Abstract
IMPORTANCE
Although consensus statements support the preoperative treatment of borderline resectable pancreatic cancer, no prospective, quality-controlled, multicenter studies of this strategy have been conducted. Existing studies are retrospective and confounded by heterogeneity in patients studied, therapeutic algorithms used, and outcomes reported.
OBJECTIVE
To determine the feasibility of conducting studies of multimodality therapy for borderline resectable pancreatic cancer in the cooperative group setting.
DESIGN, SETTING, AND PARTICIPANTS
A prospective, multicenter, single-arm trial of a multimodality treatment regimen administered within a study framework using centralized quality control with the cooperation of 14 member institutions of the National Clinical Trials Network. Twenty-nine patients with biopsy-confirmed pancreatic cancer preregistered, and 23 patients with tumors who met centrally reviewed radiographic criteria registered. Twenty-two patients initiated therapy (median age, 64 years [range, 50–76 years]; 55% female). Patients registered between May 29, 2013, and February 7,2014.
INTERVENTIONS
Patients received modified FOLFIRINOX treatment (85 mg/m2 of oxaliplatin, 180 mg/m2 of irinotecan hydrochloride, 400 mg/m2 of leucovorin calcium, and then 2400 mg/m2 of 5-fluorouracil for 4 cycles) followed by 5.5 weeks of external-beam radiation (50.4 Gy delivered in 28 daily fractions) with capecitabine (825 mg/m2 orally twice daily) prior to pancreatectomy.
MAIN OUTCOMES AND MEASURES
Feasibility, defined by the accrual rate, the safety of the preoperative regimen, and the pancreatectomy rate.
RESULTS
The accrual rate of 2.6 patients per month was superior to the anticipated rate. Although 14 of the 22 patients (64% [95% CI, 41%–83%]) had grade 3 or higher adverse events, 15 of the 22 patients (68% [95% CI, 49%–88%]) underwent pancreatectomy. Of these 15 patients, 12 (80%) required vascular resection, 14 (93%) had microscopically negative margins, 5 (33%) had specimens that had less than 5% residual cancer cells, and 2 (13%) had specimens that had pathologic complete responses. The median overall survival of all patients was 21.7 months (95% CI, 15.7 to not reached) from registration.
CONCLUSIONS AND RELEVANCE
The successful completion of this collaborative study demonstrates the feasibility of conducting quality-controlled trials for this disease stage in the multi-institutional setting. The data generated by this study and the logistical elements that facilitated the trial's completion are currently being used to develop cooperative group trials with the goal of improving outcomes for this subset of patients.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT01821612
Borderline resectable pancreatic ductal adenocarcinomas (PDACs) approximate major mesenteric blood vessels. Although radiographically localized, they are often associated with subclinical metastases.1 Following resection, cancer is found at the margins in 36% to 64% of cases, and the median duration of survival is shorter than 14 months, even when concomitant vascular resection is performed.2,3 Preoperative chemotherapy and chemoradiation are therefore often recommended in an attempt to eradicate occult systemic disease, facilitate margin-negative (RO) resection, maximize overall survival, and spare patients with evolving metastases otherwise futile surgery.4,5
Unfortunately, no high-level data support this practice. Furthermore, it has been historically difficult to conduct high-quality studies of preoperative therapy for patients with advanced PDAC; the only prospective, multi-institutional trial to evaluate this approach, the Eastern Cooperative Group Trial 1200, accrued poorly and closed prematurely.6 Treatment guidelines are therefore based on small, retrospective series confounded by heterogeneity in staging criteria, in the metrics used to characterize response to therapy, and in the criteria used to determine the role of pancreatectomy that, together, have led to an imperfect understanding of the role of preoperative therapy for patients with advanced PDAC.7,8 The reported ability of conventional cytotoxic regimens to “downstage” advanced cancers to resectable ones likely represents one direct artifact of this heterogeneity.9,10
Recognizing the importance and absence of prospective data in this setting, the Alliance for Clinical Trials in Oncology, collaborating with the Southwest Oncology Group, NRG Oncology, and the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network, sought to initiate quality-controlled, cooperative group trials of preoperative therapy for patients with borderline resectable PDAC. However, given the historical context, the National Cancer Institute (NCI) mandated this initial pilot study to demonstrate feasibility and to provide prospective outcomes data with which to inform subsequent trials.
Methods
Eligibility
This study was available to selected Alliance for Clinical Trials in Oncology, Southwest Oncology Group, NRG Oncology, and Eastern Cooperative Oncology Group-American College of Radiology Imaging Network institutions that performed at least 20 pancreatectomies yearly and had experience with vascular resections (Study Protocol in Supplement 1). Registration was accomplished in 2 phases. Eligibility criteria were confirmed during a preregistration phase and included being 18 years of age or older, an Eastern Cooperative Oncology Group performance status of 0 or 1, proof of adenocarcinoma of the pancreatic head, no remote lymphadenopathy or distant metastases, and a computed tomography (CT) or magnetic resonance imaging (MRI) study of the abdomen using a pancreatic protocol and CT or MRI of the chest demonstrating a primary tumor characterize d by 1 or more of the following relationships (intergroup criteria7): (1) a tumor-vessel interface (TVI) with the superior mesenteric vein (SMV) or portal vein (PV) measuring 180° or more of the circumference of either vein’s wall, or short-segment occlusion of either vein with a normal vein above and below the obstruction amenable to reconstruction; (2) any TVI with the common hepatic artery (CHA) with a normal artery proximal and distal to the TVI amenable to reconstruction; and (3) a TVI with the superior mesenteric artery (SMA) measuring less than 180° of the circumference of the vessel wall.
Tumors with an interface with the SMV and PV measuring less than 180° and without an interface with either the CHA or the SMA—considered borderline resectable by other guidelines-were considered resectable.11 A TVI with the SMV, PV, or CHA but without a normal vessel proximal and distal to the interface to allow reconstruction, a TVI with the SMA measuring 180° or more of that vessel’s circumference, and a TVI with the aorta were considered to represent locally advanced disease. Patients with resectable or locally advanced cancers were ineligible.
Final registration required confirmation of disease stage with central review of all radiologic images and multidisciplinary evaluation of the patient by a medical oncologist, radiation oncologist, and surgeon. Additional criteria included a granulocyte level of 2000/µL or greater, a hemoglobin level of greater than 9 g/dL [to convert to grams per liter, multiply by 10.0], a platelet count of 100 × 103/µL or greater [to convert to ×109 per liter, multiply by 1.0], an albumin level of greater than 3.0 g/dL [to convert to grams per liter, multiply by 10], a creatinine level 1.5 times or less than the upper limit of normal, aspartate transaminase and alanine transaminase levels 2.5 times or less than the upper limit of normal, and a bilirubin level of 2 mg/dL or less [to convert to micromoles per liter, multiply by 17.104]. Exclusion criteria included a peripheral neuropathy grade of 2 or higher, prior therapy for PDAC, Gilbert syndrome or homozygosity for UGT1A1*2, and any active second malignant tumor.
All patients provided written informed consent. The institutional review board at each participating institution approved the trial.
Treatment Plan
Preoperative Treatment
Modified FOLFIRINOX (85 mg/m2 of oxaliplatin, 180 mg/m2 of irinotecan hydrochloride, 400 mg/m2 of leucovorin calcium, and then 2400 mg/m2 of 5-fluorouracil for 4 cycles) was administered intravenously for 4 cycles. All patients received subcutaneous pegfilgrastim with each cycle. Following completion of the modified F0LFIRIN0X treatment, the patients’ tumors were restaged by use of CT or MRI, and those patients without evidence of metastases who had maintained a performance status of 0 or 1 underwent external-beam radiation therapy (50.4 Gy total; 28 fractions at 1.8 Gy/fraction) for 5.5 weeks concurrently with capecitabine (825 mg/m2 orally twice daily). Either a 3-dimensional conformal or intensity-modulated technique was used. The gross tumor volume included the primary tumor and any regional adenopathy seen on baseline images that was more than 10 mm in size. The clinical target volume included the gross target volume plus a 10-mm expansion. The minimum dose within the planning target volume (clinical target volume plus 20-mm cranial/caudal and 10-mm radial expansions) was mandated not to fall below or exceed 97% and 110%, respectively, of the prescribed dose. Each dosimetric plan was centrally reviewed prior to treatment.
Surgical Resection
Following chemoradiation, the patients’ tumors were re-staged by use of CT or MRI, and those patients without locally advanced or metastatic disease determined based on immediate central radiologic review and who had a performance status of 0 or 1 were required to undergo surgery within 4 to 10 weeks. The following surgical procedures were mandated: (1) skeletonization of the right lateral aspect of the SMA12; (2) venous and/or hepatic arterial resection when necessary to achieve negative margins; and (3) evaluation of the histopathologic status of the pancreatic and bile duct margins intraoperatively, with re-resection when appropriate.
Postoperative Treatment
Patients with a performance status of 0 or 1 and without evidence of residual or recurrent disease on CT or MRI scans were considered for 2 cycles of postoperative gemcitabine hydro-chloride (1000 mg/m2 intravenously on days 1, 8, and 15 every 28 days).
Assessment
Radiologic response and progression were evaluated using the Response Evaluation Criteria in Solid Tumors version 1.1 guidelines.13 Analysis of the surgical specimen was performed following recommendations of the American Joint Committee on Cancer (7th edition) and the College of American Pathologists guidelines.14,15 Histopathologic response was centrally reviewed and characterized as grade I if there were less than 5% cancer cells or grade II if there were 5% or more cancer cells in the surgical specimen.16 Histopathologic complete response was defined as the absence of cancer cells in the specimen; in such cases, the pretreatment biopsy was centrally re-reviewed. Resection status was characterized as R0, R1 (microscopic tumor at any margin), or R2 (macroscopically incomplete resection).
Adverse events were graded using the Common Terminology Criteria for Adverse Events version 4.0.17 Adverse events were recorded from enrollment to 30 days following the end of therapy.
Patients were followed up every 4 months after treatment. All visits included a history and physical examination, laboratory studies, and CT or MRI of the chest and abdomen. The appearance of any lesion with characteristics of local relapse or metastatic disease was considered recurrence.
Statistical Analysis
As mandated by the NCI, the primary objective was to demonstrate the feasibility of conducting cooperative group trials that use complex multidisciplinary treatment algorithms for patients with borderline resectable PDAC. The primary end points were (1) the accrual rate, targeted for at least 2 patients per month; (2) the safety and tolerability of the preoperative regimen, determined by the rate of grade 3 or higher treatment-related adverse events and the proportion of patients with more than 4 weeks’ treatment delay; and (3) the rate of completion of all preoperative and operative therapy, determined by the R0/R1 resection rate. At the behest of the NCI, the planned sample size was 20 patients, which would provide 82% power to detect an excessive toxicity rate of 70% (compared with 50%, which was considered reasonable in this setting) at a 1-sided significance level of 0.15. This sample size would also provide 75% power to detect an unacceptably low R0/R1 resection rate of 20% (compared with 40%, which was considered reasonable in this setting) at a 1-sided significance level of 0.08. Secondary end points included radiologic response rate (complete or partial response per the Response Evaluation Criteria in Solid Tumors), histopathologic response rate (complete or partial response per histopathologic examination), times to locoregional and distant recurrence, progression-free survival, and overall survival.
Point estimates and confidence intervals were computed for binary end points. The Fisher exact test was use to compare binary end points between subgroups.18 The Kaplan-Meier method was used to estimate the distributions of time-to-event end points.19 For overall survival analyses, the registration date was the start date of the time-to-event end points. For progression-free survival analyses based on treated patients (n = 22), the registration date was the start date of the time-to-event end points, and for analyses based on resected patients (n = 15), the surgery date was the start date. The log-rank test was used to compare time-to-event end points between subgroups. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. All analyses were based on the study database frozen on February 16, 2016. All analyses were conducted using SAS version 9 (SAS Institute Inc).
Results
Patients and Accrual
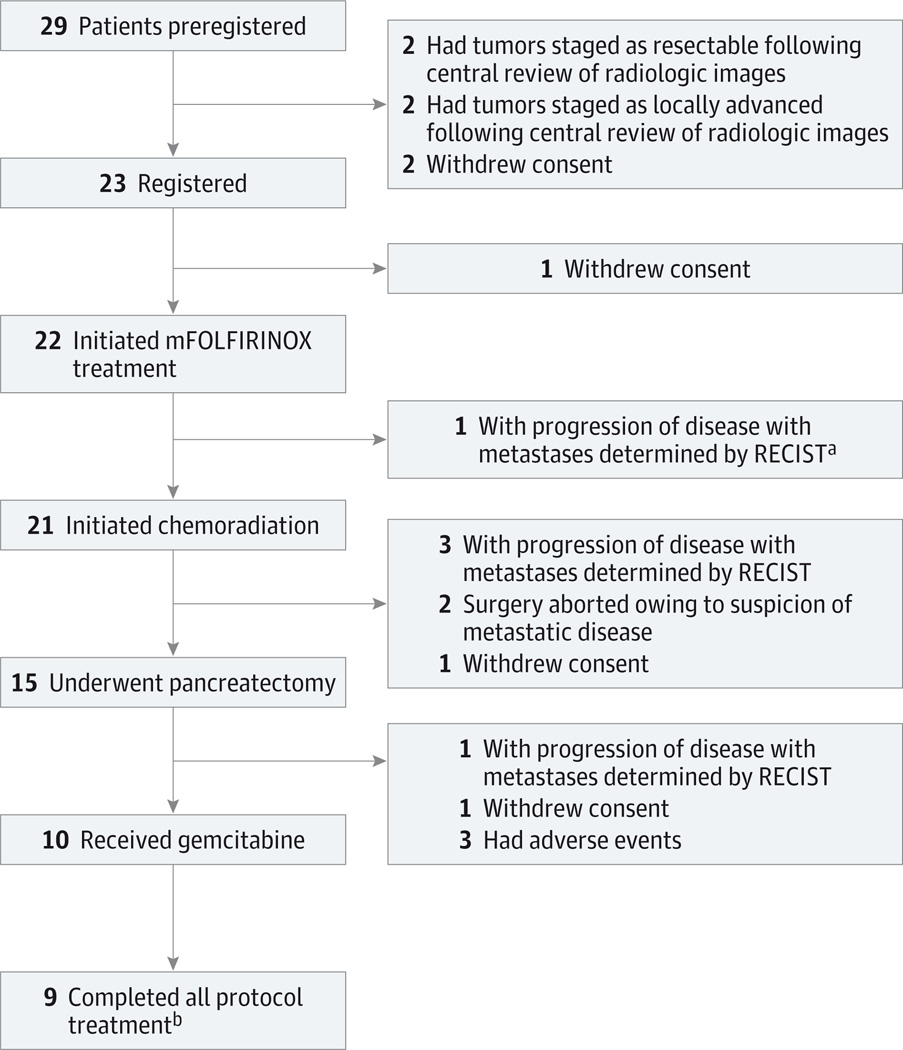
Fourteen institutions participated. Twenty-nine patients from the 14 institutions (range, 1–3 patients per site) preregistered, and 23 patients from 13 institutions (range, 1–3 patients per site) registered between May 29, 2013, and February 7, 2014. The accrual rate of 2.6 patients per month was superior to the anticipated rate of 2 patients per month. Figure 1 details all 29 preregistered patients throughout the study. Four preregistered patients were excluded following central radiologic review; 2 additional patients withdrew consent prior to registration. The baseline characteristics of the 22 patients who initiated therapy are summarized in Table 1.
Figure 1. CONSORT Diagram Including All 29 Patients Who Preregistered for the Trial.
- An additional patient had progression of disease determined by RECIST owing to isolated progression after mFOLFIRINOX treatment but, per protocol, proceeded to chemoradiation.
- Treatment was halted for 1 patient after first cycle of postoperative chemotherapy.
Table 1.
Demographic and Clinical/Radiologic Characteristics of 22 Patients at Study Entry
| Characteristic | Patients, No. (%) |
|---|---|
| Age, median (range), y | 64 (50–76) |
| Sex | |
| Male | 10 (45) |
| Female | 12 (55) |
| Race | |
| White | 21 (95) |
| Black | 1 (5) |
| ECOG performance status | |
| 0 | 14 (64) |
| 1 | 8 (36) |
| Serum CA 19-9, median (range), U/mL | 122 (0–910) |
| Tumor diameter, median (range), mm | 30 (16–49) |
| Radiographic tumor-vessel interfacea | |
| Vein onlyb | 6 (27) |
| <180° | 0 (0) |
| ≥180° | 6 (27) |
| Artery onlyc | 3 (14) |
| <180° | 3 (14) |
| ≥180° | 0 (0) |
| Artery and veinb,c | 13 (59) |
| Artery <180°, vein <180° | 6 (27) |
| Artery <180°, vein ≥180° | 6 (27) |
| Arteryd ≥180°, vein <180° | 1 (5) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; CA 19-9, carbohydrate antigen.
On central review, all vessels were patent, and no patient had a radiologic interface between the tumor and 180° or more of the superior mesenteric artery.
Portal vein and/or superior mesenteric vein.
Superior mesenteric artery, hepatic artery, or celiac trunk.
Common hepatic artery.
Preoperative Therapy
Of 22 patients, 14 (64% [95% CI, 41%–83%]) experienced at least 1 grade 3 or higher adverse event that was deemed at least possibly related to preoperative therapy. Grade 3 and higher adverse events that occurred during therapy, regardless of attribution, are listed in Table 2. No deaths occurred in association with preoperative therapy.
Table 2.
Grading of Adverse Events per Common Terminology Criteria for Adverse Events Version 4.0 (Regardless of Attribution) During Treatmenta
| Adverse Eventb | Patients, No. (%) | |
|---|---|---|
| Grade 3 | Grade 4 | |
| Preoperative mFOLFIRINOX (n = 22) | ||
| Overall | 11 (50) | 1 (5) |
| Diarrhea | 3 (14) | 0 (0) |
| Neutropenia | 2 (9) | 1 (5) |
| Lymphopenia | 0 (0) | 1 (5) |
| Dehydration | 4 (18) | 0 (0) |
| Hypokalemia | 3 (14) | 0 (0) |
| Thromboembolism | 3 (14) | 0 (0) |
| Preoperative chemoradiation (n = 21) | ||
| Overall | 9 (43) | 0 (0) |
| Lymphopenia | 3 (14) | 0 (0) |
| Pancreatectomyc (n = 15) | ||
| Overall | 8 (53) | 5 (33) |
| Anemia | 5 (33) | 0 (0) |
| Infection | 3 (20) | 1 (7) |
| Hemorrhage | 2 (13) | 0 (0) |
| ALT increased | 0 (0) | 1 (7) |
| AST increased | 0 (0) | 1 (7) |
| Thrombocytopenia | 0 (0) | 1 (7) |
| Hypoalbuminemia | 0 (0) | 1 (7) |
| Anorexia | 2 (13) | 0 (0) |
| Acute kidney injury | 0 (0) | 1 (7) |
| Atelectasis | 0 (0) | 1 (7) |
| Pleural effusion | 0 (0) | 1 (7) |
| Pulmonary edema | 0 (0) | 1 (7) |
| Respiratory failure | 0 (0) | 1 (7) |
| Postoperative gemcitabine (n = 10) | ||
| Overall | 5 (50) | 1 (10) |
| Anemia | 0 (0) | 1 (10) |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; mFOLFIRINOX, modified treatment with 85 mg/m2 of oxaliplatin, 180 mg/m2 of irinotecan hydrochloride, 400 mg/m2 of leucovorin calcium, and then 2400 mg/m2 of 5-fluorouracil for 4 cycles, concurrent with capecitabine and radiation therapy.
Overall rates of grade 3 and grade 4 adverse events during each phase of treatment, all individual grade 4 events, and individual grade 3 events observed in more than 10% of treated patients.
Calculated as the maximum severity during induction of mFOLFIRINOX, preoperative chemoradiation, pancreatectomy (within 30 postoperative days), and postoperative gemcitabine treatments, separately.
One patient died within 90 days of pancreatectomy.
Ten patients (45%) experienced a treatment delay (median delay, 1 week [range, 0.6–3.9 weeks]), and 9 patients (41%) required a dose reduction during modified FOLFIRINOX treatment. During chemoradiation, 1 patient experienced a treatment delay, 1 patient required a dose reduction of capecitabine, and 5 patients required interruptions of their radiation treatment. No patient experienced a treatment delay longer than 4 weeks during preoperative therapy.
A radiologic response to preoperative therapy was observed in 6 patients (27% [95% CI, 7%–46%]). There were 2 complete responses, both of which were observed following modified FOLFIRINOX treatment, and 4 partial responses, 2 of which were observed following modified FOLFIRINOX treatment and 2 of which were observed in response to chemoradiation. Five patients had progressive disease during preoperative therapy: 1 each with local progression and metastases following modified FOLFIRINOX treatment and 3 with metastases following chemoradiation.
Pancreatectomy
Fifteen patients (68% [95% CI, 49%–88%]) completed their preoperative therapy and underwent a pancreatoduodenectomy (n = 14) or a total pancreatectomy (n = 1). The median duration from completion of chemoradiation to pancreatectomy was 6.3 weeks (range, 4.1–11.1 weeks). Of these 15 patients, 11 (73%) required resection and reconstruction of the PV and/or SMV, and 1 (7%) required resection and reconstruction of both the PV and/or SMV and the CHA.
Fourteen of 15 patients (93%; 64% of the 22 patients who initiated therapy) underwent an R0 resection, and 1 of 15 patients (7%) underwent an R1 resection. Two patients who underwent an R0 resection had tumor cells at or within 1 mm of the inked SMA margin. The pathologic stage of resected specimens was ypT0N0 (2 patients), ypT1N0 (3 patients), ypT2N0 (1 patient), ypT3N0 (4 patients), ypT1N1 (1 patient), ypT2N1 (1 patient), ypT3N1 (2 patients), or ypT2N1M1 (1 patient, in whom a liver lesion was interpreted as benign based on intraoperative assessment but malignant based on final pathology). Five of 15 resected specimens (33%) had less than 5% viable cancer cells; 2 of 15 resected specimens (13%) had a pathologic complete response to preoperative therapy.
One or more grade 3 or higher adverse events, regardless of attribution, occurred in 8 patients (53%) within 30 days of a pancreatectomy (Table 2). One patient died following multiple adverse events within 90 days of surgery.
Postoperative Therapy
Ten patients initiated treatment with gemcitabine, and 9 patients completed it. Five of the 10 patients (50%) experienced at least 1 grade 3 adverse event during postoperative therapy (Table 2).
Disease Progression/Recurrence and Survival
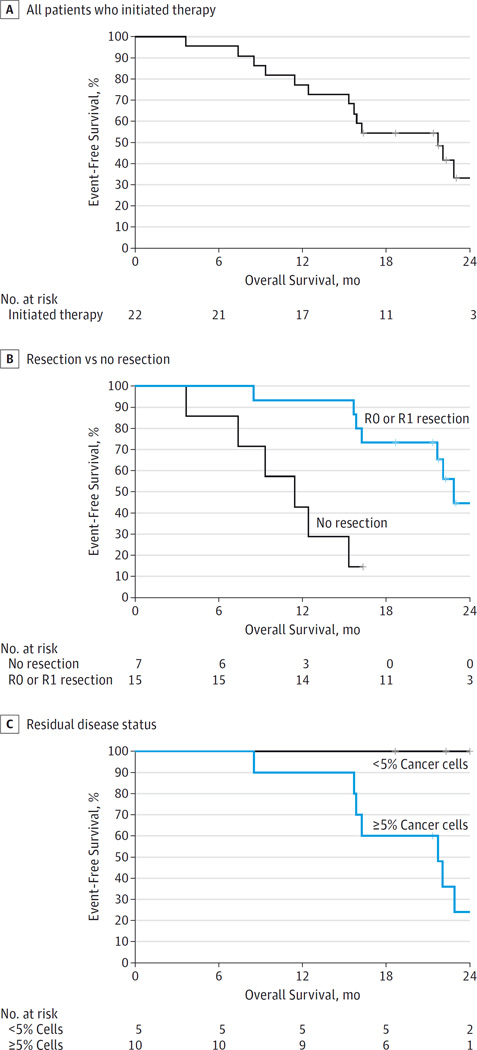
Table 3 summarizes the time-to-event end points. The median overall survival of all 22 patients was 21.7 months (95% CI, 15.7 to not reached) from registration (Figure 2A). Patients who underwent a pancreatectomy had a longer overall survival than patients who did not (18-month overall survival rate of 67% vs 43%; hazard ratio, 0.13 [95% CI, 0.03–0.48]; P = .001) (Figure 2B). Patients who underwent a pancreatectomy and had less than 5% cancer cells in their surgical specimen had a longer overall survival than patients who underwent a pancreatectomy and had 5% or more cancer cells (median overall survival not evaluable vs 21.7 months [95% CI, 15.9–25.2 months]; P = .01) (Figure 2C).
Table 3.
Summary of Survival and Disease Progression/Recurrence End Points
| End Pointa | Event | No. of Patients |
No. of Events |
Event-Free Survival Rate (95% CI) |
|
|---|---|---|---|---|---|
| At 12 mo | At 18 mo | ||||
| Overall survival | |||||
| All patients | Death from any cause | 22 | 14 | 0.77 (0.62–0.97) |
0.55 (0.37–0.80) |
| Resected patients | Death from any cause | 15 | 8 | 0.73 (0.54–1.00) |
0.43 (0.21–0.86) |
| Progression–free survival | |||||
| All patients | Progression during preoperative treatment, recurrence after surgery, or death from any cause |
22 | 15 | 0.59 (0.42–0.84) |
0.41 (0.25–0.68) |
| Resected patients | Progression during preoperative treatment, recurrence after surgery, or death from any cause |
15 | 8 | 0.53 (0.33–0.86) |
0.40 (0.19–0.84) |
| Freedom from distant recurrence |
|||||
| Resected patients | Distant recurrence after resection | 15 | 4 | 0.67 (0.45–1.00) |
0.67 (0.45–1.00) |
| Freedom from locoregional recurrence |
|||||
| Resected patients | Locoregional recurrence after resection |
15 | 2 | 0.90 (0.73–1.00) |
0.60 (0.26–1.00) |
In this table, for analyses based on all treated patients (n = 22), the registration date was the start date of the time-to-event end points; for analyses based on resected patients only (n = 15), the surgery date was the start date of the time-to-event end points.
Figure 2. Kaplan-Meier Survival Curves.
A, The median overall survival of all 22 patients was 21.7 months (95% CI, 15.7 to not reached) from registration. B, Patients who underwent a pancreatectomy had a significantly higher 18-month overall survival rate than patients who did not (67% vs 43%; hazard ratio, 0.13 [95%CI, 0.03–0.48]; P = .001). C, Patients who underwent a pancreatectomy and had less than 5% cancer cells in their surgical specimen had a longer overall survival than patients who had 5% or more cancer cells (median overall survival not evaluable vs 21.7 months [95% CI, 15.9–25.2 months]; P = .01). Hash marks represent censored data.
Discussion
This collaborative study met all of its primary end points: the 14 participating centers from all National Clinical Trials Network cooperative groups accrued patients ahead of schedule, the preoperative regimen was associated with manageable toxicity that did not preclude surgery, and 68% of patients underwent a pancreatectomy as planned. We have therefore demonstrated that a multidisciplinary regimen of coordinated, quality-controlled care can be administered to patients with borderline resectable PDAC in the context of a cooperative group trial. The quality-control mechanisms implemented in this trial, and the prospective data that it has generated, will be used in forthcoming intergroup investigations of novel therapeutic regimens for advanced PDAC.
Variability in both disease staging and treatment has represented barriers to the interpretation of retrospective studies of preoperative therapy for patients with advanced PDAC, and to the conduct of prospective clinical trials of this strategy.7,20 Several mechanistic elements of this study designed to minimize such heterogeneity are therefore worth emphasis. First, the criteria that we developed to define eligibility for this trial, and which have subsequently been endorsed by the National Comprehensive Cancer Network for use in all future prospective trials, are based on a reproducible, radiographic measurement of the circumferential interface between the tumor and each major mesenteric vessel.5,21 The definition avoids the ambiguity inherent in terms such as abutment, deformity, or involvement that were adopted in previously reported staging schemes, that may lead to inconsistent staging of disease, and that may contribute to an overestimation of the cytotoxic effects of preoperative therapy.10
Second, we used a unique, 2-phase accrual procedure for this trial. Patients were preregistered on the basis of imaging suggestive of borderline resectable disease and the expected ability to tolerate therapy, but final registration required confirmation of disease stage by a central radiologist and evaluation by the treating surgeon, medical oncologist, and radiation oncologist. This process ensured that there was a homogeneous study population and that the entire multidisciplinary team achieved consensus on the treatment plan.
Third, a strict effort was made to prospectively standardize the performance and quality of all therapeutic modalities. Radiation plans were centrally reviewed prior to initiation of therapy. Surgical quality assurance was achieved through the selection of centers with experience in the management of pancreatic tumors that involve the mesenteric vasculature. Furthermore, both the indications for surgery following the administration of preoperative therapy and the technical approach to the dissection were mandated to minimize surgical variables that might influence outcomes.22 Central review of pathology specimens was also emphasized.
The multimodality treatment regimen administered in this trial leveraged theoretical benefits associated with chemotherapy (antitumor activity on micrometastatic disease), chemoradiation (sterilization of margins), and time (selection) prior to pancreatectomy and is consistent with existing guidelines.4,5 However, because neither the value of these therapeutic components nor their optimum durations have been established, we used a basic regimen for this study that was perceived as generalizable and that would represent a foundation on which future regimens could be built. We used FOLFIRINOX based on its systemic activity but limited its duration to 4 cycles given concerns that its toxicities might prohibit the subsequent operation viewed as necessary for cure23; gemcitabine was administered postoperatively given the perception—based on data extrapolated from the post-operative setting—that all patients must receive 6 perioperative months of chemotherapy.24 Alliance for Clinical Trials in Oncology Trial A021501, a randomized phase 2 study that has been approved by the NCI for activation in 2016 and that uses many of the design elements described herein, will provide a longer course of chemotherapy and will evaluate the role of preoperative radiation by randomizing patients to either 8 cycles of modified FOLFIRINOX or 7 cycles of modified FOLFIRINOX followed by radiation.
The findings of microscopically negative margins and less than 5% residual cancer cells in 93% and 33% of resected specimens, respectively, suggest the cytotoxic activity of the preoperative regimen, even though a radiographic partial or complete response, was observed in only 27% of treated patients. Furthermore, 80% of resected patients still required vascular resection. The apparent discrepancies between a tumor’s apparent response to therapy and the change in its size and anatomic extent are consistent with the findings of previous retrospective studies that described significant “downstaging” of PDAC as rare.10,25 Surgeons must anticipate the need for vascular resection and reconstruction during a pancreatectomy for all patients with advanced cancers, even following the administration of “aggressive” preoperative regimens.21
Finally, the 21.7-month median duration of survival of all patients in this trial is remarkable given that the median overall survival of highly selected patients with resected PDAC who received postoperative chemotherapy in randomized phase 3 trials was under 24 months.24,26,27 This figure is consistent with data from retrospective, single-center reports of patients with advanced PDAC who were pretreated with FOLFIRINOX (eTable in the Supplement 2).28–33 However, these data must be taken in the context of this small, single-arm trial designed to meet feasibility, not survival, end points.
Conclusions
In conclusion, this study is the first successful cooperative group trial to evaluate patients with borderline resectable PDAC. The accrual rate exceeded the goal rate, toxicities were manageable, and 68% of patients underwent a pancreatectomy. The data generated by this study and the logistic elements inherent in its design are being used in forthcoming cooperative group trials.
Supplementary Material
Key Points.
Question
Can studies of preoperative therapy for patients with advanced pancreatic cancer be conducted within the cooperative group setting?
Findings
In this prospective, single-arm feasibility trial of modified FOLFIRINOX treatment and chemoradiation prior to pancreatectomy, 14 participating centers accrued patients ahead of schedule, preoperative toxicity did not preclude surgery, and 68% of patients underwent pancreatectomy.
Meaning
A multidisciplinary regimen of quality-controlled care can be administered to patients with pancreatic cancer within the cooperative group setting. The design elements of this trial, and the prospective data that it has generated, will be used in forthcoming investigations of therapeutic regimens for advanced disease.
Acknowledgments
Funding/Support: This work is supported in part by the National Institutes of Health (grants U10CA180821, U10CA180882, U10CA180888, and U10CA180820) and by funds from the following grants: U10CA180858, U10CA180802, U10CA180835, U10CA180850, UG1CA189870, U10CA180836, U10CA180850, U10CA180888, UG1CA189870, U10CA180820, and U10CA180799.
Role of the Funder/Sponsor: The funding agency had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dr Katz is the recipient of the 2012 Alliance for Clinical Trials in Oncology Foundation Clinical Scholars Award.
Additional Contributions: The following institutions enrolled patients in this study: Fox Chase Cancer Center, Philadelphia, PA (Lori J. Goldstein, MD); John Hopkins University Hospital, Baltimore, MD (supported by grant U10CA180802 to Julie R. Brahmer, MD); MD Anderson Cancer Center, Houston, TX (supported by grant U10CA180858 to Cathy Eng, MD); Mayo Clinic, Rochester, MN (supported by grant U10CA180790 Steven R. Alberts, MD); NorthShore University HealthSystem CCOP, Evanston, IL (David L. Grinblatt, MD); Ochsner NCI Community Oncology Research Program, New Orleans, LA (supported by grant UG1CA189870 to Jyotsna Fuloria, MD); James Graham Brown Cancer Center at University of Louisville, Louisville, KY (Robert Martin, MD); Ohio State University, Columbus, OH (supported by grant U10CA180850 to Richard M. Goldberg, MD); University of California at San Diego, San Diego, CA (supported by grant CA11789 to Barbara A. Parker, MD); University of Chicago, Chicago, IL (supported by grant U10CA180836 to Hedy L. Kindler, MD); University of Cincinnati, Cincinnati, OH (Syed A. Ahmad, MD); University of Wisconsin at Madison, Madison, WI (supported by grant U10CA180799 to Brad Kahl, MD); and Vanderbilt University, Nashville, TN (supported by grant U10CA180847 to Jordan D. Berlin, MD).
Footnotes
Author Contributions: Drs Katz and Shi had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Katz, Shi, Ahmad, Herman, Marsh, Collisson, Schwartz, Kindler, Lowy, Philip, Talamonti, LoConte, Hoffman, Venook.
Acquisition, analysis, or interpretation of data: Katz, Shi, Ahmad, Marsh, Collisson, Schwartz, Frankel, Martin, Conway, Truty, Kindler, Lowy, Bekaii-Saab, Talamonti, Cardin, Shen, Venook.
Drafting of the manuscript: Katz, Shi, Ahmad, Collisson, Schwartz, Kindler, LoConte, Venook.
Critical revision of the manuscript for important intellectual content: Katz, Shi, Ahmad, Herman, Marsh, Collisson, Frankel, Martin, Conway, Truty, Kindler, Lowy, Bekaii-Saab, Philip, Cardin, LoConte, Shen, Hoffman, Venook.
Statistical analysis: Shi, Collisson.
Obtained funding: Katz.
Administrative, technical, or material support: Katz, Shi, Ahmad, Schwartz, Frankel, Truty, Kindler, Bekaii-Saab, Talamonti, Cardin.
Study supervision: Katz, Ahmad, Herman, Schwartz, Martin, Truty, Philip, LoConte, Venook.
Conflict of Interest Disclosures: None reported.
Previous Presentation: This paper was presented at the Annual Meeting of the American Society of Clinical Oncology; May 31, 2015; Chicago, Illinois.
Supplemental content at jamasurgery.com
REFERENCES
- 1.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13(8):1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Yamada S, Fujii T, Sugimoto H, et al. Aggressive surgery for borderline resectable pancreatic cancer: evaluation of National Comprehensive Cancer Network guidelines. Pancreas. 2013;42(6):1004–1010. doi: 10.1097/MPA.0b013e31827b2d7c. [DOI] [PubMed] [Google Scholar]
- 3.Nakao A, Kanzaki A, Fujii T, et al. Correlation between radiographic classification and pathological grade of portal vein wall invasion in pancreatic head cancer. Ann Surg. 2012;255(1):103–108. doi: 10.1097/SLA.0b013e318237872e. [DOI] [PubMed] [Google Scholar]
- 4.Abrams RA, Lowy AM, O’Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16(7):1751–1756. doi: 10.1245/s10434-009-0413-9. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma, Version 2.2015. Fort Washington, PA: NCCN; 2015. [Google Scholar]
- 6.Landry J, Catalano PJ, Staley C, et al. Randomized phase II study of gemcitabine plus radiotherapy versus gemcitabine, 5-fluorouracil, and cisplatin followed by radiotherapy and 5-fluorouracil for patients with locally advanced, potentially resectable pancreatic adenocarcinoma. J Surg Oncol. 2010;101(7):587–592. doi: 10.1002/jso.21527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz MH, Marsh R, Herman JM, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol. 2013;20(8):2787–2795. doi: 10.1245/s10434-013-2886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz MH, Pisters PW, Lee JE, Fleming JB. Borderline resectable pancreatic cancer: what have we learned and where do we go from here? Ann Surg Oncol. 2011;18(3):608–610. doi: 10.1245/s10434-010-1460-y. [DOI] [PubMed] [Google Scholar]
- 9.Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7(4):e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118(23):5749–5756. doi: 10.1002/cncr.27636. [DOI] [PubMed] [Google Scholar]
- 11.Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16(7):1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 12.American College of Surgeons; Alliance for Clinical Trials in Oncology. Operative Standards for Cancer Surgery: Breast, Lung, Pancreas, Colon. Vol. 1. Philadelphia, PA: Wolters Kluwer; 2015. [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.American Joint Committee on Cancer (AJCC) AJCC Cancer Staging Manual. 7th. New York, NY: Springer; 2010. [Google Scholar]
- 15.Washington K, Berlin J, Branton P, et al. Protocol for the examination of specimens from patients with carcinoma of the exocrine pancreas. [Accessed May 9, 2016];College of American Pathologists. http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/pancreasexo-13protocol-3201.pdf. Posted October 2013. [Google Scholar]
- 16.Chatterjee D, Katz MH, Rashid A, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer. 2012;118(12):3182–3190. doi: 10.1002/cncr.26651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Common Terminology Criteria for Adverse Events (CTCAE) US Dept of Health and Human Services/National Institutes of Health/National Cancer Institute website. [Accessed May 10, 2016]; http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- 18.Altman DG. Practical Statistics for Medical Research. New York, NY: Chapman and Hall/CRC; 1991. [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 20.Varadhachary GR, Evans DB. Rational study endpoint(s) for preoperative trials in pancreatic cancer: pathologic response rate, margin negative resection, overall survival or ‘all of the above’? Ann Surg Oncol. 2013;20(12):3712–3714. doi: 10.1245/s10434-013-3165-5. [DOI] [PubMed] [Google Scholar]
- 21.Tran Cao HS, Balachandran A, Wang H, et al. Radiographic tumor-vein interface as a predictor of intraoperative, pathologic, and oncologic outcomes in resectable and borderline resectable pancreatic cancer. J Gastrointest Surg. 2014;18(2):269–278. doi: 10.1007/s11605-013-2374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz MH, Merchant NB, Brower S, et al. American College of Surgeons Oncology Group. Standardization of surgical and pathologic variables is needed in multicenter trials of adjuvant therapy for pancreatic cancer: results from the ACOSOG Z5031 trial. Ann Surg Oncol. 2011;18(2):337–344. doi: 10.1245/s10434-010-1282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conroy T, Desseigne F, Ychou M, et al. Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 24.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 25.Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261(1):12–17. doi: 10.1097/SLA.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neoptolemos JP, Stocken DD, Bassi C, et al. European Study Group for Pancreatic Cancer. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304(10):1073–1081. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 27.Neoptolemos JP, Stocken DD, Friess H, et al. European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 28.Sadot E, Doussot A, O’Reilly EM, et al. FOLFIRINOX Induction Therapy for Stage 3 Pancreatic Adenocarcinoma. Ann Surg Oncol. 2015;22(11):3512–3521. doi: 10.1245/s10434-015-4647-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christians KK, Tsai S, Mahmoud A, et al. Neoadjuvant FOLFIRINOX for borderline resectable pancreas cancer: a new treatment paradigm? Oncologist. 2014;19(3):266–274. doi: 10.1634/theoncologist.2013-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahaseth H, Brutcher E, Kauh J, et al. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas. 2013;42(8):1311–1315. doi: 10.1097/MPA.0b013e31829e2006. [DOI] [PubMed] [Google Scholar]
- 31.Boone BA, Steve J, Krasinskas AM, et al. Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer. J Surg Oncol. 2013;108(4):236–241. doi: 10.1002/jso.23392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tinchon C, Hubmann E, Pichler A, et al. Safety and efficacy of neoadjuvant FOLFIRINOX treatment in a series of patients with borderline resectable pancreatic ductal adenocarcinoma. Acta Oncol. 2013;52(6):1231–1233. doi: 10.3109/0284186X.2013.771821. [DOI] [PubMed] [Google Scholar]
- 33.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma, Version 1.2014. Fort Washington, PA: NCCN; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.