gastroenterology. 1986 Jan;90(1):80–84. doi: 10.1016/0016-5085(86)90078-8. [DOI] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Gruetter C. A., Barry B. K., McNamara D. B., Gruetter D. Y., Kadowitz P. J., Ignarro L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cyclic Nucleotide Res. 1979;5(3):211–224. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Ignarro L. J., Bush P. A., Buga G. M., Wood K. S., Fukuto J. M., Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1990 Jul 31;170(2):843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Role of oxygen metabolites in immune complex injury of lung. J Immunol. 1981 Jun;126(6):2365–2369. [PubMed] [Google Scholar]
- Kilbourn R. G., Gross S. S., Jubran A., Adams J., Griffith O. W., Levi R., Lodato R. F. NG-methyl-L-arginine inhibits tumor necrosis factor-induced hypotension: implications for the involvement of nitric oxide. Proc Natl Acad Sci U S A. 1990 May;87(9):3629–3632. doi: 10.1073/pnas.87.9.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Merrett M., Salter M., Moncada S. Differential induction of brain, lung and liver nitric oxide synthase by endotoxin in the rat. Biochem J. 1990 Sep 15;270(3):833–836. doi: 10.1042/bj2700833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J. R., Jr, Hibbs J. B., Jr EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1223–1227. doi: 10.1073/pnas.87.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey B. A., Phan S. H., Varani J., Ryan U. S., Ward P. A. Inhibition of cytotoxicity by intracellular superoxide dismutase supplementation. Free Radic Biol Med. 1990;9(4):307–314. doi: 10.1016/0891-5849(90)90005-4. [DOI] [PubMed] [Google Scholar]
- Marletta M. A. Nitric oxide: biosynthesis and biological significance. Trends Biochem Sci. 1989 Dec;14(12):488–492. doi: 10.1016/0968-0004(89)90181-3. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Seifert R., Böhme E. Formation and release of nitric oxide from human neutrophils and HL-60 cells induced by a chemotactic peptide, platelet activating factor and leukotriene B4. FEBS Lett. 1989 Feb 27;244(2):357–360. doi: 10.1016/0014-5793(89)80562-9. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Synthesis of nitrite and nitrate in murine macrophage cell lines. Cancer Res. 1987 Nov 1;47(21):5590–5594. [PubMed] [Google Scholar]
- Thiemermann C., Vane J. Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lipopolysaccharides in the rat in vivo. Eur J Pharmacol. 1990 Jul 17;182(3):591–595. doi: 10.1016/0014-2999(90)90062-b. [DOI] [PubMed] [Google Scholar]
- Varani J., Ginsburg I., Schuger L., Gibbs D. F., Bromberg J., Johnson K. J., Ryan U. S., Ward P. A. Endothelial cell killing by neutrophils. Synergistic interaction of oxygen products and proteases. Am J Pathol. 1989 Sep;135(3):435–438. [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Till G. O., Kunkel R., Beauchamp C. Evidence for role of hydroxyl radical in complement and neutrophil-dependent tissue injury. J Clin Invest. 1983 Sep;72(3):789–801. doi: 10.1172/JCI111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Warren J. S., Johnson K. J. Oxygen radicals, inflammation, and tissue injury. Free Radic Biol Med. 1988;5(5-6):403–408. doi: 10.1016/0891-5849(88)90114-1. [DOI] [PubMed] [Google Scholar]
- Warren J. S., Mandel D. M., Johnson K. J., Ward P. A. Evidence for the role of platelet-activating factor in immune complex vasculitis in the rat. J Clin Invest. 1989 Feb;83(2):669–678. doi: 10.1172/JCI113931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. S., Yabroff K. R., Mandel D. M., Johnson K. J., Ward P. A. Role of O2- in neutrophil recruitment into sites of dermal and pulmonary vasculitis. Free Radic Biol Med. 1990;8(2):163–172. doi: 10.1016/0891-5849(90)90089-2. [DOI] [PubMed] [Google Scholar]
- Warren J. S., Yabroff K. R., Remick D. G., Kunkel S. L., Chensue S. W., Kunkel R. G., Johnson K. J., Ward P. A. Tumor necrosis factor participates in the pathogenesis of acute immune complex alveolitis in the rat. J Clin Invest. 1989 Dec;84(6):1873–1882. doi: 10.1172/JCI114374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Wright C. D., Mülsch A., Busse R., Osswald H. Generation of nitric oxide by human neutrophils. Biochem Biophys Res Commun. 1989 Apr 28;160(2):813–819. doi: 10.1016/0006-291x(89)92506-0. [DOI] [PubMed] [Google Scholar]




