Abstract
We monitored the dynamic changes in the bacterial population in milk associated with refrigeration. Direct analyses of DNA by using temporal temperature gel electrophoresis (TTGE) and denaturing gradient gel electrophoresis (DGGE) allowed us to make accurate species assignments for bacteria with low-GC-content (low-GC%) (<55%) and medium- or high-GC% (>55%) genomes, respectively. We examined raw milk samples before and after 24-h conservation at 4°C. Bacterial identification was facilitated by comparison with an extensive bacterial reference database (∼150 species) that we established with DNA fragments of pure bacterial strains. Cloning and sequencing of fragments missing from the database were used to achieve complete species identification. Considerable evolution of bacterial populations occurred during conservation at 4°C. TTGE and DGGE are shown to be a powerful tool for identifying the main bacterial species of the raw milk samples and for monitoring changes in bacterial populations during conservation at 4°C. The emergence of psychrotrophic bacteria such as Listeria spp. or Aeromonas hydrophila is demonstrated.
The diversity in the microbial flora of raw milks contributes to the great differences in organoleptic characteristics among raw milk cheeses. Indeed, although many of the characteristics desired by consumers are not present in pasteurized cheeses (4, 6, 11, 20, 28), few studies address the characterization of microbial flora of raw milks. To date, identification has been limited to the enumeration of the most represented microbiological groups, with partial identification. In brief (2, 10, 12, 13, 14, 25), the dominant microflora of raw milk generally include (i) species of lactic acid bacteria (LAB; Lactococcus and/or Lactobacillus spp.), (ii) Pseudomonas spp., (iii) the group Micrococcaceae (Micrococcus and Staphylococcus spp.), and (iv) yeasts. Other microbial groups present in raw milks belong to the LAB (including Leuconostoc, Enterococcus, and Streptococcus spp.), Bacillus, Clostridium, and Listeria spp. and Enterobacteriaceae; there are also many gram-negative (Acinetobacter, Alcaligenes, Flavobacterium, and Aeromonas) and gram-positive (Arthrobacter, Corynebacterium, Brevibacterium, and Propionibacterium) species.
Many factors influence milk composition and hence the nature and abundance of the microbial load. The conditions of raw milk production, in particular the hygienic practices of farmers (e.g., washing of milking equipment and pre- and postmilking udder preparation), determine the contents in useful cheese-making and spoilage microorganisms (25). Intensive washing of milking equipment and udder preparation (individual washings) result in raw milks containing a majority of spoilage microorganisms (such as coliforms and Pseudomonas spp.) (29). In contrast, minimal hygiene around the udder preserves microorganisms, including salt-tolerant flora (such as Micrococcus, Arthrobacter, Microbacterium, Brevibacterium, and Staphylococcus spp.) and also the LAB (15), yielding raw milks in which useful cheese-making microorganisms are dominant. The health status of animals, the nature of their feed (forage, ensilage, etc.), and the storage conditions of raw milk are also important factors that determine the composition of their microbial flora. Intensive washing of milking equipment associated with storage of the raw milk at low temperatures gives higher levels of contamination by Pseudomonas spp. Fifty percent of the psychrotrophs in refrigerated raw milk (the first day) belong to the genus Pseudomonas, with Pseudomonas fluorescens being the predominant species (32). Other psychrotrophs present in refrigerated raw milk belong to the genera Acinetobacter, Alcaligenes, Flavobacterium, Aeromonas, Bacillus, Listeria, and Arthrobacter; Enterobacteriaceae such as Hafnia alvei, Citrobacter freundii, or Serratia liquefaciens are also found (12).
Until recently, the bacterial community of raw milk was described by classical microbiological methods, which are generally long and tedious, and allow only a partial inventory of the bacterial microflora. New molecular approaches based on direct analyses of DNA (or RNA) in its environment without microbial enrichment have allowed more precise descriptions of microbial dynamics in complex ecosystems. The most-developed methods are single-strand conformational polymorphism (17, 19, 30), denaturing gradient gel electrophoresis (DGGE) (18, 27, 33), temperature gradient gel electrophoresis (22, 34), and temporal temperature gel electrophoresis (TTGE) (26, 35). In all of these methods, the total DNA (or RNA) is extracted from environmental samples, and a zone corresponding to the 16S or 28S rRNA gene is PCR amplified. Nucleotide variation in these conserved sequences is the basis for separation during electrophoresis. For the low-GC-content (low-GC%) bacterial species (Tm of V3 sequence of <75°C), optimal resolution was achieved by TTGE; for bacteria with medium- or high-GC% DNA (Tm of V3 sequence of >75°C), the best separation was obtained by DGGE.
We used TTGE and DGGE here to study the evolution of the bacterial community in some raw milks upon conservation at 4°C.
MATERIALS AND METHODS
Raw milk samples.
Ten raw milk samples (A to J) were collected in the same area (Ile-de-France, France), except for sample H (Normandy, France), and were analyzed by TTGE and DGGE. Samples A, B, C, D, E, F, G, and H were sampled in farms; samples A, B, C, D, and E were nonrefrigerated, and samples F, G, and H were refrigerated at 4°C to 8°C for 12 h. Milk samples I and J, refrigerated at 4 to 8°C for 24 to 48 h, were collected from tanks in two industrial dairies. All raw milk samples (∼250 ml each) were collected in sterile conditions and carried at 4°C to the laboratory. Two DNA extractions were performed. The first was performed at the most 3 h after sampling (for all samples). The second was performed on seven raw milk samples (i.e., samples A, B, C, D, E, F, and G) after conservation at 4°C for 24 h.
DNA extraction.
To 35 ml of raw milk sample was added 50 mg of pronase (Roche Diagnostics, Meylan, France) and 100 μl of β-mercaptoethanol (Serva, Heidelberg, Germany). After 1.5 h of incubation of suspensions in a 37°C water bath, bacterial pellets, obtained by centrifugation at 12,000 × g for 15 min at 4°C, were washed once with sterile water and once with 10 ml of TES buffer (25 mM Tris-HCl, 0.1 M EDTA, 25% [wt/vol] saccharose; pH 8). Supernatants were discarded, and bacterial pellets were stored at −20°C.
Bacterial pellets were resuspended in 500 μl of TES. Bacteria were mechanically lysed with zirconium beads (diameter, 150 to 200 μm; Sigma, St. Louis, Mo.) by six cycles of 30 s of vortexing, with 1 min of storage in ice between each cycle.
DNA purification was performed as described previously (9).
PCR amplification.
The V3 region of the 16S rRNA gene is the substrate for PCR amplification. The extracted DNA (1 μl) was amplified by two successive PCR amplifications. A 700-bp fragment, including the 16S rRNA gene V3 region, was first PCR amplified as described previously (26) by using the primers W01 [5′-AGA GTT TGA TC(AC) TGG CTC-3′] and W012 [5′-TAC GCA TTT CAC C(GT)C TAC A-3′] (MWG-Biotech AG, Ebersberg, Germany). The PCR fragment containing the V3 region was then used as substrate to amplify an ∼200-bp fragment, as described previously (26). Two primers from MWG-Biotech AG were used: HDA1_GC-clamp (5′-CGC CCG GGG CGC GCC CCG GGC GGG GCG GGG GCA CGG GGG GAC TCC TAC GGG AGG CAG CAG T-3′; GC-clamp is underlined) and HDA2 (5′-GTA TTA CCG CGG CTG CTG GCA-3′). PCRs were performed by using the Gene Amp system (model 2400; Perkin-Elmer, Courtaboeuf, France). The purity and lengths of PCR products were verified on 2% agarose gels (FMC Bioproducts, Rockland, Maine) in comparison with a standard containing DNA fragments of defined lengths (λ/BstE II; Q-BIOgene, Illkirch, France). The latter fragments were used to perform TTGE and DGGE species identifications.
TTGE analysis for identification of bacteria with AT-rich genomes.
For TTGE analysis, the Dcode universal mutation detection system (Bio-Rad, Marnes la Coquette, France) was used to separate the V3 region PCR products. PCR products (5 μl) were added to 10 μl of loading buffer (100 mM EDTA, bromophenol blue [1.5 mg/ml], 40% saccharose). Samples were electrophoresed on 8% (wt/vol) polyacrylamide gels containing 6 M urea in 1.25× TAE running buffer (2 M Tris base, 1 M glacial acetic acid, 50 mM EDTA). A marker containing four reference species (Lactococcus garvieae CNRZ1323, Lactococcus raffinolactis CNRZ1214, Enterococcus faecalis CE17, and Lactococcus lactis subsp. lactis bv. diacetylactis CNRZ260) was loaded onto every gel. Migration was performed at 41 V for 16 h with a running buffer temperature of 63°C at the beginning and 70°C at the end. Gels were stained in an ethidium bromide solution (0.5 μg of 1× TAE buffer/ml) for 20 min, rinsed in 1× TAE buffer for 20 min, and photographed on a UV transillumination table. Gel photographs were converted into a file image (Photo Capt Imager Software) and analyzed by using GelCompar software (Applied Maths, Kortrijk, Belgium).
DGGE analysis for identification of bacteria with GC-rich genomes.
For DGGE analysis, the Dcode universal mutation detection system (Bio-Rad) was used to separate the V3 region PCR products. PCR products were prepared as for TTGE. Samples were electrophoresed on 8% (wt/vol) polyacrylamide gels containing a denaturating gradient from 40 to 70% urea and formamide (a 100% denaturant corresponds to 7 M urea and 40% [vol/vol] formamide) in 1.25× TAE running buffer. A marker containing six reference species (Kytococcus sedentarius CNRZ880, Arthrobacter citreus CNRZ928T, Kocuria kristinae CNRZ872, Bacillus pumilus ATCC 7725, Propionibacterium jensenii Z87, and Klebsiella oxytoca ATCC 103434T) was loaded onto every gel. Migration was performed at 92 V for 16 h, and the running buffer temperature was kept constant at 60°C. Gels were stained, photographed, and analyzed as described above.
Analysis of TTGE and DDGE gels.
GelCompar software used to analyze TTGE and DGGE gels adjusted for migration differences between gels by aligning the standardization markers included in all gels with a standard gel (31). Bacterial species isolated from dairy products were then identified by comparison with our recently reported reference dairy bacteria database (26, 26a).
Cloning and sequencing of TTGE and DGGE fragments.
In cases where new bands appeared or where assignments were ambiguous, DNA bands on TTGE and DGGE gels were excised and purified as described previously (26). The excised band (corresponding to the V3 region) was then amplified with the HDA1 primer without the GC clamp and with HDA2. PCR products were quantified on 2% agarose gels, purified by using Concert Rapid PCR purification system (Life Technologies, Gaithersburg, Md.), and cloned into the pTOPOI plasmid vector (using the TOPO TA cloning kit; Invitrogen, Carlsbad, Calif.). To verify the insert, an amplification of the V3 region searched was sequenced (ABI Prism 310; Applied Biosystems, Courtaboeuf, France) after an amplification of a region of 500 bp containing the insert with the primers M13 Reverse and M13 Forward from the TOPO TA cloning kit (Invitrogen). Sequences were compared to the Ribosomal Database Project sequences (24) for species assignment.
RESULTS
Bacterial biodiversity in raw milk samples.
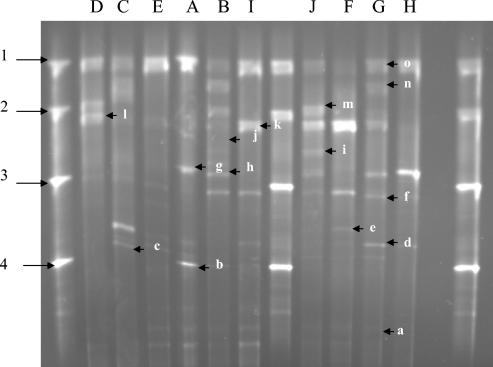
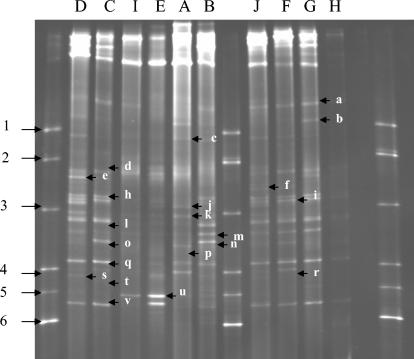
TTGE and DGGE profiles varied in complexity. In TTGE (for identification of bacteria with low-GC% genomes), some raw milk samples displayed simple profiles, with three or four bands (Fig. 1, samples A, D, E, I, and H). Other milk samples displayed were complex profiles, with as many as 10 bands (Fig. 1, samples B, C, F, G, and J). In DGGE (for identification of bacteria with medium- or high-GC% genomes), only one sample displayed simple profiles with three bands (Fig. 2, sample I). The other sample profiles displayed six or more bands. All milk samples showed the same major band (band o) in TTGE except sample F. Many common bands were present in the different milk samples, as revealed by both TTGE and DGGE.
FIG. 1.
TTGE profiles of 16S rRNA gene V3 regions obtained from different raw milk samples (as indicated above each lane). Lanes 1, 8, and 13, marker. Bands: a, unnidentified; b, Lactococcus garvieae; c, Lactobacillus plantarum/Lactobacillus pentosus; d, Listeria innocua/Listeria monocytogenes/Lactobacillus fermentum; e, Staphylococcus epidermidis; f, Pseudomonas fluorescens/Enterococcus faecium/Enterococcus durans/Enterococcus hirae/Leuconostoc carnosum; g, Leuconostoc lactis/Staphylococcus xylosus/Acinetobacter johnsonii; h, Chryseobacterium; i, Lactobacillus acidophilus group; j, Lactobacillus delbrueckii subsp. bulgaricus; k, Streptococcus uberis/Bacillus circulans; l, Staphylococcus warneri; m, Pseudomonas stutzeri; n, Streptococcus dysgalactiae/Hafnia alvei/Pseudomonas alcaligenes; o, Lactococcus lactis. Markers (arrowed): 1, Lactococcus lactis subsp. lactis bv. diacetylactis CNRZ260; 2, Enterococcus faecalis CE17; 3, Lactococcus raffinolactis CNRZ1214; 4, Lactococcus garvieae CNRZ1323.
FIG. 2.
DGGE profiles of 16S rRNA gene V3 regions obtained from different raw milk samples as indicated above each lane. Lanes 1, 8, and 13, marker. Bands: a, Escherichia coli/Klebsiella pneumoniae; b, Citrobacter freundii; c, unidentified; d, Enterobacter sakazakii/Aeromonas hydrophila/Lactobacillus reuteri; e, unidentified; f, Serratia marcescens; h, Klebsiella pneumoniae; i, Clostridium sporogenes; j, Pantoea sp.; k, unidentified; l, Kocuria rosea/Klebsiella pneumoniae; m, Brevibacterium linens; n, Arthrobacter species/Klebsiella pneumoniae/Brachybacterium tyrofermentans/Corynebacterium ammoniagenes; o, unidentified; q, Brevibacterium linens/Klebsiella pneumoniae; r, unidentified; s, unidentified; t, Brevibacterium linens; u, Kocuria kristinae/Brevibacterium linens; v, Propionibacterium acidipropionici/Kocuria sp. Markers (arrowed): 1, Bacillus pumilus ATCC 7725; 2, Klebsiella oxytoca ATCC 103434T; 3, Kytococcus sedentarius CNRZ880; 4, Arthrobacter citreus CNRZ928T; 5, Kocuria kristinae CNRZ872; 6, Propionibacterium jensenii Z87.
Most of the bands were assigned to a species or a group of species of our reference database (26, 26a). However, in some cases, e.g., when migration of V3 fragments was the same for different species (comigration as for the same V3 sequences or the same melting temperature) or to confirm an electrophoresis band, sequencing was performed to confirm the identification.
Lactococcus lactis (band o) was the major raw milk species identified by TTGE and confirmed by sequencing (see below) (Table 1). Some Staphylococcus species were also present. Staphylococcus warneri (band l) was detected as a major species in the samples B, D, and J; Staphylococcus epidermidis (band e) was also identified in four samples (samples B, C, F, and G). Several bands were assigned to a group of species of our database reference (26, 26a) (bands c, d, f, g, i, k, and n). In that case, Table 1 shows the results of sequencing, the GenBank accession number, and the percentage of identity with V3 of a known species. Band k was identified as “Streptococcus uberis” at 99% certainty for milk sample D, 100% for milk sample F and 99% for milk sample I. Band d (present in samples C and G) was ambiguously identified as being either Listeria innocua, Listeria monocytogenes, or Lactobacillus fermentum. After sequencing, it was identified as Listeria sp. at 91 and 99% certainties in samples C and G, respectively. Band f (in milk sample G) was confirmed as corresponding to Enterococcus faecium at a 100% certainty, and band n was confirmed as corresponding to Streptococcus dysgalactiae at a 99% certainty.
TABLE 1.
Identification of 16S rRNA gene (V3 region) cloned sequences
| Band | Milka | TTGE or DGGE | Species assigned by reference databaseb | Sequence analysis
|
||
|---|---|---|---|---|---|---|
| Species | GenBank accession no. | % Identity | ||||
| k | DNR | TTGE | S. uberis/B. circulans | S. uberis | AB023573 | 99 |
| d | CNR | TTGE | L. innocua/L. monocytogenes/E. casseliflavus/L. fermentum | L. monocytogenes | AJ549929 | 91 |
| L. innocua | AJ549928 | 91 | ||||
| k | FNR | TTGE | S. uberis/B. circulans | S. uberis | AB002527 | 100 |
| d | GNR | TTGE | L. innocua/L. monocytogenes/E. casseliflavus/L. fermentum | L. monocytogenes | AJ549929 | 99 |
| L. innocua | AJ549928 | 99 | ||||
| f | GNR | TTGE | P. fluorescens/E. faecium/E. durans/E. hirae/L. carnosum | E. faecium | AJ420800 | 100 |
| k | INR | TTGE | S. uberis/B. circulans | S. uberis | AB023576 | 99 |
| n | GNR | TTGE | S. dysgalactiae/H. alvei/P. alcaligenes | S. dysgalactiae | AY121362 | 99 |
| o | DNR | TTGE | L. lactis | L. lactis | AF515226 | 99 |
| d | DR | TTGE | L. innocua/L. monocytogenes/L. fermentum | L. monocytogenes | AJ508749 | 91 |
| L. innocua | AL596172 | 91 | ||||
| n | GR | TTGE | S. dysgalactiae/H. alvei/P. alcaligenes | S. dysgalactiae | AY121362 | 99 |
| o | FR | TTGE | L. lactis | L. lactis | AF515226 | 100 |
| m | GNR | DGGE | B. linens | K. pneumoniae | AF130982 | 97 |
| u | ENR | DGGE | K. kristinae/B. linens | C. bifermentans | AF320283 | 85 |
| m | BNR | DGGE | B. linens | K. pneumoniae | AF453251 | 97 |
| l | GNR | DGGE | K. rosea/K. pneumoniae | K. pneumoniae | AY291290 | 100 |
| v | DR | DGGE | P. acidipropionici/Kocuria sp. | Propionibacterium sp. | AY096033 | 99 |
| u | ER | DGGE | K. kristinae/B. linens | Kocuria sp. | AY043546 | 99 |
| q | DR | DGGE | B. linens/K. pneumoniae | K. pneumoniae | AY291290 | 98 |
| n | BR | DGGE | Arthrobacter sp./K. pneumoniae/B. tyrofermentans/C. ammoniagenes | C. bifermentans | AF320283 | 100 |
| h | DR | DGGE | K. pneumoniae | K. pneumoniae | AF390084 | 100 |
| b | DR | DGGE | C. freundii | C. freundii | AF458082 | 97 |
Some major bands were detected by DGGE. Klebsiella pneumoniae (band h) was detected as a major species in the milk sample C. Band l (in milk sample C) was identified as Kocuria rosea or Klebsiella pneumoniae. Band n (in milk sample B) was identified as Arthrobacter species, Klebsiella pneumoniae, Brachybacterium tyrofermentans, or Corynebacterium ammoniagenes. Band q was identified as Brevibacterium linens or Klebsiella pneumoniae in samples C, F, and G, and band u (in milk sample E) was identified as Kocuria kristinae or Brevibacterium linens (Fig. 2). Many of the bands gave ambiguous assignments. For example, band q was assigned to the species Brevibacterium linens or Klebsiella pneumoniae, and band n was assigned to Arthrobacter species, Klebsiella pneumoniae, Brachybacterium tyrofermentans, or Corynebacterium ammoniagenes. Sequencing clarified some of these uncertainties: band m (samples B and G) and band l (milk G) were identified as Klebsiella pneumoniae. Band u (milk E) was identified as Clostridium bifermentans.
Bacterial biodiversity of raw milk samples after conservation at 4°C for 24 h.
To determine whether bacterial dynamics in milk is affected by refrigeration, TTGE and DGGE profiles were determined for samples A, B, C, D, E, F, and G after 24 h of incubation at 4°C and then compared to profiles of nonrefrigerated samples (Fig. 3 and 4 and Tables 2 and 3). Many of the species identified after refrigeration were present in the initial sample. However, the relative proportions of bacteria were clearly altered by refrigeration. The intensities of some bands increased (e.g., in samples B, C, E, F, and G in TTGE [Table 2] and in samples A, B, D, E, F, and G in DGGE [Table 3]). Other populations decreased (for samples A, F, and G in DGGE, the band intensity showed an overall decrease). In several cases, new bands appeared after refrigeration (e.g., samples D and F in TTGE and samples A, C, E, and G in DGGE). The more pertinent changes due to refrigeration are presented below.
FIG. 3.
TTGE profiles of rRNA gene V3 regions obtained from different raw milk samples before and after conservation at 4°C for 24 h. After standardization of band migration with the GelCompar software (Applied Maths), species were identified by comparison with known species in the reference database. Bands: a, unidentified; b, Lactococcus garvieae; c, Lactobacillus plantarum/Lactobacillus pentosus; d, Listeria innocua/Listeria monocytogenes/Lactobacillus fermentum; e, Staphylococcus epidermidis; f, Pseudomonas fluorescens/Enterococcus faecium/Enterococcus durans/Enterococcus hirae/Leuconostoc carnosum; g, Leuconostoc lactis/Staphylococcus xylosus/Acinetobacter johnsonii; h, Chryseobacterium; i, Lactobacillus acidophilusgroup; j, Lactobacillus delbrueckii subsp. bulgaricus; k, Streptococcus uberis/Bacillus circulans; l, Staphylococcus warneri; n, Streptococcus dysgalactiae/Hafnia alvei/Pseudomonas alcaligenes; o, Lactococcus lactis.
FIG. 4.
DGGE profiles of rRNA gene V3 regions obtained from different raw milk samples before and after conservation at 4°C for 24 h. After standardization of band migration with the GelCompar software (Applied Maths), species were identified by comparison with known species in the reference database. Bands: a, Escherichia coli/Klebsiella pneumoniae; b, Citrobacter freundii; c, unidentified; d, Enterobacter sakazakii/Aeromonas hydrophila/Lactobacillus reuteri; e, unidentified; f, Serratia marcescens; g, Aeromonas hydrophila; h, Klebsiella pneumoniae; i, Clostridium sporogenes; j, Pantoea sp.; k, unidentified; l, Kocuria rosea/Klebsiella pneumoniae; m, Brevibacterium linens; n, Arthrobacter species/Klebsiella pneumoniae/Brachybacterium tyrofermentans/Corynebacterium ammoniagenes; o, unidentified; p, unidentified; q, Brevibacterium linens/Klebsiella pneumoniae; r, unidentified; s, unidentified; t, Brevibacterium linens; u, Kocuria kristinae/Brevibacterium linens; v, Propionibacterium acidipropionici/Kocuria sp.; w, Propionibacterium thoenii/Propionibacterium jensenii; x, unidentified; y, nonidentified.
TABLE 2.
Band intensity after storage of milk samples at 4°C for 24 h
| Milk sample | Intensitya of band:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | g | h | i | j | k | l | n | o | |
| B | − | + | = | = | + | = | D | − | = | = | ||||
| D | A | A | A | − | D | A | = | |||||||
| E | + | + | = | |||||||||||
| A | = | = | = | = | ||||||||||
| C | D | + | = | = | = | − | − | |||||||
| G | D | D | + | + | + | + | + | − | ||||||
| F | A | = | A | − | + | + | ||||||||
Key: A, appearance; D, disappearance; +, increase; −, decrease; =, no change.
TABLE 3.
Band intensity after storage of milk samples at 4°C for 24 h
| Milk sample | Intensitya of band:
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | g | h | i | j | k | l | m | n | o | p | q | r | s | t | u | v | w | x | y | |
| D | = | − | + | = | − | + | + | D | + | ||||||||||||||||
| C | = | D | A | = | = | D | A | = | D | = | A | A | |||||||||||||
| G | D | − | + | = | − | − | D | − | D | − | A | A | A | ||||||||||||
| F | D | D | + | − | D | D | D | − | D | D | |||||||||||||||
| B | = | = | = | + | = | − | = | = | + | = | − | − | = | ||||||||||||
| A | = | D | D | + | A | D | D | D | D | D | D | D | D | ||||||||||||
| E | = | = | = | + | A | + | = | = | − | ||||||||||||||||
Key: A, appearance; D, disappearance; +, increase; −, decrease; =, no change.
We detected increases in (i) Listeria innocua, Listeria monocytogenes, or Lactobacillus fermentum; (ii) Staphylococcus epidermidis; (iii) Pseudomonas fluorescens, Enterococcus faecium, Enterococcus durans, Enterococcus hirae, and/or Leuconostoc carnosum (iv) Streptococcus dysgalactiae, Hafnia alvei, and/or Pseudomonas alcaligenes (TTGE bands d, e, f, and n); (v) Serratia marcescens; (vi) Klebsiella pneumoniae; (vii) Kocuria rosea and/or Klebsiella pneumoniae; (viii) Brevibacterium linens and/or Klebsiella pneumoniae; and (ix) Propionibacterium acidipropionici and/or Kocuria sp. (DGGE bands f, h, l, q, and v). For milk sample D, bands d, e, f, and n in TTGE appeared, and the bands h, l, q, and v in DGGE intensified.
Refrigeration resulted in decreased representation of Lactococcus lactis, the major raw milk bacterial component (TTGE band o in samples C and G). Decreases in Streptococcus uberis (TTGE band k in milk sample D) and in Brevibacterium linens/Klebsiella pneumoniae (DGGE band q in milk samples F and G) were also observed. Lactobacillus plantarum/Lactobacillus pentosus, a minority species, disappeared after incubation of the raw samples at 4°C for 24 h (TTGE band c in samples C and G). In DGGE, some bands disappeared as band o (milk sample C); band m (milk sample G); bands i, l, m, o, q, r, and v (milk sample F); and bands j, k, n, q, and r (milk sample A). Bands f (milk sample F) and g (milk sample A) appeared in the majority of samples after incubation of the raw samples at 4°C for 24 h. The results of the present study reveal that refrigeration has a clear impact on the bacterial community of raw milk.
DISCUSSION
Bacterial communities and dominant populations in food products may evolve during different food fermentation processes or during storage. TTGE and/or DGGE, specifically, have been used previously for analysis of the microflora of other food systems such as artisanal cheeses (27), malt whisky (33), Mexican maize dough (3), Italian sausages (8), dairy products (26, 26a), and traditional sour cassava starch (1). In the present study, we used TTGE and DGGE to characterize the bacterial population in raw samples and to examine changes within the bacterial community due to milk refrigeration. To our knowledge, the dynamics of the bacterial population during a simple process such as raw milk conservation at 4°C for 24 h has not been studied previously.
The combined TTGE and DGGE approaches allow us to generate a global picture of the main bacterial species of raw milk samples, generally within 3 days. Strains were identified by using the reference database established by Ogier et al. (26, 26a); the database was enriched with new species in the course of this work. Overall, the results of our analyses of raw milk bacterial composition were in agreement with previous studies (12, 13, 25). The reference database we established was exhaustive and representative of the bacterial species present in raw samples. Lactococcus lactis was confirmed to be a major raw milk species.
The present study provides important information on the sanitary state of animals and the conditions of raw milk production in dairies. Bacteria known to cause mastitis, such as Streptococcus uberis, Streptococcus dysgalactiae, or Serratia marcescens were detected in many samples as majority species. Using this molecular method, detection of Listeria, a serious problem in the dairy industry particularly in raw milk cheeses, was achieved within 3 days. Some rapid classical microbiological methods (e.g., ALOA medium), immunological methods (e.g., Vidas Listeria), or molecular methods (e.g., Probelia Listeria) can also detect Listeria within 3 days, but the advantage with TTGE and DGGE is that all pathogenic bacteria can be analyzed simultaneously. To date, however, since all Listeria species have the same 16S rRNA gene, assignments go as far as genus identification. The use of more specific primers (21) will allow us to distinguish Listeria monocytogenes from other Listeria species.
TTGE and DGGE profiles of raw milk samples evolved after conservation at 4°C for 24 h. An emergence of psychrotrophic bacteria such as Listeria (samples C, D, and G) and Aeromonas hydrophila (milk sample A) was observed. The increase of psychrotroph flora in raw samples stored at 4°C was reported as requiring 48 h when tested by classical microbiological methods (5, 7, 16). Our results reveal that the time for psychrotrophic populations to increase is markedly shorter than previously reported, and such populations are present within 24 h. Furthermore, we noted that bacterial dynamics showed considerable variation between samples. This may indicate that the presence of a single different strain may have a significant effect on the microbial balance in dairy products such as milk. In some cases (e.g., samples A and F), low temperatures amplified some bacterial species that were barely detectable in the initial sample and also eliminated initially major species (see Fig. 3 and 4 and Tables 2 and 3). These results should have an impact on the storage protocols used in the future for raw milk samples.
The results obtained are of interest not only for their contribution to the knowledge on the bacterial flora of raw milk samples but also essentially for elucidating the power of these molecular approaches to rapidly and precisely describe the consequences of a simple process, milk refrigeration, on the quality of dairy products and its impact on health. Recently, a link was hypothesized between Crohn's disease and the emergence of psychrotroph bacteria during the cold chain used for foods, leading to chronic infestation of the digestive tract (23). This potential association could be detailed and confirmed by using the approach described here.
Acknowledgments
We thank the dairy industrial companies and milk producers for supplying raw samples and Arilait Recherches for their interest in the project.
REFERENCES
- 1.Ampe, F., A. Sirvent, and N. Zakhia. 2001. Dynamics of the microbial community responsible for traditional sour cassava starch fermentation studied by denaturating gradient gel electrophoresis and quantitative rRNA hybridization. Int. J. Food Microbiol. 65:45-54. [DOI] [PubMed] [Google Scholar]
- 2.Bazin, F. 1992. La qualité microbiologique des laits de qualité super A. Institut d'Etudes Supérieures d'Industrie et d'Economie Laitière, Paris, France.
- 3.Ben Omar, N., and F. Ampe. 2000. Microbial community dynamics during production of the Mexican fermented maize dough pozol. Appl. Environ. Microbiol. 66:3664-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beuvier, E., K. Berthaud, S. Cegarra, A. Dasen, S. Pochet, S. Buchin, and G. Duboz. 1997. Ripening and quality of Swiss-type cheese made from raw, pasteurized or microfiltered milk. Int. Dairy J. 7:311-323. [Google Scholar]
- 5.Brouillaud-Delattre, A., M. Maire, C. Collette, C. Mattei, and C. Lahellec. 1997. Predictive microbiology of dairy products: influence of biological factors affecting growth of Listeria monocytogenes. J. AOAC Int. 80:913-919. [PubMed] [Google Scholar]
- 6.Buchin, S., V. Delague, G. Duboz, J. L. Berdague, E. Beuvier, S. Pochet, and R. Grappin. 1998. Influence of pasteurization and fat composition of milk on the volatile compounds and flavor characteristics of a semi-hard cheese. J. Dairy Sci. 81:3097-3108. [Google Scholar]
- 7.Chatelin, Y. M., and J. Richard. 1981. Etude de quelques cas de contaminations microbiennes importantes du lait à la ferme. Lait 61:80-94. [Google Scholar]
- 8.Cocolin, L., M. Manzano, C. Cantoni, and G. Comi. 2001. Denaturing gradient gel electrophoresis analysis of the 16S rRNA gene V1 region to monitor dynamic changes in the bacterial population during fermentation of Italian sausages. Appl. Environ. Microbiol. 67:5113-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De los Reyes-Gavilan, C., G. K. Y. Limsowtin, P. Tailliez, L. Séchaud, and J. P. Accolas. 1992. A Lactobacillus helveticus-specific DNA probe detects restriction fragment length polymorphisms in this species. Appl. Environ. Microbiol. 58:3429-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demarigny, Y. 1996. Râole de la flore du lait cru et des paramètres technologiques sur l'évolution des caractéristiques biologiques, microbiologiques et sensorielles des fromages à pÂte préssée cuite. Ph.D. thesis. ENSBANA, Dijon, France.
- 11.Demarigny, Y., E. Beuvier, S. Buchin, S. Pochet, and R. Grappin. 1997. Influence of raw milk microflora on the characteristics of Swiss-type cheeses. II. Biochemical and sensory characteristics. Lait 77:151-167. [Google Scholar]
- 12.Desmasures, N. 1995. Etude de laits de haute qualité: caractérisation et aptitudes microbiologiques à la transformation en camembert au lait cru. Ph.D. thesis. Institute of Biochemistry and Applied Biology, University of Caen, Caen, France.
- 13.Desmasures, N., F. Bazin, and M. Gueguen. 1997. Microbiological composition of raw milk from selected farms in the Camembert region of Normandy. J. Appl. Microbiol. 83:53-58. [DOI] [PubMed] [Google Scholar]
- 14.Desmasures, N., and M. Gueguen. 1997. Monitoring the microbiology of high quality milk by monthly sampling over two years. J. Dairy Res. 64:271-280. [DOI] [PubMed] [Google Scholar]
- 15.Desmasures, N., W. Opportune, and M. Gueguen. 1997. Lactococcus spp., yeasts, and Pseudomonas spp. on teats and udders of milking cows as potential sources of milk contamination. Int. Dairy J. 7:643-646. [Google Scholar]
- 16.Durr, R. 1974. Le développement des bactéries psychrotrophes dans le lait cru réfrigéré dans les conditions des exploitations laitières françaises. Rev. Lait Fr. 326:913-919. [Google Scholar]
- 17.Duthoit, F., J. J. Godon, and M. C. Montel. 2003. Bacterial community dynamics during production of registered designation of origin Salers cheese as evaluated by 16S rRNA gene single-strand conformation polymorphism analysis. Appl. Environ. Microbiol. 69:3840-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ercolini, D., P. J. Hill, and C. E. R. Dodd. 2003. Bacterial community structure and location in stilton cheese. Appl. Environ. Microbiol. 69:3540-3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghozzi, R., P. Morand, A. Ferroni, J. L. Beretti, E. Bingen, C. Segonds, M. O. Husson, D. Izard, P. Berche, and J. L. Gaillard. 1999. Capillary electrophoresis single-strand conformation polymorphism analysis for rapid identification of Pseudomonas aeruginosa and other gram-negative nonfermenting bacilli recovered from patients with cystic fibrosis, J. Clin. Microbiol. 37:3374-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grappin, R., and E. Beuvier. 1997. Possible implications of milk pasteurization on the manufacture and sensory quality of ripened cheese. Int. Dairy J. 7:751-761. [Google Scholar]
- 21.Hein, I., D. Klein, A. Lehner, A. Bubert, E. Brandl, and M. Wagner. 2001. Detection and quantification of the iap gene of Listeria monocytogenes and Listeria innocua by a new real-time quantitative PCR assay. Res. Microbiol. 152:37-46. [DOI] [PubMed] [Google Scholar]
- 22.Hernan-Gomez, S., J. C. Espinosa, and J. F. Ubeda. 2000. Characterization of wine yeasts by temperature gradient gel electrophoresis (TGGE). FEMS Microbiol. Lett. 193:45-50. [DOI] [PubMed] [Google Scholar]
- 23.Hugot, J. P., C. Alberti, D. Berrebi, E. Bingen, and J. P. Cézard. 2003. Crohn's disease: the cold chain hypothesis. Lancet 362:2012-2015. [DOI] [PubMed] [Google Scholar]
- 24.Maidak, B. L., J. R. Cole, C. T. Parker, Jr., G. M. Garrity, N. Larsen, B. Li, T. G. Liburn, M. J. McCaughey, G. J. Olsen, R. Overbeek, S. Pramanik, T. M. Schmidt, J. M. Tiedje, and C. R. Woese. 1999. A new version of the RDP (Ribosomal Database Project). Nucleic Acids Res. 27:171-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michel, V., A. Hauwuy, and J. F. Chamba. 2001. La flore microbienne de laits crus de vache: diversité et influence des conditions de production. Lait 81:575-592. [Google Scholar]
- 26.Ogier, J. C., O. Son, A. Gruss, P. Tailliez, and A. Delacroix-Buchet. 2002. Identification of the bacterial microflora in dairy products by temporal temperature gradient gel electrophoresis, Appl. Environ. Microbiol. 68:3691-3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Ogier, J.-C., V. Lafarge, V. Girard, A. Rault, V. Maladen, A. Gruss, J.-Y. Leveau, and A. Delacroix-Buchet. 2004. Molecular fingerprinting of dairy microbial ecosystems by use of temporal temperature and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 70:5628-5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Randazzo, C. L., S. Torriani, A. D. L. Akkermans, W. M. de Vos, and E. E. Vaughan. 2002. Diversity, dynamics, and activity of bacterial communities during production of an artisanal Sicilian cheese as evaluated by 16S rRNA analysis. Appl. Environ. Microbiol. 68:1882-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rehman, S. U., P. L. H. McSweeney, J. M. Banks, E. Y. Brechany, D. D. Muir, and P. F. Fox. 2000. Ripening of cheddar cheese made from blends of raw and pasteurized milk. Int. Dairy J. 10:33-44. [Google Scholar]
- 29.Richard, J. 1981. Influence de diverses methodes de nettoyage des machines à traire sur la “qualité de conservation” du lait cru à basse température. Lait 61:354-369. [Google Scholar]
- 30.Schwieger, F., and C. C. Tebbe. 1998. A new approach to utilize PCR-single-strand-conformation polymorphism for 16S rRNA gene-based microbial community analysis. Appl. Environ. Microbiol. 64:4870-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tailliez, P., P. Quénée, and A. Chopin. 1996. Estimation de la diversité parmi les souches de la collection CNRZ: application de la RAPD a un groupe de lactobacilles. Lait 76:147-148. [Google Scholar]
- 32.Thomas, S. B., and B. F. Thomas. 1973. Psychrotrophic bacteria in refrigerated bulk-collected raw milk. Dairy Ind. 38:61-70. [Google Scholar]
- 33.Van Beek, S., and F. G. Priest. 2002. Evolution of the lactic acid bacterial community during malt whisky fermentation: a polyphasic study. Appl. Environ. Microbiol. 68:297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zoetendal, E. G., A. D. L. Akkermans, and W. M. De Vos. 1998. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl. Environ. Microbiol. 64:3854-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu, X. Y., T. Zhong, Y. Pandya, and R. D. Joerger. 2002. 16S rRNA-based analysis of microbiota from the cecum of broiler chickens. Appl. Environ. Microbiol. 68:124-137. [DOI] [PMC free article] [PubMed] [Google Scholar]