Abstract
Tetrapyrrole compounds, such as chlorophylls, hemes, and phycobilins, are synthesized in many enzymatic steps. For regulation of the tetrapyrrole metabolic pathway, it is generally considered that several specific isoforms catalyzing particular enzymatic steps control the flow of tetrapyrrole intermediates by differential regulation of gene expression depending on environmental and developmental factors. However, the coordination of such regulatory steps and orchestration of the overall tetrapyrrole metabolic pathway are still poorly understood. In this study, we developed an original mini-array system, which enables the expression profiling of each gene involved in tetrapyrrole biosynthesis simultaneously with high sensitivity. With this system, we performed a transcriptome analysis of Arabidopsis seedlings in terms of the onset of greening, endogenous rhythm, and developmental control. Data presented here clearly showed that based on their expression profiles at the onset of greening, genes involved in tetrapyrrole biosynthesis can be classified into four categories, in which genes are coordinately regulated to control the biosynthesis. Moreover, genes in the same group were similarly controlled in an endogenous rhythmic manner but also by a developmental program. The physiological significance of these gene clusters is discussed.
Tetrapyrrole compounds play an essential role in all living organisms. They are involved in various metabolic processes such as energy transfer, signal transduction, and catalysis (Dailey, 1990). In plants, at least three structurally and functionally distinct classes of tetrapyrroles can be distinguished, Mg-porphyrins, Fe-porphyrins, and phycobilins. Mg-porphyrins, such as chlorophylls (Chls), are the most abundant tetrapyrrole compounds on earth. As part of the photosynthetic apparatus, the Mg-porphyrin rings of Chl a and Chl b are involved in light trapping and charge separation for photosynthesis. In addition, Mg-porphyrin intermediates have been shown to regulate the transcription of nuclear-encoded photosynthetic genes in plant systems (Susek and Chory, 1992; Rodermel, 2001). Fe-porphyrins, hemes, constitute the second class of tetrapyrroles. In particular, protoheme functions as a redox-active cofactor or prosthetic group in many proteins. For instance, protoheme is bound to the various cytochromes of the plastidic and mitochondrial electron transport chains and to soluble enzymes, such as catalase and peroxidase. Apart from these functions, heme and its derivatives have also been shown to regulate a variety of cellular processes in animal and bacterial systems, such as transcription (Guarente and Mason, 1983), translation (Chen et al., 1989; Joshi et al., 1995), and posttranslational protein translocation (Lathrop and Timko, 1993). Phytochromobilin belongs to the third class of plant tetrapyrroles, the phycobilins. In contrast to Chls and hemes, phycobilins are linear pigments. Phytochromobilin is covalently bound to the phytochrome apoprotein (Lagarias and Rapoport, 1980), and this holocomplex is known to act as a photoreceptor that controls various developmental processes (Furuya, 1993).
In plants and many bacteria, all of the tetrapyrroles originate from a common biosynthetic pathway (Beale, 1999). This pathway, the so-called C5 pathway, starts at glutamyl-tRNAGlu, and the subsequently formed 5-aminolevulinic acid (ALA) is metabolized to form tetrapyrroles through a variety of reactions. All of the steps in the biosynthesis of Mg-porphyrins and Fe-porphyrins are shared up to protoporphyrin IX. As two or more products are often formed simultaneously or at different stages of development within a single organism, the process obviously requires a high degree of metabolic regulation. During the last decade, considerable progress has been made in elucidating the biosynthetic enzymes, cloning their genes, and studying their regulated expression in response to light and other environmental and developmental factors. A regulatory model has emerged as to how their expression can affect the flow of metabolites through the C5 pathway, reflecting the varying needs for tetrapyrroles in response to different environmental and developmental conditions. It is generally considered that several specific isoforms catalyzing particular enzymatic steps control the flow of tetrapyrrole intermediates via the differential regulation of gene expression depending on environmental and developmental factors, as well as the potent negative feedback regulation of enzyme activity (Reinbothe and Reinbothe, 1996; Grimm, 1998). The light-, tissue-, and developmental-dependent expression as well as diurnal and circadian expression of genes in the tetrapyrrole pathway have been described earlier (He et al., 1994; Papenbrock et al., 1999). All these conclusions, however, are based on experiments with a limited number of genes, and the coordination of such regulatory steps and orchestration of the overall tetrapyrrole metabolic pathway are still poorly understood. It is therefore necessary to test these ideas with as many genes as possible.
With the sequencing of the entire genome of Arabidopsis (Arabidopsis-Genome-Initiative, 2000), it has become feasible to study gene expression in a genome-wide fashion using high-density oligonucleotide microarrays or DNA microarrays. Unfortunately, the high cost of the necessary instruments and supplies has prevented widespread use of this technique. In addition, although an enormous amount of data obtained with microarrays is needed to elucidate physiological events in a genome-wide fashion, the amount is often too great to handle for the analysis of a particular metabolic pathway. Alternatively, cDNA macroarrays using PCR-amplified DNA fragments, such as expressed sequence tags, spotted on nylon membranes are much less expensive and use relatively standard laboratory equipment. However, the cross-hybridization of multiple labeled cDNA species cannot be identified with this method. An alternative solution to such limitations may be a mini-array membrane hybridization method using gene-specific probes from a small biosynthetic pathway. Recently, laboratory-made mini-array systems have been shown to reliably assess the expression of genes simultaneously from yeast (Hauser et al., 1998; Cox et al., 1999; Fukuzawa et al., 2001), rats and mice (Dai et al., 2002), and Arabidopsis (Matsuyama et al., 2002).
In this study, we developed a novel mini-array system to monitor the expression profile of the tetrapyrrole biosynthetic pathway in Arabidopsis. Using this system, we illustrate the profiles of changes in gene expression in terms of the onset of greening, endogenous rhythm, and developmental control. Our findings demonstrated the potential of our mini-array system for detection and analysis of the expression of genes involved in tetrapyrrole biosynthesis in a genome-wide fashion. With this system, we clearly showed that the genes corresponding to regulatory enzymes of tetrapyrrole biosynthesis can be classified into four categories, in which genes are coordinately regulated by endogenous factors to control the biosynthesis.
RESULTS
Development of the Mini-Array System
Our database search of the Arabidopsis genome identified 35 genes involved in the biosynthesis of tetrapyrrole (Table I) from numerous previous studies and a recent database search by Lange and Ghassemian (2003). These genes encode enzymes of the common pathway leading from glutamyl-tRNAGlu to protoporphyrin IX via the C5 pathway (17 genes), Mg-branch to synthesize Chl a and b (11 genes), and Fe-branch to synthesize protoheme and phytochromobilin (7 genes). Among 18 enzymatic steps tested, isoforms were found in 9 steps, although some isoforms were presumed to be pseudogenes, such as CPO2, or their functions were not identified experimentally. To monitor the quantitative expression of all 35 genes simultaneously, and, specifically, we developed an original mini-array system, which is based on the membrane-supported macroarray technique (Pietu et al., 1996).
Table I.
List of genes spotted on the mini-array membrane
| Col | Row | Gene Product | No. | Annotation | AGI Code | Comment |
|---|---|---|---|---|---|---|
| Tetrapyrrole Biosynthetic Genes | ||||||
| 1 | 1 | Glutamyl-tRNA reductase | 3 | HEMA1 | At1g58290 | |
| 1 | 2 | HEMA2 | At1g09940 | |||
| 1 | 3 | HEMA3 | At2g31250 | By homology | ||
| 1 | 4 | Glu 1-semialdehyde aminotransferase | 2 | GSA1 | At5g63570 | By homology |
| 2 | 1 | GSA2 | At3g48730 | By homology | ||
| 2 | 2 | 5-aminolevulinic acid dehydratase | 2 | ALAD1 | At1g69740 | By homology |
| 2 | 3 | ALAD2 | At1g44318 | By homology | ||
| 2 | 4 | Porphobilinogen diaminase | 1 | PBGD | At5g08280 | |
| 3 | 1 | Uroporphyrinogen III synthase | 1 | UROS | By homology, GenBank; AC002504 | |
| 3 | 2 | Uroporphyrinogen III decarboxylase | 2 | URO1 | At2g40490 | By homology |
| 3 | 3 | URO2 | At3g14930 | By homology | ||
| 3 | 4 | SAM uroporphyrinogen III methyltransferase | 1 | UPM1 | At5g40850 | Complemented yeast UPM mutant |
| 4 | 1 | Coproporphyrinogen III oxidase | 2 | CPO1 | At1g03480 | Mutant isolated as lin2 |
| 4 | 2 | CPO2 | GenBank; AC005275, Pseudogene? | |||
| 4 | 3 | CPO3 | At5g63290 | Bacterial O2-independent type | ||
| 4 | 4 | Protoporphyrinogen IX oxidase | 2 | PPO1 | At5g14220 | By homology |
| 5 | 1 | PPO2 | At4g01690 | |||
| 5 | 2 | Ferrochelatase | 2 | FC1 | At5g26030 | |
| 5 | 3 | FC2 | At2g30390 | |||
| 5 | 4 | Heme oxygenase | 4 | HO1 | At2g26670 | Mutant isolated as hy1 |
| 6 | 1 | HO2 | At2g26550 | |||
| 6 | 2 | HO3 | At1g69720 | By homology | ||
| 6 | 3 | HO4 | At1g58300 | By homology | ||
| 6 | 4 | Phytochromobilin synthase | 1 | HY2 | At3g09150 | Mutant isolated as hy2 |
| 7 | 1 | Mg-chelatase ChlI | 2 | CHLI1 | At4g18490 | |
| 7 | 2 | CHLI2 | At5g45930 | |||
| 7 | 3 | ChlH | 1 | CHLH | At5g13630 | Mutant isolated as gun5 |
| 7 | 4 | ChlD | 1 | CHLD | At1g08520 | |
| 8 | 1 | SAM Mg-protoporphyrin IX methyltransferase | 1 | CHLM | At4g25080 | |
| 8 | 2 | Mg-protoporphyrin IX monomethylester cyclase | 1 | CRD1 | At3g56940 | |
| 8 | 3 | Protochlorophyllide oxidoreductase | 3 | PORA | At5g54190 | |
| 8 | 4 | PORB | At4g27440 | |||
| 9 | 1 | PORC | At1g03630 | |||
| 9 | 2 | Chlorophyll synthetase | 1 | CHLG | At3g51820 | |
| 9 | 3 | Chlorophyll a oxygenase | 1 | CAO | At1g44446 | |
| Control Genes | ||||||
| 9 | 4 | Monogalactosyl diacylglycerol synthetase | 3 | MGD1 | At4g31780 | Chloroplast marker |
| 10 | 1 | MGD2 | At5g20410 | Chloroplast marker | ||
| 10 | 2 | MGD3 | At2g11810 | Chloroplast marker | ||
| 10 | 3 | Response regulator | 1 | ARR5 | At3g48100 | Cytokinin-responsive |
| 10 | 4 | Low temperature and salt responsive protein | 1 | RCI2a | At3g05880 | Abscisic acid responsive |
| 11 | 1 | 12-Oxophytodienoate reductase | 2 | OPR1 | At1g76680 | Jasmonate-responsive |
| 11 | 2 | OPR2 | At1g76690 | Jasmonate-responsive | ||
| 11 | 3 | Aconitate hydratase | 1 | ACO | At4g35830 | Ethylene-responsive |
| 11 | 4 | Pathogenesis related protein 1 (PR1) | 1 | PR1 | At2g14610 | Salicylate-responsive |
| 12 | 1 | Actin (housekeeping) | 1 | ACT8 | At1g49240 | House keeping |
| 12 | 2 | Polyubiquitin (UBQ10) | 1 | UBQ10 | At4g05320 | House keeping |
| 12 | 3 | Light-harvesting Chl a/b binding protein | 1 | Lhcb6 | At1g15820 | Chloroplast marker |
| 12 | 4 | Negative control (λDNA) | 1 | Negative control |
A total of 35 genes involved in tetrapyrrole biosynthesis found in the database of the complete genome sequence of Arabidopsis were spotted together with control genes. It should be noted that the annotation of isoforms may vary from those in previous publications.
To enable quantitative analysis of the expression of each gene with high sensitivity, precautions were taken to produce long (150–300 bp) DNA probes immobilized on the membrane with high gene specificity. When coding sequences with high homology were found between isoforms, we chose gene-specific regions, such as the 3′-untranslated region, and amplified them by reverse transcription (RT)-PCR (Supplemental Fig. 1, available at www.plantphysiol.org). Sequences of primers used for amplification are listed in Supplemental Table I. The specific hybridization of each RT-PCR product was confirmed by genomic southern analysis to give a single band (Supplemental Fig. 2). Resultant DNA fragments were purified and used as DNA probes for the mini-array system. Probes for ubiquitin (UBQ10; Sun and Callis, 1997) and actin (ACT8; An et al., 1996) as housekeeping genes, the light-harvesting Chl a/b complex of photosystem II (Lhcb) as a marker gene of chloroplast development, and λDNA as negative control were also spotted. We also spotted various chloroplast-related and phytohormone-responsive genes (Table I). Consequently, a total of 48 probes were spotted in duplicate in a nylon membrane, in which one set of all probes was designed to spot in one grid area (Fig. 1A). Since the amount of probe material has a strong impact on the sensitivity and reproducibility (Bertucci et al., 1999), exactly 4 ng (equivalent to 1 × 1010 molecules) of each probe was immobilized to each spot to facilitate quantitative analysis.
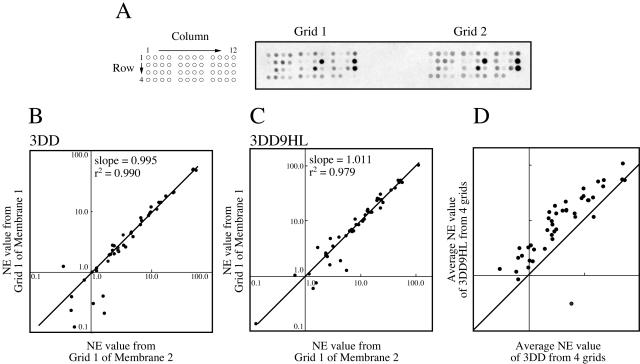
Figure 1.
Evaluation and reproducibility of the developed mini-array system. A, Design and scanned image of the mini-array. On one membrane, two grids consisting of 48 genes as described in Table I were spotted. Two scanned images from one membrane after hybridization (right). Results from two different array membranes hybridized with target DNA prepared from 3-d-old etiolated seedlings (3DD; B) and subsequently illuminated for 9 h (3DD9HL; C). NE values obtained from grid 1 of membrane 1 and 2 were plotted. A fitted line was drawn, and the obtained slope and correlation factors are described in the inset. D, Comparison between 3DD and 3DD9HL. In this plot, averaged NE values from four images of each sample are plotted. A line with slope 1 was drawn in the plot. The data showed that most genes are induced in 3DD9HL, while two spots below the line represent suppressed genes.
To evaluate the quality of this mini-array, we carried out a pilot hybridization experiment with two independent samples: total RNA isolated from 3-d-grown etiolated seedlings and subsequently 9-h-illuminated seedlings. In both samples, two mini-array membranes were hybridized simultaneously with 33P-labeled cDNA probe synthesized from each sample. Consequently, four images were obtained from each sample, which were almost indistinguishable either in the same or in another mini-array membrane (Fig. 1A). To make quantitative evaluations, the filter background, which is 0.8 times the lowest signal, was subtracted from the hybridization value, and then normalized by division with the signal intensity of the housekeeping ACT. This lower limit was selected since at levels closer to the background, a slight variation in background levels across the filter artificially affects the profile (see “Materials and Methods”). Figure 1, B and C, shows plots of two representative sets of data on row signal intensity from two samples. A good correlation was obtained in all data sets. We observed a linear correlation with a mean correlation factor of more than 0.98 and mean slope of 1.03 to 1.04 with a sd of less than 0.09. Thus, the developed mini-array system has a high degree of reproducibility and is valid for linear quantitative determination of the expression of genes of tetrapyrrole biosynthesis in a genome-wide fashion. When averaged data from two samples were compared, most of the spots showed significant changes in their expression level (Fig. 1D).
Gene Expression Profiling of Genes of Tetrapyrrole Biosynthesis at the Onset of Greening
During chloroplast development, higher plants need to synthesize Chl synchronously with Chl-binding proteins. In fact, it has been demonstrated that the expression of Chl-binding proteins and key enzymes of tetrapyrrole biosynthesis, such as glutamyl-tRNA reductase (Tanaka et al., 1996), Glu 1-semialdehyde aminotransferase (Ilag et al., 1994), and subunits of Mg-chelatase (Jensen et al., 1996), is induced by illumination. To clarify the expression profiles of the genes involved in the biosynthesis of tetrapyrrole during chloroplast development, total RNA was extracted from 3-d-old etiolated seedlings illuminated for various periods up to 24 h and analyzed with the proposed mini-array system. Hybridization experiments were carried out with triplicate independent sampling of seedlings (Supplemental Table II).
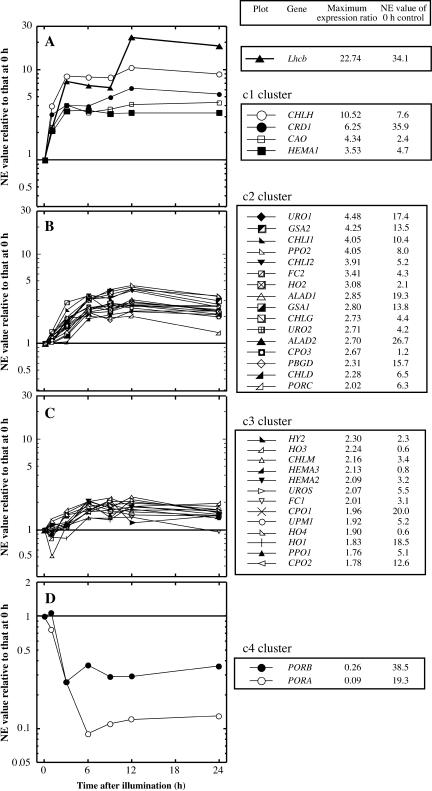
After subsequent data processing, normalized expression (NE) values (see “Materials and Methods”) were obtained for each gene. The NE values were further transformed into values relative to the NE values of a dark control (0 h), since these relative values are generally favorable for categorizing expression profiles, while the NE is valuable for evaluating absolute expression levels. Accuracy of resultant expression profiles was confirmed by northern-blot analysis (Supplemental Fig. 3). Subsequently, using the profile of the relative value from each gene, we performed clustering for all the genes using self-organizing mapping (SOM; Tamayo et al., 1999) with the GeneCluster2 software. Subsequently, genes were grouped into 4 categories from cluster 1 (c1) to cluster 4 (c4). The resulting four categories showed a good correlation with the fold changes of the maximum or minimum NE value during greening to that of the dark control, which we termed the maximum expression ratio (Fig. 2). This value indicates the level of induction or repression of gene expression during the onset of greening. The maximum expression ratio was 3.5 to 22.7 for c1, 2.0 to 4.5 for c2, 1.8 to 2.3 for c3, and less than 0.3 for c4. As shown in Figure 2A, c1 represents a group for genes the expression of which was rapidly induced by light within 1 h and reached a plateau after 3 h of illumination. The expression of Lhcb was rapidly induced within 3 h, and then a second peak appeared after 12 h of illumination. In this cluster, four genes were involved, HEMA1, an isoform of glutamyl-tRNA reductase, CHLH, a subunit of Mg-chelatase, CRD1, a putative Mg-protoporphyrin IX monomethylester cyclase, and CAO, chlorophyll(ide) a oxygenase. c2 is the biggest cluster with 16 genes, showing a gradual increase in expression and a maximum after 9 to 12 h of illumination (Fig. 2B). Interestingly, except for PORC, a light-inducible isoform of NADPH:protochlorophyllide oxidoreductase (POR), CHLG encoding Chl synthetase, and HO2 encoding heme oxygenase, all other genes encode enzymes involved in the earlier steps of tetrapyrrole biosynthesis up to the insertion of metal into protoporphyrin IX. Thirteen genes belonging to c3 show an almost constitutive expression, although the expression of some genes fluctuated slightly after illumination (Fig. 2C). These fluctuations are probably caused by a slight variation in the expression levels of genes with very low NE values. Two genes, PORA and PORB, both isoforms of POR, belonged to c4 (Fig. 2D). These were the only two genes that were negatively regulated by light. They are characteristic as they showed a high level of NE in the dark control, but this value decreased dramatically within 3 to 6 h, which is consistent with a previous study by Armstrong et al. (1995).
Figure 2.
Clustering of genes involved in tetrapyrrole biosynthesis at the onset of greening. Expression profiles of each gene at the onset of greening (0, 1, 3, 6, 9, 12, and 24 h after illumination) were clustered into four groups based on SOM analysis. In each plot, relative NE values to that of 0 h control were plotted on a semilogarithmic scale. Genes clustered in c1 and Lhcb (A), c2 (B), c3 (C), and c4 (D) are plotted. The maximum expression ratio and NE value of the 0 h control of each gene are shown in the right section.
Rhythmic Regulation of the Genes of Tetrapyrrole Biosynthesis in Mature Plants
In mature leaves that possess developed chloroplasts, the synthesis of Chl and Chl-binding proteins is closely coordinated and regulated by light and the endogenous clock for the assembly of a functional photosynthetic apparatus (Beator and Kloppstech, 1993). To determine the gene expression profiles for tetrapyrrole biosynthesis under diurnal and circadian rhythms, samplings were carried out from 3-week-old seedlings of Arabidopsis harvested after growth under a cyclic 12-h light/12-h dark regime or subsequently after a second round of continuous illumination. Total RNA isolated from the samples was analyzed using the mini-array system in duplicated hybridization experiments (Supplemental Table III).
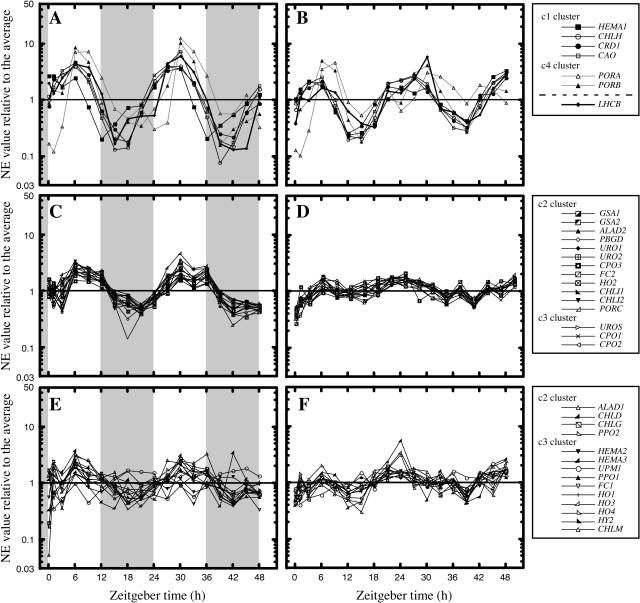
After data processing, the SOM analysis clustered genes into three categories: the first group is regulated by both diurnal and circadian rhythms, the second is only regulated by diurnal rhythm, and the third is nonrhythmic (Fig. 3). In these plots, to compare the relative changes in gene expression throughout the tested periods, the NE value from each gene was normalized by dividing by the average NE value of each gene. In this case, the resulting relative ratio gives the relative fluctuation of gene expression and the average of NE is the average expression level relative to that of ACT.
Figure 3.
Rhythmic regulation of the genes involved in tetrapyrrole biosynthesis in mature plants. Expression profiles of each gene under diurnal (A, C, and E) and circadian (B, D, and F) growth conditions were clustered into three groups based on SOM analysis. In the diurnal cycle, 3-week-old seedlings were maintained on a 12-h light/12-h dark (gray) cycle, while they were transferred under continuous light for the circadian experiment. Zeitgeber time (hours after onset of experiments) is indicated at the bottom. In each plot, the NE value relative to the average NE was plotted on a semilogarithmic scale. Genes regulated by both diurnal and circadian rhythms (A and B), genes only regulated by diurnal rhythm (C and D), and nonrhythmic genes (E and F) are plotted. Genes in each group are depicted in the right section.
The first group contained six genes. Interestingly, this group was composed of the four genes in cluster c1 and the two genes in cluster c4 (Fig. 3, A and B). The phase and amplitude of the four genes in c1 were synchronized and coincided with that of Lhcb, whereas those of the two genes in c4 (PORA and PORB) were synchronized to each other, but the peak of oscillation was somewhat delayed compared to that of c1. These results suggest that four genes in c1, which are dramatically induced by illumination at the onset of greening, are also important for coordinating the assembly of a functional photosynthetic apparatus with the endogenous clock, and two genes in c4 may be differentially regulated by the endogenous clock to maintain Chl biosynthesis. As shown in Figure 3, C and D, 19 genes were clustered into the second group, which was regulated only by diurnal rhythm. This group was composed of the c2 and c3 clusters and 16 genes corresponding to the earlier steps of tetrapyrrole biosynthesis up to the metal insertion stage. Moreover, the phase and amplitude of all genes coincided well under diurnal light/dark cycles (Fig. 3C). When plants were transferred to continuous light, the rhythmic expression of these genes disappeared, although some fluctuations remained (Fig. 3D). The remaining genes involved in the c2 and c3 cluster did not show a pronounced change in expression under diurnal or circadian growth conditions (Fig. 3, E and F).
Development-Dependent Expression of the Genes of Tetrapyrrole Biosynthesis
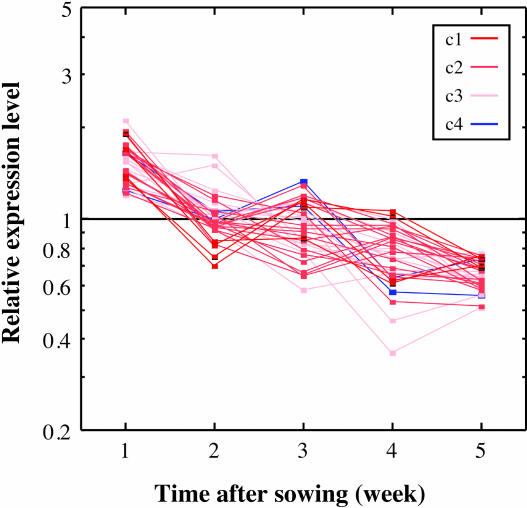
Finally, we determined the development-dependent control of the expression of these genes. Whole plants were sampled every week after sowing and analyzed using the mini-array system (Supplemental Table IV). The relative expression level in each developmental stage was calculated by dividing the NE value of each developmental stage by the average NE value for all the stages. In this experiment, regardless of the clusters of light responsiveness, all genes basically showed a similar profile of expression (Fig. 4). The highest level of gene expression was observed after 1 week of germination, after which there was a decline to the average level, which was retained until week 4. Although several genes showed a peak of expression after 3 weeks of germination, we could not find definite correlation of these genes with the gene clusters. The level then declined after 5 weeks of germination. Thus, it is likely that the genes of tetrapyrrole biosynthesis tested with this mini-array system are similarly controlled by development, although they markedly differed in the light-responsive and rhythmic control of their expression.
Figure 4.
Development-dependent expression of the genes of tetrapyrrole biosynthesis. Development-dependent gene expression profiles are plotted. Samples were obtained every week after sowing. In each plot, the NE value relative to the average NE was plotted on a semilogarithmic scale. Since all genes basically showed a similar profile of expression, they are plotted in one chart.
DISCUSSION
In this study, we developed a mini-array system to monitor the genes of tetrapyrrole biosynthesis in Arabidopsis. While the technical principle is not new, owing to precautions taken to prepare DNA probes of 150–350 bp with high gene specificity and to immobilize an exact quantity (4 ng) of highly purified probe to nylon membranes, the data presented here show that this mini-array method has good reproducibility and sensitivity (Fig. 1). It has been reported that the sensitivity of mini-arrays was dependent on the concentration of probe on the filter (Bertucci et al., 1999), and 4 × 109 molecules/spot are necessary for a quantitative analysis using the mini-array method with colorimetrical detection (Quere et al., 2002). In this study, 4 ng of probe DNA is equivalent to 1 × 1010 target molecules/spot, if one assumes that the average probe size is 300 bp and all the materials spotted bind to the membrane and are available for hybridization. Thus, this mini-array system is considered to have a sensitivity comparable to that of other macro- and microarray systems. Furthermore, a significant advantage in cost enabled gene expression analysis of various physiological events, such as greening, rhythmic control, and developmental control. We have confirmed that it is possible to reuse membranes several times after stripping the target DNA (data not shown). Thus, this method is effective and inexpensive for quantitative analysis of the expression of each gene involved in tetrapyrrole biosynthesis in Arabidopsis.
Using this system, we obtained information on the absolute value (NE) and the value relative to appropriate control levels of gene expression, which is important to evaluate the exact contribution of each gene and the profiles of each expression, respectively. The calculation of NE value comprises two steps, subtraction from an appropriate background value and normalization to that of ACT. The choice of background was critical as the profiles of genes expressed at very low levels were greatly affected by slight variations in the background level. We found that a background 0.8 times the level of the lowest signal usually gave a linear correlation and high reproducibility, as in the case of cDNA macroarray experiments (Obayashi et al., 2004).
Clustering of Expression Profiles by SOM
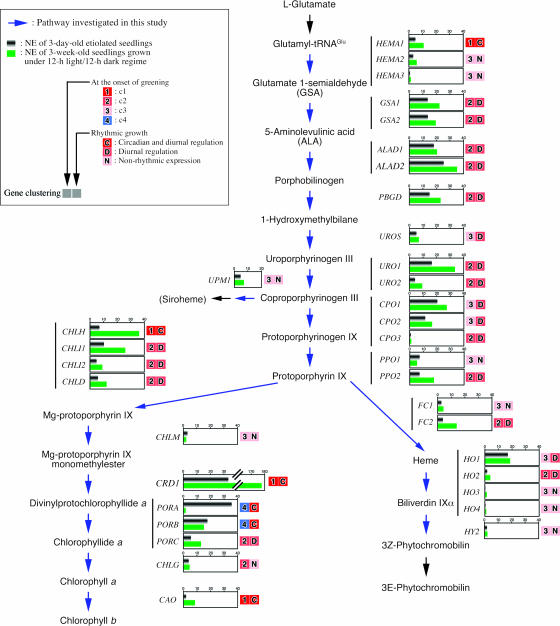
So far, several algorithms are available for clustering array data, such as one-dimensional and two-dimensional SOM. In this study, we use one-dimensional SOM for clustering, since this procedure is simple to understand and appropriate for categorizing small groups of genes. In clustering, a smaller number of categories is better for understanding the physiological characteristics in each cluster. In this study, the genes of tetrapyrrole biosynthesis were clustered into four major groups based on their expression profiles during greening. Genes in c1 and c4 were clearly distinguishable as having pronounced changes in expression, although the classification of genes in c2 and c3 was somewhat obscure. We carried out repetitive SOM analyses by modifying the number of categories, and finally determined four gene clusters. These four categories showed a good correlation with the maximum expression ratio. Moreover, genes in the same group were similarly controlled in an endogenous rhythm. A summary of gene clustering and the NE value of each gene in 3-d-old etiolated and 3-week-old mature seedlings are shown in Figure 5.
Figure 5.
Summary of gene expression analysis with the mini-array system. The tetrapyrrole biosynthetic pathway is depicted. Genes investigated in this study are shown by blue arrows in the pathway. For each gene, the NE values of 3-d-old etiolated seedlings (black bar) and 3-week-old seedlings grown under 12-h light/12-h dark regime (green bar) are shown. Results of gene clustering at the onset of greening, and rhythmic growth are shown on the right.
The c1 Cluster Comprises the Most Important Regulatory Genes in Tetrapyrrole Biosynthesis
Four genes in the c1 cluster were rapidly induced by light at the onset of greening and synchronously fluctuated under diurnal and circadian rhythms. It should be noted that the oscillation and amplitude of the rhythmic regulation of these genes are synchronized to those of Lhcb. The pronounced regulation of these genes suggests that they are the most important regulatory gene group for the biosynthesis and flow into the Chl branch pathway of tetrapyrroles.
The first gene in c1 was HEMA1 encoding glutamyl-tRNA reductase, which is the first committed enzyme of ALA biosynthesis. The formation of ALA is the rate-limiting step for tetrapyrrole biosynthesis and is the primary determinant of the rate of Chl synthesis in light-grown plants (Beale and Weinstein, 1991). Among three isoforms of HEMA, HEMA1 showed the highest NE values in young and mature tissues. Consistent with our results, the expression of HEMA1 of Arabidopsis is reported to be positively regulated by light (Ilag et al., 1994) through phytochrome (McCormac et al., 2001), while that of HEMA2 is independent of light (Kumar et al., 1996; Ujwal et al., 2002). Thus, it is apparent that the level of HEMA1 expression primarily determines the flow of total tetrapyrroles in photosynthetic tissues.
The second gene in c1 was CHLH encoding a subunit of Mg-chelatase, which is the branch point of Chl and heme biosynthesis. Mg-chelatase is composed of three subunits, which are commonly referred to as ChlI, ChlD, and ChlH. CHLH is demonstrated to be the protoporphyrin IX-binding subunit (Gibson et al., 1995; Karger et al., 2001). The expression of the CHLH gene is known to be induced by light in Arabidopsis (Gibson et al., 1996), soybean (Nakayama et al., 1998), and barley (Jensen et al., 1996). Thus, CHLH is the determinant of the flux of protoporphyrin IX into Chl biosynthesis at the branch point of the tetrapyrrole biosynthetic pathway. Furthermore, experiments with the Arabidopsis mutant GUN5 revealed the involvement of the CHLH subunit in the plastid-to-nucleus signal transduction. Currently, it is thought that Mg-protoporphyrin IX accumulation triggers an intracellular signaling pathway that regulates nuclear gene expression (Rodermel and Park, 2003; Strand et al., 2003). Therefore, it is likely that CHLH is important for the coordinated assembly of nuclear-encoded proteins with Chls and plastid-encoded proteins (Mochizuki et al., 2001; Rodermel, 2001).
The third gene in c1 was CRD1, which had the highest NE value among all the genes tested. This gene was first isolated from pea as a mesophyll-specific cDNA that is regulated by a phytochrome and circadian rhythm (Zheng et al., 1998). Then, this gene was isolated from Chlamydomonas reinhardtii by screening for mutants that failed to accumulate photosystem I and light-harvesting complex I under copper-deficient conditions (Moseley et al., 2000). It is reported that the expression of CRD1 in Chlamydomonas is increased in copper-deficient or anaerobic conditions (Moseley et al., 2002). Subsequently, the disruption of this gene in Rubrivivax gelatinosus (acsF) resulted in the accumulation of Mg-protoporphyrin IX monomethylester under aerobic conditions (Pinta et al., 2002), indicating that the CRD1 gene encodes an oxidative Mg-protoporphyrin IX monomethylester cyclase. Recently, the physiological function of CRD1 has been confirmed in Arabidopsis (Tottey et al., 2003). As the CRD1 gene showed a similar expression profile to other genes in c1, it is likely that the Mg-protoporphyrin IX monomethylester cyclase is also responsible for the regulation of tetrapyrrole biosynthesis. One possibility is that CRD1 is responsible for the plastid-to-nucleus signal transduction like the CHLH protein, since Mg-protoporphyrin IX monomethylester is also known to function as a plastid-derived signal (Kropat et al., 1997). Further analysis is definitely necessary to clarify the regulatory role of CRD1.
The fourth gene in c1 is CAO encoding chlorophyll(ide) a oxygenase, which is responsible for Chl b biosynthesis (Tanaka et al., 1998). It was presumed that the biosynthesis of Chl b, which binds to the light-harvesting Chl antenna complex, is regulated by environmental conditions, such as light intensity, rather than coordinated control with other regulatory genes. In fact, it has been reported that the expression of Arabidopsis CAO decreased under dim-light conditions (Espineda et al., 1999). Thus, it is possible that the expression of CAO is synchronized to that of other genes in c1 to regulate the assembly of the light-harvesting Chl antenna during greening and in matured leaves, and in addition is regulated by light conditions to fine-tune the size of the antenna.
Characteristics of the c2 and c3 Clusters
Most genes in the biggest cluster c2 were involved in the earlier steps of tetrapyrrole biosynthesis up to the insertion of metal. All genes in c2 were induced by light at the onset of greening and showed a diurnal fluctuation in mature leaves. Thus, it is likely that the genes in c2 encoding the enzymes of tetrapyrrole biosynthesis are similarly controlled to provide tetrapyrrole intermediates for the production of Chl and heme in photosynthetic tissues. As these genes were not regulated by circadian rhythm, light may be the primary determinant for the gene expression. A POR isoform, PORC, and CHLG encoding Chl synthetase were in this group. The light-induced induction of PORC was previously reported (Oosawa et al., 2000), but currently we do not know whether such light-regulation is coordinated with the early steps of tetrapyrrole biosynthesis.
The cluster c3 comprised genes that were constitutively expressed with high NE values, such as HO1, CPO1, and CPO2, and with very low NE values, such as HEMA3, HO3, and HO4. It is probable that some genes in this group are pseudogenes or their functional contribution is not significant. Alternatively, it is possible that they are induced under particular environmental conditions, such as stress. In fact, the expression of HEMA2 and FC1 in Arabidopsis is known to be induced by a cytoplasmic protein synthesis inhibitor, cycloheximide (data not shown), as well as cucumber (Suzuki et al., 2002). A characteristic of this cluster is that all genes in the heme branch, except for FC2 and HO2, both of which are in c2, were in the c3 cluster. These results indicate the ubiquitous biosynthesis of heme in higher plants, which is independent of environmental changes. Such a constant heme supply may facilitate the assembly of heme- and phycobiliprotein, such as phytochrome, to sense the environmental conditions.
The c4 Cluster Comprises Negatively Light-Regulated POR Isoforms, PORA and PORB
Two genes in the cluster c4 were well-known isoforms of PORA and PORB, which were negatively regulated by light. The proteins encoded by these two genes are known to be the major components of prolamellar bodies in etioplasts of dark-grown seedlings (Masuda et al., 2003). It has been reported that both mRNAs are accumulated in etiolated seedlings but only PORB mRNA continues to accumulate in light-grown plants, while PORA mRNA rapidly disappeared after illumination. This study, however, showed that the expression of PORA persisted in mature leaves under circadian rhythmic control, as well as that of PORB, although the NE value of PORA was 7 to 15 times lower than that of PORB. Thus, it is likely that PORA, as well as PORB, persists in its expression in mature leaves with rhythmic fluctuation to provide a way to adjust the synthesis of POR to meet the varying needs of fully green plants for Chl. It should be noted that oscillations of PORA and PORB were somewhat delayed to those of genes in c1. Database searches of plant cis-acting regulatory DNA elements (PLACE; Higo et al., 1999) detected a conserved motif, the nine nucleotide AAAATATCT “evening element” in the 5′-upstream regions of PORA and PORB but not in c1 (Harmer et al., 2000). Thus, it is likely that these two genes are differentially controlled to maintain Chl biosynthesis.
CONCLUSION
In summary, we have developed a mini-array of the genes involved in the biosynthesis of tetrapyrrole in Arabidopsis. The system is reasonably cheap, is highly sensitive, and can be used to monitor the expression profiles of all genes specifically and simultaneously. All these parameters will contribute to the study of other metabolic or regulatory genes of interest. With this system, we could group genes into four categories based on their expression profile during greening, although c2 and c3 have insufficient features to classify further. Clustering of c1 and c2 was also applicable to the rhythmic expression of the genes of tetrapyrrole biosynthesis. Interestingly, a good coordination of gene expression was observed in each group, suggesting that each group of genes is regulated by common regulatory machinery. Among them, the c1 cluster contained the most important genes that control the biosynthesis and efflux of tetrapyrroles in Arabidopsis. Further analysis with the aid of this system will contribute to the elucidation of the complete regulatory circuit of tetrapyrrole biosynthesis in Arabidopsis.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Seed stocks of Arabidopsis L. Heynh from the Columbia (Col) ecotype were used for all experiments. Surface-sterilized seeds were plated on a 0.8% (w/v) agar medium containing Murashige and Skoog (1962) salts. Plates were placed at 4°C in darkness for 3 d prior to receiving 1 h of white light irradiation to synchronize germination. Then, plates were exposed to white fluorescent light (50 μmol m−2 s−1). For rhythm experiments, seedlings were grown on the same medium supplemented with 1% (w/v) Suc at 23°C in an environmental growth chamber under a 12-h light/12-h dark cycle. For development experiments, seedlings were grown under continuous white light illumination.
Preparation of Probes and Production of Mini-Array Membranes
Total RNA was prepared from 3-d-old etiolated or 2- to 4-week-old mature plants with an RNeasy Mini Kit (Qiagen, Chartsworth, CA) as recommended by the manufacturer. RT-PCR was carried out with RNA PCR kit (Avian Myeloblastosis Virus) version 2.1 (Takara, Otsu, Japan) with 1 μg of total RNA according to the manufacturer's instruction. RT-PCR amplification was carried out with cognate gene-specific primers (Supplemental Table I) as follows: 30 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 1 min. The DNA fragments were checked by agarose electrophoresis (Supplemental Fig. 1), cloned into a TA-cloning vector, and subsequently sequenced using a Thermo Sequenase Sequencing kit (Amersham Bioscience, Piscataway, NJ). The inserted DNA fragment of each clone was amplified by PCR with universal M13 forward and reverse primers. PCR products were purified with a Qiaquick PCR Purification kit (Qiagen, Valencia, CA), and their DNA concentration was determined by spectrometry and agarose electrophoresis. Using the spotting machines, the Hydra Microdispenser and Tango Liquid Handling system (Shieh et al., 2002), 4 ng of each probe was spotted onto a nylon membrane (1.5 cm × 8 cm) in duplicate and fixed with UV-crosslinking (Labo, Tokyo).
Genomic Southern Hybridization Analysis
The gene specificity of the DNA probes was confirmed by genomic southern hybridization analysis (Supplemental Fig. 2). Genomic DNA was isolated from Arabidopsis seedlings according to Murray and Thompson (1980). The DNA (3 μg) was digested with restriction enzymes, separated on agarose gel, transferred onto nylon membrane, and hybridized with 32P-labeled probes. The labeling of probes was carried out with Random Primer DNA Labeling kit Ver.2 (Takara). After overnight hybridization at 65°C, blots were washed twice with 0.2× SSC, 0.1% SDS at 65°C, and then exposed to an imaging plate (Fuji Film, Tokyo) for detection.
Mini-Array Hybridization and Image Analysis
Total RNA was prepared from samples as described above. With the resultant RNA sample (10 μg) as a template, the labeling of the target DNA was carried out by RT in the presence of [α-33P] dCTP and 1 μg of oligo(dT)16–18 primer with a SuperScript First-Strand Synthesis system (Invitrogen) according to the manufacturer's directions. The labeled cDNA was denatured and used as target DNA for hybridization. Hybridization with the labeled target was performed in the presence of 0.5 m Na2HPO4, 1 mm EDTA, and 7% SDS (Church and Gilbert, 1984) at 65°C for 16 h. After incubation, the membranes were washed twice with 0.2× SSC, and 0.1% SDS at 65°C, and then exposed to an imaging plate (Fuji Film) for detection.
Radioactive images were obtained with a high-resolution scanner (Storm, Amersham Bioscience), and the signal intensity was quantified with Array Vision software (Amersham Bioscience). Quantitative analysis of hybridization with the same probe resulted in high degree of reproducibility (Fig. 1). To normalize the hybridization signal intensities of each membrane, it was first necessary to subtract the appropriate background value. The subtraction of a real value, such as the signal intensity of nonspotted area or λDNA spotted area, as a background from the intensity of each spot sometimes caused large fluctuations of particularly low intensity signals. According to our experiments using cDNA macroarray data (Obayashi et al., 2004), we adopted 0.8 times the lowest signal intensity of each membrane as the appropriate background value. Then, the value of each spot was normalized by that of ACT, a housekeeping control, as percentage of ACT. The average of this value from replicated experiments was calculated, and resultant value was defined as the NE of each gene.
Clustering of Gene Expression Profiles
For the clustering of gene expression profiles, one-dimensional SOM was carried out using GeneCluster2 released by the Whitehead Institute (http://www-genome.wi.mit.edu/cancer/software/software.html). As a result of a trial altering the number of categories, four major clusters were obtained by the SOM of gene profiles during greening. Similarly, analysis was carried out in terms of profiles of rhythmic and development-dependent expression.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes.
Supplementary Material
Acknowledgments
We are grateful to Hiroshi Shimada for valuable discussions. We would like to thank Nozomi Taki for technical assistance in preparing the radio-labeled cDNA targets for hybridization.
This work was supported by a grant from the 21st Century COE Program, Ministry of Education, Culture, Sports, Science and Technology, and by a project titled Development of Fundamental Technologies for Controlling the Process of Material Production of Plants based on funds provided by the Ministry of Ecology, Trade and Industry in Japan.
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.042408.
References
- An YQ, McDowell JM, Huang S, McKinney EC, Chambliss S, Meagher RB (1996) Strong, constitutive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. Plant J 10: 107–121 [DOI] [PubMed] [Google Scholar]
- Arabidopsis-Genome-Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815 [DOI] [PubMed] [Google Scholar]
- Armstrong GA, Runge S, Frick G, Sperling U, Apel K (1995) Identification of NADPH:protochlorophyllide oxidoreductases A and B: a branch pathway for light-dependent chlorophyll biosynthesis in Arabidopsis thaliana. Plant Physiol 108: 1505–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale SI (1999) Enzymes of chlorophyll biosynthesis. Photosynth Res 60: 43–73 [Google Scholar]
- Beale SI, Weinstein JD (1991) Biochemistry and regulation of photosynthetic pigment formation in plants and algae. In PM Jordan, ed, Biosynthesis of Tetrapyrrole. Elsevier, Amsterdam, pp 155–235
- Beator J, Kloppstech K (1993) The circadian oscillator coordinates the synthesis of apoproteins and their pigments during chloroplast development. Plant Physiol 103: 191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertucci F, Bernard K, Loriod B, Chang YC, Granjeaud S, Birnbaum D, Nguyen C, Peck K, Jordan BR (1999) Sensitivity issues in DNA array-based expression measurements and performance of nylon microarrays for small samples. Hum Mol Genet 8: 1715–1722 [DOI] [PubMed] [Google Scholar]
- Chen JJ, Yang JM, Petryshyn R, Kosower N, London IM (1989) Disulfide bond formation in the regulation of eIF-2 alpha kinase by heme. J Biol Chem 264: 9559–9564 [PubMed] [Google Scholar]
- Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci USA 81: 1991–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KH, Pinchak AB, Cooper TG (1999) Genome-wide transcriptional analysis in S. cerevisiae by mini-array membrane hybridization. Yeast 15: 703–713 [DOI] [PubMed] [Google Scholar]
- Dai G, Lu L, Tang S, Peal MJ, Soares MJ (2002) Prolactin family miniarray: a tool for evaluating uteroplacental-trophoblast endocrine cell phenotypes. Reproduction 124: 755–765 [DOI] [PubMed] [Google Scholar]
- Dailey HA (1990) Biosynthesis of heme and chlorophylls. McGraw-Hill, New York
- Espineda CE, Linford AS, Devine D, Brusslan JA (1999) The AtCAO gene, encoding chlorophyll a oxygenase, is required for chlorophyhll b synthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 96: 10507–10511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzawa T, Fukuma M, Yano K, Sakurai H (2001) A genome-wide analysis of transcriptional effect of Gal11 in Saccharomyces cerevisiae: an application of “mini-array hybridization technique”. DNA Res 8: 23–31 [DOI] [PubMed] [Google Scholar]
- Furuya M (1993) Phytochromes: their molecular species, gene families, and functions. Annu Rev Plant Physiol Plant Mol Biol 44: 617–645 [Google Scholar]
- Gibson LCD, Marrison JL, Leech RM, Jensen PE, Bassham DC, Gibson M, Hunter CN (1996) A putative Mg chelatase subunit from Arabidopsis thaliana cv C24. Plant Physiol 111: 61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson LCD, Willows RD, Kannangara CG, von Wettstein D, Hunter CN (1995) Magnesium-protoporphyrin chelatase of Rhodobacter sphaeroides: reconstitution of activity by combining the products of the bchI, -H and -D genes expressed in Escherichia coli. Proc Natl Acad Sci USA 92: 1941–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm B (1998) Novel insights in the control of tetrapyrrole metabolism of higher plants. Curr Opin Plant Biol 1: 245–250 [DOI] [PubMed] [Google Scholar]
- Guarente L, Mason T (1983) Heme regulates transcription of the CYC1 gene of S. cerevisiae via an upstream activation site. Cell 32: 1279–1286 [DOI] [PubMed] [Google Scholar]
- Harmer SL, Hogenesch JB, Straume M, Chang HS, Han B, Zhu T, Wang X, Kreps JA, Kay SA (2000) Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290: 2110–2113 [DOI] [PubMed] [Google Scholar]
- Hauser NC, Vingron M, Scheideler M, Krems B, Hellmuth K, Entian KD, Hoheisel JD (1998) Transcriptional profiling on all open reading frames of Saccharomyces cerevisiae. Yeast 14: 1209–1221 [DOI] [PubMed] [Google Scholar]
- He Z-H, Li J, Sundqvist C, Timko MP (1994) Leaf developmental age controls expression of genes encoding enzymes of chlorophyll and heme biosynthesis in pea (Pisum sativum L.). Plant Physiol 106: 537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27: 297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilag LL, Kumar AM, Soll D (1994) Light regulation of chlorophyll biosynthesis at the level of 5-aminolevulinate formation in Arabidopsis. Plant Cell 6: 265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PE, Willows RD, Petersen BL, Vothknecht UC, Stummann BM, Kannangara CG, von Wettstein D, Henningsen KW (1996) Structural genes for Mg-chelatase subunits in barley: xantha-f, -g and -h. Mol Gen Genet 250: 383–394 [DOI] [PubMed] [Google Scholar]
- Joshi B, Morley SJ, Rhoads RE, Pain VM (1995) Inhibition of protein synthesis by the heme-controlled eIF-2 alpha kinase leads to the appearance of mRNA-containing 48S complexes that contain eIF-4E but lack methionyl-tRNA(f). Eur J Biochem 228: 31–38 [PubMed] [Google Scholar]
- Karger GA, Reid JD, Hunter CN (2001) Characterization of the binding of deuteroporphyrin IX to the magnesium chelatase H subunit and spectroscopic properties of the complex. Biochemistry 40: 9291–9299 [DOI] [PubMed] [Google Scholar]
- Kropat J, Oster U, Rüdiger W, Beck CF (1997) Chlorophyll precursors are signals of chloroplast origin involved in light induction of nuclear heat-shock genes. Proc Natl Acad Sci USA 94: 14168–14172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Csankovszki G, Soll D (1996) A second and differentially expressed glutamyl-tRNA reductase gene from Arabidopsis thaliana. Plant Mol Biol 30: 419–426 [DOI] [PubMed] [Google Scholar]
- Lagarias JC, Rapoport H (1980) Chromopeptides from phytochrome. The structure and linkage of the PR form of the phytochrome chromophore. J Am Chem Soc 102: 4821–4828 [Google Scholar]
- Lange BM, Ghassemian M (2003) Genome organization in Arabidopsis thaliana: a survey for genes involved in isoprenoid and chlorophyll metabolism. Plant Mol Biol 51: 925–948 [DOI] [PubMed] [Google Scholar]
- Lathrop JT, Timko MP (1993) Regulation by heme of mitochondrial protein transport through a conserved amino acid motif. Science 259: 522–525 [DOI] [PubMed] [Google Scholar]
- Masuda T, Fusada N, Oosawa N, Takamatsu K, Yamamoto YY, Ohto M, Nakamura K, Goto K, Shibata D, Shirano Y, et al (2003) Functional analysis of isoforms of NADPH:protochlorophyllide oxidoreductase (POR), PORB and PORC, in Arabidopsis thaliana. Plant Cell Physiol 44: 963–974 [DOI] [PubMed] [Google Scholar]
- Matsuyama T, Tamaoki M, Nakajima N, Aono M, Kubo A, Moriya S, Ichihara T, Suzuki O, Saji H (2002) cDNA microarray assessment for ozone-stressed Arabidopsis thaliana. Environ Pollut 117: 191–194 [DOI] [PubMed] [Google Scholar]
- McCormac AC, Fischer A, Kumar AM, Soll D, Terry MJ (2001) Regulation of HEMA1 expression by phytochrome and a plastid signal during de-etiolation in Arabidopsis thaliana. Plant J 25: 549–561 [DOI] [PubMed] [Google Scholar]
- Mochizuki N, Brusslan JA, Larkin R, Nagatani A, Chory J (2001) Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc Natl Acad Sci USA 98: 2053–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley J, Quinn J, Eriksson M, Merchant S (2000) The Crd1 gene encodes a putative di-iron enzyme required for photosystem I accumulation in copper deficiency and hypoxia in Chlamydomonas reinhardtii. EMBO J 19: 2139–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley JL, Page MD, Alder NP, Eriksson M, Quinn J, Soto F, Theg SM, Hippler M, Merchant S (2002) Reciprocal expression of two candidate di-iron enzymes affecting photosystem I and light-harvesting complex accumulation. Plant Cell 14: 673–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8: 4321–4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Masuda T, Bando T, Yamagata H, Ohta H, Takamiya K (1998) Cloning and expression of the soybean chlH gene encoding a subunit of Mg-chelatase and localization of the Mg2+ concentration-dependent ChlH protein within the chloroplast. Plant Cell Physiol 39: 275–284 [DOI] [PubMed] [Google Scholar]
- Obayashi T, Okegawa T, Sasaki-Sekimoto Y, Shimada H, Masuda T, Asamizu E, Nakamura Y, Shibata D, Tabata S, Takamiya K, et al (2004) Distinct feature of plant organs characterized by global analysis of gene expression in Arabidopsis. DNA Res 11: 11–25 [DOI] [PubMed] [Google Scholar]
- Oosawa N, Masuda T, Awai K, Fusada N, Shimada H, Ohta H, Takamiya K (2000) Identification and light-induced expression of a novel gene of NADPH-protochlorophyllide oxidoreductase isoform in Arabidopsis thaliana. FEBS Lett 474: 133–136 [DOI] [PubMed] [Google Scholar]
- Papenbrock J, Mock H-P, Kruse E, Grimm B (1999) Expression studies in tetrapyrrole biosynthesis: inverse maxima of magnesium chelatase and ferrochelatase activity during cyclic photoperiods. Planta 208: 264–273 [Google Scholar]
- Pietu G, Alibert O, Guichard V, Lamy B, Bois F, Leroy E, Mariage-Sampson R, Houlgatte R, Soularue P, Auffray C (1996) Novel gene transcripts preferentially expressed in human muscles revealed by quantitative hybridization of a high density cDNA array. Genome Res 6: 492–503 [DOI] [PubMed] [Google Scholar]
- Pinta V, Picaud M, Reiss-Husson F, Astier C (2002) Rubrivivax gelatinosus acsF (previously orf358) codes for a conserved, putative binuclear-iron-cluster-containing protein involved in aerobic oxidative cyclization of Mg-protoporphyrin IX monomethylester. J Bacteriol 184: 746–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quere R, Commes T, Marti J, Bonami JR, Piquemal D (2002) White spot syndrome virus and infectious hypodermal and hematopoietic necrosis virus simultaneous diagnosis by miniarray system with colorimetry detection. J Virol Methods 105: 189–196 [DOI] [PubMed] [Google Scholar]
- Reinbothe S, Reinbothe C (1996) The regulation of enzymes involved in chlorophyll biosynthesis. Eur J Biochem 237: 323–343 [DOI] [PubMed] [Google Scholar]
- Rodermel S (2001) Pathways of plastid-to-nucleus signaling. Trends Plant Sci 6: 471–478 [DOI] [PubMed] [Google Scholar]
- Rodermel S, Park S (2003) Pathways of intracellular communication: tetrapyrroles and plastid-to-nucleus signaling. Bioessays 25: 631–636 [DOI] [PubMed] [Google Scholar]
- Shieh J, To C, Carramao J, Nishimura N, Maruta Y, Hashimoto Y, Wright D, Wu HC, Azarani A (2002) High-throughput array production using precision glass syringes. Biotechniques 32: 1360–1362, 1364–1365 [DOI] [PubMed] [Google Scholar]
- Strand A, Asami T, Alonso J, Ecker JR, Chory J (2003) Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrinIX. Nature 421: 79–83 [DOI] [PubMed] [Google Scholar]
- Sun CW, Callis J (1997) Independent modulation of Arabidopsis thaliana polyubiquitin mRNAs in different organs and in response to environmental changes. Plant J 11: 1017–1027 [DOI] [PubMed] [Google Scholar]
- Susek RE, Chory J (1992) A tale of two genomes: role of a chloroplast signal in coordinating nuclear and plastid genome expression. Aust J Plant Physiol 19: 387–399 [Google Scholar]
- Suzuki T, Masuda T, Singh DP, Tan FC, Tsuchiya T, Shimada H, Ohta H, Smith AG, Takamiya K (2002) Two types of ferrochelatase in photosynthetic and nonphotosynthetic tissues of cucumber: their difference in phylogeny, gene expression, and localization. J Biol Chem 277: 4731–4737 [DOI] [PubMed] [Google Scholar]
- Tamayo P, Slonim D, Mesirov J, Zhu Q, Kitareewan S, Dmitrovsky E, Lander E, Golub TR (1999) Interpreting patterns of gene expression with self-organizing maps: methods and application to hematopoietic differentiation. Proc Natl Acad Sci USA 96: 2907–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Ito H, Tanaka R, Tanaka NK, Yoshida K, Okada K (1998) Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Proc Natl Acad Sci USA 95: 12719–12723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Yoshida K, Nakayashiki T, Masuda T, Tsuji H, Inokuchi H, Tanaka A (1996) Differential expression of two hemA mRNAs encoding glutamyl-tRNA reductase proteins in greening cucumber seedlings. Plant Physiol 110: 1223–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottey S, Block MA, Allen M, Westergren T, Albrieux C, Scheller HV, Merchant S, Jensen PE (2003) Arabidopsis CHL27, located in both envelope and thylakoid membranes, is required for the synthesis of protochlorophyllide. Proc Natl Acad Sci USA 100: 16119–16124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujwal ML, McCormac AC, Goulding A, Kumar AM, Soll D, Terry MJ (2002) Divergent regulation of the HEMA gene family encoding glutamyl-tRNA reductase in Arabidopsis thaliana: expression of HEMA2 is regulated by sugars, but is independent of light and plastid signalling. Plant Mol Biol 50: 83–91 [DOI] [PubMed] [Google Scholar]
- Zheng CC, Porat R, Lu P, O'Neill SD (1998) PNZIP is a novel mesophyll-specific cDNA that is regulated by phytochrome and the circadian rhythm and encodes a protein with a leucine zipper motif. Plant Physiol 116: 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.