Abstract
A protein that imitates the sequence of a highly conserved segment predicted to line the pore of dihydropyridine-sensitive L-type calcium channels was designed and synthesized. Single-channel conductance properties were studied in planar lipid bilayers. The synthetic protein emulates the ionic conductance, ionic selectivity, and pharmacological properties of the authentic calcium channel, including the stereospecific action of agonist and antagonist enantiomers of the dihydropyridine BayK 8644. The identified sequence is identical in L-type calcium channels from skeletal muscle and isoforms of cardiac muscle, brain, and aorta. It is plausible that this structural motif represents the molecular blueprint for the pore-forming structure of voltage-gated calcium channels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biel M., Ruth P., Bosse E., Hullin R., Stühmer W., Flockerzi V., Hofmann F. Primary structure and functional expression of a high voltage activated calcium channel from rabbit lung. FEBS Lett. 1990 Sep 3;269(2):409–412. doi: 10.1016/0014-5793(90)81205-3. [DOI] [PubMed] [Google Scholar]
- Bolger G. T., Marcus K. A., Daly J. W., Skolnick P. Local anesthetics differentiate dihydropyridine calcium antagonist binding sites in rat brain and cardiac membranes. J Pharmacol Exp Ther. 1987 Mar;240(3):922–930. [PubMed] [Google Scholar]
- Catterall W. A., Seagar M. J., Takahashi M. Molecular properties of dihydropyridine-sensitive calcium channels in skeletal muscle. J Biol Chem. 1988 Mar 15;263(8):3535–3538. [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Ellis S. B., Williams M. E., Ways N. R., Brenner R., Sharp A. H., Leung A. T., Campbell K. P., McKenna E., Koch W. J., Hui A. Sequence and expression of mRNAs encoding the alpha 1 and alpha 2 subunits of a DHP-sensitive calcium channel. Science. 1988 Sep 23;241(4873):1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- Greenblatt R. E., Blatt Y., Montal M. The structure of the voltage-sensitive sodium channel. Inferences derived from computer-aided analysis of the Electrophorus electricus channel primary structure. FEBS Lett. 1985 Dec 2;193(2):125–134. doi: 10.1016/0014-5793(85)80136-8. [DOI] [PubMed] [Google Scholar]
- Hess P. Calcium channels in vertebrate cells. Annu Rev Neurosci. 1990;13:337–356. doi: 10.1146/annurev.ne.13.030190.002005. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986 Sep;88(3):293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay S. D., Ellis S. B., McCue A. F., Williams M. E., Vedvick T. S., Harpold M. M., Campbell K. P. Primary structure of the gamma subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1990 Apr 27;248(4954):490–492. doi: 10.1126/science.2158672. [DOI] [PubMed] [Google Scholar]
- Josephson I. R. Lidocaine blocks Na, Ca and K currents of chick ventricular myocytes. J Mol Cell Cardiol. 1988 Jul;20(7):593–604. doi: 10.1016/s0022-2828(88)80117-2. [DOI] [PubMed] [Google Scholar]
- Koch W. J., Hui A., Shull G. E., Ellinor P., Schwartz A. Characterization of cDNA clones encoding two putative isoforms of the alpha 1 subunit of the dihydropyridine-sensitive voltage-dependent calcium channel isolated from rat brain and rat aorta. FEBS Lett. 1989 Jul 3;250(2):386–388. doi: 10.1016/0014-5793(89)80761-6. [DOI] [PubMed] [Google Scholar]
- Lansman J. B., Hess P., Tsien R. W. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J Gen Physiol. 1986 Sep;88(3):321–347. doi: 10.1085/jgp.88.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Montal M. Molecular anatomy and molecular design of channel proteins. FASEB J. 1990 Jun;4(9):2623–2635. doi: 10.1096/fasebj.4.9.1693348. [DOI] [PubMed] [Google Scholar]
- Montal M., Montal M. S., Tomich J. M. Synporins--synthetic proteins that emulate the pore structure of biological ionic channels. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6929–6933. doi: 10.1073/pnas.87.18.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
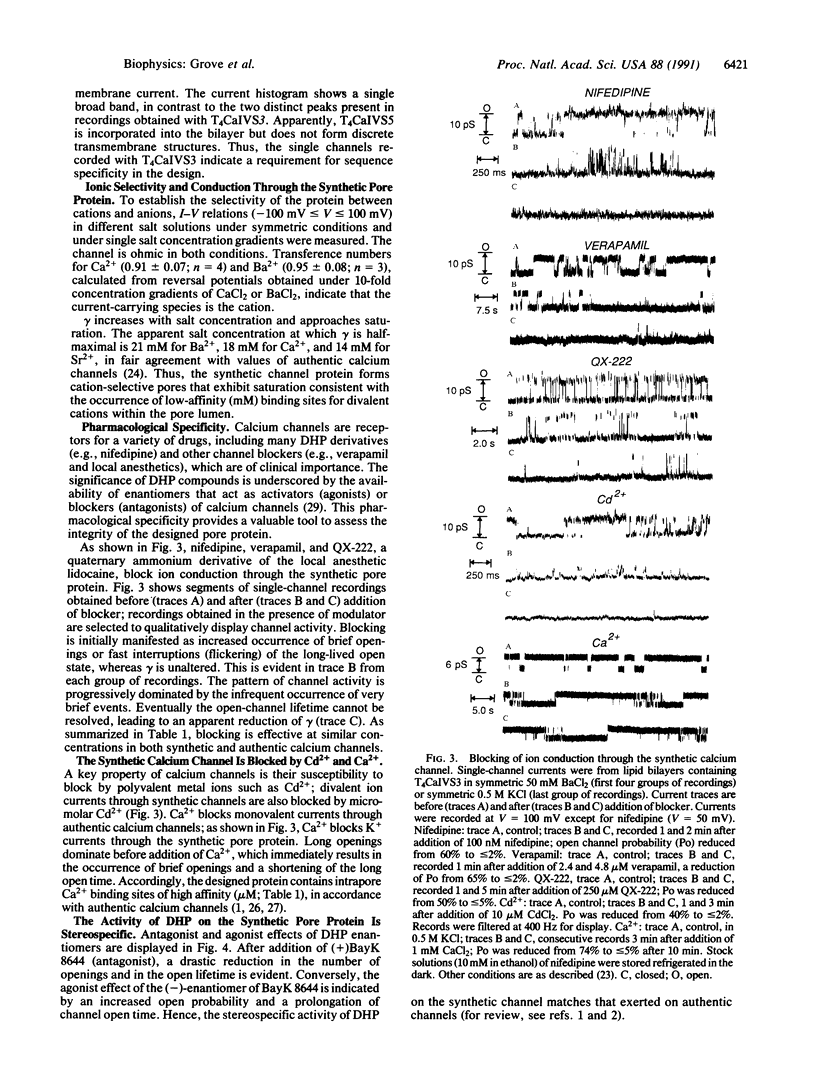
- Mutter M., Hersperger R., Gubernator K., Müller K. The construction of new proteins: V. A template-assembled synthetic protein (TASP) containing both a 4-helix bundle and beta-barrel-like structure. Proteins. 1989;5(1):13–21. doi: 10.1002/prot.340050104. [DOI] [PubMed] [Google Scholar]
- Oiki S., Danho W., Montal M. Channel protein engineering: synthetic 22-mer peptide from the primary structure of the voltage-sensitive sodium channel forms ionic channels in lipid bilayers. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2393–2397. doi: 10.1073/pnas.85.7.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oiki S., Madison V., Montal M. Bundles of amphipathic transmembrane alpha-helices as a structural motif for ion-conducting channel proteins: studies on sodium channels and acetylcholine receptors. Proteins. 1990;8(3):226–236. doi: 10.1002/prot.340080305. [DOI] [PubMed] [Google Scholar]
- Palade P. T., Almers W. Slow calcium and potassium currents in frog skeletal muscle: their relationship and pharmacologic properties. Pflugers Arch. 1985 Sep;405(2):91–101. doi: 10.1007/BF00584528. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E., Kim H. S., Lacerda A. E., Horne W., Wei X. Y., Rampe D., Campbell K. P., Brown A. M., Birnbaumer L. Induction of calcium currents by the expression of the alpha 1-subunit of the dihydropyridine receptor from skeletal muscle. Nature. 1989 Jul 20;340(6230):233–236. doi: 10.1038/340233a0. [DOI] [PubMed] [Google Scholar]
- Regulla S., Schneider T., Nastainczyk W., Meyer H. E., Hofmann F. Identification of the site of interaction of the dihydropyridine channel blockers nitrendipine and azidopine with the calcium-channel alpha 1 subunit. EMBO J. 1991 Jan;10(1):45–49. doi: 10.1002/j.1460-2075.1991.tb07919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. L., Hess P., Tsien R. W. Cardiac calcium channels in planar lipid bilayers. L-type channels and calcium-permeable channels open at negative membrane potentials. J Gen Physiol. 1988 Jul;92(1):27–54. doi: 10.1085/jgp.92.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruth P., Röhrkasten A., Biel M., Bosse E., Regulla S., Meyer H. E., Flockerzi V., Hofmann F. Primary structure of the beta subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1989 Sep 8;245(4922):1115–1118. doi: 10.1126/science.2549640. [DOI] [PubMed] [Google Scholar]
- Snutch T. P., Leonard J. P., Gilbert M. M., Lester H. A., Davidson N. Rat brain expresses a heterogeneous family of calcium channels. Proc Natl Acad Sci U S A. 1990 May;87(9):3391–3395. doi: 10.1073/pnas.87.9.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striessnig J., Glossmann H., Catterall W. A. Identification of a phenylalkylamine binding region within the alpha 1 subunit of skeletal muscle Ca2+ channels. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9108–9112. doi: 10.1073/pnas.87.23.9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Isla B. A., Wan K., Lindstrom J., Montal M. Single-channel recordings from purified acetylcholine receptors reconstituted in bilayers formed at the tip of patch pipets. Biochemistry. 1983 May 10;22(10):2319–2323. doi: 10.1021/bi00279a003. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Powell J. A., Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988 Nov 10;336(6195):134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Triggle D. J., Langs D. A., Janis R. A. Ca2+ channel ligands: structure-function relationships of the 1,4-dihydropyridines. Med Res Rev. 1989 Apr-Jun;9(2):123–180. doi: 10.1002/med.2610090203. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Hess P., McCleskey E. W., Rosenberg R. L. Calcium channels: mechanisms of selectivity, permeation, and block. Annu Rev Biophys Biophys Chem. 1987;16:265–290. doi: 10.1146/annurev.bb.16.060187.001405. [DOI] [PubMed] [Google Scholar]
- Vaghy P. L., Williams J. S., Schwartz A. Receptor pharmacology of calcium entry blocking agents. Am J Cardiol. 1987 Jan 23;59(2):9A–17A. doi: 10.1016/0002-9149(87)90170-6. [DOI] [PubMed] [Google Scholar]
- Yatani A., Brown A. M. The calcium channel blocker nitrendipine blocks sodium channels in neonatal rat cardiac myocytes. Circ Res. 1985 Jun;56(6):868–875. doi: 10.1161/01.res.56.6.868. [DOI] [PubMed] [Google Scholar]
- Yatani A., Kunze D. L., Brown A. M. Effects of dihydropyridine calcium channel modulators on cardiac sodium channels. Am J Physiol. 1988 Jan;254(1 Pt 2):H140–H147. doi: 10.1152/ajpheart.1988.254.1.H140. [DOI] [PubMed] [Google Scholar]