Abstract
A novel restriction-modification system, designated LlaJI, was identified on pNP40, a naturally occurring 65-kb plasmid from Lactococcus lactis. The system comprises four adjacent similarly oriented genes that are predicted to encode two m5C methylases and two restriction endonucleases. The LlaJI system, when cloned into a low-copy-number vector, was shown to confer resistance against representatives of the three most common lactococcal phage species. This phage resistance phenotype was found to be strongly temperature dependent, being most effective at 19°C. A functional analysis confirmed that the predicted methylase-encoding genes, llaJIM1 and llaJIM2, were both required to mediate complete methylation, while the assumed restriction enzymes, specified by llaJIR1 and llaJIR2, were both necessary for the complete restriction phenotype. A Northern blot analysis revealed that the four LlaJI genes are part of a 6-kb operon and that the relative abundance of the LlaJI-specific mRNA in the cells does not appear to contribute to the observed temperature-sensitive profile. This was substantiated by use of a LlaJI promoter-lacZ fusion, which further revealed that the LlaJI operon appears to be subject to transcriptional regulation by an as yet unidentified element(s) encoded by pNP40.
Restriction-modification (R/M) systems are the most abundant bacteriophage resistance mechanism found in bacteria thus far. These systems are classified into four groups (designated type I, II, III, or IV) on the basis of their cofactor requirements, subunit composition, recognition sequence structure, and cleavage site relative to the recognition sequence (51). Members of type I are composed of the products of three distinct structural genes, which encode the subunits of a hetero-oligomeric enzyme complex that is required for restriction (R), modification (M), and specificity (S). Type I enzymes are present in two different oligomeric forms in vivo. The M2S oligomeric enzyme is capable of methylation in the presence of S-adenosylmethionine (AdoMet) and Mg2+, while the R2M2S oligomer is responsible for the restriction of unmethylated DNA in the presence of ATP, AdoMet, and Mg2+. Type I system representatives cleave target DNAs at sites that are distant from an asymmetrical recognition sequence. A model for cleavage by type I endonucleases has been proposed by Bourniquel and Bickle (4), who suggested that the endonuclease binds to its unmethylated recognition site, forming a dimer of two R2M2S oligomers. In the presence of ATP, all four HsdR subunits of this dimer complex promote the independent translocation of DNA directed toward the bound complex, and cleavage occurs when a physical barrier forces the translocation process to stop.
A typical R/M system belonging to type II, which is the simplest group with respect to genetic structure and cofactor requirements, is composed of two distinct gene products, one of which acts as a Mg2+-dependent sequence-specific endonuclease (REase) while the other functions as a cognate AdoMet-requiring methyltransferase (MTase). Type II MTases typically modify a nucleotide within the same symmetrical recognition site as the endonuclease by methyl transfer, thus concealing the endonuclease target. Type II endonucleases generally act as homodimers bound to each other in opposite orientations, which permits essentially simultaneous double-stranded DNA scission. In a recent classification scheme proposed by Roberts et al. (51), the criteria for type II systems have been modified to include additional kinds of enzymes, with various atypical characteristics. Indeed, 11 subtypes of the type II group have now been described to accommodate the >3,500 REases belonging to this class. Representatives of one such subtype, designated type IIS, cleave at least one strand of the target DNA at a fixed distance from the asymmetrical recognition site and have an additional Mg2+ requirement for methylase activity. However, most frequently, cleavage of both strands occurs outside of the recognition sequence, which essentially remains intact. Typically, these systems are composed of one REase and two MTases, with one MTase specific for each strand of the asymmetric target sequence, although exceptions have been noted. Restriction enzymes of the type II group (unlike type I and type III representatives) do not normally exhibit sequence similarity (3, 67, 68). In contrast, type II DNA methylases show extensive sequence homologies (29, 67, 68) and can be grouped into three classes corresponding to their modification specificities, i.e., m4C, m5C, or m6A.
Type III R/M systems are composed of two distinct structural genes (mod and res), which encode subunits of a multifunctional restriction complex that possesses both restriction and modification activities. The holoenzyme, composed of two Res and two Mod subunits, cleaves DNA at a fixed distance on one side of an asymmetric target site in the presence of ATP and Mg2+ and is stimulated by AdoMet. The Res subunit has no enzyme activity on its own, in contrast to the Mod subunit, which may form stable dimers that are capable of methylation in the presence of AdoMet. Cleavage by type III endonucleases is somewhat similar to that observed for representatives of the type I system, involving ATP-dependent translocation and the cooperative interaction of the Res subunits from two Res2Mod2 complexes. Type III restriction enzymes require the presence of two unmodified asymmetric target sites positioned in inverse orientations with respect to each other. Cleavage results after the collision and consequent dimerization of two convergently translocating Res2Mod2 complexes (4, 26).
Type IV R/M systems are specified by either one or two structural genes encoding proteins with specificities for methylated, hydroxymethylated, or glucosyl-hydroxymethylated bases in the target DNA molecule.
Hybrid systems have also been identified that share features of more than one group described above. For example, the LlaI system, present on pTR2030 (24), involves a single type IIS methylase and requires the action of three other gene products for restriction activity (49). The second of these three gene products, designated ORF2, contains a potential ATP/GTP binding site, indicating an energy requirement for restriction. Therefore, LlaI shares characteristics of both type I and type IIS groups (49). Similarly, the LlaGI system of pEW104 (36), which is comprised of a single polypeptide with both restriction and modification activities, exhibits hybrid features of both type I and type III systems and is expected to be a variant of type I systems (36).
To date, at least 19 mostly plasmid-encoded lactococcal R/M systems have been described, with more than half belonging to the type II or IIS group (12, 18, 24, 27, 34-37, 41, 45, 46, 55-57, 60, 64). The recognition sequences of approximately half of these systems have been determined, while in only three cases a model for the regulation of expression has been proposed (5, 10, 48). Here we report the presence of a novel R/M system, designated LlaJI, present on the conjugative lactococcal plasmid pNP40.
MATERIALS AND METHODS
Bacteria, bacteriophages, and plasmids.
Details of the bacterial strains, bacteriophages, and plasmids used for this study are summarized in Table 1.
TABLE 1.
Bacteria, phages, and plasmids used for this study
| Strain, phage, and plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| MC1000 | Cloning host for pPTPL | 6 |
| EC101 | Cloning host, Kanr | 30 |
| L. lactis | ||
| MG1614 | Plasmid-free derivative of L. lactis subsp. lactis 712, c2 and 936 species host | 22 |
| MG1614/pNP40 | MG1614 transconjugant containing pNP40 | DPC collectiona |
| MG1614/pNP10 | Spontaneous mutant of pNP40 with ISLL6 insertion in llaJIR2 | This study |
| NCK690KP1 | Partially cured derivative of NCK203, host strain for phages Q30, Q33, and U136 | 17 |
| NZ9000 | MG1363, pepN::nisRK, nisin-inducible overexpression host | 28 |
| Lactococcal phages | ||
| c2 | Prolate-headed phage for MG1614 | 23 |
| 952 | Prolate-headed phage for MG1614 | 11 |
| 712 | Small, isometric-headed phage for MG1614 | 22 |
| sk1 | Small, isometric-headed phage for MG1614 | 8 |
| q30 | P335 lytic phage for NCK690 KP1 | 43 |
| q33 | P335 lytic phage for NCK690 KP1 | 43 |
| ul36 | P335 lytic phage for NCK690 KP1 | 42 |
| Plasmids | ||
| pMTL22 | E. coli cloning vector, Ampr | 7 |
| pPTP | E. coli-L. lactis shuttle vector, tetK, pIL252, pSC101 | This study |
| pPTPi | Low-copy-number E. coli-L. lactis shuttle vector for cloning, Tcr, PnisA, pPTP derivative | This study |
| pPTPL | Promoter-screening vector containing promoterless lacZ gene, Tcr, pPTP derivative | This study |
| pJO-J | pPTPi derivative containing 6.2-kb fragment from pNP40 encompassing entire LlaJI system | This study |
| pJO-M1 | pPTPi derivative containing 1.8-kb fragment from pNP40 encompassing promoter and llaJIM1 gene | This study |
| pJO-M1M2 | pPTPi derivative containing 3.0-kb fragment from pNP40 encompassing promoter and llaJIM1 and llaJIM2 genes | This study |
| pJO-M1M2R1 | pPTPi derivative containing 4.6-kb fragment from pNP40 encompassing promoter and llaJIM1, llaJIM2, and llaJIR1 genes | This study |
| pJPL | pPTPL derivative containing 500-bp fragment from pNP40 encompassing LlaJI promoter | This study |
| pNZ8020 | High-copy-number L. lactis-E. coli shuttle vector, PnisA, Cmr | 14 |
| pNZ-M1 | pNZ8020 derivative containing llaJIM1 | This study |
| pNZ-M2 | pNZ8020 derivative containing llaJIM2 | This study |
| pNZ-M1M2 | pNZ8020 derivative containing llaJIM1M2 | This study |
DPC, Dairy Products Centre, Moorepark, Fermoy, Co Cork, Ireland.
Media and growth conditions.
All Lactococcus lactis strains were grown in M17 broth (Oxoid Ltd., Hampshire, United Kingdom) containing 0.5% glucose at 30°C (unless otherwise stated). Escherichia coli was grown at 37°C in Luria-Bertani medium (53). When appropriate, antibiotics were added as follows: for L. lactis, 5 μg of tetracycline ml−1 and 10 μg of chloramphicol ml−1, and for E. coli, 50 μg of kanamycin ml−1 and 10 μg of tetracycline ml−1. Recombinant E. coli cells containing pPTPL were selected on Luria-Bertani agar containing tetracycline and supplemented with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (40 mg ml−1). Induction of the nisin-inducible promoter of pNZ8020 in the NZ9000 host background was achieved by the inclusion of nisin (5 ng ml−1) in the growth media.
β-Galactosidase assays.
β-Galactosidase assays were performed as described by Miller (40). All values represent the means of at least three replicates from which the background expression from the promoter probe vector was subsequently deducted.
Bacteriophage propagation and assays.
Lactococcal phages were propagated on their relevant host strains (Table 1). Briefly, strains were grown to early log phase (optical density at 600 nm, 0.3 to 0.5) in 10 ml of GM17 broth, CaCl2 (10 mM) and phage (100 μl of a 109 PFU ml−1 stock) were added, and incubation continued until lysis occurred. The lysates were clarified by filtration (0.45-μm pore size) and stored at 4°C until required. When necessary, phage were concentrated by CsCl2 density gradient centrifugation as previously described (53). Plaque assays were conducted as described by Lillehaug (31), and the efficiency of plaquing (EOP) was calculated as the ratio of the number of plaques formed on a tested host to those formed on a sensitive host.
Molecular cloning and plasmid DNA isolation.
E. coli plasmid DNA was isolated by use of an SV Wizard plasmid miniprep kit (Promega, Madison, Wis.) according to the manufacturer's instructions. Lactococcal plasmid DNA was isolated as described by O'Sullivan and Klaenhammer (47). Restriction enzymes and T4 DNA ligase were purchased from Roche (Mannheim, Germany) and were used according to the manufacturer's instructions. Electroporation of plasmid DNA into E. coli was performed as described by Sambrook et al. (53) and that into L. lactis was performed as described by Wells et al. (66). Total DNA preparations were used as templates in PCRs. For routine PCR applications, Taq DNA polymerase (Qiagen, West Sussex, United Kingdom) was used. For plasmid construction when a high fidelity was required, PCRs were conducted with a combination of Taq DNA polymerase (Qiagen) and Proofstart DNA polymerase (Qiagen) according to the manufacturer's instructions.
Construction of pPTPL.
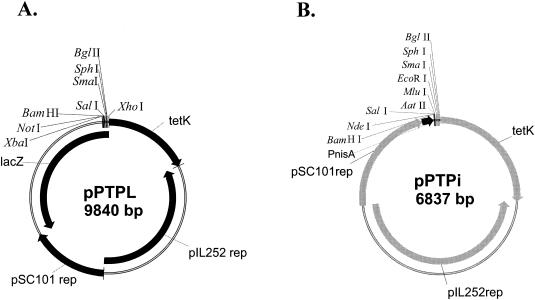
A new promoter probe vector for L. lactis, pPTPL, which contains the pIL252 minimal replicon, a promoterless β-galactosidase operon derived from E. coli, and a gene for tetracycline resistance derived from Staphylococcus aureus, was constructed as follows. The low-copy-number pSC101 origin of replication was amplified by PCR from the plasmid pWSK29 (65) as a 1.6-kb XhoI-PstI fragment. The tetracycline resistance gene tetK was amplified from the plasmid pGhost8 (38) as a 1.8-kb XhoI-PstI fragment. The resulting 3.4-kb plasmid, pPT, obtained after ligation of the pSC101 and tetK PCR products, was capable of replication in E. coli. The low-copy-number pIL252 origin of replication was amplified as a 3.2-kb PstI fragment from the plasmid pIL252 (58) and was introduced into the unique PstI site of pPT. The resulting 6.6-kb plasmid, pPTP, was capable of replication in both E. coli and L. lactis. The multiple cloning site and promoterless β-galactosidase operon were amplified as a 3.2-kb XhoI-SpeI fragment from the plasmid pORI13 (54) and then introduced into the XhoI-XbaI sites of pPTP, resulting in the 9.8-kb low-copy-number promoter probe vector pPTPL (Fig. 1A).
FIG. 1.
Physical maps of the promoter probe vector pPTPL (A) and the cloning vector pPTPi (B). Polylinkers are indicated.
Construction of LlaJI subclones.
All clones and subclones were generated by PCR amplification of regions of interest by the use of synthetic oligonucleotides (with suitable restriction sites incorporated at their 5′ ends) followed by direct cloning into relevant vectors as described below (Table 1). The coordinates and oligonucleotide sequences used are listed in Table 2. For amplification of the complete LlaJI system, primers Pr/m and R/mR1 were used. Subclones containing llaJIM1, llaJIM1M2, and llaJIM1M2R1 were produced by amplification with the primer combinations Pr/m and R/mR2, Pr/m and R/mR3, and Pr/m and R/mR4, respectively. The low-copy-number cloning vector pPTPi was constructed as follows. The nisin-inducible promoter of pNZ8020 was produced by PCR amplification and cloned into the BamHI site of the cloning vector pMTL22. The cloned nisin promoter and the multiple cloning site of pMTL22 were excised as a single XhoI fragment and then cloned into pPTP, generating plasmid pPTPi (Fig. 1B). Initial attempts to clone the LlaJI system under the control of the nisin-inducible promoter in plasmids pNZ8020 and pPTPi failed due to the instability of the constructs. Successful cloning of the complete LlaJI system was only achieved when the system was under the control of its native promoter.
TABLE 2.
Coordinates of oligonucleotides used for LlaJI subclone construction
| Oligonucleotide | Start position | Sequencea |
|---|---|---|
| Pr/m | 1 | GGGAGATCTGGTGAGATGAGAATCTTG CTTG |
| R/mR1 | 6234 | GGGAGATCTGACATAATGGGAAGTTAT GACTAG |
| R/mR2 | 1859 | GGGAGATCTCTCCCTTATACTAATTTCT CAAAAAC |
| R/mR3 | 3010 | GGGAGATCTGTCCTCCTATATGCGATTA ATTAATT |
| R/mR4 | 4784 | GGGAGATCTGAGCCATCCTCTATTCTTC AGTG |
| Pr/mFnew | 539 | GGGTCTAGACTGGAGACTATATTGCTC ACTG |
| M1F | 441 | GGGGGATCCCTATTGAGGTGATAGAAT GATTG |
| M1R | 1859 | GGGTCTAGACCCTTATACTAATTTCTCA AAAAC |
| M2F | 1853 | GGGGGATCCAAGGGAGTATATATGTCT AAATTA |
| M2R | 3010 | GGGTCTAGAGTCCTCCTATATGCGATTA ATT |
Restriction sites are shown in italics.
All PCR products were purified with a Jetquick PCR purification kit (Genomed, Lohne, Germany). Fragments encompassing the complete LlaJI system or fragments thereof (but always including the promoter region) were digested with BglII and cloned into the BglII site of pPTPi. The nisin-inducible promoter present on pPTPi was not used for the expression of these cloned fragments, however, as all constructs included the native LlaJI promoter region. In the case of the LlaJI promoter-lacZ transcriptional fusion present in plasmid pJPL, the LlaJI promoter-containing DNA fragment was amplified by PCR (with primers Pr/m and Pr/mFnew), digested with XbaI (one site incorporated by an oligonucleotide and an internal restriction site in the PCR product), and cloned into the XbaI site of pPTPL. For expression analysis, the methylase-encoding genes llaJIM1 and llaJIM2 and a fragment encompassing llaJIM1M2 were generated by PCRs with the primer combinations M1F and M1R, M2F and M2R, and M1F and M2R, respectively, and were subsequently cloned into the BamHI-XbaI site of pNZ8020, which placed the cloned LlaJI genes under the control of the nisin-inducible promoter (13). The correct orientation and integrity of all constructs were verified by sequencing.
DNA sequencing and analysis.
Sequence determination was performed at MWG Biotech (Ebersberg, Germany). Sequence assembly, verification, and analysis were achieved with the Seqman program from the DNASTAR package (DNASTAR, Madison, Wis.). Database searches were performed with the BLAST suite of programs (2) and the REBASE database (52).
RNA isolation and Northern blot analysis.
RNAs were prepared from lactococcal cultures essentially as described by McGrath et al. (39). Denaturing formaldehyde gel electrophoresis was performed by standard techniques (53). After electrophoresis, the RNAs were transferred to a Hybond N+ membrane (Amersham) by capillary transfer using 10 mM NaOH (53). A llaJIM1-specific probe was obtained by PCR amplification with the primers 5′-CTATTGAGGTGATAGAATGAT-3′ and 5′-TACTAATTTCTCAAAAACTTTTTT-3′ (coordinates 441 to 1853), purified with a Jetquick PCR purification kit (Genomed) according to the manufacturer's instructions, and end labeled with [γ-32P]ATP by the use of polynucleotide kinase (New England Biolabs, Hertfordshire, United Kingdom). Hybridizations were performed in Ultrahyb sensitive hybridization buffer (Ambion, Huntington, Cambridgeshire, United Kingdom) according to the manufacturer's instructions.
Primer extension.
The 5′ end of the LlaJI transcript was determined by primer extension with the synthetic oligonucleotide 5′-GTATAACTCGTTATTTTCCCAAGG-3′ (coordinate 589).
Fifty micrograms of total RNA was mixed with 10 pmol of primer (end labeled with [γ-32P]ATP) and 3 μl of annealing buffer (1 M Tris-HCl [pH 7.5], 100 mM MgCl2, 160 mM dithiothreitol) in a final volume of 15 μl. The mixture was denatured at 65°C for 10 min and incubated for 1 h at 55°C. Avian myeloblastosis virus reverse transcriptase (Roche) was used for the extension reaction. Two microliters of reverse transcriptase, 2 μl of deoxynucleoside triphosphate mix (20 mM), and 5 μl of reverse transcription buffer (250 mM Tris-HCl, 40 mM MgCl2, 150 mM KCl, 5 mM dithiothreitol, pH 8.5) were added to the RNA-primer mix, and the final volume was brought up to 25 μl with RNA-grade water. The reaction was incubated for 1.5 h at 42°C. The reaction mix was subsequently extracted with phenol-chloroform, and the cDNA was precipitated overnight at −20°C after the addition of a 1/10 volume of 3 M sodium acetate (pH 4.8) and 3 volumes of 96% ethanol. Centrifugation for 30 min at 14,000 rpm (Eppendorf F-45-30-11 rotor) was performed to pellet the cDNA, which was washed in 70% ethanol and resuspended in 8 μl of stop solution from a sequencing kit (USB Corporation, Cleveland, Ohio). A sequence ladder was generated by use of the oligonucleotide described above (end labeled with [γ-32P]ATP) and a T7 sequencing kit (USB Corporation) according to the manufacturer's instructions. The products of the sequence reactions and the extension products were separated by electrophoresis in a 9% polyacrylamide-urea gel for 2.5 h at 1,600 V, 50 W, and 50 mA.
Nucleotide sequence accession number.
The nucleotide sequence of the LlaJI R/M system has been deposited in the GenBank database under accession number AY530537.
RESULTS
Sequence, genetic organization, and amino acid analysis of LlaJI R/M system.
The lactococcal plasmid pNP40 was previously shown to mediate complete resistance to φc2 and φ712 (20). However, the two Abi systems present on this plasmid, AbiE and AbiF, do not appear to fully account for this observed resistance (20), and evidence has been presented which suggests the presence of a DNA penetration blocking system (21). A spontaneous mutant of pNP40, designated pNP10, was isolated and displayed an altered restriction profile, in which a pNP40-derived AccI fragment was enlarged by 1.5 kb (data not shown), and a significant decrease in the level of phage resistance relative to that of pNP40 (Table 3). Sequence analysis of the altered AccI fragment of pNP10 revealed the presence of an insertion sequence (IS) element, which displayed 97% homology to ISLL6 from the lactococcal plasmid pWV04 (32) and which is absent from the corresponding position in pNP40. Sequencing and homology searches of the pNP40 region into which ISLL6 had integrated to produce pNP10 revealed that the insertion had occurred within the most distal gene of a tetracistronic operon (see below) (Fig. 2A). The complete sequence of this operon, designated LlaJI, was determined. The G+C content for the sequence is 33%, which is in reasonable agreement with the 36% reported G+C content for lactococci (44). The first gene of the LlaJI system, llaJIM1, codes for a potential protein with a calculated molecular mass of 53.9 kDa. Database searches revealed that this protein shows 40 and 34% identities with the HgaIA and HgaIB cytosine-specific methyltransferases of Haemophilus gallinarum, respectively (61). Within the deduced amino acid sequence, all 10 conserved motifs characteristic of known 5-methylcytosine methyltransferases are present (29). Interestingly, M1.LlaJI appears to contain an extended N-terminal region, roughly encompassing the first 85 residues of this protein. No conserved motifs or domains and no significant similarity to known protein sequences could be discerned within this region of M1.LlaJI.
TABLE 3.
Observed phage resistance conferred by pNP40, pNP10, and the cloned LlaJI R/M system against lactococcal phages of c2, 936, and P335 species at 30°C
| Phage | Value for indicated strain
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MG1614/pNP40
|
MG1614/pNP10
|
MG1614
|
MG1614/pJO-J
|
NCK690KP1
|
NCK690KP1/pJO-J
|
|||||||
| EOP | Plaque size (mm) | EOP | Plaque size (mm) | EOP | Plaque size (mm) | EOP | Plaque size (mm) | EOP | Plaque size (mm) | EOP | Plaque size (mm) | |
| c2 | NPa | 1 × 10−8 | Pinpoint | 1 | 5 | 1 × 10−4 | 3 | |||||
| 952 | NP | NPa | 1 | 5 | 3 × 10−4 | 3 | ||||||
| 712 | NP | 8 × 10−5 | Pinpoint | 1 | 1 | 5 × 10−5 | 1 | |||||
| sk1 | 7 × 10−4 | Pinpoint | 3 × 10−1 | 1 | 1 | 3 | 3 × 10−3 | 3 | ||||
| q30 | 1 | 3 | 4 × 10−2 | 2-3 | ||||||||
| q33 | 1 | 2 | 2 × 10−2 | 1-2 | ||||||||
| ul36 | 1 | 1 | 3 × 10−2 | 1 | ||||||||
NP, no plaques (complete resistance).
FIG. 2.
(A) Genetic organization of 6.2-kb LlaJI gene cluster. The ISLL6 insertion site, approximately 280 bp downstream of the ATG start codon of llaJIR2 on plasmid pNP10, is indicated. (B) Nucleotide sequences of the promoter region and the 5′ end of llaJIM1 of the LlaJI operon. The ribosome binding site, preceding llaJIM1, is doubly underlined. The extended −10 and −35 sequences of the promoter region are highlighted. A 24-bp perfect inverted repeat structure is indicated by opposing arrows, and a 14-bp direct repeat sequence is shown by broken arrows. The determined transcriptional start site is denoted in lowercase italics.
The second ORF, llaJIM2, codes for a 43.7-kDa protein whose deduced amino acid sequence shows 41 and 35% identity to the HgaIA and HgaIB methylases, respectively. As for M1.LlaJI, the 10 conserved motifs of 5mC-MTases were all present in M2.LlaJI. In both LlaJI-encoded methylases, high sequence similarities were observed within motif I, which is involved in AdoMet binding, and motif IV, which contains the catalytic active site. Furthermore, extensive similarities exist between the LlaJI and HgaI methylase sequences in the region between motifs VIII and IX, known as the target recognition domain.
The HgaI methylases have been shown to be responsible for the modification of asymmetric but complementary sequences in the target DNA (5′-GACGC-3′ by HgaIBM and 3′-CTGCG-5′ by HgaIAM) (61). Due to the extensive similarity of the LlaJI-encoded methylases to those of the HgaI system, it is plausible that M1.LlaJI and M2.LlaJI may operate in a similar manner.
The third gene of the LlaJI operon, llaJIR1, is predicted to encode a protein of 67.2 kDa. The deduced amino acid sequence exhibits 51% identity to a putative restriction enzyme of Helicobacter pylori J99 (accession no. AAD05745) (1) and 36% identity to LlaI.2, the second component required for restriction by the LlaI R/M system (49). A conserved domain was identified within the protein sequence that is homologous to McrB and was also identified within LlaI.2 (16, 49). The proposed domain includes three motifs responsible for GTP binding and hydrolysis and has been observed in many GTPases (15). Motif I [GXXXXGK(S/T)] is the phosphate binding loop followed by the switch II region (DXXG), with motif III (NTAD) acting as the guanine recognition loop (50). The presence of this domain suggests that restriction by R1.LlaJI requires energy, possibly to translocate DNA through the enzyme to facilitate restriction at a putative site that is distant from the recognition sequence.
The fourth gene of the LlaJI system, llaJIR2, specifies a 48.8-kDa protein with no obvious conserved domains, although the protein shows 26% identity to a predicted type II R/M system restriction subunit of Bacillus cereus ATCC 14579 (accession no. AAP07927) (25).
Cloning of LlaJI and efficacy against lactococcal phages.
The complete LlaJI system, including its transcriptional signals (see below), was amplified by PCR and cloned into the low-copy-number vector pPTPi. The resulting construct, designated pJO-J, was analyzed for its ability to confer phage resistance against seven lactococcal phages belonging to the c2, 936, and P335 species at 30°C (Table 3). LlaJI was shown to confer a broad level of resistance against all phages tested, being most effective against representatives of the c2 and 936 species. The resistance conferred against members of the P335 species was slightly less pronounced, with EOPs of the order of 10−2 under the conditions tested. Full protection against LlaJI was obtained for all phages propagated on a strain bearing pJO-J, while this protection was subsequently lost after the propagation of such phages on a host devoid of pJO-J, thus conforming to the classical R/M phenotype (data not shown). Interestingly, besides the reduction in EOPs, LlaJI also affects the plaque sizes of representatives of the c2 and P335 species, which is a phenotype that is not normally associated with an R/M system. Phages φc2 and φ952, which form plaques of 5 mm in diameter on the sensitive MG1614 host at 30°C, were found to produce 3-mm-diameter plaques when challenged with the LlaJI+ strain (Table 3). Similarly, phages φQ30 and φQ33, which produce plaques of 3 and 2 mm in diameter, respectively, on the sensitive NCK690KP1 host, were found to produce, in addition to wild-type-size plaques, smaller plaques when challenged with the LlaJI+ host. As was hypothesized for mutants of the LlaI system (49), a variable plaque phenotype could be attributed to incomplete restriction or to suboptimal conditions for these particular enzymes.
Characterization of temperature-sensitive profile exhibited by LlaJI.
During the course of this work, we found that the resistance level conferred by LlaJI was strongly dependent on the temperature at which the plaque assays were performed (data not shown). To determine the optimal temperature for the LlaJI system, we determined the EOP of φsk1 as a function of temperature for MG1614 harboring pJO-J. As shown in Fig. 3, the optimum temperature for restriction was observed to be about 19°C (EOP, 10−7). At temperatures above this optimum, a steady drop in the level of resistance up to 37°C was observed, at which temperature the resistance was completely abolished. Similarly, at temperatures below 19°C, a decrease, although less dramatic, in the potency of the LlaJI system was observed. Furthermore, our findings also indicated a far less dramatic temperature dependency profile for methylation than that for restriction, as modification of the phage was still detectable to a certain extent at 37°C. At this temperature, phage propagated on a strain harboring pJO-J were found to afford a 2-log increase in EOP (relative to phage propagated on the relevant sensitive host alone at 37°C) when subsequently challenged with an LlaJI+ host at 19°C (data not shown).
FIG. 3.
Temperature-dependent phage resistance profile exhibited by LlaJI system. The EOP of φsk1 was determined as a function of the temperature for MG1614 containing the LlaJI system (in plasmid pJO-J).
Functional characterization of individual genes of LlaJI operon.
To elucidate the contribution of each open reading frame (ORF) to the LlaJI phenotype, we amplified various portions of the LlaJI operon, cloned them into the low-copy-number cloning vector pPTPi, and analyzed them for the ability to confer phage resistance at 19°C. The results are summarized in Table 4 and show that all four ORFs were required to mediate full resistance. In the absence of llaJIR2, as in construct pJO-M1M2R1, a dramatic effect on the EOP was observed, with a significant reduction in the level of resistance in all cases. The variable plaque phenotype, as observed for the complete LlaJI system (in construct pJO-J), remained, which was indicative of some residual phage resistance activity. A plasmid containing only the assumed LlaJI methylase-encoding genes, pJO-M1M2, resulted in a complete loss of phage resistance, and plaque sizes identical to those obtained on the sensitive host were observed. As expected, no phage resistance was evident for the deletion derivative containing llaJIM1 alone (present in plasmid pJO-M1; results not shown).
TABLE 4.
Functional characterization of the four ORFs encoded by the LlaJI R/M system
| Phage | Value for indicated strain
|
|||||||
|---|---|---|---|---|---|---|---|---|
| MG1614
|
MG1614/pJO-J
|
MG1614/pJO-M1M2R1
|
MG1614/pJO-M1M2
|
|||||
| EOP | Plaque size(mm) | EOP | Plaque size (mm) | EOP | Plaque size (mm) | EOP | Plaque size (mm) | |
| sk1 | 1 | 2-3 | 1 × 10−7 | 1-2 | 1 | 1-2 | 1 | 2-3 |
| c2 | 1 | 2 | >10−10 | NPa | 2 × 10−1 | 1 and pinpoint | 1 | 2 |
| 712 | 1 | 2 | 8 × 10−9 | 2 | 1 | 2 | 1 | 2 |
| 952 | 1 | 2 | >10−10 | NP | 2 × 10−1 | 1 and pinpoint | 1 | 2 |
NP, no plaques (complete resistance). Plaque assays were conducted at 19°C.
To evaluate the components responsible for methylation, we passaged φsk1 through each of the deletion derivatives at 19°C. These potentially modified phages were subsequently challenged with the complete LlaJI system (pJO-J) at 19°C. Full protection was observed only when phages had been passaged through a strain harboring both llaJIM1 and llaJIM2 (data not shown). For further investigation of this observation, llaJIM1 and llaJIM2 were cloned individually and in tandem, as they are organized on pNP40, into the nisin-inducible expression vector pNZ8020, producing plasmids pNZ-M1, pNZ-M2, and pNZ-M1M2, respectively. φsk1 was subsequently propagated on strains expressing the individual or combined methylases. The methylation status of the phage progeny was assessed by plaque analysis on a host that harbored either plasmid pJO-J expressing the complete LlaJI system or its derivative, pJO-M1M2R1, at 19°C (Table 5). As expected, full protection against LlaJI was observed only when phage had been propagated on a host expressing both M1.LlaJI and M2.LlaJI (through the presence of pNZ-M1M2). Phage propagated on a host expressing either methylase alone were only partially protected when challenged with LlaJI, with a persistence in the variable plaque phenotype and a slight decrease in the EOP. Phage propagated on the host expressing M1.LlaJI alone (pNZ-M1) were found to still be sensitive to restriction by MG1614/pJO-M1M2R1 (observed as a variable plaque phenotype), suggesting that M1.LlaJI may not be involved in methylating the R1.LlaJI target site. To further clarify this observation, we propagated φsk1 on MG1614/pJO-M1 (expressing M1.LlaJI in the low-copy-number vector pPTPi). The phage was found to afford a 2-log increase in EOP when challenged with a LlaJI+ host (when compared to the EOP observed for unmodified φsk1), confirming that (partial) methylation had occurred. These modified phage were also found to be sensitive to the phenotype exhibited by a host bearing pJO-M1M2R1 (as seen for unmodified φsk1 in Tables 4 and 5), supporting the data presented above (results not shown). Furthermore, phage propagated on the host expressing M2.LlaJI alone (pNZ-M2) appeared to be insensitive to pJO-M1M2R1, with plaque sizes identical to those of the sensitive host (Table 5), suggesting that M2.LlaJI methylates the R1.LlaJI target site. From these data, it appears as if the methylation component of the LlaJI system is encoded by llaJIM1 and llaJIM2, whereas llaJIR1 and llaJIR2 are responsible for the restriction phenotype.
TABLE 5.
In vivo LlaJI methylation assaya
| Propagating host | Value for indicated strain
|
|||||
|---|---|---|---|---|---|---|
| MG1614
|
MG1614/pJO-J
|
MG1614/pJO-M1M2R1
|
||||
| EOP | Plaque size (mm) | EOP | Plaque size (mm) | EOP | Plaque size (mm) | |
| NZ9000/pNZ8020 | 1 | 2-3 | 1 × 10−7 | 1-2 | 1 | 1-2 |
| NZ9000/pNZ-M1 | 1 | 2-3 | 3 × 10−1 | 1-2 | 1 | 1-3 |
| NZ9000/pNZ-M2 | 1 | 2 | 4 × 10−1 | 1-2 | 1 | 2 |
| NZ9000/pNZ-M1M2 | 1 | 2-3 | 1 | 2-3 | 1 | 2-3 |
φsk1 was propagated on strains overexpressing LlaJI methylases separately and in tandem and was subsequently analyzed for its insensitivity to restriction by pJO-J and pJO-M1M2R1 at 19°C.
Northern blot analysis and examination of the promoter activity of the LlaJI operon.
In order to conduct a transcriptional analysis of the LlaJI gene cluster, we performed Northern blot studies. Total RNAs were isolated from MG1614 and MG1614/pJO-J at 25, 30, and 37°C to determine if the variable phage resistance phenotype found at these temperatures was transcriptionally related. Northern blot analysis using a llaJIM1-specific probe revealed an RNA species of approximately 6 kb which appeared to be intrinsically unstable (Fig. 4A). An additional, but much fainter, signal was detected representing a transcript of over 6 kb, which may be attributable to incomplete transcriptional termination. The RNA from MG1614 did not reveal any signal, confirming that the signal detected with RNA from MG1614 containing pJO-J was specific to the LlaJI mRNA. A Northern blot analysis using probes specific to the other ORFs of the LlaJI cluster showed that they hybridized to the same RNA species (data not shown), confirming that LlaJI is transcribed as a single polycistronic mRNA. Surprisingly, as shown in Fig. 4A, the highest level of LlaJI-encompassing mRNA appeared to be present at 37°C, which is the temperature at which the phage resistance conferred by LlaJI is completely lost. This result was confirmed by use of a LlaJI transcriptional fusion to a reporter gene (see below).
FIG. 4.
(A) Northern blot analysis of total RNAs isolated from MG1614 and MG1614/pJO-J at three different temperatures and hybridized to an llaJIM1-specific probe. Lanes 1 and 2, RNAs isolated from MG1614 and MG1614/pJO-J, respectively, at 25°C; lanes 3 and 4, RNAs isolated from MG1614 and MG1614/pJO-J, respectively, at 30°C; lanes 5 and 6, RNAs isolated from MG1614 and MG1614/pJO-J, respectively, at 37°C. The positions of the 16S and 23S rRNAs as well as the RNA markers are indicated. (B) Primer extension analyses of total RNAs isolated from MG1614/pNP40 (lane 1), MG1614 (lane 2), and MG1614/pJO-J (lane 3) at 30°C identified the transcriptional start site as a guanine residue (see Fig. 2B).
A 500-bp PCR product encompassing the assumed promoter region was generated and cloned into the promoter probe vector pPTPL to generate construct pJPL. This plasmid was introduced into two genetic backgrounds, MG1614 and MG1614 containing plasmid pNP40, to characterize the activity of the LlaJI promoter by measuring the β-galactosidase activity.
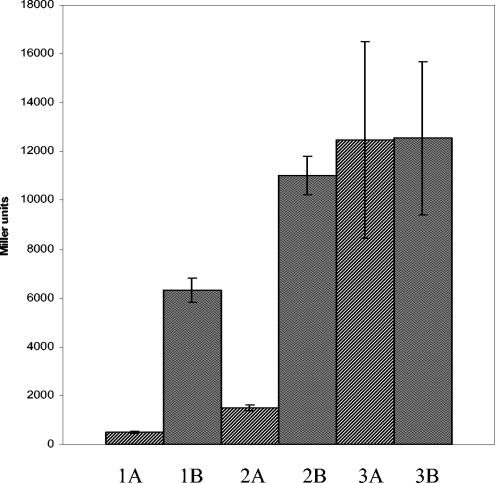
As shown in Fig. 5, at 30°C the LlaJI promoter exhibited about sevenfold more activity in the MG1614 background than in the MG1614/pNP40 background, which suggests transcriptional regulation of this promoter by one or more factors encoded by pNP40. At 25°C, the overall level of promoter activity was significantly lower than that at 30°C in the MG1614 background. A 12-fold lower (relative to MG1614) activity was detected in the MG1614/pNP40 background at this temperature. This is in agreement with Northern blot analysis, which showed lower levels of LlaJI transcription at 25°C. At 37°C, the promoter activity in the MG1614 background was similar to that observed at 30°C. There was no significant difference observed between this level and that of the pNP40-containing background at this temperature, suggesting a loss of the transcriptional repression which was previously evident. This observation is consistent with the findings of the Northern blot analysis, which showed a higher level of LlaJI-specific mRNA at 37°C. These results confirm that the LlaJI operon is transcriptionally regulated by unknown elements encoded by pNP40.
FIG. 5.
β-Galactosidase assays of LlaJI promoter-lacZ fusion in MG1614 and MG1614/pNP40 backgrounds conducted at 25, 30, and 37°C. 1A, MG1614/pNP40/pJPL at 25°C; 1B, MG1614/pJPL at 25°C; 2A, MG1614/pNP40/pJPL at 30°C; 2B, MG1614/pJPL at 30°C; 3A, MG1614/pNP40/pJPL at 37°C; 3B, MG1614/pJPL at 37°C.
Primer extension analysis to map the precise location of the LlaJI promoter.
To determine the 5′ end of the LlaJI mRNA species, we conducted primer extension analysis on total RNAs isolated from MG1614/pNP40, MG1614, and MG1614/pJO-J by using a primer that was complementary to the 5′ region of llaJIM1. The migration of the extension product with a sequence reaction of LlaJI that used the same primer identified the transcriptional start site to be a guanine residue located 28 bp upstream of the ATG start codon of llaJIM1 (Fig. 4B; also see Fig. 2B). This result was confirmed by multiple replicates of the experiment.
A single promoter was identified within this region. As shown in Fig. 2B, extended −10 (TGTAGAAT) and −35 (TTGACG) sequences were identified which were very similar to the consensus promoter sequences for lactococci (44). Several putative Rho-independent transcriptional terminator sequences were identified downstream of llaJIR2; however, as of yet, the exact terminator for the operon remains unidentified. Interestingly, a perfect 24-bp inverted repeat structure was found to straddle the putative −10 and −35 boxes of the promoter. Such a structure may serve as an operator for the binding of a transcriptional regulator. Overlapping the end of this large inverted repeat, a 14-bp perfect direct repeat sequence was identified that encompasses the −10 box, part of the llaJIM1 ribosomal binding site, and the first two codons of the llaJIM1 gene (Fig. 2B). This structural feature may also serve a regulatory purpose.
DISCUSSION
This paper describes the identification of a novel R/M system, LlaJI, which represents the fourth resistance mechanism to be specified by the lactococcal plasmid pNP40. The LlaJI system was found to confer resistance against members of the c2, 936, and P335 quasi-phage species. The two previously characterized pNP40-encoded Abi systems, AbiE and AbiF, do not confer resistance against representatives of the P335 species (63), and therefore this is the first evidence of pNP40-mediated resistance against members of this phage species. The resistance observed was less potent than that obtained for members of the c2 and 936 species, which may reflect differences in the numbers of LlaJI recognition sequences between the phages tested, since the EOP is known to decrease logarithmically as the number of recognition sites on the phage genome targeted by a given R/M system increases (41, 68). The resistance phenotype observed was shown to be temperature dependent and was optimally functional at 19°C. Interestingly, LlaJI produces a variable plaque phenotype, similar to that previously observed for mutants of LlaI (49). This may be a consequence of suboptimal restriction, although at the apparent optimum temperature for restriction, the variable plaque phenotype was actually found to be more pronounced.
A functional characterization of the LlaJI operon revealed that llaJIM1 and llaJIM2 are both required to mediate complete methylation. Similarly, llaJIR1 and llaJIR2 both appear to be required for full restriction to occur, since the removal of llaJIR2 (in plasmid pJO-M1M2R1) significantly reduced the restriction phenotype. The initial overexpression individually of M1.LlaJI and M2.LlaJI demonstrated that both proteins are capable of methylation; however, as stated, both methylases were required for complete protection against LlaJI.
The similarities between the HgaI (61) methylases and those of the LlaJI system extend into the region between motifs VIII and IX, known as the target recognition domain. The individual HgaI methylases have been demonstrated to recognize an asymmetric but complementary sequence in the target DNA, and it is conceivable that M1.LlaJI and M2.LlaJI act in a similar manner. R1.LlaJI shows significant homology to LlaI.2 (49) and McrB (16), particularly with respect to a conserved ATP/GTP binding domain. This suggests that this enzyme requires energy for some aspect of its function, possibly to pull the DNA through the enzyme to facilitate cleavage, which occurs at a site that is distant from the recognition sequence and is a property of type I enzymes (reviewed in reference 4). In light of these findings, we propose a working model for the LlaJI system, which is in agreement with the suggestions of Szybalski et al. for enzymes of the type IIS class (62) and consistent with observations for the HgaI (61), LlaI (49), and FokI systems (59). The LlaJI restriction enzymes may recognize different asymmetric (and possibly complementary) sequences, introducing individual single-stranded cuts at sites that are distant from the recognition sequences. The two restriction enzymes are accompanied by two cognate methylases specific for a single endonuclease recognition target, which protect the host cell by the methylation of appropriate bases of their respective recognition sequences. The residual phage resistance exhibited by a host expressing R1.LlaJI alone (pJO-M1M2R1) may indicate that this gene product introduces a single-stranded cut in the target DNA, with subsequent double-stranded scission achieved only in the joint presence of R2.LlaJI. However, the possibility for a multisubunit restriction complex comprised of the products of llaJIR1 and llaJIR2, with separate cleavage capabilities for both strands in the target DNA, cannot be entirely excluded. Given the putative energy requirement for DNA translocation by R1.LlaJI, facilitating cleavage distant from the recognition sequence, it seems probable that the LlaJI system, like the LlaI system of pTR2030 (49), has diverged from its putative type IIS origin, acquiring a characteristic of type I enzymes. Both the LlaJI and LlaI systems are located on large conjugative lactococcal plasmids, facilitating horizontal transfer, which may have contributed to this evolutionary divergence.
A Northern blot analysis confirmed that the LlaJI system is transcribed as an operon, while the transcript appeared to be intrinsically unstable. The latter is not an unusual feature of large polycistronic transcripts and has been described previously (9, 19, 33). Of the three sample temperatures analyzed, the highest level of the major 6-kb LlaJI transcript was detected at 37°C, which is paradoxically the temperature at which there is no phage resistance phenotype. Furthermore, the dramatic increase in phage resistance observed as the temperature decreased appears to be accompanied by a decrease in the levels of LlaJI-specific mRNA in the cells. This was confirmed by an analysis using a transcriptional fusion of the LlaJI promoter region with a lacZ reporter, which showed that LlaJI was subject to transcriptional regulation by one or more factors encoded by pNP40, which also appeared to be temperature sensitive. However, in agreement with the Northern hybridization analysis, this did not contribute to the temperature-dependent profile observed for restriction. From these results, we can conclude that the LlaJI operon is transcribed, even under circumstances in which no restriction phenotype is apparent, and that the temperature-induced relative abundance of the LlaJI mRNA species in the cell has no effect on the potency of the phage resistance phenotype. It seems most likely that the RNA profiles are attributable to the temperature sensitivity of a transcriptional regulator, causing a higher level of transcription at 37°C due to the loss of transcriptional repression. The LlaJI system itself is also highly temperature sensitive, with no observed phage resistance activity at 37°C. Interestingly, as stated, the observed regulation is lost under conditions in which restriction, and to a lesser extent, modification, is also lost, which may implicate components of the R/M machinery in the transcriptional regulation of LlaJI.
The regulated expression of the components of the LlaJI operon thus poses an intriguing enigma for future consideration. The prodigious nature of the LlaJI challenge, in combination with the previously characterized phage resistance mechanisms encoded by pNP40 and its conjugal capacity, further highlights this replicon's usefulness in improving the fitness of industrial starter cultures in a food-grade manner.
Acknowledgments
This work was funded by Science Foundation Ireland (02/IN1/B198).
We thank Todd Klaenhammer for supplying L. lactis NCK690 KP1, Sylvain Moineau for supplying phages Q30, Q33, and UL36, and Michel Kleerebezem for supplying plasmid pNZ8020 and L. lactis NZ9000. We also thank Kayla Polzin for preliminary work on pNP40.
REFERENCES
- 1.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bickle, T. A., and D. H. Kruger. 1993. Biology of DNA restriction. Microbiol. Rev. 57:434-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourniquel, A. A., and T. A. Bickle. 2002. Complex restriction enzymes: NTP-driven molecular motors. Biochimie 84:1047-1059. [DOI] [PubMed] [Google Scholar]
- 5.Butler, D., and G. F. Fitzgerald. 2001. Transcriptional analysis and regulation of expression of the ScrFI restriction-modification system of Lactococcus lactis subsp. cremoris UC503. J. Bacteriol. 183:4668-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadaban, M. J., and S. N. Cohen. 1980. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138:179-207. [DOI] [PubMed] [Google Scholar]
- 7.Chambers, S. P., S. E. Prior, D. A. Barstow, and N. P. Minton. 1988. The pMTL nic-cloning vectors. I. Improved pUC polylinker regions to facilitate the use of sonicated DNA for nucleotide sequencing. Gene 68:139-149. [DOI] [PubMed] [Google Scholar]
- 8.Chandry, P. S., B. E. Davidson, and A. J. Hillier. 1994. Temporal transcription map of the Lactococcus lactis bacteriophage sk1. Microbiology 140:2251-2261. [DOI] [PubMed] [Google Scholar]
- 9.Chen, L. H., S. A. Emory, A. L. Bricker, P. Bouvet, and J. G. Belasco. 1991. Structure and function of a bacterial mRNA stabilizer: analysis of the 5′ untranslated region of ompA mRNA. J. Bacteriol. 173:4578-4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen, L. L., and J. Josephsen. 2004. The methyltransferase from the LlaDII restriction-modification system influences the level of expression of its own gene. J. Bacteriol. 186:287-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coveney, J. A. 1989. Characterisation of Lactococcal bacteriophages based on morphology, host range, DNA restriction endonuclease patterns, DNA hybridisation and structural protein profiles. Ph.D. thesis. University College Cork, Cork, Ireland.
- 12.Deng, Y. M., C. Q. Liu, and N. W. Dunn. 2000. LldI, a plasmid-encoded type I restriction and modification system in Lactococcus lactis. DNA Sequence 11:239-245. [DOI] [PubMed] [Google Scholar]
- 13.de Ruyter, P. G., O. P. Kuipers, M. M. Beerthuyzen, I. van Alen-Boerrigter, and W. M. de Vos. 1996. Functional analysis of promoters in the nisin gene cluster of Lactococcus lactis. J. Bacteriol. 178:3434-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Ruyter, P. G., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dever, T. E., M. J. Glynias, and W. C. Merrick. 1987. GTP-binding domain: three consensus sequence elements with distinct spacing. Proc. Natl. Acad. Sci. USA 84:1814-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dila, D., E. Sutherland, L. Moran, B. Slatko, and E. A. Raleigh. 1990. Genetic and sequence organization of the mcrBC locus of Escherichia coli K-12. J. Bacteriol. 172:4888-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Djordjevic, G. M., and T. R. Klaenhammer. 1997. Bacteriophage-triggered defense systems: phage adaptation and design improvements. Appl. Environ. Microbiol. 63:4370-4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzgerlad, G. F., C. Daly, L. R. Brown, and T. R. Gingeras. 1982. ScrFI: a new sequence-specific endonuclease from Streptococcus cremoris. Nucleic Acids Res. 10:8171-8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gamper, M., B. Ganter, M. R. Polito, and D. Haas. 1992. RNA processing modulates the expression of the arcDABC operon in Pseudomonas aeruginosa. J. Mol. Biol. 226:943-957. [DOI] [PubMed] [Google Scholar]
- 20.Garvey, P., G. F. Fitzgerald, and C. Hill. 1995. Cloning and DNA sequence analysis of two abortive infection phage resistance determinants from the lactococcal plasmid pNP40. Appl. Environ. Microbiol. 61:4321-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garvey, P., C. Hill, and G. Fitzgerald. 1996. The lactococcal plasmid pNP40 encodes a third bacteriophage resistance mechanism, one which affects phage DNA penetration. Appl. Environ. Microbiol. 62:676-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins, D. L., R. B. Sanozky-Dawes, and T. R. Klaenhammer. 1988. Restriction and modification activities from Streptococcus lactis ME2 are encoded by a self-transmissible plasmid, pTN20, that forms cointegrates during mobilization of lactose-fermenting ability. J. Bacteriol. 170:3435-3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill, C., L. A. Miller, and T. R. Klaenhammer. 1991. In vivo genetic exchange of a functional domain from a type II A methylase between lactococcal plasmid pTR2030 and a virulent bacteriophage. J. Bacteriol. 173:4363-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanova, N., A. Sorokin, I. Anderson, N. Galleron, B. Candelon, V. Kapatral, A. Bhattacharyya, G. Reznik, N. Mikhailova, A. Lapidus, L. Chu, M. Mazur, E. Goltsman, N. Larsen, M. D'Souza, T. Walunas, Y. Grechkin, G. Pusch, R. Haselkorn, M. Fonstein, S. D. Ehrlich, R. Overbeek, and N. Kyrpides. 2003. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature 423:87-91. [DOI] [PubMed] [Google Scholar]
- 26.Janscak, P., U. Sandmeier, M. D. Szczelkun, and T. A. Bickle. 2001. Subunit assembly and mode of DNA cleavage of the type III restriction endonucleases EcoP1I and EcoP15I. J. Mol. Biol. 306:417-431. [DOI] [PubMed] [Google Scholar]
- 27.Kong, J., J. Jytte, and G. R. Ma. 2001. Cloning and structure analysis of a restriction and modification system, LlaBIII from Lactococcus lactis subsp. cremoris W56. Sheng Wu Gong Cheng Xue Bao 17:663-668. [PubMed] [Google Scholar]
- 28.Kuipers, O. P., P. G. G. A. de Ruyter, M. Kleerebezem, and W. M. de Vos. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J. Biotechnol. 64:15-21. [Google Scholar]
- 29.Kumar, S., X. Cheng, S. Klimasauskas, S. Mi, J. Posfai, R. J. Roberts, and G. G. Wilson. 1994. The DNA (cytosine-5) methyltransferases. Nucleic Acids Res. 22:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Law, J., G. Buist, A. Haandrikman, J. Kok, G. Venema, and K. Leenhouts. 1995. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J. Bacteriol. 177:7011-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lillehaug, D. 1997. An improved plaque assay for poor plaque-producing temperate lactococcal bacteriophages. J. Appl. Microbiol. 83:85-90. [DOI] [PubMed] [Google Scholar]
- 32.Liu, C. Q., P. Charoechai, N. Khunajakr, Y. M. Deng, Widodo, and N. W. Dunn. 2002. Genetic and transcriptional analysis of a novel plasmid-encoded copper resistance operon from Lactococcus lactis. Gene 297:241-247. [DOI] [PubMed] [Google Scholar]
- 33.Liu, J., W. O. Barnell, and T. Conway. 1992. The polycistronic mRNA of the Zymomonas mobilis glf-zwf-edd-glk operon is subject to complex transcript processing. J. Bacteriol. 174:2824-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madsen, A., and J. Josephsen. 1998. Characterization of LlaCI, a new restriction-modification system from Lactococcus lactis subsp. cremoris W15. Biol. Chem. 379:443-449. [DOI] [PubMed] [Google Scholar]
- 35.Madsen, A., and J. Josephsen. 1998. Cloning and characterization of the lactococcal plasmid-encoded type II restriction/modification system, LlaDII. Appl. Environ. Microbiol. 64:2424-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madsen, A., and J. Josephsen. 2001. The LlaGI restriction and modification system of Lactococcus lactis W10 consists of only one single polypeptide. FEMS Microbiol. Lett. 200:91-96. [DOI] [PubMed] [Google Scholar]
- 37.Madsen, A., C. Westphal, and J. Josephsen. 2000. Characterization of a novel plasmid-encoded HsdS subunit, S.LlaW12I, from Lactococcus lactis W12. Plasmid 44:196-200. [DOI] [PubMed] [Google Scholar]
- 38.Maguin, E., H. Prevost, S. D. Ehrlich, and A. Gruss. 1996. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J. Bacteriol. 178:931-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGrath, S., G. F. Fitzgerald, and D. van Sinderen. 2001. Improvement and optimization of two engineered phage resistance mechanisms in Lactococcus lactis. Appl. Environ. Microbiol. 67:608-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 41.Moineau, S., S. A. Walker, E. R. Vedamuthu, and P. A. Vandenbergh. 1995. Cloning and sequencing of LlaDCHI [corrected] restriction/modification genes from Lactococcus lactis and relatedness of this system to the Streptococcus pneumoniae DpnII system. Appl. Environ. Microbiol. 61:2193-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moineau, S., J. Fortier, H. W. Ackermann, and S. Pandian. 1992. Characterisation of lactococcal bacteriophages from Quebec cheese plants. Can. J. Microbiol. 38:857-882. [Google Scholar]
- 43.Moineau, S., M. Borkaev, B. J. Holler, S. A. Walker, J. K. Kondo, E. R. Vedamuthu, and P. A. Vandenbergh. 1996. Isolation and characterisation of lactococcal bacteriophages from US buttermilk plants. J. Dairy Sci. 79:2104-2111. [Google Scholar]
- 44.Nilsson, D., and E. Johansen. 1994. A conserved sequence in tRNA and rRNA promoters of Lactococcus lactis. Biochim. Biophys. Acta 1219:141-144. [DOI] [PubMed] [Google Scholar]
- 45.Nyengaard, N., F. K. Vogensen, and J. Josephsen. 1993. LlaAI and LlaBI, two type-II restriction endonucleases from Lactococcus lactis subsp. cremoris W9 and W56 recognizing, respectively, 5′-/GATC-3′ and 5′-C/TRYAG-3′. Gene 136:371-372. [DOI] [PubMed] [Google Scholar]
- 46.Nyengaard, N., F. K. Vogensen, and J. Josephsen. 1995. Restriction-modification systems in Lactococcus lactis. Gene 157:13-18. [DOI] [PubMed] [Google Scholar]
- 47.O'Sullivan, D., and T. Klaenhammer. 1993. Rapid mini-prep isolation of high-quality plasmid DNA from Lactococcus and Lactobacillus spp. Appl. Environ. Microbiol. 59:2730-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Sullivan, D. J., and T. R. Klaenhammer. 1998. Control of expression of LlaI restriction in Lactococcus lactis. Mol. Microbiol. 27:1009-1020. [DOI] [PubMed] [Google Scholar]
- 49.O'Sullivan, D. J., K. Zagula, and T. R. Klaenhammer. 1995. In vivo restriction by LlaI is encoded by three genes, arranged in an operon with llaIM, on the conjugative Lactococcus plasmid pTR2030. J. Bacteriol. 177:134-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pieper, U., T. Schweitzer, D. H. Groll, F. U. Gast, and A. Pingoud. 1999. The GTP-binding domain of McrB: more than just a variation on a common theme? J. Mol. Biol. 292:547-556. [DOI] [PubMed] [Google Scholar]
- 51.Roberts, R. J., M. Belfort, T. Bestor, A. S. Bhagwat, T. A. Bickle, J. Bitinaite, R. M. Blumenthal, S. Degtyarev, D. T. Dryden, K. Dybvig, K. Firman, E. S. Gromova, R. I. Gumport, S. E. Halford, S. Hattman, J. Heitman, D. P. Hornby, A. Janulaitis, A. Jeltsch, J. Josephsen, A. Kiss, T. R. Klaenhammer, I. Kobayashi, H. Kong, D. H. Kruger, S. Lacks, M. G. Marinus, M. Miyahara, R. D. Morgan, N. E. Murray, V. Nagaraja, A. Piekarowicz, A. Pingoud, E. Raleigh, D. N. Rao, N. Reich, V. E. Repin, E. U. Selker, P. C. Shaw, D. C. Stein, B. L. Stoddard, W. Szybalski, T. A. Trautner, J. L. Van Etten, J. M. Vitor, G. G. Wilson, and S. Y. Xu. 2003. A nomenclature for restriction enzymes, DNA methyltransferases, homing endonucleases and their genes. Nucleic Acids Res. 31:1805-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roberts, R. J., T. Vincze, J. Posfai, and D. Macelis. 2003. REBASE: restriction enzymes and methyltransferases. Nucleic Acids Res. 31:418-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 54.Sanders, J. W., G. Venema, J. Kok, and K. Leenhouts. 1998. Identification of a sodium chloride-regulated promoter in Lactococcus lactis by single-copy chromosomal fusion with a reporter gene. Mol. Gen. Genet. 257:681-685. [DOI] [PubMed] [Google Scholar]
- 55.Schouler, C., F. Clier, A. L. Lerayer, S. D. Ehrlich, and M. C. Chopin. 1998. A type IC restriction-modification system in Lactococcus lactis. J. Bacteriol. 180:407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schouler, C., M. Gautier, S. D. Ehrlich, and M. C. Chopin. 1998. Combinational variation of restriction modification specificities in Lactococcus lactis. Mol. Microbiol. 28:169-178. [DOI] [PubMed] [Google Scholar]
- 57.Seegers, J. F., D. van Sinderen, and G. F. Fitzgerald. 2000. Molecular characterization of the lactococcal plasmid pCIS3: natural stacking of specificity subunits of a type I restriction/modification system in a single lactococcal strain. Microbiology 146:435-443. [DOI] [PubMed] [Google Scholar]
- 58.Simon, D., and A. Chopin. 1988. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie 70:559-566. [DOI] [PubMed] [Google Scholar]
- 59.Skowron, P., T. Kaczorowski, J. Tucholski, and A. J. Podhajska. 1993. Atypical DNA-binding properties of class-IIS restriction endonucleases: evidence for recognition of the cognate sequence by a FokI monomer. Gene 125:1-10. [DOI] [PubMed] [Google Scholar]
- 60.Su, P., H. Im, H. Hsieh, A. S. Kang, and N. W. Dunn. 1999. LlaFI, a type III restriction and modification system in Lactococcus lactis. Appl. Environ. Microbiol. 65:686-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sugisaki, H., K. Yamamoto, and M. Takanami. 1991. The HgaI restriction-modification system contains two cytosine methylase genes responsible for modification of different DNA strands. J. Biol. Chem. 266:13952-13957. [PubMed] [Google Scholar]
- 62.Szybalski, W., S. C. Kim, N. Hasan, and A. J. Podhajska. 1991. Class-IIS restriction enzymes—a review. Gene 100:13-26. [DOI] [PubMed] [Google Scholar]
- 63.Tangney, M., and G. F. Fitzgerald. 2002. Effectiveness of the lactococcal abortive infection systems AbiA, AbiE, AbiF and AbiG against P335 type phages. FEMS Microbiol. Lett. 210:67-72. [DOI] [PubMed] [Google Scholar]
- 64.Twomey, D. P., L. L. McKay, and D. J. O'Sullivan. 1998. Molecular characterization of the Lactococcus lactis LlaKR2I restriction-modification system and effect of an IS982 element positioned between the restriction and modification genes. J. Bacteriol. 180:5844-5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195-199. [PubMed] [Google Scholar]
- 66.Wells, J. M., P. W. Wilson, and R. W. Le Page. 1993. Improved cloning vectors and transformation procedure for Lactococcus lactis. J. Appl. Bacteriol. 74:629-636. [DOI] [PubMed] [Google Scholar]
- 67.Wilson, G. G. 1991. Organization of restriction-modification systems. Nucleic Acids Res. 19:2539-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson, G. G., and N. E. Murray. 1991. Restriction and modification systems. Annu. Rev. Genet. 25:585-627. [DOI] [PubMed] [Google Scholar]