Abstract
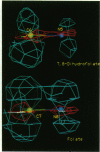
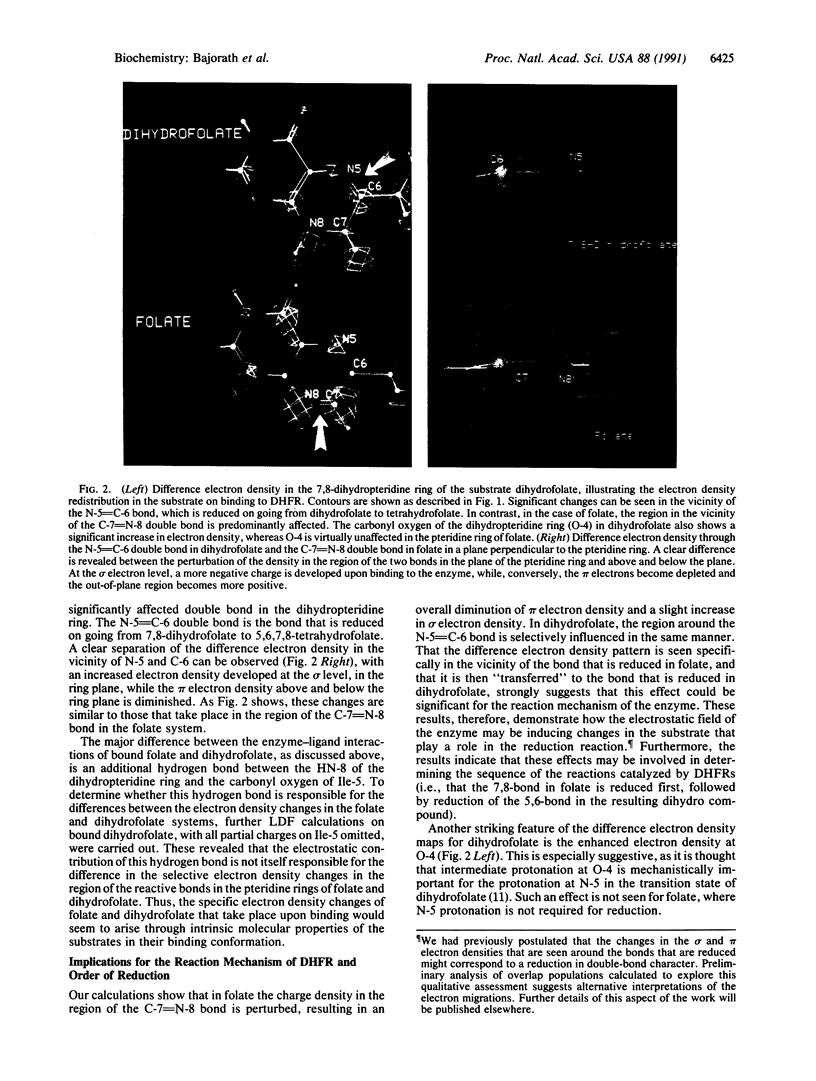
We have applied local density functional theory, an ab initio quantum mechanical method, to study the shift in the spatial electron density of the substrate dihydrofolate that accompanies binding to the enzyme dihydrofolate reductase. The results shed light on fundamental electronic effects due to the enzyme that may contribute to catalysis. In particular, the enzyme induces a long-range polarization of the substrate that perturbs its electron density distribution in a specific and selective way in the vicinity of the bond that is reduced by the enzyme. Examination of the electron density changes that occur in folate reveals that a similar effect is seen but this time specifically at the bond that is reduced in this substrate. This suggests that the polarization effect may be implicated in the reaction mechanism and may play a role in determining the sequence whereby the 7,8-bond in folate is reduced first, followed by reduction of the 5,6-bond in the resulting dihydro compound.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajorath J., Kitson D. H., Fitzgerald G., Andzelm J., Kraut J., Hagler A. T. Electron redistribution on binding of a substrate to an enzyme: folate and dihydrofolate reductase. Proteins. 1991;9(3):217–224. doi: 10.1002/prot.340090307. [DOI] [PubMed] [Google Scholar]
- Bystroff C., Oatley S. J., Kraut J. Crystal structures of Escherichia coli dihydrofolate reductase: the NADP+ holoenzyme and the folate.NADP+ ternary complex. Substrate binding and a model for the transition state. Biochemistry. 1990 Apr 3;29(13):3263–3277. doi: 10.1021/bi00465a018. [DOI] [PubMed] [Google Scholar]
- Davies J. F., 2nd, Delcamp T. J., Prendergast N. J., Ashford V. A., Freisheim J. H., Kraut J. Crystal structures of recombinant human dihydrofolate reductase complexed with folate and 5-deazafolate. Biochemistry. 1990 Oct 9;29(40):9467–9479. doi: 10.1021/bi00492a021. [DOI] [PubMed] [Google Scholar]
- Rullmann J. A., Bellido M. N., van Duijnen P. T. The active site of papain. All-atom study of interactions with protein matrix and solvent. J Mol Biol. 1989 Mar 5;206(1):101–118. doi: 10.1016/0022-2836(89)90527-5. [DOI] [PubMed] [Google Scholar]
- Warshel A., Levitt M. Theoretical studies of enzymic reactions: dielectric, electrostatic and steric stabilization of the carbonium ion in the reaction of lysozyme. J Mol Biol. 1976 May 15;103(2):227–249. doi: 10.1016/0022-2836(76)90311-9. [DOI] [PubMed] [Google Scholar]
- Warshel A., Sussman F., Hwang J. K. Evaluation of catalytic free energies in genetically modified proteins. J Mol Biol. 1988 May 5;201(1):139–159. doi: 10.1016/0022-2836(88)90445-7. [DOI] [PubMed] [Google Scholar]
- Weiner S. J., Seibel G. L., Kollman P. A. The nature of enzyme catalysis in trypsin. Proc Natl Acad Sci U S A. 1986 Feb;83(3):649–653. doi: 10.1073/pnas.83.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]