Abstract
Conifer stem pest resistance includes constitutive defenses that discourage invasion and inducible defenses, including phenolic and terpenoid resin synthesis. Recently, methyl jasmonate (MJ) was shown to induce conifer resin and phenolic defenses; however, it is not known if MJ is the direct effector or if there is a downstream signal. Exogenous applications of MJ, methyl salicylate, and ethylene were used to assess inducible defense signaling mechanisms in conifer stems. MJ and ethylene but not methyl salicylate caused enhanced phenolic synthesis in polyphenolic parenchyma cells, early sclereid lignification, and reprogramming of the cambial zone to form traumatic resin ducts in Pseudotsuga menziesii and Sequoiadendron giganteum. Similar responses in internodes above and below treated internodes indicate transport of a signal giving a systemic response. Studies focusing on P. menziesii showed MJ induced ethylene production earlier and 77-fold higher than wounding. Ethylene production was also induced in internodes above the MJ-treated internode. Pretreatment of P. menziesii stems with the ethylene response inhibitor 1-methylcyclopropene inhibited MJ and wound responses. Wounding increased 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase protein, but MJ treatment produced a higher and more rapid ACC oxidase increase. ACC oxidase was most abundant in ray parenchyma cells, followed by cambial zone cells and resin duct epithelia. The data show these MJ-induced defense responses are mediated by ethylene. The cambial zone xylem mother cells are reprogrammed to differentiate into resin-secreting epithelial cells by an MJ-induced ethylene burst, whereas polyphenolic parenchyma cells are activated to increase polyphenol production. The results also indicate a central role of ray parenchyma in ethylene-induced defense.
Resistance in conifer stems to invasion by bark beetles, wood borers, and fungal pathogens includes constitutive defenses that deter initial invasion and inducible responses that may include mass oleoresin secretion and increased phenolic synthesis surrounding the wound zone following invasion. The bark (periderm and secondary phloem) is the first line of defense against stem-invading organisms and in many conifers contains basal levels of compartmentalized phenolic and terpenoid compounds. However, little is known about the activity of cells in the secondary phloem with respect to defense response mechanisms.
Polyphenolic parenchyma (PP) cells are a common component of the secondary phloem of all conifers and are active in the constitutive synthesis, storage, and modification of various phenolic compounds (Franceschi et al., 1998; Krekling et al., 2000). Following abiotic or biotic damage, PP cells give rise to the wound periderm and are activated to accumulate and release phenolics around the wound site, but these cells can also be induced to accumulate phenolics 10 to 30 cm away from damaged tissue (Franceschi et al., 1998, 2000; Hudgins et al., 2003a; Krekling et al., 2004). A number of studies have revealed qualitative alterations in phenolic compounds following fungal inoculations, including increased activity of the flavonoid pathway (Brignolas et al., 1995; Lieutier et al., 1996; Bois and Lieutier, 1997; Bonello et al., 2003), and accumulation of phenolics is considered a significant part of induced defense responses in the bark (Nicholson and Hammerschmidt, 1992; Schultz et al., 1992; Viiri et al., 2001).
Many, but not all, conifers also contain resin-producing structures in the secondary phloem or xylem as a constitutive defense (Langenheim, 2003). Some Pinaceae species (e.g. Pinus, Picea) constitutively produce xylem resin ducts (Wu and Hu, 1997) but can be induced to form additional xylem traumatic resin ducts (TD) following herbivory, pathogen invasion, or wounding (Reid et al., 1967; Alfaro, 1995; Franceschi et al., 1998, 2000; Tomlin et al., 1998; Nagy et al., 2000; Hudgins et al., 2003a; McKay et al., 2003), while others (Abies, Cedrus) only develop xylem resin ducts in response to injury (Bannan, 1936; Fahn et al., 1979). The resin formed from inducible TDs may be more toxic or fungistatic because of the addition of phenolics and compositional changes of terpene content (Nagy et al., 2000; Fäldt et al., 2003; Krokene et al., 2003). Copious resin production provides an effective defense system that functions in flushing the wound, discouraging herbivory and sealing the injury through evaporation of the volatile turpentine fractions (Katoh and Croteau, 1997; Phillips and Croteau, 1999).
While there is considerable information about the anatomical and biochemical defenses in conifer stems, there is little known about the regulation of constitutive and inducible defenses. Recent work has shown that PP cell activation and TD development can be strongly elicited in the absence of wounding with exogenous treatments of methyl jasmonate (MJ) in the Pinaceae (Franceschi et al., 2002; Martin et al., 2002, 2003; Fäldt et al., 2003; Hudgins et al., 2003a) and Taxodiaceae (Hudgins et al., 2004). While there is little information on natural jasmonates in conifers, jasmonic acid has been shown to be induced in conifer cell culture (Taxus baccata) following elicitation with fungal cell wall fragments (Mueller et al., 1993). By contrast, jasmonates are known to modulate many activities in angiosperms, including defense responses (see Creelman and Mullet, 1997; Cheong and Choi, 2003; Farmer et al., 2003). A few investigations have also shown that MJ is capable of inducing terpenoid biosynthetic enzymes and their associated genes in Norway spruce (Picea abies; Martin et al., 2002, 2003; Fäldt et al., 2003) and chalcone synthase, a key enzyme in the phenylpropanoid pathway, in white spruce (Picea glauca; Richard et al., 2000). Thus, MJ appears to be involved in signal transduction pathways for both the phenylpropanoid and terpenoid defense responses in conifer stems. A question that remains is whether MJ directly induces TD development and PP cell activation or if it activates other factors that ultimately induce these responses.
A potential clue to the factors directly involved in TD formation may be inferred from the developmental features of these structures. TD formation occurs as a result of reprogramming of cambial cell derivatives that would normally develop into tracheids (Werker and Fahn, 1969; Nagy et al., 2000; Krekling et al., 2004), but mechanisms controlling this process are unknown. The phytohormone ethylene is involved in a diverse array of plant growth and developmental processes (Abeles et al., 1992; Kende, 1993; Bleecker and Kende, 2000) and is a possible candidate effector. In conifers, a number of studies have indicated that ethylene evolution is positively correlated with cambial zone activity, primarily with tracheid production and compression wood formation (Barker, 1979; Telewski and Jaffe, 1986; Eklund, 1990, 1991; Ingemarsson et al., 1991; Eklund and Little, 1995, 1996, 1998; Little and Pharis, 1995; Dolan, 1997; Little and Eklund, 1999; Klintborg et al., 2002; Andersson-Gunneras et al., 2003; Du and Yamamoto, 2003). Reports have also indicated that monoterpene synthesis and ethylene production are correlated with stem damage following fungal inoculations and wounding in several species of Pinus (Peters, 1977; Popp et al., 1995). Katoh and Croteau (1997) found that application of Ethrel, in addition to wounding, enhanced monoterpene synthase activity over wounding alone in Abies grandis (Pinaceae) saplings. Exogenous application of Ethrel has also been shown to induce enhanced resin exudation in a member of the Cupressaceae (Kusumoto and Suzuki, 2001), and an increase in the number (linear density) of resin ducts in seedlings of loblolly pine (Pinus taeda) and Pinus densiflora (Telewski et al., 1983; Yamamoto and Kozlowski, 1987). However, the direct effect of ethylene on resin production in conifers is not clear since many studies have included wounding as part of the application method, or continued application over many days.
A link between jasmonates and ethylene has been shown in a number of systems (Farmer et al., 2003). MJ has been shown to induce ethylene production in such diverse organs as apple and tomato fruits and olive leaves (Saniewski, 1997; Fan et al., 1998). In Arabidopsis, responses to different pathogens have been shown to include a synergistic effect of jasmonate and ethylene for induction of defense-related genes (Xu et al., 1994; Penninckx et al., 1998). For conifers, information on jasmonates has only recently become available but is limited, and a correlation between jasmonate and ethylene has not been established.
Another phytohormone that needs to be considered with respect to PP cell activation and TD formation in conifers is salicylic acid (SA), a known mediator of the expression of various defense-related genes (Ryals et al., 1996; Sticher et al., 1997). In conifers, SA has been shown to accumulate in response to pathogen challenge (Franich et al., 1986; Kozlowski and Metraux, 1998; Kozlowski et al., 1999), and Davis et al. (2002) showed that following either pathogen challenge or salicylic acid treatments, Pinus species were induced to express three chitinase homologs. Kozlowski et al. (1999) found that MJ induces the accumulation of salicylic acid in Norway spruce seedlings, establishing a link between the MJ and SA pathways in conifers. Although evidence indicates SA to be an important activator of genes encoding pathogenesis-related proteins (Durner et al., 1997), various proteins induced in the host plant in response to pathogens (van Loon et al., 1994), there is no information for its role in induction of other defense responses important to conifers, such as terpene and phenolic synthesis.
The purpose of this study was to determine if MJ directly induces PP cell activation and TD formation, as well as their systemic induction in untreated regions, or if it is an intermediate signaling compound. We used non-wounding exogenous applications of MJ, methyl salicylate (MS), and ethylene to assess stem responses in two diverse conifers: Douglas fir (Pseudotsuga menziesii Mirbel Franco), which has constitutive resin ducts, and giant redwood (Sequoiadendron giganteum Lindl. Buchholz), which only produces TDs. Our results demonstrate that a major part of the MJ-induced response is due to increased ethylene production, and the sites of ethylene production are identified. The results are relevant to mechanisms of induced resistance in conifers, as previous studies have demonstrated that wounding or MJ pretreatment of mature Norway spruce stems induces these same responses and also confers local resistance to the bark beetle-vectored fungal pathogen, Ceratocystis polonica (Franceschi et al., 2002; Krokene et al., 2003).
RESULTS
Methyl Jasmonate and Ethylene Induce Resin Accumulation and Morphogenic Changes
Following application of MJ or ethylene to Douglas fir and giant redwood, resin accumulated on the bark of the treated internode of all the saplings within 21 d but never appeared on the bark of MS or control saplings (data not shown; see Hudgins et al., 2004). Observations of stem surfaces indicated that resin exudation was considerably greater with 100 mm MJ than lower MJ concentrations and ethylene, which although not quantified correlates with the cross-sectional area of TDs formed at the treated internode at each concentration (see Figs. 3 and 4 for quantitative values).
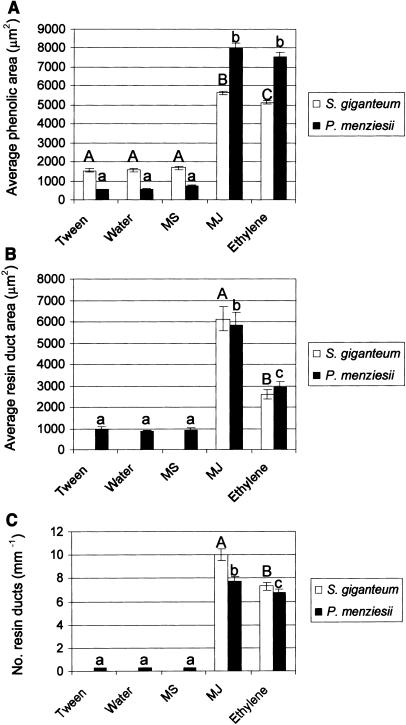
Figure 3.
Analysis of change in secondary phloem PP cell phenolics and xylem resin ducts 28 d after application of 0.1% Tween 20 and water (controls), and 100 mm MS, 100 mm MJ, and ethylene in giant redwood and Douglas fir. Shown are total mean polyphenolic body area (A), mean xylem resin duct cross sectional area (B), and mean number of resin ducts per millimeter of xylem (C). Measurements (μm2/mm) represent means from cross-sections from three individual replicate saplings. Means are of three replicates ± se. Forty PP cells and 10 xylem resin ducts were analyzed per section. Different letters indicate significant differences (P < 0.05) for each treatment within a species. Significant differences were found between MJ and ethylene, and MJ and ethylene and Tween 20, water, and MS treatments. Significant differences were not found among Tween 20, water, and MS treatments.
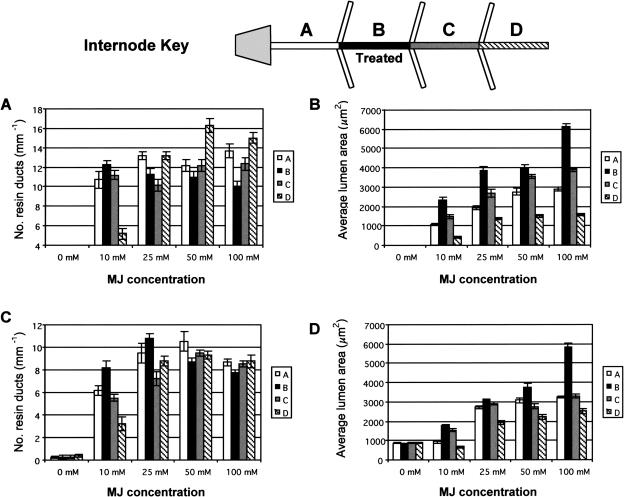
Figure 4.
Response of giant redwood (A and B) and Douglas fir (C and D) following application of 10, 25, 50, and 100 mm MJ. Number of TD per millimeter ± se (A and C) and TD lumen area ± se (B and D), as viewed in cross-section, formed at the treated internode (B), internode below the treated internode (A), internode above treated internode (C), and two internodes above treated internode (D). A dose response is evident with respect to application concentration and distance from treated internode. Refer to text for description of significant differences.
Normal anatomy of Douglas fir secondary phloem, as seen in cross-section, consists of sieve cells and associated albuminous cells, PP cells, radial ray parenchyma, and scattered radial resin ducts. In control tissue (Tween 20 and water), PP cells generally occurred as semicomplete tangential rows with 5 to 10 sieve cells between rows, though individual PP cells were occasionally scattered among the sieve cells (Fig. 1A). All PP cells contained small phenolic bodies. Twenty-eight days after treatment with MJ or ethylene, PP cells appeared in an activated stage (Franceschi et al., 2000), which included swelling and massive accumulation of phenolics (Fig. 1, B and C; quantified in Fig. 3). PP cells showed about a 2-fold increase in size after 100 mm MJ and ethylene treatments versus Tween 20, MS, and water treatments (138 ± 17 μm2 0.1% Tween 20 versus 220 ± 37 μm2 100 mm MJ). MJ and ethylene also induced early lignification of stone cells (sclereids) in the secondary phloem (data not shown). PP cell and stone cell changes were not observed following Tween 20 (Fig. 1A), MS (Fig. 1D), or water treatments (Fig. 3).
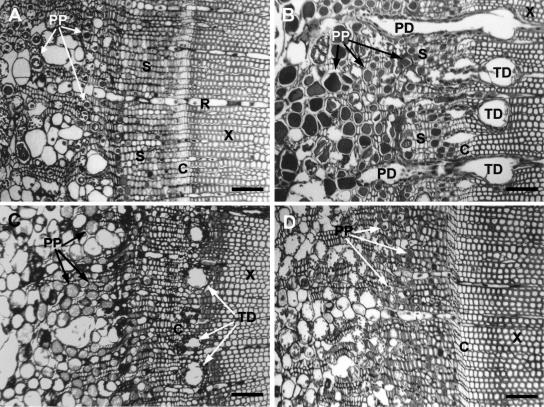
Figure 1.
Douglas fir stem cross-sections 28 d after application of 0.1% Tween 20 (A), 100 mm MJ (B), ethylene (C), and 100 mm MS (D). Figures include similar region from each treatment with secondary phloem, cambium, and xylem for comparison. Bars = 200 μm. C, Cambial zone; PP, PP cell; PD, phloem resin duct; R, ray parenchyma; S, sieve cells; X, xylem. A, Normal anatomy of Douglas fir (Tween 20 control) with rows of PP cells, normal cambium, and the absence of constitutive axial xylem ducts. B, MJ-induced PP cell swelling, phenolic accumulation, and formation of radial phloem ducts and large axial xylem TDs. C, Ethylene treatment-induced PP cell swelling and phenolic deposition and a single row of TDs in the xylem. D, MS treatment did not induce anatomical changes.
Following MJ treatments to Douglas fir saplings, a tangential row of large axial xylem TDs developed proximal to the cambium, and radial resin ducts in secondary phloem and xylem were activated to accumulate resin as indicated by expansion and increase in lumen size (Fig. 1B). The radial ducts of the secondary phloem were often seen to be connected with the induced xylem TDs (Fig. 1B). These same responses were found at all four internodes from which sections were collected, although the response was reduced in magnitude at both internodes above the treatment site (Fig. 4). Ethylene also induced xylem TDs, but the ducts were smaller than observed with MJ treatments (Figs. 1C and 3). Copper acetate staining indicated that induced axial and radial TDs contained resin acids (data not shown). Control treatments (Figs. 1A and 3) or MS (Figs. 1D and 3) did not induce axial or radial TDs.
Stems of giant redwood have a considerably different anatomy than Douglas fir and do not normally produce resin ducts, which is why we chose it to compare responses to ethylene. The secondary phloem of giant redwood consist of a highly organized structure with the following tangential pattern: fiber cells followed by sieve cells, followed by PP cells, then sieve cells, and then another layer fiber cell to repeat the pattern (Fig. 2A). Tangential layers of mature (thickened) fibers occur in the secondary phloem layers older than 2 years, while the fiber walls appear weakly lignified in the first two layers near the cambium. MJ treatment strongly induced PP cell activation as seen by both swelling of PP cells and the accumulation of phenolics (Figs. 2B and 3). Early lignification of young fiber cells also occurred in response to MJ treatment (Fig. 2B). Ethylene treatment also strongly induced PP cell activation and lignification of fiber cells (Figs. 2C and 3), while MS had no apparent effect on PP cells or fiber lignification (Fig. 2D). Differences were significant at P < 0.05.
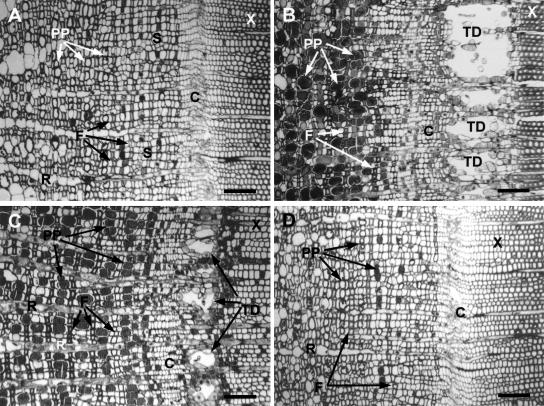
Figure 2.
Giant redwood stem cross-sections 28 d after application of 0.1% Tween 20 (A), 100 mm MJ (B), ethylene (C), and 100 mm MS (D). Figures include similar region from each treatment with secondary phloem, cambium, and xylem for comparison. Bars = 200 μm. C, Cambial zone; F, fiber; PP, PP cell; R, ray parenchyma; S, sieve cells; X, xylem. A, Tween 20 control has normal anatomy of giant redwood, with regular secondary phloem pattern of a row of PP cells followed by sieve cells, fibers, and another row of sieve cells, and the absence of constitutive axial xylem ducts. B, MJ-induced PP cell swelling, phenolic accumulation, lignification of young fibers, and formation of axial xylem TDs. C, Ethylene treatment induced PP cell swelling and phenolic deposition and a row of TDs in the xylem. D, MS treatment did not induce anatomical changes.
Some species of Pinaceae but no species of Taxodiaceae that have been studied produce constitutive axial xylem resin ducts (Hudgins et al., 2004); however, MJ and ethylene both induced TD formation in giant redwood, a member of the Taxodiaceae (Fig. 2, B and C). The TDs that developed in giant redwood in response to 100 mm MJ were approximately 2.5-fold larger (in cross-sectional area) than those formed in response to exogenous ethylene treatments (Fig. 3C). Copper acetate stained the MJ- and ethylene-induced TD lumens blue, indicating the TDs produced resin acids and were functional following both treatments. Phenolic accumulation (data not shown) and TD formation (Fig. 4) were also found at the internodes above and below treatment sites for both MJ and ethylene treatments, although the magnitude of the remote response was reduced compared with the response at the application site. TD induction was not observed with MS (Fig. 2D) or control treatments (Fig. 2A).
Dose Response to Methyl Jasmonate
A differential dose response was observed with respect to TD size and numbers, and the amount of MJ applied to giant redwood and Douglas fir (Fig. 4). In both species, TD size generally increased with increasing MJ concentration in the treated internode as well as internodes immediately above and below the treatment site. For a given MJ concentration, the size of the TDs decreased moving from the treated internode to the internode immediately above, and then again to the second internode above. TD size also decreased in the internode below the treated internode. Previous studies showed that gaseous MJ alone was not able to penetrate the bark at a level that gave a response (Hudgins et al., 2004), and, thus, the effects in distant internodes are due to internal transport of a signal.
A statistical analysis was done on the number (linear density in mm−1) and size (area in μm2) of MJ-induced TDs at the internode below the treated internode (internode A), the treated internode (B), and both internodes above the treatment site (C and D; 10 and 20 cm above, respectively). In giant redwood, significant differences were found only between the number of induced TDs at internodes A, B, C, and D within 10 and 50 mm MJ treatments (P < 0.0001; Fig. 4A). Significant differences with 25 mm MJ were found at internodes A and C (P = 0.0037) and internodes C and D (P = 0.0037; Fig. 4A). For 100 mm MJ, the number of TDs was significant between internodes B and A (P = 0.0007), C (P = 0.0168), and D (P < 0.0001). Within each MJ concentration in giant redwood, TD lumen sizes also showed significant differences (P < 0.0001; Fig. 4B). In Douglas fir, significant differences occurred between the number of induced TDs at all internode locations except A and C with 10 mm MJ treatment (P = 0.3339), while with 25 mm MJ differences were found between internodes A and C (P = 0.0017), B and C (P < 0.0001), B and D (P = 0.006), and C and D (P = 0.0198; Fig. 4C). The number of induced TDs were not significantly different within 50 and 100 mm MJ treatments at internodes A, C, and D (P > 0.06 in all cases); however, internode B was significantly different from A (P = 0.011) at 50 mm MJ. Lumen sizes of the induced TDs in Douglas fir at 10 mm MJ treatment were significant between internodes A and B, B and D, and C and D (all P = 0.0372; Fig. 4D). For 25 mm MJ, significant differences were found between A and B (P = 0.0372), A and D, B and D, and C and D at P < 0.0001. With 50 mm MJ, significant differences were found between internodes A and B (P = 0.0002), C and D (P = 0.0025), B and C (P < 0.0001), B and D (P = 0.0001), and B and D (P < 0.0001). For 100 mm MJ treatments, significant differences (P < 0.0001) were found between all internodes except A and C (P = 0.81). Data on the number of TDs formed is somewhat misleading, especially with highest MJ concentration. This is because at high MJ concentrations the TDs were so large that they tended to inhibit formation of multiple smaller TDs, as occurs in internodes above or below the treated internode or with lower MJ (Fig. 4). This inverse relationship between TD size and number is purely due to physical restraints.
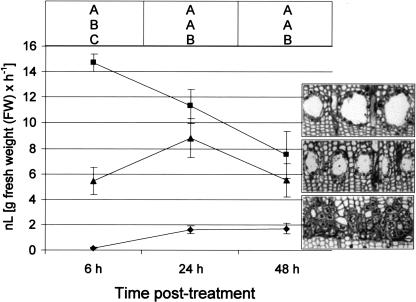
Methyl Jasmonate and Wounding Induce Ethylene Production
Exogenous MJ treatment on Douglas fir stems induced ethylene production at both the treated internode and the untreated internode above (Fig. 5). The response was rapid, with a 77-fold increase of ethylene at 6 h over wound-induced ethylene at the same time point (significant at P < 0.0001). In the treated internode, there was a decrease in ethylene production from 6 to 24 to 48 h after treatment. The ethylene production by the untreated internode peaked at 24 h, indicating a lag phase probably associated with transport of the signal from the treated internode. Although significantly lower, ethylene above control levels was detected at wounded internodes after 6 h and increased at 24 h, and remained at this level up to 48 h (Fig. 5). An ethylene signal was observed at the internode above the wound; however, it remained below the quantifiable limits of the calibration curve. Statistical analysis revealed MJ treatments were significantly higher (P < 0.0001) over wounding at all sample time points, but differences between the treated and untreated internodes at 24 (P = 0.131) and 48 h (P = 0.2211) with MJ treatments were not significantly different. Ethylene was not detected in internodes of the Tween 20-treated control trees.
Figure 5.
Ethylene biosynthesis in Douglas fir stems at different times following wounding or 100 mm MJ treatment. Samples were collected at 6, 24, and 48 h. Means of three replicates ± se are indicated. Data are for MJ-treated internode (▪), internode above MJ treatment (▴), and wounded internode (♦). Ethylene was detected at the internode above the wound, although it was below the quantifiable limits of the calibration curve and, thus, is not shown on this scale. Ethylene was not detected after Tween 20 control treatments. Images to the right of each curve are representative of the appearance of the new sapwood 4 weeks after treatment. Different letters indicate significant differences (P < 0.05) between treatments at each time point.
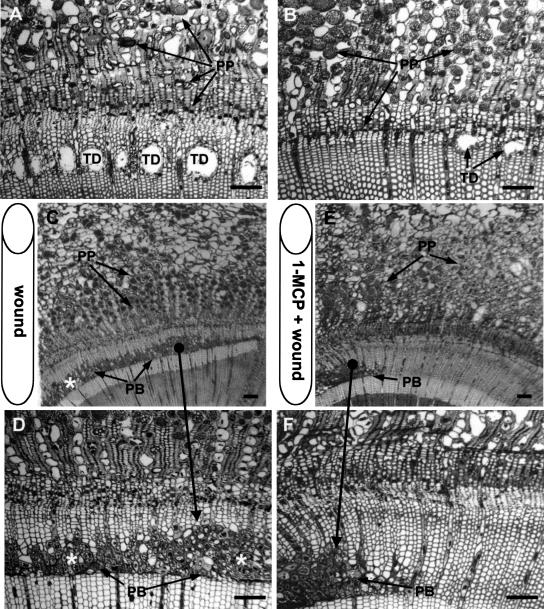
The Ethylene Antagonist, 1-Methylcyclopropene, Reduces the MJ and Wound Response
We sought to clarify and differentiate the role of MJ and ethylene by using 1-methylcyclopropene (1-MCP) to inhibit the ethylene response in Douglas fir. As found previously, in the absence of 1-MCP, MJ-treated stems developed activated PP cells and a tangential band of xylem TD at the treated internode (Fig. 6A) and the internode directly above the treatment site (data not shown). In the presence of 1-MCP, only a few small scattered xylem TD were formed at the treated internode (Fig. 6B), and resin ducts were not formed at the internode above the treatment site. Quantitative analysis showed that 1-MCP reduced MJ-induced TD area to only 16% of that formed by MJ treatment without 1-MCP (908 ± 74 versus 5,850 ± 408 μm2/mm) in the treated internode, and no TDs were formed in the internode above the treated internode when 1-MCP was used. The TDs in Figure 6B are shown as an example of the anatomy of the few TDs formed in the presence of 1-MCP, and it must be noted that this picture represents a rarity, as in most of the stem no TDs could be found. However, PP cells did appear to be activated by MJ in the presence of 1-MCP (Fig. 6B), though it was not as extensive as in the absence of 1-MCP. Following wounding with a cork borer, a band of 10 to 15 phenolic filled cells and undifferentiated TDs formed in the xylem in a circumferential band some distance from the wound (Fig. 6, C and D). In saplings treated with 1-MCP and wounding, the same response only occurred directly below the wound and not in adjacent tissue (Fig. 6, E and F). Use of 1-MCP did not produce observable negative effects on growth of control saplings.
Figure 6.
Anatomical changes in Douglas fir stems following 100 mm MJ application or wounding in the presence or absence of the ethylene response inhibitor 1-MCP 3 weeks after treatment. Bars = 200 μm. PB, Phenolic band; PP, PP cell. A, Typical response of Douglas fir following exogenous application of MJ. PP cell swelling and a single tangential band of xylem TDs were formed. B, Pretreatment with 1-MCP substantially reduced the MJ effect with only a rare TD and some PP cell swelling. C and D, Cork borer wounding to the cambium (position indicated by cylinder) results in PP cell swelling and development of a band of phenolic cells and undifferentiated TDs (*) in the xylem. The responses extend well beyond the wound. D, Enlarged area showing response of secondary phloem and xylem. E and F, Cork borer wounding of saplings exposed to 1-MCP. E, Image of stem section showing that the wound response is restricted to the xylem immediately adjacent to the wound in the presence of 1-MCP. F, Enlarged area showing limited response of current year phloem and xylem tissue to wounding after 1-MCP treatment, indicating that ethylene is involved in the wound response.
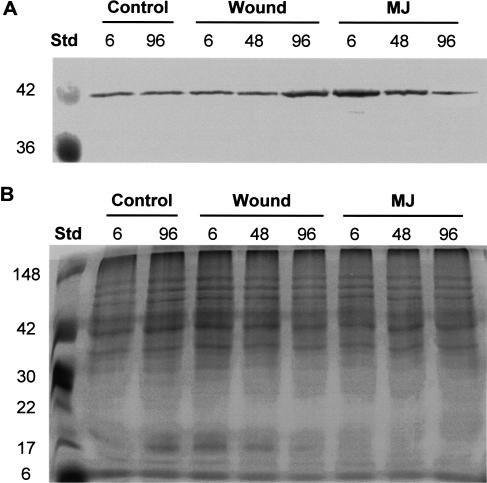
Expression and Localization of ACC Oxidase
To determine which cells may be involved in the MJ-induced ethylene production, we used immunochemical techniques and an antibody to 1-aminocyclopropane-carboxylic acid (ACC) oxidase. Western immunoblots demonstrate that the antibody recognized ACC oxidase from Douglas fir, as indicated by a single band of the correct molecular mass of approximately 40 kD (Fig. 7). This enzyme is shown here to be constitutively expressed, as it is present in control tissue. The enzyme is also inducible; both wounding and MJ treatment results in an increase in the amount of protein (Fig. 7). A clear increase can be seen 96 h after wounding, whereas in MJ-treated plants, the increase can be seen at 6 and 48 h, with a subsequent decrease at 96 h after treatment (Fig. 7). These data are consistent with the data on ethylene production under similar treatments (Fig. 5).
Figure 7.
ACC oxidase protein in Douglas fir bark 6, 48, and 96 h after wounding, MJ, or control treatment. A, Western-blot analysis of ACC oxidase following Tween 20 control treatment (lanes 2 and 3), cork borer wounding (lanes 4–6) and MJ treatment (lanes 7–9). A total of 18 μg of total protein was loaded per lane, separated by SDS-PAGE, blotted to a PVDF membrane, and probed with an anti-ACC oxidase antibody. Prestained Mr (MW) markers were loaded in lane 1. B, Complementary SDS-PAGE stained with Coomassie Blue to verify equal protein loading. Lane 1 was loaded with MW standards.
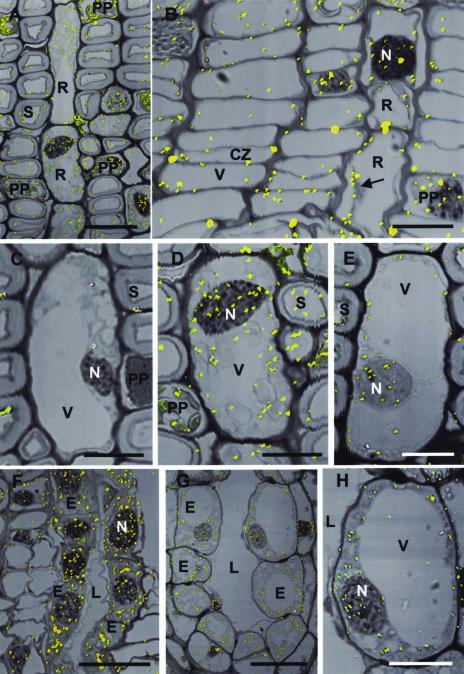
Immunocytochemical localization demonstrated that ACC oxidase was most highly expressed within the cytoplasm of ray parenchyma cells of Douglas fir followed by cambial and PP cells (Fig. 8, A–E). Label was also abundant in secretory epithelial cells of resin ducts, including secondary phloem radial resin ducts (Fig. 8F), cortical axial resin ducts (Fig. 8, G and H), and TD (data not shown). Some label was associated with the nucleus (Fig. 8, A and D–G). The enzyme was present in control (Fig. 8E), wound (Fig. 8G), and MJ-treated (Fig. 8D) tissue, but the greatest amount of labeling occurred with wounding and MJ treatments. Labeling for ACC oxidase was associated with the cytoplasm and nucleus but not with the cell wall, vacuole, or other organelles. Nonimmune serum controls did not give any appreciable labeling of any cell type (Fig. 8C).
Figure 8.
Immunocytochemical localization of ACC oxidase in resin-embedded Douglas fir stem sections. MJ treatments were 100 mm as described in “Materials and Methods.” Reflected/transmitted images of silver-enhanced gold labeling (yellow particles). CZ, Cambial zone; E, resin duct epithelial cell; L, resin duct lumen; N, nucleus; PP, PP cell; R, ray parenchyma cell; S, sieve cell; V, vacuole. A, Secondary phloem of MJ-treated stem. Label is abundant in ray parenchyma, and some label is in young PP cells. Bar = 40 μm. B, Cambial zone from MJ-treated stem. Label is associated with the thin cytoplasm of the cambial zone cells and the cytoplasm and nucleus of ray parenchyma. Arrow points to where cytoplasm has pulled away from the wall, demonstrating label is in cytoplasm and not in the wall or vacuole. Bar = 10 μm. C, Section of a ray parenchyma cell from MJ-treated stem treated with nonimmune serum. Label is absent. Bar = 20 μm. D, Ray parenchyma cell from MJ-treated stem section treated with ACC oxidase antibody showing abundant label in cytoplasm and nucleus. Bar = 20 μm. E, Section of a ray parenchyma cell from control stem treated with ACC oxidase antibody. Bar = 10 μm. F, Section from MJ-treated stem showing labeling of the epithelial cells, including nuclei, surrounding a radial resin duct lumen. Bar = 10 μm. G, Section from wounded stem showing labeling of the epithelial cells of a constitutive cortical resin duct. Bar = 30 μm. H, Enlargement of an epithelial cell from (G) showing label in the cytoplasm and nucleus but not in the vacuole or wall. Bar = 15 μm.
DISCUSSION
Constitutive phenolic and terpenoid compounds are important barriers to pests attacking the stems of conifers, but once compromised, additional defenses, some of which require extensive anatomical changes (Christiansen et al., 1999; Franceschi et al., 2000; Nagy et al., 2000), are elicited. The signals involved in eliciting these defenses are not understood. Here, it is demonstrated that exogenous applications of ethylene can elicit similar defense responses as MJ treatment in stems of two diverse conifers, Douglas fir and giant redwood. The results indicate that ethylene is a downstream signaling agent from MJ and likely the ultimate elicitor of the extensive responses seen involving phenolics and especially terpenoids (Fig. 9). These MJ/ethylene-induced responses are relevant to induced resistance of conifer trees, as both wounding and MJ pretreatments can make mature Norway spruce completely resistant to the bark beetle-vectored fungal pathogen C. polonica (Franceschi et al., 2002; Krokene et al., 2003). MS was ruled out as a signaling agent for activation of phenol and terpenoid synthesis and the alteration of cambial cell programming required for TD development. However, there are many other defense responses we have not yet explored, such as pathogenesis-related protein induction, where MS may play a role.
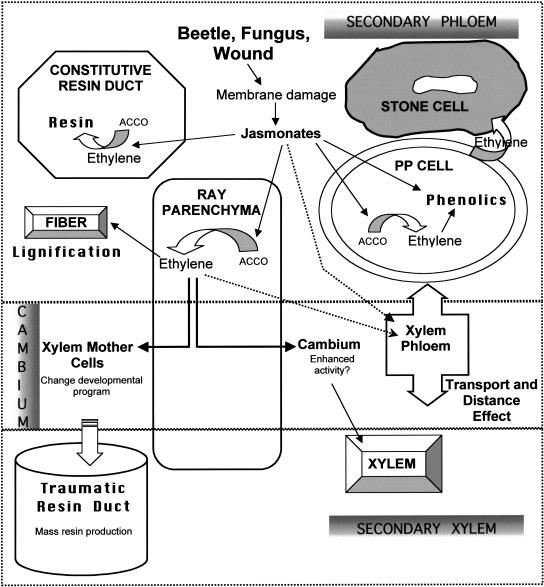
Figure 9.
Model of potential interaction of jasmonates and ethylene with secondary phloem and cambium resulting in anatomically based defense responses in conifer stems. Bark invasion generates jasmonates that induce ACC oxidase (ACCO) and ethylene synthesis in ray parenchyma, cambium, and resin duct epithelia. Ethylene is transported radially by rays and to adjacent cells tangentially, enhancing ethylene levels in PP cells, fibers, cambial cells, and constitutive resin ducts. Ethylene promotes phenolic and resin synthesis and early lignification of fibers. A high-level ethylene burst induces reprogramming of xylem mother cells to form TDs and PP cells to form stone cells. Transport of ethylene or jasmonates by secondary phloem and xylem results in similar responses at some distance from attack, giving rise to a form of systemic resistance.
A major site for phenolic-based defenses in conifer stems is found in the PP cells of the secondary phloem (Franceschi et al., 1998, 2000; Krekling et al., 2000; Nagy et al., 2000; Kusumoto and Suzuki, 2003). Previous studies comparing MJ treatments and wounding found MJ is capable of activating PP cells similar to what was previously shown for Norway spruce in response to wounding or bark beetle attack (Franceschi et al., 2000), in all conifer lineages tested (Franceschi et al., 2002; Hudgins et al., 2003a, 2004). This study indicates that MJ-induced ethylene is likely one direct signal to PP cells eliciting the phenolic response. This is supported by results showing that MJ strongly induces production of ethylene in stems, ethylene alone elicits PP cell activation, and that the ethylene response inhibitor 1-MCP can significantly reduce MJ-induced PP cell activation. Ethylene was also shown to affect lignification, another process involving the phenylpropanoid pathway. Lignificaton is a common response to pathogen invasion (Vance et al., 1980) and is also part of the constitutive defenses in conifers (Hudgins et al., 2003b). Some conifer lineages have multiple tangential rows of fibers in the secondary phloem (Hudgins et al., 2003b, 2004), and lignification of these fibers was accelerated by exogenous MJ application (Hudgins et al., 2004). In this study, early lignification of developing fiber rows was found to occur in giant redwood in response to ethylene alone. MJ and ethylene also induced lignification in Douglas fir in the form of secondary phloem sclerieds (stone cells). Scleried formation typically occurs progressively beginning in 3- to 5-year-old phloem in the few Pinaceae species that have been examined (Janakowski and Golinowski, 2000; T. Krekling and V.R. Franceschi, unpublished data), but the current studies indicate they can be induced to form earlier. A relationship between jasmonates and ethylene in lignification has also been shown in Arabidopsis, in which application of jasmonic acid or ACC (ethylene precursor) caused extensive lignification (Cano-Delgado et al., 2003), suggesting, as shown here, that ethylene may be proximal to the signal transduction events for wound-induced lignification.
Increased production of terpenoid resins is a well-known response to stem injury or MJ treatment of conifers (Bohlman and Croteau, 1999; Trapp and Croteau, 2001; Franceschi et al., 2002; Martin et al., 2002; Hudgins et al., 2003a, 2004). This study indicates ethylene may be ultimately responsible for induced resin production via its effect on the cambial zone as well as on existing constitutive phloem and xylem resin duct epithelial cells (Fig. 9). Formation of axial TD in the xylem is from cells of the cambial zone that would ordinarily form tracheids (Franceschi et al., 2000; Nagy et al., 2000; Hudgins et al., 2004) and that appear to exist as a discrete symplasmic domain as indicated by synchronous TD formation (Krekling et al., 2004). Given our results that ethylene alone can induce TDs and the ethylene response inhibitor can inhibit TD induction by MJ and by wounding, it appears that ethylene is directly inducing reprogramming of cambial zone xylem mother cell differentiation from tracheid (Lachaud et al., 1999; Savidge, 2001) into secretory epithelial cells. While basal levels of ethylene in concert with other growth hormones (indole-3-acetic acid; Little and Pharis, 1995; Eklund and Little, 1998) control normal tracheid formation, a high-level ethylene burst induced by upstream signaling agents dramatically alters cell programming. Because the ethylene burst is dissipated in a day or so, as shown by our ethylene measurements, the subsequently produced derivatives from the cambial initial cells resume their regular developmental programming under a normal ethylene level and differentiate into tracheids, resulting in the TDs being embedded in xylem.
In a study of Norway spruce cuttings, significant increases in ethylene occurred 6 h after wounding (Ingemarsson and Bollmark, 1997), consistent with our results, and this included a turnover of ACC, the substrate for ACC oxidase (Kende, 1993; Bleecker and Kende, 2000). In conifers, ACC oxidase activity has been characterized in stems (Plomion et al., 2000) and needles (Ievinsh and Tillberg, 1995) of a couple of species. Here, it is shown that ACC oxidase is constitutively expressed in conifer stems but that wounding and, especially, exogenous MJ induce a large increase in levels of this enzyme. This increase is rapid and is correlated with the time course of MJ-induced enhancement of ethylene production in stems. Immunolabeling shows the enzyme is highest in the ray parenchyma and cambial zone cells. In addition, epithelial cells of radial resin ducts and axial cortical resin ducts have abundant labeling for the enzyme, which could help explain the rapid induction of resin flow by wounding, shown by Ruel et al. (1998), which occurs too quickly to be accounted for by TD formation. The ray parenchyma cells may be particularly important for ethylene signaling since they are involved in radial flow between secondary phloem and cambium/xylem (Fig. 9), and the cambium is considered to be supplied primarily by the rays (Lev-Yadun and Aloni, 1995). They are also linked anatomically to a tangential pathway in the secondary phloem of the stem via the rows of PP cells to which they are connected by abundant plasmodesmata in the interconnecting radial walls (Krekling et al., 2000).
There is conflicting information about the subcellular location of ACC oxidase. Various reports place it in the cell wall/apoplast (Rombaldi et al., 1994; Gomez-Jimenez et al., 2001), the external face of the plasma membrane (Ramassamy et al., 1998), and the cytosol (Chung et al., 2002). Our results show that the enzyme is present in the cytoplasm and the nucleus but not in other compartments. Nuclear localization in plants and animal cells has been shown for a number of different types of enzymes, including some in the glycolytic and gluconeogenic pathways (Yanez et al., 2003), Man-6-P reductase (Everard et al., 1993), plant hemoglobin (Seregelyes et al., 2000), apyrase (Shibata et al., 2002), lipoxygenase (Brock et al., 2001), and glutathione peroxidase (Orbea et al., 2000). Ethylene-responsive transcription factors are present in the plant nucleus (Johnson and Ecker, 1998; Ohta et al., 2000; Lee and Kim, 2003), and the nuclear localization of ACC oxidase is likely related to ethylene regulation of transcription.
A few investigations have shown a correlation between wounding, ethylene, and monoterpene accumulation in conifers (Peters, 1977; Popp et al., 1995). Increased production of ethylene is also often associated with plant-microbial interactions (Boller, 1991). Popp et al. (1995) demonstrated that following inoculations with pathogenic fungi, the rate of ethylene production and the monoterpene concentrations were closely associated in slash pine (Pinus elliottii) and loblolly pine. Additionally, the authors showed that inoculations with a virulent fungus increased ethylene production over nonpathogenic fungal inoculations, indicating that higher levels of ethylene production were likely a result of continuing fungal growth through stem tissues. Popp et al. (1997) also found that loblolly pine cell cultures exposed to virulent fungus showed increased levels of ethylene production, further demonstrating the relationship between fungal invasion and elicitation of ethylene production. It is possible that fungal toxins or enzymes may result in oxidative processes leading to the formation of jasmonates, which are known to elicit ethylene formation and ACC oxidase activity as shown here and by others (Kim et al., 1998; Watanabe and Sakai, 1998; Ievinsh et al., 2001), but this remains to be tested.
The similarity of responses to MJ and ethylene in the absence of wounding implies these compounds may both be directly involved in the inducible defense response pathway of conifers, similar to the genetically defined defense pathways of Arabidopsis (Pieterse et al., 1998; McDowell and Dangl, 2000). In angiosperms, several models have been proposed where jasmonates and ethylene produce a synergistic effect on certain defense related genes (Xu et al., 1994; O'Donnell et al., 1996; Penninckx et al., 1998) or resistance to multiple organisms (Bostock, 1998). The connection between MJ and ethylene may involve several potential pathways, but evidence provided here indicates that MJ acts upstream of ethylene. We propose a general model for the role of jasmonates and ethylene in the anatomical-based defense responses that we have been investigating (Fig. 9), and this will provide a basis for further investigations resolving the signaling pathway and transduction mechanisms and cellular response in the bark and cambial zone of conifers.
Our previous studies demonstrated that MJ or wounding can elicit a response in the untreated regions above or below the treated regions of mature trees or saplings (Franceschi et al., 2000, 2002; Hudgins et al., 2003a, 2004; Krekling et al., 2004), which is important to systemic resistance in conifers. Krekling et al. (2004) suggested a diffusible signal was responsible for a developmental wave away from a wound that moved at 2.5 cm per day with respect to TD formation. This study shows that ethylene can elicit the same systemic response as wounding and MJ. Branches do not respond, indicating gaseous MJ or ethylene diffusing from the treated internodes is not at a high enough level to penetrate the periderm and is, thus, not responsible for the effect seen in untreated internodes. These observations support the concept that an internal signal is generated that can travel some distance from the region of initial elicitation, via the phloem (downward) and xylem (upward). Ethylene is more soluble than MJ or jasmonic acid in aqueous solutions, and we suggest it may be the primary signaling agent. However, given the constitutive levels of ACC oxidase, it is also possible that ACC (or a conjugate) may be transported as suggested by O'Neill et al. (1993), and a rapid flux of ACC to distant parts of the stem gives rise to a transient ethylene burst. These alternatives remain to be tested.
CONCLUSIONS
This study provides evidence for the activity of MJ and ethylene as key signaling compounds for inducible defense mechanisms and systemic defense involving the phenylpropanoid and terpenoid resin pathways in conifers. This up-regulation parallels at least part of the wound response that is seen in a number of conifers following abiotic or biotic injury and is relevant to acquired resistance against bark beetles and pathogenic fungi. Interestingly, exogenous MJ, ethylene, or MS applications did not induce lesion formation or characteristic tissue damage associated with a hypersensitive response, which implies that other signaling agents are required for the establishment of necrotic tissue and the necrophylactic periderm. When taken together, the data on exogenous ethylene induction of anatomical defense responses, MJ induction of ethylene synthesis and ACC oxidase protein, and 1-MCP inhibition of these responses provide a strong case for ethylene as the immediate signal for PP cell activation and cambial zone reprogramming for TD formation. These studies provide valuable new information on defense response mechanisms and experimental approaches for further work to resolve the signaling and signal transduction pathways of inducible defenses in conifer stems.
MATERIALS AND METHODS
Plant Materials and Greenhouse Conditions
Four-year-old Douglas fir (Pseudotsuga menziesii) and giant redwood (Sequoiadendron giganteum) saplings from a commercial nursery (Forest Farm, Williams, OR) were grown in a Washington State University-Pullman greenhouse. They were approximately 1 m in height, with a diameter of 1.5 to 2.0 cm at the first internode. Saplings were grown in 3-L containers at 23°C day and 18°C night temperatures, under a 14-h-light/10-h-dark regime for 3 months prior to treatments. They received 500 mL of water daily and fertilizer weekly [Peters fertilizer (20:20:20 N:P:K); Scotts Horticulture, Marysville, OH].
Methyl Jasmonate and Methyl Salicylate Treatments
On April 26, 2002, three replicate saplings of each species were treated with 10, 25, 50, or 100 mm MJ (Sigma-Aldrich, St. Louis; provided by Dr. J. Gershenzon) in Millipore (Billerica, MA) Nanopure water with 0.1% (v/v) Tween 20 (Fischer Biotech, Fairlawn, NJ), or 10, 25, 50, or 100 mm MS (Sigma-Aldrich) in water with 0.1% (v/v) Tween 20. Three control trees from each species were treated with 0.1% (v/v) Tween 20 in water. The solutions were applied evenly around the second internode using a small brush and repeated after 5 min to ensure a uniform coating. A small rubber tube was tightened around the bottom of all treated internodes to ensure fluid did not reach the lower untreated internode. Tween 20 is a biologically nonactive detergent used to emulsify MJ and MS and to act as a surfactant to evenly spread the solution on the bark surface (Franceschi et al., 2002). On May 24, 2002, stem samples were collected for microscopy from the middle of the internode below the treated internode, the treated internode, and both internodes (10 and 20 cm, respectively) immediately above the treatment site. Samples were prepared for microscopy as described below. Representative images and statistical analysis are only presented for the application internode from 100 mm MJ, 100 mm MS, Ethaphon, and 0.1% Tween 20 treatments described above for Figures 1 to 3. Statistical analysis was conducted on all MJ concentrations for the dose-response study presented in Figure 4.
Ethylene Treatments
Three saplings of each species were treated with exogenous application of an Ethaphon (Carolina Biological, Burlington, NC) solution, and three received water (control). Ethaphon releases ethylene in the presence of water. At the first internode of all trees, a 5-cm length of stem was wrapped with thin cheesecloth and then wrapped twice with plastic (Saran Wrap: polyvinylidene chloride). Rubber bands were cut and tensioned over the plastic wrap at both ends to create a tight seal without injuring the stem. Ethylene treatments were applied by injecting 5 mL of Ethapon:water solution (1:1, v/v) under the plastic wrap with a small gauge hypodermic needle. Following application, the treated area was wrapped a second time with plastic. Saplings were treated for 12 h, and the apparatus was then removed. Control (water) treatments used the same method. Stem samples were taken 28 d after treatment and were prepared for microscopy as described below.
Ethylene Inhibitor Treatment on Douglas Fir
To examine the potential role of ethylene as a downstream signaling agent from MJ, nine saplings of Douglas fir (three replicates of each of three treatments) were exposed to the ethylene response inhibitor 1-MCP (EthylBloc; Rohm & Haas, Philadelphia) prior to subsequent treatments. Three groups of three saplings were treated separately and sequentially in a sealed 225-L Plexiglas growth chamber. Each group of three saplings was first exposed to 1-MCP at 9 ppm for 12 h, and then the three saplings from each group were treated with Tween 20 (control), 100 mm MJ, or a 5-mm cork borer wound into the sapwood. Along with the treatment, a second dose of 1-MCP of the same concentration was released in the container, and after an additional 12 h of exposure the plants were transferred to a greenhouse. Control, MJ, and wound treatments were conducted independently to exclude volatile effects from wound-induced ethylene or volatile MJ. Identical treatments (Tween 20, MJ, wounding; three saplings for each) were conducted in the absence of 1-MCP as controls. Three weeks after treatment, samples from the first through third internode of each plant were prepared for microscopy as described below.
Ethylene Analysis in Douglas Fir
Twenty-seven Douglas fir saplings were treated with 100 mm MJ in 0.1% Tween 20 (9 trees), 0.1% Tween 20 (9 trees), or wounded twice with a 2-mm cork borer (9 trees) around the stem along a 6-cm length of the first internode. Two 6-cm intact stem sections from each treatment were collected at 6, 24, and 48 h at the first and second internodes, and from three replicate-treated saplings at each time point. Needles were removed from stem sections; the sections were weighed and placed individually in a 10-mL glass vial with a rubber septum stopper. Following incubation times of 2 or 4 h at 22°C, 0.5 cm3 gas samples were drawn from the head space with a tuberculin syringe and injected into a Hewlett-Packard 5890 GC-MS (Hewlett-Packard, Palo Alto, CA) with a J&W Scientific CARBONPLT column (30 m × 0.53 mm; Folsom, CA) and a flame ionization detector. Chromatography was conducted with a prepurified nitrogen gas flow adjusted to 8 mL/min. Injector/detector and oven temperatures were set at 150°C and 200°C, respectively. Ethylene production was determined as nL [g fresh weight (FW) × h]−1. Following sampling, distilled water was added to vials with tissue to determine total vial gas volume.
Protein Extraction and Western-Immunoblot Analysis and Immunocytochemical Analysis of Douglas Fir
Twenty-seven Douglas fir stems were treated as described above for ethylene analysis. Three saplings were collected following each treatment at 6, 48, and 96 h. Stem secondary phloem and cambial zone tissue was separated from the xylem, frozen in liquid nitrogen, and stored at −80°C. Protein extractions were conducted as described previously by Martin et al. (2002), with the addition of the protease inhibitors phenylmethylsulfonyl fluoride (0.5 mm) and Complete Tablets (Roche, Nutley, NJ). Total protein concentrations were determined using a Protein Assay kit (Pierce Biotechnology, Rockford, IL) with bovine serum albumin (BSA) as a standard. Equal masses (18 μg) of protein were loaded in each lane, and the samples were subjected to SDS-PAGE using 10% polyacrylamide gels. Gels were either stained with Coomassie Brilliant Blue R-250 for imaging, or proteins were transferred to a PVDF membrane (Bio-Rad, Hercules, CA). The membrane was incubated for 1 h with TBST + BSA [Tris-buffered saline-Tween 20; 10 mm Tris, 250 mm NaCl, 0.1% (v/v) Tween 20 + 1% (w/v) BSA, pH 7.2] and then treated with a 1:500 dilution of an affinity-purified polyclonal anti-Arabidopsis ACC oxidase (aN-19, raised in goat against a peptide mapping at the amino terminus; Santa Cruz Biotechnology, Santa Cruz, CA) for 3 h. The membrane was rinsed with TBST + BSA (4 × 15 min), and then treated with rabbit anti-goat alkaline phosphatase-tagged secondary antibody (Sigma-Aldrich) diluted 1:25,000 in TBST + BSA for 1 h. After washing, the membrane was allowed to equilibrate in reaction buffer (100 mm Tris, 100 mm NaCl, 10 mm MgCl2, pH 9.5), and protein immunodetection was visualized by a reaction with p-Nitro-Blue tetrazolium chloride (NBT) and X-Phosphate in the reaction buffer (45 μL of NBT and 35 μL of X-Phosphate/10 mL of buffer).
Light Microscopy Staining
Copper Acetate Resin Staining
Samples from experiments were routinely stained to determine resin acid production and location. Tissue was fixed as described below, rinsed (four times) in 50 mm buffer [l-piperazine-N-N′-bis (2-ethane sulfonic) acid, pH 7.2] and hand cut into semithin sections (0.1–0.5 mm). Sections were then soaked 12 h in a saturated (aqueous) copper acetate solution, which stains resin acids blue. Sections were rinsed with distilled water and examined by light microscopy to determine if resin acid accumulation was present.
Sampling and Microscopy Preparation of Stem Samples
Intact stem section samples (3 cm) were collected and directly placed in fixative solution [2% (v/v) paraformaldehyde and 1.25% (v/v) glutaraldehyde buffered in 50 mm l-piperazine-N-N′-bis (2-ethane sulfonic) acid, pH 7.2]. Each sample was subsequently cut into 2.5-mm2 blocks and allowed to fix for 24 h at 4°C. Blocks were subsampled and rinsed with the same buffer solution, dehydrated with an ethanol series, and infiltrated with L.R. White acrylic resin (London Resin, Reading, UK). Samples were polymerized in fresh resin at 62°C for 12 h.
General Anatomy Staining
Sections (1 μm thick) were cut with a diamond knife on a Sorvall MT 5000 Ultramicrotome (Asheville, NC) and dried from a drop of water onto gelatin-coated slides. Stevenel's blue staining (Del Cerro et al., 1980) was used for general observation of tissue structure.
Image Analysis
Scion Image 1.62 imaging software (Scion, Frederick, MD; www.scioncorp.com) was used to make measurements of PP cells and TD from stem cross-sections of each of three replicate saplings for each treatment. The cross-sectional areas of PP cells and their polyphenolic inclusions were measured, whereas both the number of TDs and their cross-sectional areas were determined. Forty to 50 PP cells per section were measured from three different sections and combined to represent one treated internode per sapling. Eight to 10 TDs per section were measured from three different sections and combined to represent a treated internode per sapling. For statistical analysis, data was calculated based on combined averages from three individual saplings (n = 3).
Immunocytochemistry
Stem section tissue was collected from MJ-, wound-, and control (water)-treated Douglas fir following treatments at 6 and 96 h for immunocytochemical comparison with western-blot analysis. Tissue was fixed and embedded in L.R. White as described above and was sectioned at 1 μm. Tissue was first blocked for nonspecific binding of antibodies by incubation for 1 h in TBST + BSA containing 0.05% (w/v) polyvinylpyrrolidone (10,000 molecular weight). Sections were incubated for 4 h at room temperature with goat anti-Arabidopsis ACC oxidase polyclonal antibody diluted 1:75 in TBST + BSA. Following incubation with the primary antibody, the sections were rinsed with TBST + BSA (4 × 15 min) and incubated for 1 h at room temperature with rabbit anti-goat gold-tagged secondary antibody diluted 1:150 with TBST + BSA. After sequential rinsing with TBST + BSA, TBST, and distilled water, the sections were silver enhanced (Amersham Silver Enhancement kit; Amersham Life Science, Buckinghamshire, UK) and counter-stained with 0.5% (w/v) aqueous Safranin O. The sections were coverslipped with immersion oil as a mounting media and imaged with a confocal microscope (Bio-Rad 1024), using a combination of reflected and transmitted imaging.
Statistical Analysis
All statistical analyses were conducted with SAS software (SAS Institute, Cary, NC) with significance judged at P < 0.05 in all cases. Dose-response studies were treated as two-way analysis of variance (ANOVA) in a completely randomized design with protected lsd to compare number of resin ducts and internode location within each MJ concentration. The same analysis was conducted to compare resin duct lumen size and internode location within each MJ concentration. Natural log transformation was performed on the number of ducts because of nonhomogeneous variances among treatment concentrations. For phenolic area, one-way ANOVA in a completely randomized design with protected lsd was used to compare differences among treatment means. For effect of various compounds on giant redwood, resin duct area and number of resin ducts were analyzed by two sample t tests because water, Tween 20, and MS treatments had zero resin ducts. Satterthwaite adjustments were made to the t test due to unequal variances between ethylene and MJ treatment groups. For effect of various compounds on Douglas fir, analysis of resin duct area and number of resin ducts were performed with one-way ANOVA in a completely randomized design with protected lsd to compare between treatment groups. Ethylene production studies following control, wound, and MJ application were treated as two-way ANOVA in a completely randomized design with protected lsd to compare internode location at 6, 24, and 48 h time points.
Acknowledgments
We thank Dr. J. Gershenzon (Max Planck Institute of Chemical Ecology, Jena, Germany) for the generous supply of MJ, Dr. J. Fellman, Ines Müller, and Greg Hoffman (Department of Horticulture and Landscape Architecture, Washington State University) for use of equipment and technical assistance with GC analyses, and Dr. E. Christiansen (Skogforsk, Aas, Norway) and an anonymous reviewer for very helpful comments on the manuscript. Confocal microscopy was done in the Electron Microscopy Center, Washington State University. Dr. R. Alldredge (Department of Statistics, Washington State University) provided consultation for the statistical analysis. This work was conducted as part of an international scientific study on conifer defense mechanisms (CONDEF).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.037929.
References
- Abeles FB, Morgan PW, Saltveit ME (1992) Ethylene in Plant Biology, Ed 2. Academic Press, San Diego, pp 26–55
- Alfaro RI (1995) An induced defense reaction in white spruce to attack by the white pine weevil (Pissodes strobi). Can J For Res 25: 1725–1730 [Google Scholar]
- Andersson-Gunneras S, Hellgren JM, Bjorklund S, Regan S, Moritz T, Sundberg B (2003) Asymmetric expression of a poplar ACC oxidase controls ethylene production during gravitational induction of tension wood. Plant J 34: 339–349 [DOI] [PubMed] [Google Scholar]
- Bannan MW (1936) Vertical resin ducts in the secondary wood of the Abietineae. New Phytol 35: 11–46 [Google Scholar]
- Barker JE (1979) Growth and wood properties of Pinus radiata in relation to applied ethylene. N Z J For Sci 9: 15–19 [Google Scholar]
- Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16: 1–18 [DOI] [PubMed] [Google Scholar]
- Bohlman J, Croteau R (1999) Diversity and variability of terpenoid defenses in conifers: molecular genetics, biochemistry and evolution of the terpene synthase gene family in grand fir (Abies grandis). In DJ Chadwick, JA Goode, eds, Insect Plant Interactions and Induced Plant Defense. John Wiley and Sons, West Sussex, UK, pp 132–146 [DOI] [PubMed]
- Bois E, Lieutier F (1997) Phenolic response of Scots pine clones to inoculation with Leptographium wingfieldii, a fungus associated with Tomicus piniperda. Plant Physiol Biochem 35: 819–825 [Google Scholar]
- Boller T (1991) Ethylene in pathogenesis and disease resistance. In AK Mattoo, JC Suttle, eds, The Plant Hormone Ethylene. CRC Press, Boca Raton, FL, pp 293–314
- Bonello P, Storer AJ, Gordon TR, Wood DL (2003) Systemic effects of Heterobasidion annosum on ferulic acid glucoside and lignin of presymptomatic ponderosa pine phloem, and potential effects on bark-beetle-associated fungi. J Chem Ecol 29: 1167–1182 [DOI] [PubMed] [Google Scholar]
- Bostock RM (1998) Signal conflicts and synergies in induced resistance to multiple attackers. Physiol Mol Plant Pathol 55: 99–109 [Google Scholar]
- Brignolas F, Lacroix B, Lieutier F, Sauvard D, Drouet A, Claudot A-C, Yart A, Berryman AA, Christiansen E (1995) Induced responses in phenolic metabolism in two Norway spruce clones after wounding and inoculation with Ophiostoma polonicum, a bark beetle-associated fungus. Plant Physiol 109: 821–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock TG, Maydanski E, McNish RW, Peters-Golden M (2001) Co-localization of leukotriene A(4) hydrolase with 5-lipoxygenase in nuclei of alveolar macrophages and rat basophilic leukemia cells but not neutrophils. J Biol Chem 276: 35071–35077 [DOI] [PubMed] [Google Scholar]
- Cano-Delgado A, Penfield S, Smith C, Catley M, Bevan M (2003) Reduced cellulose synthesis invokes lignification and defense responses in Arabidopsis thaliana. Plant J 34: 351–362 [DOI] [PubMed] [Google Scholar]
- Cheong JJ, Choi YD (2003) Methyl jasmonate as a vital substance in plants. Trends Genet 19: 409–413 [DOI] [PubMed] [Google Scholar]
- Christiansen E, Franceschi VR, Nagy NE, Krekling T, Berryman AA, Krokene P, Solheim H (1999) Traumatic resin duct formation in Norway spruce after wounding or infection with a bark beetle associated blue-stain fungus Ceratocystis polonica. In F Lieuter, WJ Mattson, MR Wagner, eds, Physiology and Genetics of Tree-Phytophage Interactions. Les Colloques De I'INRA, INRA Editions, Versailles, France, pp 77–89
- Chung MC, Chou SJ, Kuang LY, Charng YY, Yang SF (2002) Subcellular localization of 1-aminocyclopropane-1-carboxylic acid oxidase in apple fruit. Plant Cell Physiol 43: 549–554 [DOI] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE (1997) Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol 48: 355–381 [DOI] [PubMed] [Google Scholar]
- Davis JM, Wu H, Cooke JEK, Reed J, Luce KS, Michler CH (2002) Pathogen challenge, salicylic acid and jasmonic acid regulate expression of chitinase gene homologs in pine. Mol Plant Microbe Interact 15: 380–387 [DOI] [PubMed] [Google Scholar]
- Del Cerro M, Cogen J, Del Cerro C (1980) Stevenel's blue, an excellent stain for optical microscopical study of plastic embedded tissue. Microsc Acta 83: 117–121 [PubMed] [Google Scholar]
- Dolan L (1997) The role of ethylene in the development of plant form. J Exp Bot 48: 201–210 [Google Scholar]
- Durner J, Shah J, Klessig DF (1997) Salicylic acid and disease resistance in plants. Trends Plant Sci 7: 266–274 [Google Scholar]
- Du S, Yamamoto F (2003) Ethylene evolution changes in the stems of Metasequoia glyptostroboides and Aesculus turbinata seedlings in relation to gravity-induced reaction wood formation. Trees (Berl) 17: 522–528 [Google Scholar]
- Eklund L (1990) Endogenous levels of oxygen, carbon dioxide and ethylene in stems of Norway spruce trees during one growing season. Trees (Berl) 4: 150–154 [Google Scholar]
- Eklund L (1991) Hormone levels in the cambial region of Picea abies during the onset of cambial activity. Physiol Plant 82: 385–388 [Google Scholar]
- Eklund L, Little CHA (1995) Interaction between indole-3-acetic acid and ethylene in the control of tracheid production in detached shoots of Abies balsamea. Tree Physiol 15: 27–34 [DOI] [PubMed] [Google Scholar]
- Eklund L, Little CHA (1996) Laterally applied Ethrel causes local increases in radial growth and indole-3-acetic acid concentration in Abies balsamea shoots. Tree Physiol 16: 509–513 [DOI] [PubMed] [Google Scholar]
- Eklund L, Little CHA (1998) Ethylene evolution, radial growth and carbohydrate concentrations in Abies balsamea shoots ringed with Ethrel. Tree Physiol 18: 383–391 [DOI] [PubMed] [Google Scholar]
- Everard JD, Franceschi VR, Loescher WH (1993) Mannose 6-phosphate reductase, a key enzyme in photoassimilate partitioning, is abundant and located in the cytoplasm of photosynthetically active cells of celery (Apium graveolens L.) source leaves. Plant Physiol 102: 345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn A, Werker E, Ben-Zur P (1979) Seasonal effects of wounding and growth substances on development of traumatic resin ducts in Cedrus libani. New Phytol 82: 537–544 [Google Scholar]
- Fäldt J, Martin D, Miller B, Rawat S, Bohlmann J (2003) Traumatic resin defense in Norway spruce (Picea abies): methyl jasmonate-induced terpene synthase gene expression, and cDNA cloning and functional characterization of (+)-3-carene synthase. Plant Mol Biol 51: 119–133 [DOI] [PubMed] [Google Scholar]
- Fan X, Mattheis JP, Fellman JK (1998) A role for jasmonates in climacteric fruit ripening. Planta 204: 444–449 [Google Scholar]
- Farmer EE, Almeras E, Krishnamurthy V (2003) Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr Opin Plant Biol 6: 372–378 [DOI] [PubMed] [Google Scholar]
- Franceschi VR, Krekling T, Berryman AA, Christiansen E (1998) Specialized phloem parenchyma cells in Norway spruce (Pinaceae) bark are an important site of defense reactions. Am J Bot 85: 601–615 [PubMed] [Google Scholar]
- Franceschi VR, Krokene P, Krekling T, Berryman AA, Christiansen E (2000) Phloem parenchyma cells are involved in local and distant defense responses to fungal inoculation or bark-beetle attack in Norway spruce (Pinaceae). Am J Bot 87: 314–326 [PubMed] [Google Scholar]
- Franceschi VR, Krekling T, Christiansen E (2002) Application of methyl jasmonate on Picea abies (Pinaceae) stems induces defense-related responses in phloem and xylem. Am J Bot 89: 578–586 [DOI] [PubMed] [Google Scholar]
- Franich RA, Carson MJ, Carson SD (1986) Synthesis and accumulation of benzoic acid in Pinus radiata needles in response to tissue-injury by dothistromin and correlation with resistance of Pinus radiata families to Dothistroma pini. Physiol Mol Plant Pathol 28: 267–286 [Google Scholar]
- Gomez-Jimenez MD, Garcia-Olivares E, Matilla AJ (2001) 1-aminocyclopropane-1-carboxylate oxidase from embryonic axes of germinating chick-pea (Cicer arietinum L.) seeds: cellular immunolocalization and alterations in its expression by indole-3-acetic acid, abscisic acid and spermine. Seed Sci Res 11: 243–253 [Google Scholar]
- Hudgins JW, Christiansen E, Franceschi VR (2003. a) Methyl jasmonate induces changes mimicking anatomical defenses in diverse members of the Pinaceae. Tree Physiol 23: 361–371 [DOI] [PubMed] [Google Scholar]
- Hudgins JW, Christiansen E, Franceschi VR (2004) Induction of anatomically based defense responses in stems of diverse conifers by methyl jasmonate: a phylogenetic treatment. Tree Physiol 24: 251–264 [DOI] [PubMed] [Google Scholar]
- Hudgins JW, Krekling T, Franceschi VR (2003. b) Distribution of calcium oxalate crystals in the secondary phloem of conifers: a constitutive defense mechanism? New Phytol 159: 677–690 [DOI] [PubMed] [Google Scholar]
- Ievinsh G, Dreibante G, Kruzmane D (2001) Changes of 1-aminocyclopropane-1-carboxylic acid oxidase activity in stressed Pinus sylvestris needles. Biol Plant 44: 233–237 [Google Scholar]
- Ievinsh G, Tillberg E (1995) Stress-induced ethylene biosynthesis in pine needles: a search for the putative 1-aminocyclopropane-1-carboxylic acid-independent pathway. J Plant Physiol 145: 308–314 [Google Scholar]
- Ingemarsson BS, Bollmark M (1997) Ethylene production and 1-aminocyclopropane-1-carboxylic acid turnover in Picea abies hypocotyls after wounding. J Plant Physiol 151: 711–715 [Google Scholar]
- Ingemarsson BS, Lundqvist ME, Eliasson L (1991) Seasonal variation in ethylene concentration in the wood of Pinus sylvestris L. Tree Physiol 8: 273–279 [DOI] [PubMed] [Google Scholar]
- Janakowski S, Golinowski W (2000) Sclerification in the bark tissues of common fir (Abies alba Mill.). Acta Soc Bot Pol 69: 11–20 [Google Scholar]
- Johnson PR, Ecker JR (1998) The ethylene gas signal transduction pathway: a molecular perspective. Annu Rev Genet 32: 227–254 [DOI] [PubMed] [Google Scholar]
- Katoh S, Croteau R (1997) Individual variation in constitutive and induced monoterpene biosynthesis in grand fir. Phytochemistry 47: 577–582 [Google Scholar]
- Kende H (1993) Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol 44: 283–307 [Google Scholar]
- Kim YS, Choi D, Lee MM, Lee SH, Kim WT (1998) Biotic and abiotic stress-related expression of 1-aminocyclopropane-1-carboxylate oxidase gene family in Nicotiana glutinosa L. Plant Cell Physiol 39: 565–573 [DOI] [PubMed] [Google Scholar]
- Klintborg A, Eklund L, Little CHA (2002) Ethylene metabolism in Scots pine (Pinus sylvestris) shoots during the year. Tree Physiol 22: 59–66 [DOI] [PubMed] [Google Scholar]
- Kozlowski G, Metraux JP (1998) Infection of Norway spruce (Picea abies (L.) Karst.) seedlings with Pythium irregulare Buism. and Pythium ultimum Trow.: histological and biochemical responses. Eur J Plant Pathol 104: 225–234 [Google Scholar]
- Kozlowski GA, Buchala A, Metraux JP (1999) Methyl jasmonate protects Norway spruce [Picea abies (L) Karst.] seedlings against Pythium ultimum Trow. Physiol Mol Plant Pathol 55: 53–58 [Google Scholar]
- Krekling T, Franceschi VR, Berryman AA, Christiansen E (2000) The structure and development of polyphenolic parenchyma cells in Norway spruce (Picea abies) bark. Flora 195: 354–369 [Google Scholar]
- Krekling T, Franceschi VR, Krokene P, Solheim H (2004) Differential anatomical response of Norway spruce stem tissues to sterile and fungus infected inoculations. Trees (Berl) 18: 1–9 [Google Scholar]
- Krokene P, Solheim H, Krekling T, Christiansen E (2003) Inducible anatomical defense responses in Norway spruce stems and their possible role in induced resistance. Tree Physiol 23: 191–197 [DOI] [PubMed] [Google Scholar]
- Kusumoto D, Suzuki K (2001) Induction of traumatic resin canals in Cupressaceae by ethrel application. Mokuzai Gakkaishi 47: 1–6 [Google Scholar]
- Kusumoto D, Suzuki K (2003) Spatial distribution and time-course of polyphenol accumulation as a defense response induced by wounding in the phloem of Chamaecyparis obtuse. New Phytol 10: 1469–1481 [DOI] [PubMed] [Google Scholar]
- Lachaud S, Catesson AM, Bonnemain JL (1999) Structure and functions of the vascular cambium. C R Acad Sci Ser III 322: 633–650 [DOI] [PubMed] [Google Scholar]
- Langenheim JH (2003) Plant Resins: Chemistry, Evolution, Ecology, and Ethnobotany. Timber Press, Portland, OR
- Lee JH, Kim WT (2003) Molecular and biochemical characterization of VR-EILs encoding Mung bean ETHYLENE INSENSITIVE3-LIKE proteins. Plant Physiol 132: 1475–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Yadun S, Aloni R (1995) Differentiation of the ray system in woody-plants. Bot Rev 61: 45–84 [Google Scholar]
- Lieutier F, Sauvard D, Brignolas F, Picron V, Yart A, Bastien C, Jay-Allemand C (1996) Changes in phenolic metabolites of Scots pine phloem induced by Ophiostoma brunneo-cilatum, a bark beetle-associated fungus. Eur J For Pathol 26: 145–158 [Google Scholar]
- Little CHA, Eklund L (1999) Ethylene in relation to compression wood formation in Abies balsamea shoots. Trees (Berl) 13: 173–177 [Google Scholar]
- Little CHA, Pharis RP (1995) Hormonal control of radial and longitudinal growth in the tree stem. In BL Gartner, ed, Plant Stems: Physiology and Functional Morphology. Academic Press, San Diego, pp 281–319
- Martin D, Gershenzon J, Bohlmann J (2003) Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce. Plant Physiol 132: 1586–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Tholl D, Gershenzon J, Bohlmann J (2002) Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol 129: 1003–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JM, Dangl JL (2000) Signal transduction in the plant immune response. Trends Biochem Sci 25: 79–82 [DOI] [PubMed] [Google Scholar]
- McKay SAB, Hunter WL, Godard K-A, Wang SW, Martin DM, Bohlmann J, Plant AL (2003) Insect attack and wounding induce traumatic resin duct development and gene expression of (–)-pinene synthase in Sitka spruce. Plant Physiol 133: 368–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MJ, Wilhelm B, Spannagl E, Meinhart HZ (1993) Signaling in the elicitation process is meditated through the octadecanoid pathway leading to jasmonic acid. Proc Natl Acad Sci USA 90: 7490–7494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy NE, Franceschi VR, Solheim H, Krekling T, Christiansen E (2000) Wound-induced traumatic resin duct development in stems of Norway spruce (Pinaceae): anatomy and cytochemical traits. Am J Bot 87: 302–313 [PubMed] [Google Scholar]
- Nicholson RL, Hammerschmidt R (1992) Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol 30: 369–389 [Google Scholar]
- O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ (1996) Ethylene as a signal mediating the wound response of tomato plants. Science 274: 1914–1917 [DOI] [PubMed] [Google Scholar]
- Ohta M, Ohme-Takagi M, Shinshi H (2000) Three ethylene-responsive transcription factors in tobacco with distinct transactivation functions. Plant J 22: 29–38 [DOI] [PubMed] [Google Scholar]
- O'Neill SD, Nadeau JA, Zhang XS, Bui AQ, Halevy AH (1993) Interorgan regulation of ethylene biosynthetic genes by pollination. Plant Cell 5: 419–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbea A, Fahimi HD, Cajaraville MP (2000) Immunolocalization of four antioxidant enzymes in digestive glands of mollusks and crustaceans and fish liver. Histochem Cell Biol 114: 393–404 [DOI] [PubMed] [Google Scholar]
- Penninckx IAMA, Thomma BPHJ, Buchala A, Metraux J-P, Broekaert W (1998) Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10: 2103–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters WJ (1977) Ethrel-bipyridilium synergism in slash pine. In Proceedings of the Fourth Lightwood Research Coordinating Council, Annual Meeting, January 18–19, 1977. Energy Research and Development Program, pp 78–83
- Phillips MA, Croteau R (1999) Resin-based defenses in conifers. Trends Plant Sci 5: 184–190 [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, van Wees SCM, van Pelt JA, Knoester M, Laan R, Gerrits H, Weisbeek PJ, van Loon LC (1998) A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10: 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomion C, Pionneau C, Brach J, Costa P, Bailleres H (2000) Compression wood-responsive proteins in developing xylem of maritime pine (Pinus pinaster Ait.). Plant Physiol 123: 959–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp M, Johnson JD, Lesney M (1995) Changes in ethylene production and monoterpene concentration in slash pine and loblolly pine following inoculation with bark beetle vectored fungi. Tree Physiol 15: 807–812 [DOI] [PubMed] [Google Scholar]
- Popp MP, Lesney MS, Davis JM (1997) Defense responses elicited in pine cell suspension cultures. Plant Cell Tissue Organ Cult 47: 199–206 [Google Scholar]
- Ramassamy S, Olmos E, Bouzayen M, Pech JC, Latche A (1998) 1-aminocyclopropane-1-carboxylate oxidase of apple fruit is periplasmic. J Exp Bot 49: 1909–1915 [Google Scholar]
- Reid RW, Whitney HS, Watson JA (1967) Reactions of lodgepole pine to attack of Dendroctonus ponderosae Hopkins and blue stain fungus. Can J Bot 45: 1115–1126 [Google Scholar]
- Richard S, Drevet C, Jouanin L, Seguin A (2000) Induction of chalcone synthase expression in white spruce by wounding and jasmonate. Plant Cell Physiol 41: 982–987 [DOI] [PubMed] [Google Scholar]
- Rombaldi C, Lelievre JM, Latche A, Petitprez M, Bouzayen M, Pech JC (1994) Immunocytolocalization of 1-aminocyclopropane-1-carboxylic acid oxidase in tomato and apple fruit. Planta 192: 453–460 [DOI] [PubMed] [Google Scholar]
- Ruel JJ, Ayres MP, Lorio PL (1998) Loblolly pine responds to mechanical wounding with increased resin flow. Can J For Res 28: 596–602 [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8: 1809–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saniewski M (1997) The role of jasmonates in ethylene biosynthesis. In AK Kanellis, C Chang, H Kende, D Grierson, eds, Biology and Biotechnology of the Plant Hormone Ethylene. Kluwer Academic Publishers, NATO ASI Series, Dordrecht, The Netherlands, pp 39–45
- Savidge RA (2001) Intrinsic regulation of cambial growth. J Plant Growth Regul 20: 52–77 [Google Scholar]
- Schultz TP, Boldin WD, Fischer TH, Nicholas DD, McMurtrey KD, Pobanz K (1992) Structure-fungicidal properties of some 3- and 4-hydroxylated stilbenes and bibenzyl analogues. Phytochemistry 31: 3801–3806 [Google Scholar]
- Seregelyes C, Mustardy L, Ayaydin F, Sass L, Kovacs L, Endre G, Lukacs N, Kovacs I, Vass I, Kiss GB, et al (2000) Nuclear localization of a hypoxia-inducible novel non-symbiotic hemoglobin in cultured alfalfa cells. FEBS Lett 482: 125–130 [DOI] [PubMed] [Google Scholar]
- Shibata K, Abe S, Yoneda M, Davies E (2002) Sub-cellular distribution and isotypes of a 49-kDa apyrase from Pisum sativum. Plant Physiol Biochem 40: 407–415 [Google Scholar]
- Sticher L, Mauch-Mani B, Metraux J-P (1997) Systemic acquired resistance. Annu Rev Phytopathol 35: 235–270 [DOI] [PubMed] [Google Scholar]
- Telewski FW, Jaffe MJ (1986) Thigmomorphogenesis: the role of ethylene in the response of Pinus taeda and Abies fraseri to mechanical perturbation. Physiol Plant 66: 227–233 [DOI] [PubMed] [Google Scholar]
- Telewski FW, Wakefield AH, Jaffe MJ (1983) Computer-assisted image analysis of tissues of ethrel-treated Pinus taeda seedlings. Plant Physiol 72: 177–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlin ES, Alfaro RI, Borden JH, He F (1998) Histological response of resistant and susceptible white spruce to simulated white pine weevil damage. Tree Physiol 18: 21–28 [DOI] [PubMed] [Google Scholar]
- Trapp S, Croteau R (2001) Defensive resin biosynthesis in conifers. Annu Rev Plant Physiol Plant Mol Biol 52: 689–724 [DOI] [PubMed] [Google Scholar]
- Vance CP, Kirk TK, Sherwood RT (1980) Lignification as a mechanism of disease resistance. Annu Rev Phytopathol 18: 259–288 [Google Scholar]
- Viiri H, Annila E, Kitunen V, Niemelia P (2001) Induced responses in stilbenes and terpenes in fertilized Norway spruce after inoculation with blue-stain fungus, Ceratocystis polonica. Trees (Berl) 15: 112–122 [Google Scholar]
- Watanabe T, Sakai S (1998) Effects of active oxygen species and methyl jasmonate on expression of the gene for a wound-inducible 1-aminocyclopropane-1-carboxylate synthase in winter squash (Cucurbita maxima). Planta 206: 570–576 [Google Scholar]
- Werker E, Fahn A (1969) Resin ducts of Pinus halepensis Mill. – their structure, development and pattern of arrangement. Bot J Linn Soc 62: 379–411 [Google Scholar]
- Wu H, Hu Z (1997) Comparative anatomy of resin ducts of the Pinaceae. Trees (Berl) 11: 135–143 [Google Scholar]
- Xu Y, Chang PFL, Liu D, Narasimhan ML, Raghothama KG, Hasegawa PM, Bressan RA (1994) Plant defense genes are synergistically induced by ethylene and methyl jasmonate. Plant Cell 6: 1077–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto F, Kozlowski TT (1987) Effects of flooding, tilting of stems, and Ethrel application on growth, stem anatomy and ethylene production of Pinus densiflora seedlings. J Exp Bot 38: 293–310 [Google Scholar]
- Yanez AJ, Bertinat R, Concha II, Slebe JC (2003) Nuclear localization of liver FBPase isoenzyme in kidney and liver. FEBS Lett 550: 35–40 [DOI] [PubMed] [Google Scholar]
- van Loon LC, Pierpoint WS, Boller T, Conejero V (1994) Recommendations for naming plant pathogenesis related proteins. Plant Mol Biol Rep 12: 245–264 [Google Scholar]