Abstract
Background
Primary prevention of cardiovascular disease (CVD) focuses on treatment of risk factors, including hypercholesterolemia, hypertension, and type 2 diabetes mellitus. We investigated whether a healthy diet in adolescence prevents development of clinical risk factors or incidence of CVD in adulthood.
Methods and Results
We examined the time to the first development of ≥1 clinical risk factor (hypercholesterolemia, hypertension, or type 2 diabetes mellitus) or CVD in relation to a high school Alternative Healthy Eating Index (HS‐AHEI) within the Nurses’ Health Study II. Among those who completed a food frequency questionnaire about their high school diet and adult diet (mean age 42 years), 27 406 women free of clinical risk factors and 42 112 women free of CVD in 1998 were followed to June 2011. Hazard ratios (HRs) and 95% CIs were adjusted for potential confounders in high school and adulthood. We documented 11 542 first diagnoses of clinical risk factors and 423 CVD events. The HS‐AHEI was associated with a lower rate of risk factors (HR highest versus lowest quintiles 0.82; 95% CI, 0.77–0.87 [P trend <0.001]), was inversely associated with risk of developing ≥1 clinical risk factor in women with a low, medium, and high AHEI score during adulthood (HR high HS‐AHEI/high adult AHEI versus low/low 0.79 [95% CI, 0.74–0.85]), but was not statistically significantly associated with incident CVD.
Conclusions
A healthy diet during adolescence is associated with lower risk of developing CVD risk factors. As diet tracks throughout life, and adult diet prevents CVD, healthy dietary habits that begin early are important for primordial prevention of CVD.
Keywords: diet, prevention, risk factor
Subject Categories: Diet and Nutrition, Epidemiology, Primary Prevention, Women
Introduction
Primary prevention efforts for cardiovascular disease (CVD) focus on the identification and modification of 3 established risk factors (hypercholesterolemia, hypertension, and type 2 diabetes mellitus),1 which are associated with substantial long‐term risk of CVD. For example, adults with one risk factor at midlife have a lifetime risk of CVD of 40% to 50%.2, 3, 4 Successful pharmacological treatment lowers, but does not completely eliminate, this elevated risk.5 In contrast, adults who reach middle age with ideal levels of all 3 risk factors have a lifetime risk of CVD of 5% to 8%.2, 3 Thus, the prevention or delay of risk factor development through healthy lifestyle factors, or primordial prevention, is of paramount importance to minimize an individual's long‐term CVD risk.
In adults, greater adherence to a healthy dietary pattern defined by the Alternative Healthy Eating Index (AHEI), which includes high intake of fruits and vegetables, fish, whole grains and nuts, and low intake of red and processed meats, sodium, and trans fats, is associated with lower risk of type 2 diabetes mellitus6 and CVD.7, 8, 9 The AHEI was developed a priori based on knowledge of dietary factors associated with CVDs in adults in the published literature.7 However, data on the association between diet quality earlier in life and the long‐term development of CVD risk factors are sparse.10 In the Nurses’ Health Study (NHS) II, we previously collected information on dietary intake during high school.11 In this study, we investigated whether overall diet quality in adolescence assessed using the AHEI, was associated with cardiovascular risk factors in adulthood. We hypothesized that a high‐quality diet was associated with lower incidence of hypercholesterolemia, hypertension, type 2 diabetes mellitus, and CVD during adulthood among women in the NHS II.
Materials and Methods
Study Population
The NHS II is a prospective cohort study of 116 430 female registered nurses aged 25 to 42 years when they completed questionnaires about their lifestyle and medical history at recruitment in 1989. The women completed biennial follow‐up questionnaires to update this information, and response proportions have been ≥90% in each follow‐up cycle.12 Dietary intakes are assessed every 4 years by validated self‐administered food frequency questionnaires (FFQs) of ≈131 items. In 1997, participants were asked whether they would complete a supplemental high school FFQ (HS‐FFQ) about their diets in that period. Approximately 55% of the cohort agreed (n=64 380), of whom 73% (n=47 355) returned the HS‐FFQ in 1998.
We excluded women who reported implausible daily energy intakes during high school (<500 or >5000 kcal/d; n=1181) or adulthood (<600 or >3500 kcal/d; n=1283); left ≥10 HS‐FFQ items blank (n=226); were lost to follow‐up (n=201); or reported CVD (including myocardial infarction [MI], stroke, angina, and revascularization procedures; n=915) or cancer (except nonmelanoma skin cancer; n=1437) prior to return of the HS‐FFQ. There were 42 112 women available for the analysis of CVD risk. For analyses of clinical risk factor outcomes, we further excluded women who reported diagnoses of hypercholesterolemia, hypertension, or type 2 diabetes mellitus prior to return of the HS FFQ (n=14 710), leaving 27 406 women. All participants provided informed consent at entry to the cohort study, and this study protocol was approved by the institutional review board of Brigham and Women's Hospital.
Assessment of High School and Adult Dietary Intakes
Dietary intake during adolescence was assessed using the 124‐item HS‐FFQ, designed to capture foods commonly consumed between 1960 and 1982, when study participants attended high school. Women were asked to indicate how often, on average, they had consumed specific portion sizes of each food item when they were in high school (grades 9–12).11 Nutrient intake was calculated by multiplying the frequency of intake of each food by its nutrient content, and summing across all food items. The nutrient database was constructed from US Department of Agriculture information about foods consumed in that period. Secular changes in food formulations were accounted for by the use of different nutrient profiles for specific foods depending on individual participants’ birth years. The high school period was chosen in part to provide participants with a defined phase of their lives to guide their memories. It has demonstrated reasonable reproducibility in 333 randomly selected NHS II participants who completed 2 HS‐FFQs within a 4‐year period and validity when comparing dietary intakes reported by mothers of 272 NHS II participants with intakes reported on the HS‐FFQ.13, 14 The mean Spearman rank correlation for food intakes between repeated questionnaires 4 years apart was 0.60 (range, 0.37–0.77); the mean Pearson correlation for nutrient intakes was 0.65 (range, 0.50–0.77).
We used the FFQ administered in 1995 to represent diet in adulthood because this was the most recent dietary questionnaire prior to the HS‐FFQ. We used the most recent dietary database to calculate adult nutrient intake.15 If a woman was missing the 1995 FFQ, we carried forward dietary intake previously reported in 1991. The validity and reliability of FFQs similar to those used here, and in similar populations, have been previously described.16, 17
High School Alternative Healthy Eating Index
We defined diet quality based on the AHEI‐2010, which was previously developed based on foods and nutrients predictive of chronic disease risk.7 Information on some nutrients in the AHEI‐2010 were not available from the HS‐FFQ. Therefore, we modified the AHEI based on the available data (Table 1). We used servings per week of dark meat fish as a surrogate for long‐chain omega‐3 polyunsaturated fatty acids (PUFAs). Because sodium intake is poorly estimated on the FFQ, we calculated a “high sodium food” variable based on the top 10 contributors to sodium intake among 14‐ to 18‐year‐old women in the United States, including bread, pizza, cheese, and snacks.18 Finally, we did not include the alcohol component, as high school students are below the legal drinking age in the United States. The high school AHEI (HS‐AHEI) consisted of 10 components: fruits, vegetables, whole grains, sugar‐sweetened beverages and juices, nuts and legumes, red/processed meat, trans fats, dark meat fish, PUFA, and high sodium foods. Each component contributed 0 to 10 points to the total score; a score of 10 signified optimal dietary behavior, whereas a score of 0 represents the least healthy dietary behavior. Intermediate intakes were scored proportionately between 0 and 10. The total score ranged from 0 (nonadherence) to 100 (complete adherence).
Table 1.
Components of the High School AHEIa
| AHEI Components | Criteria for Minimum Score (0) | Criteria for Maximum Score (10) |
|---|---|---|
| Vegetables, servings/d | 0 | ≥5 |
| Fruit, servings/d | 0 | ≥4 |
| Whole grains, g/d | 0 | ≥75 |
| Nuts and legumes, servings/d | 0 | ≥1 |
| Polyunsaturated fatty acids (% of energy) | ≤2 | ≥10 |
| Dark meat fish, servings/wkb | 0 | 2 |
| Sugar‐sweetened beverages and juices, servings/d | >1 | 0 |
| Red/processed meats, servings/d | ≥1.5 | 0 |
| Trans fats (% of energy) | ≥4 | ≤0.5 |
| High sodium foods, servings/dc | Highest decile | Lowest decile |
| Total score possible | 0 | 100 |
Alcohol is not included in the score.
All vegetables on the food frequency questionnaire were included in the vegetable component, except for potatoes, and ≥5 servings/d were considered ideal based on current dietary guidelines. One serving is 0.5 cup of vegetables or 1 cup of green leafy vegetables (1 cup=236.6 g). Only whole fruit were included in the fruit component, not fruit juice. We considered ≥4 servings/d to be ideal based on current dietary guidelines. One serving is 1 medium piece of fruit or 0.5 cup of berries. One serving of a 100% whole grain product (ie, 0.5 cup of oatmeal) contains around 15 to 20 g of whole grains (per dry weight). We considered 75 g/d to be optimal (around 5 servings/d) for women based on current guidelines for total grains. Intakes of sugar‐sweetened carbonated beverages and fruit juice were combined in one component, and ≥1 serving/d was considered the least optimal. One serving is 8 oz (1 oz=28.4 g). We considered 1 serving/d of nuts to be ideal on the basis of the American Heart Association recommendations and the current literature. One serving is 1 oz of nuts or 1 tablespoon (15 mL) of peanut butter. For red meat and processed meats, >1 serving/mo was considered to be ideal, with an upper limit of ≥1.5 servings/d. One serving is 4 oz of unprocessed meat or 1.5 oz of processed meat. For trans fats of fatty acids, cutoffs are consistent with original Alternative Healthy Eating Index (AHEI) cutoffs. The cutoff for optimal intake of eicosapentaenoic acid+docosahexaenoic acid (EPA+DHA; 250 mg/d) in the AHEI‐2010 score can be achieved by consuming two 4‐oz servings of fish/wk, which is consistent with current guidelines. We considered dark meat fish, such as tuna, mackerel, salmon, or sardines, relevant. The highest scores in the polyunsaturated fatty acid component, which did not include EPA or DHA intake, were given to individuals with ≥10% of total energy intake from polyunsaturated fatty acid. High sodium foods consisted of breads, pizza, chicken products, chowders, cheese, pasta, and snacks such as popcorn and potato chips. The cutoffs for sodium were based on deciles of distribution in the study population, inversed so that the highest score was awarded to the lowest decile of intake.
AHEI‐2010 component EPA and DHA (mg/d) 0 to 250.
AHEI‐2010 component sodium (mg/d) highest decile to lowest decile.
Adult diet quality was assessed by the AHEI‐2010 score. We excluded alcohol from the adult AHEI for comparability with the HS‐AHEI.
Outcome Assessment
We considered type 2 diabetes mellitus, hypertension, and hypercholesterolemia as outcomes, representing targets for primordial prevention and subsequent CVD risk reductions based on recent guidelines.19 Our primary outcome was the diagnosis of the first of any one of hypercholesterolemia, hypertension, or type 2 diabetes mellitus. We also investigated each individual clinical risk factor separately.
We defined type 2 diabetes mellitus as a self‐report of incident diabetes mellitus confirmed by a validated supplemental questionnaire using 1997 American Diabetes Association criteria.20 Participants reported physician‐diagnosed hypertension and hypercholesterolemia, along with the calendar year of diagnosis, or the use of blood pressure– or cholesterol‐lowering medications on the biennial questionnaires. As previously described, we estimated the date of diagnosis based on reported calendar time and month of questionnaire return.21 In sensitivity analyses, we defined hypertension and hypercholesterolemia by self‐report and medication use.
Our secondary outcome was CVD, defined as the first event of nonfatal MI, fatal coronary heart disease, nonfatal and fatal ischemic stroke (thrombotic or embolic occlusion of a cerebral artery), and coronary revascularization procedures (coronary artery bypass grafting surgery or percutaneous transluminal coronary angioplasty). All nonfatal events were confirmed through review of medical records by study investigators blinded to the participant's risk factor status. MI was defined according to World Health Organization criteria based on symptoms plus diagnostic ECG changes or elevated cardiac enzymes.22 Strokes were confirmed if data in the medical records fulfilled the National Survey of Stroke criteria requiring evidence of a neurologic deficit with sudden or rapid onset that persisted for >24 hours or until death.23 Deaths were reported by next of kin, postal authorities, or through the National Death Index and confirmed through reviews of medical records or autopsy reports. Self‐reported revascularization was almost 100% specific in another population of health professionals.24
Assessment of Covariates
Information on age, height, BMI at age 18, smoking between 15 and 19 years, physical activity between 15 and 19 years, family history of type 2 diabetes mellitus, and family history of hypertension was obtained from the questionnaire at recruitment in 1989. Information on current weight, smoking status, physical activity, oral contraceptive use, postmenopausal hormone use, aspirin use, and alcohol intake was obtained from the 1997 follow‐up questionnaire. BMI was calculated as the ratio of weight in 1997 (in kilograms) to squared height (in meters squared), with height being assessed at baseline only. Physical activity was computed as metabolic equivalents per week using the duration per week of various forms of exercise after weighting each activity by its intensity level in metabolic equivalents.
Statistical Analysis
We categorized the HS‐AHEI scores into quintiles and estimated person‐time of follow‐up for each participant from 1997 until the date of diagnosis of a clinical risk factor (hypercholesterolemia, hypertension, or type 2 diabetes mellitus), date of death or June 2011, whichever came first.
We used Cox proportional hazards regression to estimate hazard ratios (HRs) and 95% CIs for each quintile of the HS‐AHEI compared with the referent (lowest) quintile. To test for a linear trend, we calculated the median quintile score values and modeled them as a continuous variable. Models were first adjusted for age. We then adjusted for high school levels of nondietary risk factors for hypercholesterolemia, hypertension, and type 2 diabetes mellitus (multivariable model 1): BMI at age 18, total energy intake, smoking between 15 and 19 years, physical activity, and family history of type 2 diabetes mellitus and family history of hypertension. In multivariable model 2, we further adjusted for established adult period risk factors for hypercholesterolemia, hypertension, type 2 diabetes mellitus, and CVD including total energy, smoking status, physical activity, oral contraceptive use, postmenopausal hormone use, aspirin use, and alcohol intake. We used information collected on the 1997 questionnaire, which was the questionnaire administered closest in time to the return of the HS‐FFQ. We additionally adjusted for adult AHEI score and adult BMI, which are potential mediators of the association between high school diet and CVD risk factors. In sensitivity analyses, we adjusted for parity and ancestry, as surrogates for socioeconomic status.
To investigate the cumulative effect of lifetime diet as previously described,25 we cross‐classified tertiles of the HS‐AHEI and adult AHEI scores into a single variable with 9 categories. Multivariable HRs and 95% CIs for any clinical risk factor were estimated for each category in relation to women in the lowest tertile for both high school and adult diet.
In secondary analyses, we explored incident CVD. Person‐time of follow‐up was calculated as above until the date of diagnosis of CVD, date of death, or June 2011, whichever came first. We adjusted for hypercholesterolemia, hypertension, or type 2 diabetes mellitus reported at any time prior to return of the HS‐FFQ, in addition to confounders listed above.
The proportional hazards assumption was investigated by the Wald test after modelling an interaction term between the HS‐AHEI and age. All models fulfilled this assumption.
We used SAS version 9.3 (SAS Institute, Cary, NC), P values were 2‐sided, and a P value <0.05 was considered statistically significant.
Results
Women in the highest quintile of HS‐AHEI had slightly higher AHEI‐2010 scores, were more likely to be nonsmokers in high school and in adulthood, and were less likely to report a family history of MI, diabetes mellitus, or hypertension than those in the lowest quintile (Table 2). HS‐AHEI and AHEI‐2010 scores in adulthood were only modestly correlated (Pearson correlation coefficient 0.40; P<0.001).
Table 2.
Age‐Standardized Characteristics of the Whole Study Population of Nurses and by Quintiles of Adherence to the AHEI in High School (N=27 406)
| Total Study Population | Quintiles of High School AHEI | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| No. | 27 406 | 5481 | 5481 | 5481 | 5481 | 5482 |
| High school AHEI score, median | 41.9 | 29.9 | 36.6 | 41.6 | 46.7 | 55.6 |
| Range | 11.3–87.9 | 11.3–33.8 | 33.9–39.1 | 39.2–43.9 | 44.0–49.6 | 49.7–87.9 |
| Age at 1997 questionnaire return, ya | 42.0 (4.6) | 41.5 (4.6) | 42.0 (4.6) | 42.1 (4.6) | 42.3 (4.6) | 42.3 (4.7) |
| High school AHEI component scoresb | ||||||
| Vegetables | 4.9 (2.5) | 3.3 (1.7) | 4.2 (2.0) | 4.7 (2.2) | 5.6 (2.4) | 6.9 (2.5) |
| Fruit | 3.5 (2.2) | 2.3 (1.5) | 2.9 (1.7) | 3.4 (1.9) | 4.0 (2.1) | 5.2 (2.6) |
| Whole grains | 3.0 (2.0) | 1.9 (1.3) | 2.4 (1.6) | 2.8 (1.7) | 3.3 (2.0) | 4.5 (2.5) |
| Nuts and legumes | 2.2 (3.1) | 1.0 (2.2) | 1.8 (2.8) | 2. 2 (3.0) | 2.6 (3.2) | 3.3 (3.8) |
| PUFAs | 5.4 (3.1) | 3.5 (2.6) | 4.7 (2.9) | 5.5 (3.0) | 6.1 (3.0) | 7.1 (2.9) |
| Dark meat fish | 2.0 (2.4) | 1.0 (1.6) | 1.5 (2.0) | 1.8 (2.2) | 2.2 (2.4) | 3.4 (3.0) |
| SSBs and juices | 4.8 (1.9) | 3.9 (2.0) | 4.4 (1.9) | 4.7 (1.8) | 5.1 (1.8) | 5.7 (1.7) |
| Red/processed meats | 5.8 (1.6) | 5.3 (1.5) | 5.6 (1.5) | 5.8 (1.6) | 5.9 (1.6) | 6.2 (1.7) |
| Trans fats | 5.0 (3.1) | 4.1 (3.0) | 4.8 (3.1) | 5.1 (3.1) | 5.4 (3.2) | 5.8 (3.1) |
| High sodium foods | 5.4 (3.6) | 2.7 (2.8) | 4.5 (3.3) | 5.7 (3.3) | 6.5 (3.3) | 7.6 (4.0) |
| High school characteristics | ||||||
| BMI at age 18 y, kg/m² | 20.9 (2.9) | 20.8 (2.9) | 20.8 (2.9) | 20.9 (2.8) | 21.0 (2.9) | 20.9 (2.8) |
| Smoking, % | 22 | 22 | 23 | 22 | 22 | 20 |
| Physical activity, MET/wk | 54.1 (17.5) | 52.0 (17.4) | 53.1 (17.5) | 53.9 (17.4) | 55.0 (17.5) | 56.7 (17.3) |
| Energy intake, kcal/d | 2725 (781) | 2789 (719) | 2720 (758) | 2732 (791) | 2711 (805) | 2664 (819) |
| Adult characteristics | ||||||
| AHEI‐2010 score | 45.3 (10.2) | 39.9 (9.4) | 43.4 (9.5) | 44.8 (9.4) | 47.1 (9.3) | 51.3 (9.7) |
| Energy intake, kcal/d | 1814 (548) | 1803 (534) | 1801 (549) | 1813 (552) | 1829 (547) | 1819 (558) |
| Alcohol intake, 0 g/d, % | 39 | 42 | 39 | 38 | 36 | 37 |
| 0.1 to 4.9 g/d, % | 38 | 38 | 39 | 38 | 39 | 39 |
| ≥5 g/d, % | 23 | 20 | 22 | 24 | 24 | 24 |
| BMI, kg/m² | 24.6 (4.9) | 24.8 (5.0) | 24.6 (5.0) | 24.7 (4.9) | 24.6 (5.0) | 24.2 (4.8) |
| Physical activity, MET/wk‡ | 18.2 (15.5) | 15.5 (14.4) | 17.0 (15.1) | 17.8 (15.3) | 19.1 (15.6) | 21.7 (16.3) |
| Current smoking, % | 8 | 9 | 9 | 9 | 8 | 7 |
| Parity, no children, % | 19 | 17 | 18 | 18 | 20 | 23 |
| 1 to 2 children, % | 53 | 54 | 51 | 54 | 53 | 53 |
| 3 to 4 children, % | 26 | 28 | 29 | 26 | 26 | 23 |
| 5+ children, % | 2 | 2 | 2 | 2 | 2 | 2 |
| Current oral contraceptive use, % | 9 | 9 | 9 | 9 | 9 | 9 |
| Postmenopausal, % | 7 | 7 | 7 | 7 | 7 | 8 |
| Current postmenopausal hormone use, % | 15 | 15 | 15 | 15 | 15 | 15 |
| Current aspirin use, % | 12 | 12 | 13 | 11 | 13 | 12 |
| African ancestry, % | 1 | 0 | 1 | 0 | 1 | 1 |
| Hispanic ancestry, % | 1 | 0 | 1 | 1 | 1 | 3 |
| Asian ancestry, % | 1 | 0 | 0 | 1 | 1 | 3 |
| Other non‐Caucasian ancestry, % | 2 | 2 | 2 | 2 | 2 | 3 |
| Family history of MI, % | 28 | 29 | 29 | 28 | 27 | 25 |
| Family history of diabetes mellitus, % | 14 | 16 | 15 | 14 | 14 | 13 |
| Family history of hypertension, % | 49 | 51 | 50 | 50 | 48 | 47 |
Values are expressed as means (SD), median, or percentages. Alternate Healthy Eating Index indicates AHEI; BMI, body mass index; MET, metabolic equivalent of task hours; MI, myocardial infarction; Q, quartile.
Value is not age‐adjusted.
Scores were 0 to 10 with 10 being the highest intake for vegetables, fruits, whole grains, nuts and legumes, polyunsaturated fatty acids (PUFAs), and dark meat fish, and 10 being the lowest intake for sugar‐sweetened beverages (SSBs) and juices, red/processed meats, trans fats, and high sodium foods.
High School Diet and Clinical CVD Risk Factor Development
Over 13 years, 11 542 women were diagnosed with ≥1 risk factor. Higher HS‐AHEI scores were associated with lower risk of developing at least one risk factor (hypercholesterolemia, hypertension, or type 2 diabetes mellitus) after adjusting for confounders during high school and adulthood (HR comparing the highest with the lowest quintile: 0.82; 95% CI, 0.77–0.87 [P trend <0.001]) (Table 3). Further adjustment for adult diet quality and adult BMI did not alter the results (HR comparing the highest with the lowest quintile: 0.89; 95% CI, 0.83–0.94 [P trend <0.001]). Results were not appreciably altered when we restricted the definition of hypercholesterolemia and hypertension to self‐report and medication use (data not shown), nor when we adjusted for socioeconomic factors (HR comparing the highest with the lowest quintile: 0.88; 95% CI, 0.83–0.94 [P trend <0.001]).
Table 3.
HRs and 95% CIs for Associations Between Quintiles of High School Diet Scores and Diagnosis With Clinical CVD Risk Factors Among Women With No Preexisting Disease
| Model | Q1 | Q2 | Q3 | Q4 | Q5 | P Linear Trend | HR for a 10‐Point Increment |
|---|---|---|---|---|---|---|---|
| Person‐years | 53 654 | 53 808 | 54 754 | 54 211 | 56 156 | ||
| HS‐AHEI, median | 29.9 | 36.7 | 41.6 | 46.5 | 54.2 | ||
| ≥1 clinical CVD risk factor | |||||||
| No. of events | 2399 | 2423 | 2281 | 2386 | 2053 | ||
| Age‐adjusted | 1.0 (ref) | 0.98 (0.93–1.04) | 0.89 (0.84–0.95) | 0.93 (0.88–0.99) | 0.77 (0.73–0.82) | <0.001 | 0.91 (0.89–0.93) |
| Multivariable 1 | 1.0 (ref) | 0.98 (0.93–1.04) | 0.89 (0.84–0.94) | 0.94 (0.89–0.99) | 0.78 (0.73–0.82) | <0.001 | 0.91 (0.89–0.93) |
| Multivariable 2 | 1.0 (ref) | 1.00 (0.94–1.06) | 0.91 (0.86–0.97) | 0.97 (0.92–1.03) | 0.82 (0.77–0.87) | <0.001 | 0.93 (0.91–0.95) |
| Hypercholesterolemiaa | |||||||
| No. of events | 1713 | 1786 | 1628 | 1684 | 1477 | ||
| Age‐adjusted | 1.0 (ref) | 1.01 (0.95–1.08) | 0.89 (0.83–0.95) | 0.92 (0.86–0.98) | 0.79 (0.74–0.85) | <0.001 | 0.91 (0.89–0.93) |
| Multivariable 1 | 1.0 (ref) | 1.02 (0.95–1.09) | 0.89 (0.83–0.96) | 0.92 (0.86–0.99) | 0.80 (0.75–0.86) | <0.001 | 0.91 (0.89–0.94) |
| Multivariable 2 | 1.0 (ref) | 1.03 (0.97–1.11) | 0.91 (0.85–0.98) | 0.96 (0.90–1.03) | 0.84 (0.78–0.90) | <0.001 | 0.93 (0.91–0.96) |
| Hypertensionb | |||||||
| No. of events | 1266 | 1250 | 1200 | 1264 | 1036 | ||
| Age‐adjusted | 1.0 (ref) | 0.95 (0.88–1.03) | 0.90 (0.83–0.98) | 0.95 (0.87–1.02) | 0.76 (0.70–0.82) | <0.001 | 0.91 (0.88–0.94) |
| Multivariable 1 | 1.0 (ref) | 0.95 (0.88–1.03) | 0.89 (0.82–0.96) | 0.94 (0.87–1.02) | 0.75 (0.69–0.82) | <0.001 | 0.91 (0.88–0.93) |
| Multivariable 2 | 1.0 (ref) | 0.96 (0.89–1.04) | 0.91 (0.84–0.99) | 0.97 (0.90–1.05) | 0.80 (0.74–0.87) | <0.001 | 0.93 (0.90–0.96) |
| Type 2 diabetes mellitusc | |||||||
| No. of events | 132 | 143 | 155 | 137 | 107 | ||
| Age‐adjusted | 1.0 (ref) | 1.05 (0.83–1.33) | 1.14 (0.90–1.43) | 0.99 (0.78–1.26) | 0.77 (0.60–1.00) | 0.05 | 0.91 (0.83–1.00) |
| Multivariable 1 | 1.0 (ref) | 1.05 (0.83–1.34) | 1.13 (0.90–1.43) | 0.96 (0.76–1.23) | 0.75 (0.58–0.98) | 0.02 | 0.90 (0.82–0.99) |
| Multivariable 2 | 1.0 (ref) | 1.12 (0.88–1.42) | 1.22 (0.97–1.54) | 1.07 (0.84–1.36) | 0.88 (0.68–1.14) | 0.35 | 0.96 (0.87–1.05) |
N=27 406 (11 542 events of ≥1 clinical cardiovascular disease [CVD] risk factor, 8288 events of hypercholesterolemia alone, 6016 events of hypertension alone, and 674 events of type 2 diabetes mellitus alone). Multivariable model 1=age, body mass index (BMI) at age 18 years (≤18.5, 18.5 to <25, 25 to <30, or ≥30 kg/m2), total energy intake in high school (quintiles, kcal/d), smoking between ages 15 and 19 years (none or 1–4, 5–14, or 15+ cigarettes/d), and high school physical activity (quintiles, metabolic equivalent of task hours [MET]/wk), family history of hypertension (yes/no), and family history of diabetes mellitus (yes/no). Multivariable model 2=model 1+adult energy intake (quintiles, kcal/d), adult smoking status (never, past, current: 1–14 cigarettes/d, ≥15 cigarettes/d), adult physical activity (quintiles, MET/wk), adult oral contraceptive use (never, past, current), postmenopausal hormone use (never, past, current), adult aspirin use (yes/no), and adult alcohol intake (0, 0.1–4.9, 5–14.9, 15–29.9, ≥30 g/d). HR indicates hazard ratio; HS‐AHEI, high school Alternative Healthy Eating Index.
Not adjusted for family history of disease.
Adjusted for family history of hypertension.
Adjusted for family history of diabetes mellitus.
Within each tertile of AHEI‐2010 scores, higher HS‐AHEI scores were associated with a lower risk of developing at least one risk factor (Figure). Women with higher AHEI scores in both high school and adulthood had the lowest risk of developing at least one risk factor compared with women with low scores at both periods, independent of high school or adult confounders (HR, 0.79; 95% CI, 0.74–0.85 [Figure 1]). Further adjustment for adult BMI did not alter this association (HR, 0.83; 95% CI, 0.77–0.89).
Figure 1.
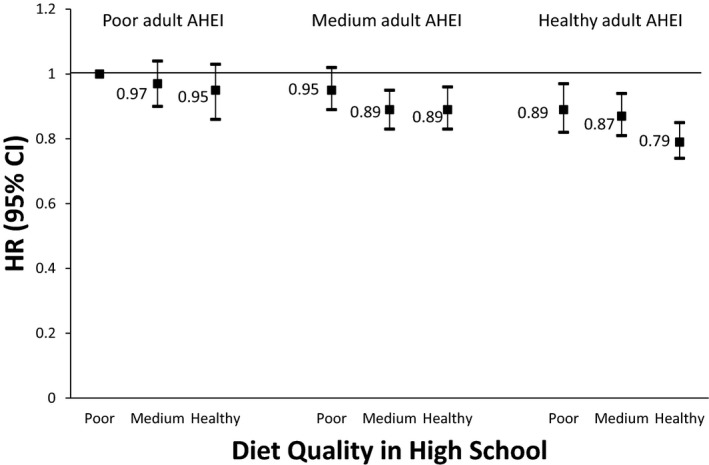
Hazard ratios (HRs) for associations between tertiles of Alternative Healthy Eating Index (AHEI) adherence in high school and adulthood and diagnosis with ≥1 clinical cardiovascular disease (CVD) risk factor. (N=27 406, 11 543 events). Cox proportional hazards model adjusted for age, body mass index at age 18 years (≤18.5, 18.5 to <25, 25 to <30, or ≥30 kg/m2), total energy intake in high school (quintiles, kcal/d), smoking between ages 15 and 19 years (none or 1–4, 5–14, or 15+ cigarettes/d), high school physical activity (quintiles, metabolic equivalent of task hours [MET]/wk), family history of hypertension, family history of diabetes mellitus, adult smoking status (never, past, current: 1–14 cigarettes/d, ≥15 cigarettes/d), adult physical activity (quintiles, MET/wk), adult oral contraceptive use (never, past, current), postmenopausal hormone use (never, past, current), adult energy intake (quintiles, kcal/d), adult alcohol intake (0, 0.1–4.9, 5–14.9, 15–29.9, ≥30 g/d), and adult aspirin use (yes/no).
Higher HS‐AHEI scores were inversely associated with risk of hypercholesterolemia (HR comparing the highest with the lowest quintile: 0.84; 95% CI, 0.78–0.90) and hypertension (HR comparing the highest with the lowest quintile: 0.80; 95% CI, 0.74–0.87) after adjustment for confounders during high school and adulthood (Table 3). Higher HS‐AHEI scores were associated with lower risk of type 2 diabetes mellitus after adjustment for confounders during high school (HR comparing the highest with the lowest quintiles, 0.75; 95% CI, 0.58–0.98). After adjustment for confounders during adulthood, this association was attenuated (HR, 0.88; 95% CI, 0.68–1.14). Adult physical activity was the strongest confounder (not shown).
Incident CVD
Over 13 years of follow‐up, 423 CVD events occurred among 42 112 women at risk of developing CVD. There was a suggestion of an association between HS‐AHEI scores and risk of CVD after adjustment for confounders during high school (HR comparing extreme quintiles: 0.80; 95% CI, 0.59–1.09 [P trend=0.16]) (Table 4). However, this association was attenuated after adjustment for adulthood confounders (HR: 0.92; 95% CI, 0.68–1.25 [P trend=0.68]).
Table 4.
HRs and 95% CIs for Associations Between Quintiles of High School Diet Scores and Risk of CVD
| Model | Q1 | Q2 | Q3 | Q4 | Q5 | P Linear Trend | HR for a 10‐Point Increment |
|---|---|---|---|---|---|---|---|
| No. of events | 89 | 79 | 96 | 80 | 79 | ||
| Age‐adjusted | 1.0 (ref) | 0.84 (0.62–1.14) | 1.01 (0.75–1.34) | 0.82 (0.61–1.11) | 0.80 (0.59–1.08) | 0.16 | 0.92 (0.82–1.03) |
| Multivariable 1 | 1.0 (ref) | 0.84 (0.62–1.14) | 1.02 (0.76–1.36) | 0.82 (0.61–1.11) | 0.80 (0.59–1.09) | 0.18 | 0.92 (0.82–1.04) |
| Multivariable 2 | 1.0 (ref) | 0.88 (0.65–1.19) | 1.07 (0.8–1.43) | 0.91 (0.67–1.23) | 0.92 (0.68–1.25) | 0.68 | 0.98 (0.87–1.1) |
N=42 112 (423 events). Multivariable model 1=age, body mass index at age 18 years (≤18.5, 18.5 to <25, 25 to <30, or ≥30 kg/m2), total energy intake in high school (quintiles, kcal/d), smoking between ages 15 and 19 years (none or 1–4, 5–14, or 15+ cigarettes/d), and high school physical activity (quintiles, metabolic equivalent of task hours [MET]/wk) and family history of myocardial infarction (MI). Multivariable model 2=model 1+adult smoking status (never, past, current: 1–14 cigarettes/d, ≥15 cigarettes/d), adult physical activity (quintiles, MET/wk), adult oral contraceptive use (never, past, current), postmenopausal hormone use (never, past, current), adult energy intake (quintiles, kcal/d), adult aspirin use (yes/no) and adult alcohol intake (0, 0.1–4.9, 5–14.9, 15–29.9, ≥30 g/d). CVD indicates cardiovascular disease; HR hazard ratio; HS‐AHEI, high school Alternative Healthy Eating Index.
Discussion
A high‐quality diet in adolescence, as measured by adherence to the AHEI during high school, is associated with lower risk of developing CVD risk factors during mid‐adulthood. In particular, women with high HS‐AHEI scores had a 16% lower risk of hypercholesterolemia and 20% lower risk of hypertension compared with women with low HS‐AHEI scores. Importantly, women who maintained a high‐quality diet from adolescence to mid‐adulthood had the lowest incidence of risk factor development.
Data on the role of a healthy diet in adolescence and cardiovascular health are sparse. However, results from prior investigations support a high‐quality diet as part of a healthy lifestyle in young (18–30 years)26, 27 and middle adulthood (27–44 years)8 for the maintenance of cardiovascular health. For example, participants in the CARDIA study (Coronary Artery Risk Development in Young Adults) who adhered to a modified Mediterranean diet in young adulthood were at lower risk of developing the metabolic syndrome in middle age.26 Adherence to healthy dietary and lifestyle factors was also associated with lower risk of developing CVD risk factors in adulthood.27 The results of the current study suggest that healthy dietary patterns earlier in life (during adolescence) may also play an important role in the primordial prevention of CVD.
In our study, adolescent diet was strongly related to lower risk of hypercholesterolemia and hypertension, but not with risk of diabetes mellitus or CVD. Similarly, an index based on the 2005 Dietary Guidelines for Americans was associated with favorable changes in blood pressure and high‐density lipoprotein cholesterol, but not with risk of diabetes mellitus, among young adults in the CARDIA study.28 Although higher AHEI scores during adulthood are associated with lower risk of type 2 diabetes mellitus and CVD in adults,6, 7 the “prudent” diet pattern in adolescence was not associated with risk of adulthood diabetes mellitus, either.25 There are several plausible explanations for these discrepant findings. First, dyslipidemia, hypercholesterolemia, and hypertension often precede type 2 diabetes mellitus as a risk factor for CVD.29 Therefore, adolescent diet may play a stronger role early in the CVD development process. Further, dietary modifications after a diagnosis of hypertension or hypercholesterolemia may prevent development of diabetes mellitus and CVD. Additionally, the HS‐AHEI may not encompass all dietary factors relevant for long‐term prevention of type 2 diabetes mellitus, such as dairy intake.30 Similarly, moderate alcohol intake, consistently associated with lower risk of CVD in adults,31 was not included in the HS‐AHEI. Finally, in this relatively young population of women and only 423 cases, we may have lacked sufficient power to detect an association with CVD risk. However, our CIs were broad and contained values of clinically significant benefit (up to 42% lower risk of CVD).
Few adolescents meet all 7 ideal cardiovascular health behaviors identified by the American Heart Association: not smoking, eating a healthy diet, being physically active, having normal weight, and ideal levels of blood pressure, blood glucose, and total cholesterol levels.19 The prevalence of a healthy diet is particularly low, with >80% of US adolescents classified as having a poor diet.32, 33 Similar trends are seen among children younger than 12 years.34 Hypertension, hyperinsulinemia, and dyslipidemia began clustering in childhood in the Bogalusa Heart Study,35 and this cluster tracks through early adulthood. Healthy dietary patterns also track from childhood through adolescence to adulthood and are associated with beneficial vascular changes such as lower arterial stiffness.10, 36, 37 Because hypertension and hypercholesterolemia do not usually present acute symptoms, but do increase risk of CVD,2 prevention of these conditions through a healthy diet from a young age may be particularly beneficial in the primordial prevention of CVD. Further, a life‐course approach to prevention of CVD is considered cost‐effective; thus, the focus on prevention is imperative.38 Additional research is needed to determine whether dietary improvements among children and adolescents can prevent the clustering of preclinical disease into adulthood.
The AHEI favors a diet rich in foods and nutrients that have been shown to be beneficial for cardiovascular health. Few studies have investigated the AHEI pattern on risk of hypertension or hypercholesterolemia directly; however, AHEI components that may play a role in the prevention of hypertension and hypercholesterolemia include polyphenols found in nuts, which may modulate plasma nitric oxide levels to reduce blood pressure39 and improve plaque stability40; dietary fibers in whole grains, fruits, vegetables, and legumes, which lower low‐density lipoprotein cholesterol and improve satiety41; and the reduced sodium content of the diet.42 The components of the AHEI pattern are similar to the components of the DASH and Mediterranean diets and clinical trials of these diet patterns have been effective in reducing blood pressure, low‐density lipoprotein cholesterol, and major cardiovascular events.42, 43
Study Strengths and Limitations
Our study is uniquely suited to investigate adolescent diet in relation to adult disease risk prospectively in a large study population. We followed participants at risk of disease for over 13 years and extensively adjusted for potential confounders. However, there are some potential limitations of our study that warrant discussion. First, while hypercholesterolemia and hypertension were self‐reported, these outcomes have been previously validated.44, 45 Information on high school diet was collected during adulthood and is likely recalled with some error. Given the prospective design of this study, such error is likely nondifferential with respect to the outcome and would bias the results towards the null and underestimate the true association between HS‐AHEI and development of risk factors and CVD. For our results to be overestimated due to recall bias, women with very healthy habits as adults would have had to systematically over‐report the healthfulness of their high school diet. The correlation between adult AHEI and HS‐AHEI was modest and adult AHEI score did not vary across quintiles of HS‐AHEI scores. Therefore, the potential bias of adolescent diet by current dietary habits is likely minimal. The incidence of risk factor development in this population of young health professionals may be lower than the general population and might lead to underassessment of the true association between adolescent diet quality and adult disease. As in all observational studies, residual confounding cannot be excluded. These findings among predominantly white female nurses may not be generalizable to more racially diverse populations or men. Finally, our analysis of incident CVD was consistent with findings for CVD risk factors but was limited by the few cases among these younger women.
Conclusions
A high‐quality diet in adolescence is associated with a significantly lower risk of developing at least one clinical risk factor for CVD, particularly among women who maintained a healthy diet into middle adulthood. Focusing efforts on adopting and maintaining a healthy diet earlier in life may enhance primordial prevention of CVD.
Sources of Funding
This research was funded by a Carlsbergfondet Postdoc Travel Grant; the Danish Ministry of Science, Innovation and Higher Education International Network Programme; the Danish Council for Strategic Research project DIPI (Diet and Prevention of Ischemic Heart Disease: A Translational Approach); and National Institutes of Health grant UM1 CA176726.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003583 doi: 10.1161/JAHA.116.003583)
References
- 1. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 2. Lloyd‐Jones DM, Leip EP, Larson MG, D'Agostino RB, Beiser A, Wilson PW, Wolf PA, Levy D. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–798. [DOI] [PubMed] [Google Scholar]
- 3. Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van HL, Tracy RP, Lloyd‐Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd‐Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA. 2012;308:1795–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Howard G, Banach M, Cushman M, Goff DC, Howard VJ, Lackland DT, McVay J, Meschia JF, Muntner P, Oparil S, Rightmyer M, Taylor HA. Is blood pressure control for stroke prevention the correct goal? The lost opportunity of preventing hypertension. Stroke. 2015;46:1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Koning L, Chiuve SE, Fung TT, Willett WC, Rimm EB, Hu FB. Diet‐quality scores and the risk of type 2 diabetes in men. Diabetes Care. 2011;34:1150–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chomistek AK, Chiuve SE, Eliassen AH, Mukamal KJ, Willett WC, Rimm EB. Healthy lifestyle in the primordial prevention of cardiovascular disease among young women. J Am Coll Cardiol. 2015;65:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwingshackl L, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the dietary approaches to stop hypertension score, and health outcomes: a systematic review and meta‐analysis of cohort studies. J Acad Nutr Diet. 2015;115:780–800. [DOI] [PubMed] [Google Scholar]
- 10. Kaikkonen JE, Mikkila V, Raitakari OT. Role of childhood food patterns on adult cardiovascular disease risk. Curr Atheroscler Rep. 2014;16:443. [DOI] [PubMed] [Google Scholar]
- 11. Linos E, Willett WC, Cho E, Colditz G, Frazier LA. Red meat consumption during adolescence among premenopausal women and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:2146–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20‐year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. [DOI] [PubMed] [Google Scholar]
- 13. Maruti SS, Feskanich D, Colditz GA, Frazier AL, Sampson LA, Michels KB, Hunter DJ, Spiegelman D, Willett WC. Adult recall of adolescent diet: reproducibility and comparison with maternal reporting. Am J Epidemiol. 2005;161:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maruti SS, Feskanich D, Rockett HR, Colditz GA, Sampson LA, Willett WC. Validation of adolescent diet recalled by adults. Epidemiology. 2006;17:226–229. [DOI] [PubMed] [Google Scholar]
- 15. Giovannucci E, Stampfer MJ, Colditz G, Rimm EB, Willett WC. Relationship of diet to risk of colorectal adenoma in men. J Natl Cancer Inst. 1992;84:91–98. [DOI] [PubMed] [Google Scholar]
- 16. Willett WC, Lennart E. Reproducibility and validity of food‐frequency questionnaires In: Willett WC, ed. Nutritional Epidemiology. 3rd ed New York, NY: Oxford University Press; 2013:96–141. [Google Scholar]
- 17. Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–796. [DOI] [PubMed] [Google Scholar]
- 18. Applied Research Program Web site, National Cancer Institute . Sources of Sodium among the US Population, 2005–06. 11‐4‐2014. 23‐5‐2015.
- 19. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van HL, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 20. Report of the expert committee on the diagnosis, classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. [DOI] [PubMed] [Google Scholar]
- 21. Flint AJ, Hu FB, Glynn RJ, Jensen MK, Franz M, Sampson L, Rimm EB. Whole grains and incident hypertension in men. Am J Clin Nutr. 2009;90:493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rose GA, Blackburn H, Gillum RF, Prineas RJ. Cardiovascular Survey Methods. Geneva, Switzerland: World Health Organization; 1982:162–165. WHO Monograph Series [56]. [PubMed] [Google Scholar]
- 23. Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke. 1981;12:I13–I44. [PubMed] [Google Scholar]
- 24. Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–468. [DOI] [PubMed] [Google Scholar]
- 25. Malik VS, Fung TT, van Dam RM, Rimm EB, Rosner B, Hu FB. Dietary patterns during adolescence and risk of type 2 diabetes in middle‐aged women. Diabetes Care. 2012;35:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steffen LM, Van HL, Daviglus ML, Zhou X, Reis JP, Loria CM, Jacobs DR, Duffey KJ. A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Br J Nutr. 2014;112:1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu K, Daviglus ML, Loria CM, Colangelo LA, Spring B, Moller AC, Lloyd‐Jones DM. Healthy lifestyle through young adulthood and the presence of low cardiovascular disease risk profile in middle age: the Coronary Artery Risk Development in (Young) Adults (CARDIA) study. Circulation. 2012;125:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zamora D, Gordon‐Larsen P, He K, Jacobs DR Jr, Shikany JM, Popkin BM. Are the 2005 Dietary Guidelines for Americans Associated With reduced risk of type 2 diabetes and cardiometabolic risk factors? Twenty‐year findings from the CARDIA study. Diabetes Care. 2011;34:1183–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paynter NP, Kiefe CI, Lewis CE, Loria CM, Goff DC, Lloyd‐Jones DM. Accumulation of metabolic cardiovascular risk factors in black and white young adults over 20 years. J Am Heart Assoc. 2015;4:e001548 doi: 10.1161/JAHA.114.001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malik VS, Sun Q, van Dam RM, Rimm EB, Willett WC, Rosner B, Hu FB. Adolescent dairy product consumption and risk of type 2 diabetes in middle‐aged women. Am J Clin Nutr. 2011;94:854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta‐analysis. BMJ. 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang Q, Yuan K, Gregg EW, Loustalot F, Fang J, Hong Y, Merritt R. Trends and clustering of cardiovascular health metrics among U.S. adolescents 1988–2010. J Adolesc Health. 2014;55:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shay CM, Ning H, Daniels SR, Rooks CR, Gidding SS, Lloyd‐Jones DM. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005–2010. Circulation. 2013;127:1369–1376. [DOI] [PubMed] [Google Scholar]
- 34. Ning H, Labarthe DR, Shay CM, Daniels SR, Hou L, Van HL, Lloyd‐Jones DM. Status of cardiovascular health in US children up to 11 years of age: the National Health and Nutrition Examination Surveys 2003–2010. Circ Cardiovasc Qual Outcomes. 2015;8:164–171. [DOI] [PubMed] [Google Scholar]
- 35. Bao W, Srinivasan SR, Wattigney WA, Berenson GS. Persistence of multiple cardiovascular risk clustering related to syndrome X from childhood to young adulthood. The Bogalusa Heart Study. Arch Intern Med. 1994;154:1842–1847. [PubMed] [Google Scholar]
- 36. Mikkila V, Rasanen L, Raitakari OT, Pietinen P, Viikari J. Consistent dietary patterns identified from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. Br J Nutr. 2005;93:923–931. [DOI] [PubMed] [Google Scholar]
- 37. Aatola H, Hutri‐Kahonen N, Juonala M, Laitinen TT, Pahkala K, Mikkila V, Telama R, Koivistoinen T, Lehtimaki T, Viikari JS, Raitakari OT, Kahonen M. Prospective relationship of change in ideal cardiovascular health status and arterial stiffness: the Cardiovascular Risk in Young Finns Study. J Am Heart Assoc. 2014;3:e000532 doi: 10.1161/JAHA.113.000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weintraub WS, Daniels SR, Burke LE, Franklin BA, Goff DC Jr, Hayman LL, Lloyd‐Jones D, Pandey DK, Sanchez EJ, Schram AP, Whitsel LP. Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation. 2011;124:967–990. [DOI] [PubMed] [Google Scholar]
- 39. Medina‐Remon A, Tresserra‐Rimbau A, Pons A, Tur JA, Martorell M, Ros E, Buil‐Cosiales P, Sacanella E, Covas MI, Corella D, Salas‐Salvado J, Gomez‐Gracia E, Ruiz‐Gutierrez V, Ortega‐Calvo M, Garcia‐Valdueza M, Aros F, Saez GT, Serra‐Majem L, Pinto X, Vinyoles E, Estruch R, Lamuela‐Raventos RM. Effects of total dietary polyphenols on plasma nitric oxide and blood pressure in a high cardiovascular risk cohort. The PREDIMED randomized trial. Nutr Metab Cardiovasc Dis. 2015;25:60–67. [DOI] [PubMed] [Google Scholar]
- 40. Sala‐Vila A, Romero‐Mamani ES, Gilabert R, Nunez I, de la Torre R, Corella D, Ruiz‐Gutierrez V, Lopez‐Sabater MC, Pinto X, Rekondo J, Martinez‐Gonzalez MA, Estruch R, Ros E. Changes in ultrasound‐assessed carotid intima‐media thickness and plaque with a Mediterranean diet: a substudy of the PREDIMED trial. Arterioscler Thromb Vasc Biol. 2014;34:439–445. [DOI] [PubMed] [Google Scholar]
- 41. Satija A, Hu FB. Cardiovascular benefits of dietary fiber. Curr Atheroscler Rep. 2012;14:505–514. [DOI] [PubMed] [Google Scholar]
- 42. Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER III, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–2464. [DOI] [PubMed] [Google Scholar]
- 43. Estruch R, Ros E, Salas‐Salvado J, Covas MI, Corella D, Aros F, Gomez‐Gracia E, Ruiz‐Gutierrez V, Fiol M, Lapetra J, Lamuela‐Raventos RM, Serra‐Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez‐Gonzalez MA. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. [DOI] [PubMed] [Google Scholar]
- 44. Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. [DOI] [PubMed] [Google Scholar]
- 45. Forman JP, Curhan GC, Taylor EN. Plasma 25‐hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52:828–832. [DOI] [PMC free article] [PubMed] [Google Scholar]


