MON1/CCZ1-mediated vacuolar transport of tapetum degradation-related cysteine proteases affects tapetal programmed cell death and pollen development in Arabidopsis.
Abstract
Programmed cell death (PCD)-triggered degradation of plant tapetum is essential for microspore development and pollen coat formation; however, little is known about the cellular mechanism regulating tapetal PCD. Here, we demonstrate that Rab7-mediated vacuolar transport of tapetum degradation-related cysteine proteases is crucial for tapetal PCD and pollen development in Arabidopsis (Arabidopsis thaliana), with the following evidence: (1) The monensin sensitivity1 (mon1) mutants, which are defective in Rab7 activation, showed impaired male fertility due to a combined defect in both tapetum and male gametophyte development. (2) In anthers, MON1 showed preferential high level expression in tapetal cell layers and pollen. (3) The mon1 mutants exhibited delayed tapetum degeneration and tapetal PCD, resulting in abnormal pollen coat formation and decreased male fertility. (4) MON1/CALCIUM CAFFEINE ZINC SENSITIVITY1 (CCZ1)-mediated Rab7 activation was indispensable for vacuolar trafficking of tapetum degradation-related cysteine proteases, supporting that PCD-triggered tapetum degeneration requires Rab7-mediated vacuolar trafficking of these cysteine proteases. (5) MON1 mutations also resulted in defective pollen germination and tube growth. Taken together, tapetal PCD and pollen development require successful MON1/CCZ1-mediated vacuolar transport in Arabidopsis.
Proper pollen development is essential for successful reproduction of flowering plants. The development of fertile pollen requires nutritive contributions from the surrounding cell layers (Xie et al., 2014; Zhang et al., 2014). The tapetum is of particular importance in this regard by providing proteins, lipids, and other materials. Through proper programmed cell death (PCD)-mediated tapetum degeneration, the tapetum provides proteins and lipids for the development of microspores into mature pollen grains (Varnier et al., 2005; Ito et al., 2007; Phan et al., 2011; McCormick, 2013). Premature or delayed tapetal PCD results in sterile pollen due to disruption of nutrient supply (Ku et al., 2003; Millar and Gubler, 2005; Kawanabe et al., 2006; Vizcay-Barrena and Wilson, 2006; W. Zhang et al., 2006). Proper tapetal PCD is regulated by a set of transcription factors as well as their downstream proteolytic enzymes, including aspartic and Cys proteases, which play important roles in the execution of plant tapetal PCD (Li et al., 2006; Yang et al., 2007; Xu et al., 2010; Richau et al., 2012; Niu et al., 2013). Many of these proteolytic enzymes are activated in an acid pH environment and perform their function in tapetal PCD (Lam and del Pozo, 2000; Kuroyanagi et al., 2002; Hatsugai et al., 2004; Zhang et al., 2014). Despite the importance of these proteolytic enzymes to plant reproduction, little is known about the cellular basis in regulating their transport and contribution to tapetal PCD and pollen development.
In animals, the PCD is executed by caspases (Cys-containing Asp-specific proteases). In plants, several studies suggest that proteolytic enzymes, including aspartic and Cys proteases, have caspase-like activity and function in PCD, although plants have no direct homologs to animal caspases (Solomon et al., 1999; Lam and del Pozo, 2000; Hatsugai et al., 2004; Hatsugai et al., 2015). In Arabidopsis (Arabidopsis thaliana), Cys proteases are synthesized in the endoplasmic reticulum (ER) as the proprotein precursor form, and self-catalytically converted into the mature form in an acidic environment such as the vacuole (Kuroyanagi et al., 2002; Hatsugai et al., 2004; Gu et al., 2012; Zhang et al., 2014; Hatsugai et al., 2015; Chung et al., 2016). It has been speculated that the maturation process of these Cys proteases occurs in vacuoles during the PCD. For example, the mature form of VPE (vacuolar processing enzyme) released from ruptured vacuoles initiates the proteolytic cascade that leads to PCD in the plant immune response as well as tissue development (Hatsugai et al., 2015). Although PCD has been well studied for its essential function in plant development and defense response, the role of vacuoles and vacuolar transport in regulating PCD remains elusive (Hatsugai et al., 2009, 2015; Hatsugai and Hara-Nishimura, 2010).
In plant cells, the vacuole often occupies almost 90% of cellular volume in mature tissue cells and plays diverse cellular functions (Rojo et al., 2001). Vacuolar transport of proteins is tightly controlled by a series of machinery proteins, including Rab GTPases, which function as key molecular switches to coordinate vesicular transport (Cui et al., 2016). Previous studies showed that activation of Rab7 by the MONENSIN SENSITIVITY1 (MON1)/CALCIUM CAFFEINE ZINC SENSITIVITY1 (CCZ1) complex is essential for prevacuolar compartment (PVC)-to-vacuole trafficking and plant growth (Cui et al., 2014; Ebine et al., 2014; Singh et al., 2014). Here, we further demonstrate that Rab7-mediated vacuolar trafficking of tapetum degradation-related Cys proteases plays important roles in proper tapetal PCD, pollen coat formation, and plant reproduction. This study has thus unveiled a previously unidentified regulatory mechanism of Rab7-mediated vacuolar transport in regulating tapetal PCD and pollen development in plants.
RESULTS
Abnormal Deposition of Pollen Coat Materials Occurs in mon1 Mutants
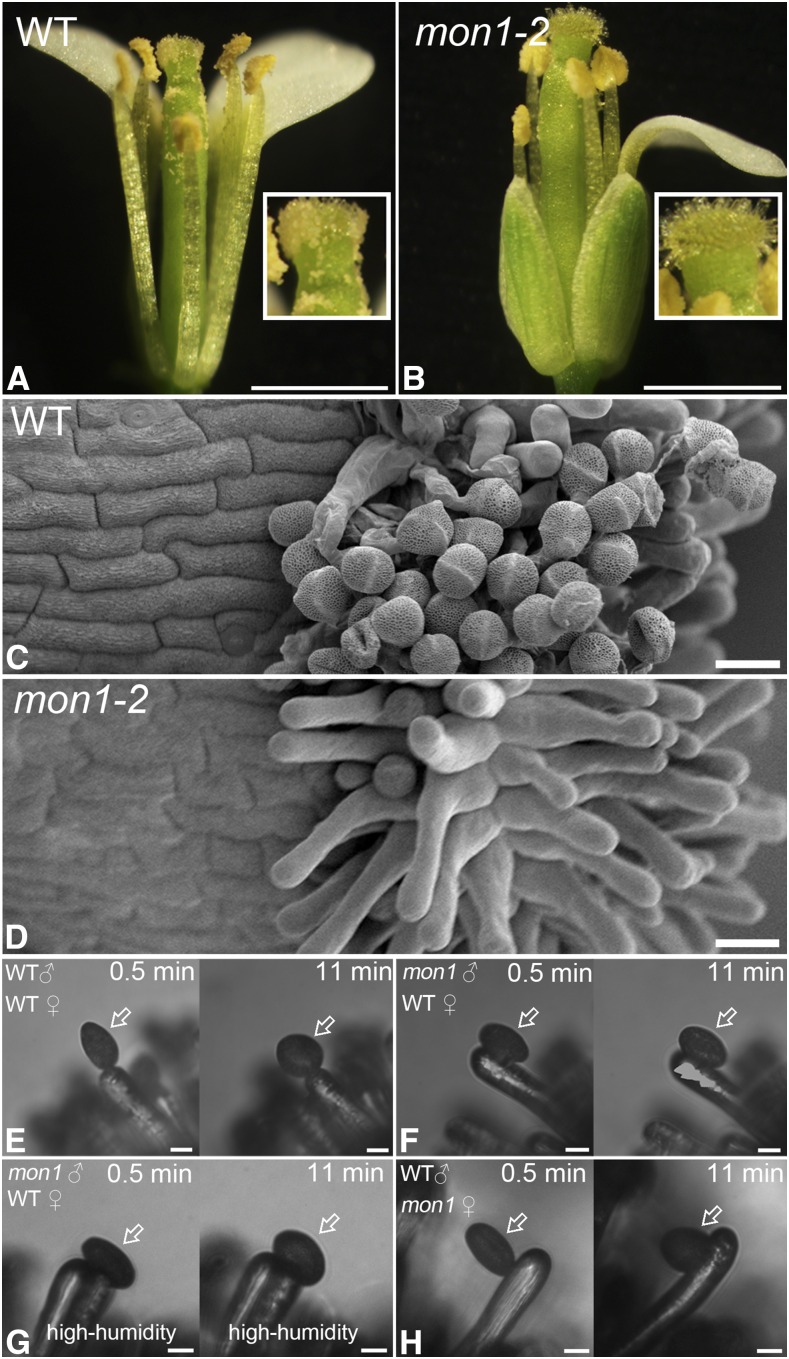
We recently found two MON1 knockout lines, mon1-1 and mon1-2, showing a dwarf phenotype (Supplemental Fig. S1, A and B; Cui et al., 2014). In this report, we further identify and characterize male sterile phenotype of mon1, which was caused by defective pollen coat formation based on the following evidence: (1) The mon1 mutants exhibited reduced fertility as indicated by nonelongated siliques, inside which no mature embryo was formed, and this phenotype was recovered by GFP-MON1 back transfer (Fig. 1, A–H; Supplemental Fig. S1, C–E). (2) A substantial portion (65%) of mature pollen from mon1 homozygous mutants showed irregular pollen coat patterns and a deformed shape, compared with the wild type, heterozygous mon1+/−, and GFP-MON1/mon1-2−/− (Fig. 1, I–L; Supplemental Fig. S2). This suggests a function of MON1 in pollen coat formation. Further transmission electron microscopy (TEM) analysis using pollen grains at the same developmental stages also showed that the pollen coats of mon1-2−/− have defects compared with the wild type, mon1-2+/−, and GFP-MON1/mon1-2−/− (Fig. 1, M–P). (3) In mon1 mutants, due to the pollen coat defects, fewer mature pollen grains were released from the anther and adhered to the stigma (Fig. 2, A–D; Supplemental Fig. S1, F and G). The reduced fertility was not caused by the shorter filaments observed in mon1 mutant, because compared with the wild type, fewer pollen grains from mon1-2 were attached to stigmas when hand-pollinated to wild-type stigma (Supplemental Fig. S1F). (4) Compared with the wild type (90%), fewer pollen grains from mon1 (50%) showed complete hydration at 11 min after pollination on wild-type stigmatic papillae (Fig. 2, E and F), indicating a defective hydration process in mon1 pollen grains. High humidity partially rescued this defect of mon1 (Fig. 2G). The mon1 stigmatic papillae showed no defect for accepting wild-type pollen (Fig. 2H). (5) The floral organs of mon1-2 mutants were around 25% smaller compared with the wild type but showed no obvious flower developmental defect (Supplemental Fig. S3, A–D). Using Alexander and 4′,6-diamidino-2-phenylindole (DAPI) staining, no difference was observed regarding pollen viability between the wild type and mon1-2 (Supplemental Fig. S3, E–H), indicating that MON1 did not regulate flower development and pollen viability. Taken together, these results demonstrated that MON1 is required for pollen coat formation and plant reproduction.
Figure 1.
The mon1 mutants exhibit defects in pollen coat formation, causing decreased male fertility. A to C, Impaired fertility in mon1 was indicated by nonelongated siliques of representative primary inflorescences. Nonelongated siliques were found in mon1-2 mutants (B). Bars = 1 cm. D to G, Nonelongated siliques from mon1-2 mutants did not contain any mature embryos. Bars = 1 mm. H, Significant decrease of mon1 fertility indicated by quantitative analysis of silique developmental phenotype. I to P, SEM and TEM images showed mon1-2−/− mutants (K and O) had abnormal pollen coat compared with the wild type (I and M), mon1-2+/− (J and N), and GFP-MON1/mon1-2−/− (L and P). The arrows in K and O indicate abnormal coat formation in mon1-2−/− mutants. Bars = 10 μm in I to L and 2 μm in M to P. WT, Wild type.
Figure 2.
Defective pollen coats of mon1 result in decreased pollen adhesion to the stigma and a slower pollen hydration process. A to D, Fewer pollen grains were released and attached to the stigma in the mon1 mutant as shown by light and SEM imaging. Bars = 1 mm in A and B and 20 μm in C and D. E to H, Compared with the wild type (E), pollen (indicated by arrows) from the mon1 mutant (F) showed a slower hydration process when pollinated on the wild-type stigmatic papilla under low-humidity conditions (<40%). High humidity (>80%) partially rescued this defect of mon1 (G). Wild-type pollen showed a normal hydration process when pollinated on the mon1 stigmatic papilla under low-humidity conditions (H). Bars = 10 μm. WT, Wild type.
Preferential Expression of MON1 in Tapetal Cell Layers Is Essential for Tapetum Degeneration
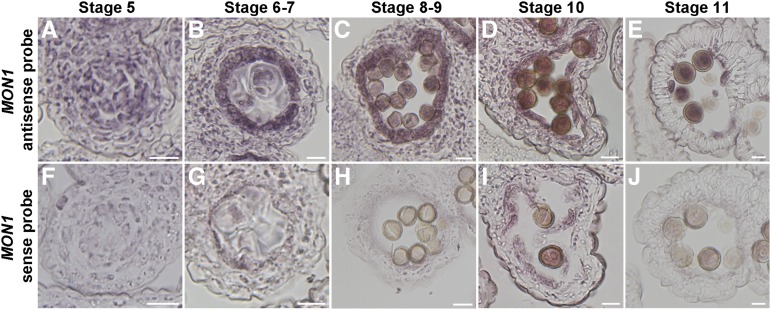
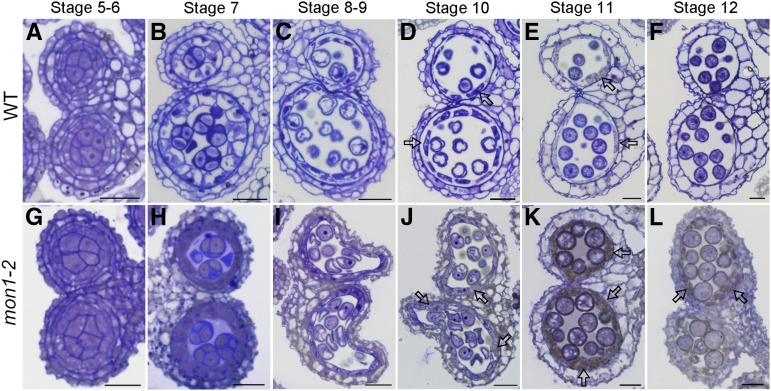
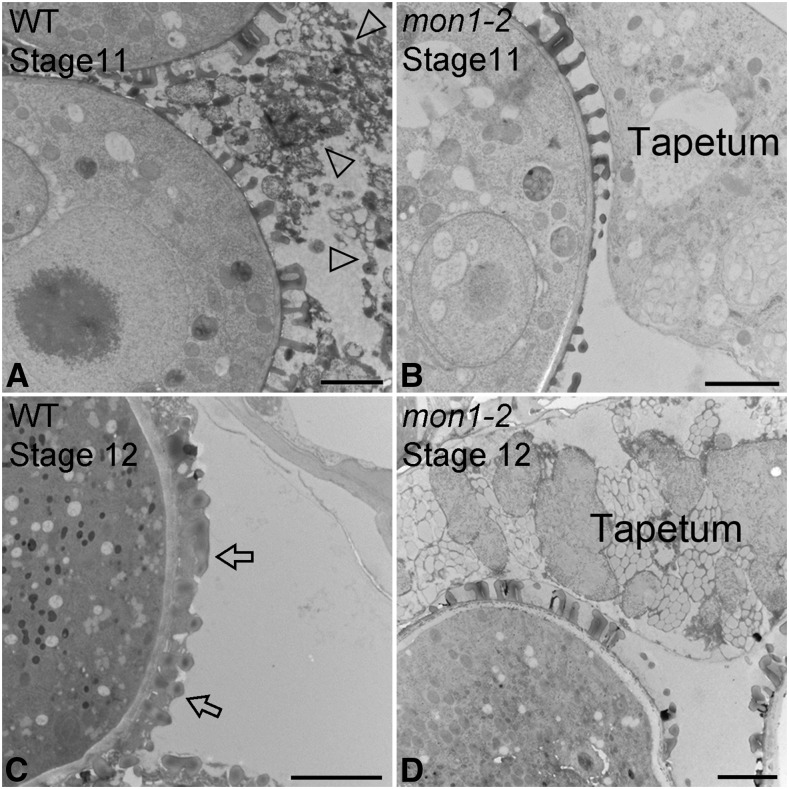
In plants, production of viable pollen in anther locules depends on both the proper development of microspores and tapetum degeneration that provides materials for pollen coat formation (Li et al., 2006; Ito et al., 2007; Yang et al., 2007; Zhang et al., 2014). Here, we have discovered that expression of MON1 in the tapetal cell layers is essential for tapetum degeneration. The Arabidopsis genome encodes one MON1. Based on the Arabidopsis eFP Browser, MON1 showed relative high expression in various organs and tissues, including the flower and pollen (Supplemental Fig. S4; Winter et al., 2007). RNA in situ hybridization analysis showed that MON1 is highly expressed in the tapetal cell layers during anther developmental stages 5 to 11 as well as in pollen grains (Fig. 3). The specific expression of MON1 in tapetum suggests that MON1 was involved in pollen coat formation, while MON1 expression in pollen grains indicates a function in pollen germination and tube growth. To further define MON1 function in pollen coat formation, we performed histological analyses using wild-type and mon1-2 anthers at stages 5 to 12. Compared with the wild type, delayed tapetum degeneration was observed in mon1-2 mutants from stages 10 to 12 (Fig. 4). Wild-type tapetal cells began to shrink at stage 10 and gradually degenerate into a thin and broken layer at stage 11, and disappeared completely at stage 12 (Fig. 4, d–f). However, mon1 tapetum remained intact and became slightly hypertrophic until stage 11, and at stage 12 a thin and broken layer of tapetum was still visible (Fig. 4, K and L). TEM analysis also showed a delayed degeneration of tapetal cells in mon1 compared with the wild type, suggesting the notion that MON1 is involved in tapetum degeneration (Fig. 5).
Figure 3.
Preferential expression of MON1 in tapetum and pollen. High expression of MON1 in tapetum and pollen was detected by RNA in situ hybridization in anthers at different development stages as indicated using MON1 antisense (A–E) and MON1 sense (F–J) probes. All sections are transverse. The pink-purple color indicates the hybridization signals. The strong signals were detected in tapetum and pollen grains from stage 5 to 11 (A–E). Control hybridizations with sense probes for MON1 do not show any signals above background (F–J). Bars = 10 μm.
Figure 4.
MON1 mutation results in delayed tapetum degeneration. Semithin sections showed delayed tapetum degeneration in mon1-2 compared with the wild type (WT). Transverse semithin sections of wild-type (A–F) and mon1-2 (G–L) anthers at indicated stages were represented. The tapetal cells were completely degenerated at stage 12 in the wild type (F); however, residue from degenerating tapetal cells remained at stage 12 in mon1-2 (L). The arrows in D, E, and J to L indicate tapetal cells. Bars = 20 μm.
Figure 5.
The mon1-2 shows delayed tapetum degeneration in TEM analysis. High-pressure frozen/freeze-substituted anthers at stages 11 and 12 were examined. TEM images of 70-nm-thick sections showed close-up views of tapetal cells and pollen coat structures in the wild type (WT; A and C) and mon1-2 (B and D). The arrowheads in A indicate the degradation of tapetal cells in the wild type. The arrow in C shows the fully packed pollen coat in the wild type. Bars = 2 μm.
The MON1 Mutation Causes Delayed PCD in Tapetal Cells
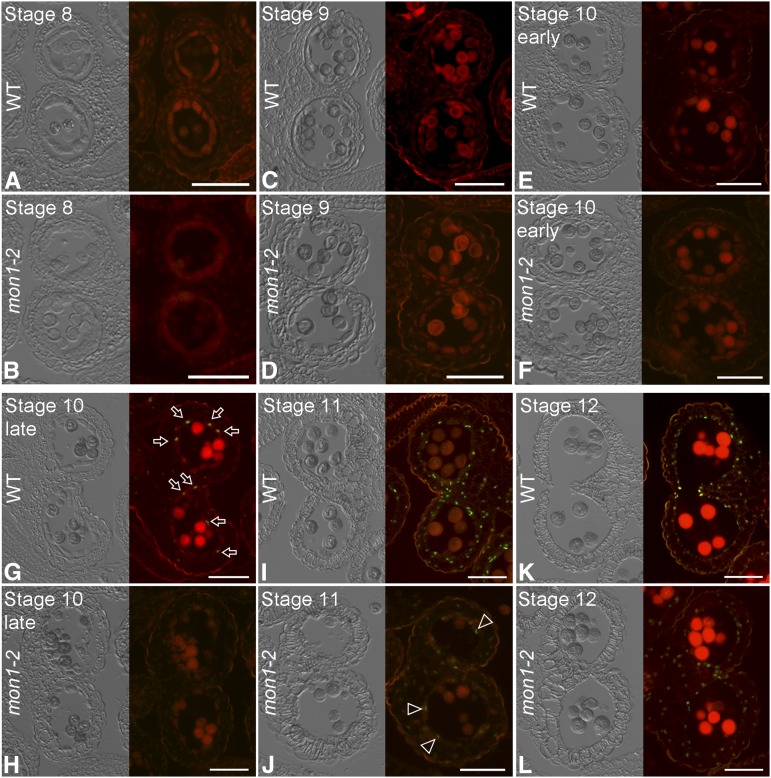
Tapetum degeneration relies mainly on tapetal PCD, which consists of sequential cytological events, including shrinkage of cytoplasm and nuclear, fragmentation of DNA, rupture of the vacuole, and ER swelling (Wilson and Zhang, 2009; Chang et al., 2011). The delayed tapetum degeneration in mon1 may be due to a delayed tapetal PCD. To test this hypothesis, we performed terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assays on wild-type and mon1 anthers at stages 8 to 12, in which positive signals indicate cells that undergo massive DNA fragmentation (Fig. 6). In the wild type, tapetal cells undergo PCD for tapetum degeneration from late stage 10 (Fig. 6G; Xie et al., 2014). By contrast, in the mon1 mutant, weak TUNEL-positive signals began to be detected at stage 11 and 12, showing delayed PCD in mon1 (Fig. 6, J and L). No TUNEL-positive signal was detected before stage 10 in the wild-type and mon1 mutants (Fig. 6, A–F). Tapetum-specific organelles, including the tapetosomes and elaioplasts, showed no difference in the wild type and mon1, excluding the possible involvement of MON1 in their biogenesis (Supplemental Fig. S5, B and C; Quilichini et al., 2014). These results suggest that MON1 is crucial for the proper timing of tapetal PCD. To know whether MON1 dependent PCD is general, we have performed the root cap PCD experiments according to the previous study (Fendrych et al., 2014). The results showed no significant difference between the wild type and mon1 in root cap PCD process (Supplemental Fig. S6). This led us to conclude that the MON1-mediated PCD is not involved in the root cap PCD process.
Figure 6.
MON1 loss of function results in delayed tapetal PCD. Delayed PCD was detected in mon1 compared with the wild type (WT) using the TUNEL assay. A to L, Fluorescence microscopy of DNA fragmentation detected using the TUNEL assays in sections of wild-type and mon1-2 anthers at indicated stages. Tapetal PCD did not occur until stage 11 (J) in mon1 as indicated by a weak TUNEL signal (indicated by arrowheads), while in the wild type the tapetal PCD occurred from late stage 10 (G). The arrows indicate examples of the TUNEL signals showing up in wild-type tapetal cell layers at late stage 10. Bars = 50 μm.
Tapetal PCD Requires Cys Protease Transport to the Vacuole
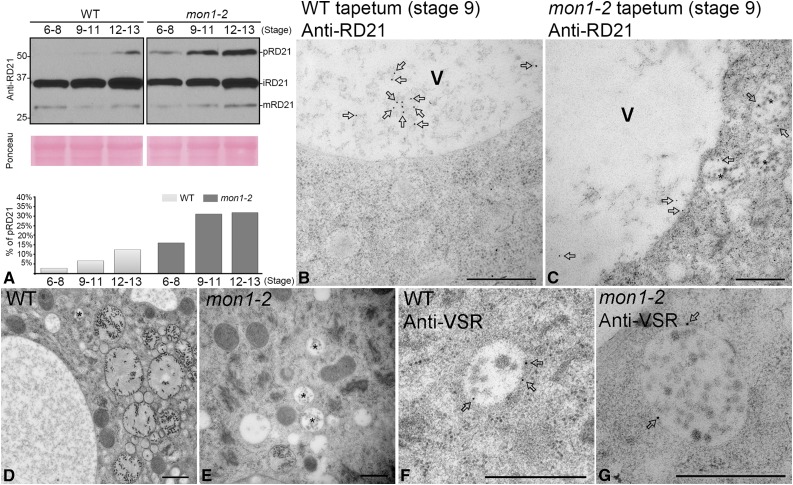
The observation of enlarged PVCs in mon1 tapetal cells indicates that vacuolar transport may contribute to tapetal PCD (Fig. 7). Several Cys proteases, including RD21, RD19, RDL1, and AT4G32940, were reported to show coordinated down-regulation of expression in the ms1 or ams mutants and were speculated to play significant roles in tapetal PCD (Yang et al., 2007; Xu et al., 2010). MON1/CCZ1-mediated Rab7 activation contributes to vacuolar transport of most soluble cargo proteins, including storage proteins and lytic vacuole markers (Cui et al., 2014; Ebine et al., 2014; Singh et al., 2014). We thus hypothesized that MON1/CCZ1-mediated vacuolar transport plays a key role in the regulation of maturation of tapetum degradation-related Cys proteases. To test this hypothesis, we analyzed vacuolar processing of Cys protease RD21 using flower buds from the wild type and mon1 mutants. RD21 was detected as three forms: proform (pRD21), intermediate isoform (iRD21); and mature RD21 (mRD21; Hayashi et al., 2001; Gu et al., 2012). pRD21 showed more accumulation in mon1-2 mutants than in the wild type, supporting that MON1 loss of function inhibited maturation of newly synthesized proteases, which is likely caused by inhibited vacuolar trafficking of RD21 in mon1 (Fig. 7A). We speculate that MON1/CCZ1-mediated Rab7 activation is essential for RD21 transport to the vacuole, which provides an acidic environment for enzyme maturation. Further immunogold TEM analysis showed that the Cys protease RD21 accumulated in enlarged PVCs at stage 9 in mon1-2 tapetal cells. By contrast, in the wild type, RD21 successfully reached the vacuole, supporting that vacuolar transport of RD21 was essential for protease maturation, which was inhibited in mon1 tapetum (Fig. 7, B and C). The high and low magnification images and quantitative analysis of PVCs in the wild type and mon1 mutant showed that the size of PVCs in mon1 is significantly enlarged compared with the wild type (Fig. 7, D–G; Supplemental Fig. S5A).
Figure 7.
The mon1 mutant has impaired vacuolar processing of Cys protease RD21 with accumulated proform. A, Immunoblot and quantitative analysis of total anther protein extracts from the wild type (WT) and mon1-2 at indicated stages with the antibody against Cys protease RD21 showed that the proform of RD21 accumulated in mon1-2. RD21 was detected as three forms: proform (pRD21), intermediate isoform (iRD21), and mature RD21 (mRD21). B and C, Immunogold electron microscopy images show that at stage 9, Cys protease RD21 accumulated in enlarged PVCs (indicated by stars) in mon1-2 (C), while in wild type RD21 mainly reached the vacuole for processing (B). The arrows indicate the labeled gold particles using anti-RD21. V, Vacuole. Bars = 500 nm. D to G, TEM images showed PVC morphology in wild-type (D and E) and mon1-2 (F and G) tapetum. PVCs were labeled by anti-VSR (E and G). The stars indicate the PVCs. The arrows indicate the labeled gold particles using anti-VSR. Bars = 500 nm.
Vacuolar Trafficking of Tapetum Degeneration-Related Cys Proteases Requires Rab7 Activation by MON1/CCZ1 Complex
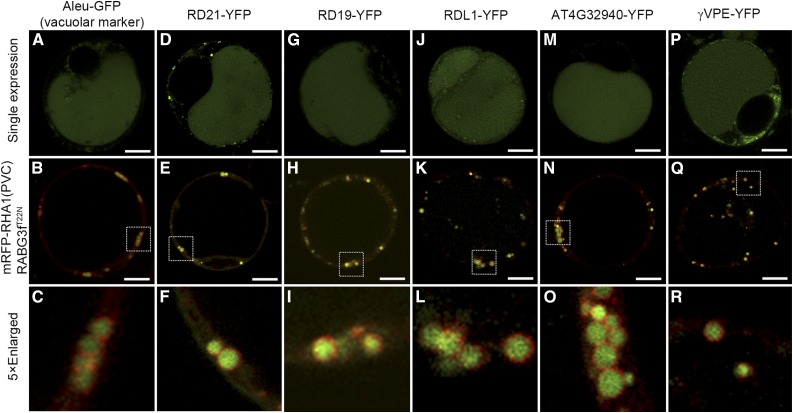
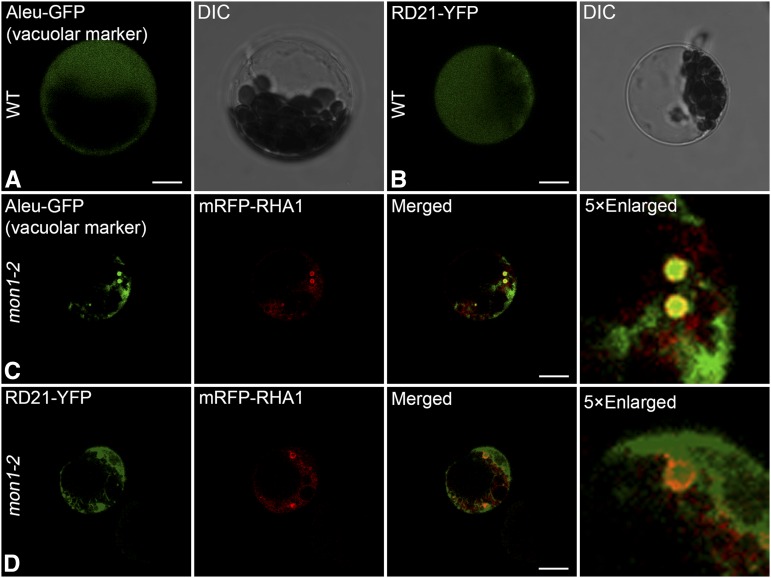
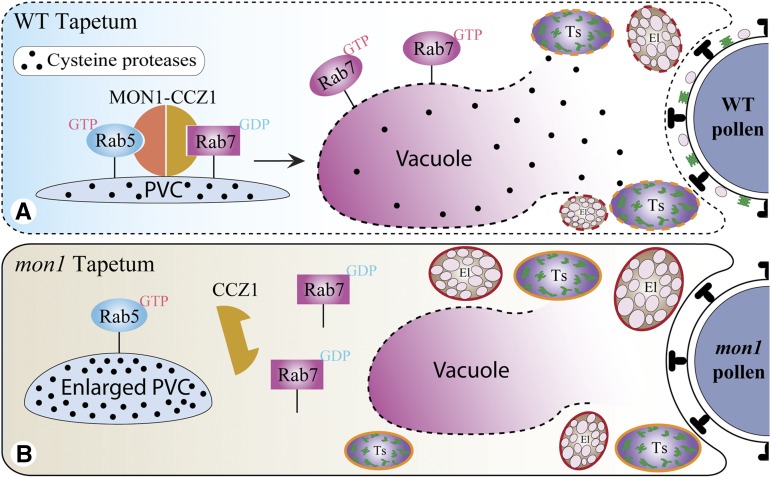
In Arabidopsis protoplasts, vacuolar trafficking of the soluble vacuolar marker Aleu-GFP was inhibited when coexpressed with dominant negative Rab7 (Fig. 8, A–C). When expressed individually in Arabidopsis protoplasts, RD21 showed a vacuolar pattern; however, when coexpressed with the dominant negative Rab7, the vacuolar trafficking of RD21 was inhibited as they were accumulated inside the enlarged PVCs (Fig. 8, D–F). These results demonstrate that vacuolar trafficking of the Cys protease RD21 requires Rab7 activation by MON1/CCZ1. In addition, we further tested three other Cys proteases (RD19, RDL1, and AT4G32940), which were down-regulated in ms1 or ams mutants, as well as a putative Cys protease γVPE, which was reported to participate in PCD for the plant immune response and proposed to be involved in reproductive development (Hatsugai et al., 2004; Yang et al., 2007; Xu et al., 2010; Hatsugai et al., 2015). Here, single expression of these Cys proteases, including RD19, RDL1, AT4G32940 and γVPE, all showed vacuole localization; however, when coexpressed with dominant negative Rab7, the vacuolar trafficking of these Cys proteases was inhibited (Fig. 8, G–R). We have also performed the transient expression in leaf protoplasts of the wild type and mon1. The results consistently showed that vacuolar transport of Aleu-GFP and RD21-GFP was dependent on MON1, similar to the situation when coexpressing with the dominant negative Rab7 mutant (Fig. 9). These results further support the conclusion that dominant negative Rab7 has equal effect as MON1 mutation. In summary, based on these results we propose that tapetal PCD requires successful vacuolar transport of Cys proteases through RAB5/RAB7 sequential action on PVCs. In wild-type tapetum, mature enzymes were released and triggered the tapetal PCD after vacuole rupture. Tapetal cell contents, including the tapetosomes and elaioplasts, then contributed to the pollen coat formation (Fig. 10A). However, MON1 mutation inhibited vacuolar transport of Cys proteases and their maturation, resulting in delayed tapetal PCD and abnormal pollen coat formation (Fig. 10B).
Figure 8.
Dominant negative Rab7 mutant (RABG3fT22N) inhibits the vacuolar trafficking of tapetum degradation-related Cys proteases as well as soluble vacuolar marker. Vacuolar transport of soluble vacuolar marker (A–C) and Cys proteases (D–R) as indicated was inhibited as they were accumulated inside the enlarged PVCs when coexpressed with RABG3fT22N in protoplasts. Images were collected using a confocal microscope from cells at 12 to 18 h after transfection. Bars = 10 μm.
Figure 9.
In mon1-2 mutant leaf protoplasts, vacuolar trafficking of soluble vacuolar marker and RD21-YFP was inhibited. Aleu-GFP (A) and RD21-YFP (B) showed vacuolar pattern in wild-type (WT) leaf protoplasts. However, in mon1-2 mutant protoplasts, Aleu-GFP (C) and RD21-YFP (D) showed inhibited vacuolar transport and were accumulated in the enlarged PVCs as indicated by the PVC marker mRFP-RHA1. Images were collected using a confocal microscope from cells at 12 to 18 h after transfection. Bars = 10 μm. DIC, Differential interference contrast.
Figure 10.
Working model for the essential role of MON1/CCZ1-mediated Rab7 activation in regulating tapetal PCD. Tapetal PCD requires Rab7-mediated vacuolar transport of tapetum degradation-related Cys proteases. Vacuole rupture releases mature Cys proteases, which triggers the PCD in wild-type (WT) tapetum. The cell contents, including the tapetosomes and elaioplasts, then contribute to the formation of pollen coats (A). However, MON1 mutation inhibits vacuolar transport of Cys proteases and their maturation, resulting in delayed tapetal PCD and formation of abnormal pollen coats (B). Ts, Tapetosome; El, elaioplast.
MON1 Mutation Results in Defective Pollen Germination and Tube Growth
Proper pollen germination and tube growth are a prerequisite of double fertilization and seed formation in flowering plants (Lu et al., 2011; Dresselhaus and Franklin-Tong, 2013; Duan et al., 2014; Dresselhaus et al., 2016). To further test whether pollen coat defects would lead to reduced pollen germination and tube growth, we perform the in vitro and in vivo pollen germination assay. The germination rate of the pollen grains in vitro decreased significantly in homozygous mon1-2 (46% ± 9%) compared with that in the wild type (85% ± 2%), heterozygous mon1-2 (75% ± 6%), and GFP-MON1/mon1-2 (82% ± 3%), suggesting that MON1 regulates pollen germination (Supplemental Fig. S7). MON1 also regulates pollen tube growth because in vivo aniline blue staining on emasculated wild-type pistils hand-pollinated with pollen grains from either the wild type or mon1-2 showed decreased pollen tube growth of mon1. At 9 h after pollination (HAP), wild-type pollen tubes reached to the middle part of a pistil, while pollen tubes of mon1 just emerged from the style (Supplemental Fig. S8, A and B). At 12 HAP, most wild-type pollen tubes had reached to the bottom of a pistil (Supplemental Fig. S8C). By contrast, pollen tubes of mon1 just reached the middle part of a pistil (Supplemental Fig. S8D). The decreased pollen tube growth of mon1 could be caused by either pollen coat defects or pollen defects or both. To find out, we performed reciprocal crosses between mon1+/− and the wild type. Pollen transmission rate of mon1-1 (mon1-1+/−:wild type = 42:62) and mon1-2 (mon1-2+/−:wild type = 44:60) were slightly lower than expected (1:1), while female gametophytes were not affected (Supplemental Table S1). In addition, limited pollination assay with limited pollen grains from the wild type, mon1+/−, or mon1−/− manually pollinated onto emasculated wild-type pistils showed lower ratio of fertilization of ovules from mon1−/− (65%) compared with the wild type (95%) and mon1+/− (89%). When limited pollen grains from the wild type were manually pollinated onto emasculated the wild type or mon1−/− pistils, they showed comparable ratio of fertilization of ovules from wild-type (95%) and mon1−/− (91%) pistils. These reciprocal crosses further support that the reduced fertility in mon1 mutant is caused by MON1 function in pollen coat formation and pollen development. Taken together, these results suggest that abnormal pollen coats in mon1 contribute mainly to the decreased pollen germination and tube growth. MON1 showed high expression in both tapetum and pollen, indicating MON1 function in both tapetal cell layers and mature pollen grains (Fig. 3). Previous studies show that proper endomembrane trafficking is essential for pollen tube growth (de Graaf et al., 2005; Zhang et al., 2010; Zhang and McCormick, 2010; Li et al., 2013; Zhao et al., 2013). Our results also showed impaired vacuolar trafficking in mon1 mutant pollen tubes. In wild-type germinated pollen tube, MON1 showed a punctate pattern and Rab7 (RABG3f) localized to endosomes and tonoplast, similar to that in roots (Supplemental Fig. S9, A–F; Cui et al., 2014; Ebine et al., 2014; Singh et al., 2014). In mon1 germinated pollen tube, the GFP-RABG3f lost its tonoplast pattern and showed punctate dots, which were separated with the mCherry-RHA1 positive PVCs, indicating that in mon1 mutant pollen tubes protein transport between PVCs and the vacuole was also inhibited, which may also contribute to the pollen tube growth defect in mon1 (Supplemental Fig. S9, G and H).
DISCUSSION
In plants, tapetal PCD is a prerequisite for normal pollen development and successful reproduction (Xie et al., 2014; Zhang et al., 2014; Dresselhaus et al., 2016). Through tapetal PCD process, tapetum provides its cellular contents, including lipids and proteins, for pollen coat formation (Zhang et al., 2014). In the past decades, much has been known about plant PCD. Distinct from animals, most mutant plants with single-gene mutations of conserved PCD components are viable, indicating the regulations of plant PCD are usually quantitative or are regulated in response to both developmental cues and environmental signals (Bozhkov and Lam, 2011). Recently, promoter-reporter lines, including PASPA3, RNS3, SCPL48, CEP1, and DMP4, have been successfully generated for visualizing cells ready for developmentally regulated PCD, which will largely facilitate the temporal and spatial observation of plant PCD process during development (Olvera-Carrillo et al., 2015). However, the underlying cellular mechanisms in regulating tapetal PCD remain elusive. Here, we provide evidence demonstrating that MON1-mediated PVC-to-vacuole trafficking is crucial for tapetal PCD and pollen development (Fig. 10). Previous studies in Arabidopsis show that MON1-mediated Rab7 activation is essential for PVC-to-vacuole trafficking and plant growth. MON1 forms a MON1/CCZ1 GEF complex to bind and activate Rab7. Plants without functional MON1 contain enlarged PVCs and show abnormal vacuolar trafficking, resulting in arrested plant development and growth (Cui et al., 2014; Ebine et al., 2014; Singh et al., 2014). In this study, we observed abnormal pollen coats in both mon1-1 and mon1-2 mutants, which were recovered in GFP-MON1/mon1 plants (Fig. 1, I–P; Supplemental Fig. S2). In mon1 mutants, abnormal pollen coats caused reduced adhesion to stigma and a slower hydration process, resulting in reduced fertility as mon1 exhibited shorter or nonelongated siliques (Figs. 1, A–H, and 2; Supplemental Fig. S1). Increased humidity partially overcame the hydration defects of mon1 pollen (Fig. 2G) when pollen hydration experiments were performed under high humidity condition, further supporting the conclusion that abnormal mon1 pollen coat caused reduced hydration process. These phenotypic observations suggest that MON1 also functions in pollen coat formation and pollen development. The results from pollination experiments using wild-type pollen and mon1 mutant stigmas (Fig. 2H) showed no decreased stigma receptivity of mon1 compared with the wild type, which is consistent with the conclusion that reduced mon1 fertility was not due to decreased stigma receptivity. Shorter filaments were observed in mon1 mutant but were not the cause for the fertility problem, because (1) when hand-pollinated to wild-type stigma, mon1-2 mutant pollen grains showed less attachment to wild-type stigma (Supplemental Fig. S1F), and (2) mon1 pollen grains showed significant reduction (mon1 65% versus wild type 95%) in fertilization when we performed the limited pollination assay using wild-type stigma. Indeed, RNA in situ hybridization detected preferential expression of MON1 in tapetal cell layers as well as pollen in developed anthers (Fig. 3), indicating MON1 may function in tapetum. In mon1 mutants, significantly delayed tapetal PCD resulted in delayed tapetum degeneration, supporting the notion that MON1 is involved in regulating tapetal PCD (Figs. 4, 5, and 6). In the early stages, we also observed that the development of middle layer and endothecium was compromised in mon1 mutant (Fig. 4). Endothecial cells, together with epidermal cells, provide structural support to the anther development. The observed defect in endothecial cells may also contribute to the reduced male fertility in mon1, but the precise role of the middle layer remains unknown. The delayed tapetal PCD is the major reason for the defective mon1 pollen coat formation because tapetum directly provides materials for pollen coat formation. Thus, we conclude that MON1-mediated vacuolar trafficking regulates tapetal PCD.
Tapetum PCD is usually triggered by developmental cues for pollen coat formation (Van Hautegem et al., 2015; Van Durme and Nowack, 2016). Proper timing of tapetal PCD depends on tightly transcriptional regulation of downstream genes by key tapetum-specific transcription factors, such as MS1 and AMS (Yang et al., 2007; Xu et al., 2010). Cys proteases acting downstream of these transcription factors have been identified as executor in tapetal PCD (Zhang et al., 2014). Vacuolar transport of proteases has been postulated to be essential for their function in PCD, yet with little evidence (Zhang et al., 2014). The mon1 tapetal cells contained enlarged PVCs (Fig. 7, D–G, Supplemental Fig. S5A), suggesting that defective PVC-to-vacuole transport may contribute to delayed tapetal PCD in mon1. Usually plant PCD relies on a tightly regulated amount of PCD executors, including proteases (Van Durme and Nowack, 2016). Such factors like RD21 were kept inactive until they reached an acidic environment in the vacuole for maturation and activation (Shindo et al., 2012). Here, through analyzing the vacuolar processing of tapetum degradation-related Cys proteases in mon1 mutant, we revealed a cellular basis that MON1-mediated vacuolar transport of these Cys proteases is essential for proper tapetal PCD (Fig. 10). Considerably decreased vacuolar processing of Cys protease RD21 was detected in mon1 anther, supporting that complete vacuolar transport machinery is in need for RD21 maturation (Fig. 7A). Abnormal accumulation of RD21 in enlarged PVCs in mon1 further supports that vacuolar processing of RD21 relies on MON1-mediated PVC-to-vacuole trafficking (Fig. 7, B and C). In Arabidopsis, there are nine members in RD21 or RD21A-LIKE PROTEASE1 subfamily. Two previous independent reports using two individual RD21 null mutants found that single mutant showed no obvious developmental phenotype (Gu et al., 2012; Shindo et al., 2012). It is possible that single mutation of individual has no phenotype due to their functional redundancy. In addition to RD21, vacuolar transport of other putative Cys proteases, including RD19, RDL1, and AT4G32940 that were down-regulated in ms1 or ams mutants, depends on MON1-mediated Rab7 activation (Fig. 8, A–O). Notably, vacuolar transport of another putative Cys protease γVPE, which was reported to function in plant immune response PCD, also requires MON1-mediated Rab7 activation (Fig. 8, P–R). Similar inhibited vacuolar transport of RD21 and Aleu-GFP was observed in mon1 mutant protoplast, further supporting that dominant negative Rab7 has equal effect as MON1 mutation (Fig. 9). Different from the above Cys proteases, CEP1 is a KDEL-tailed Cys protease, and cep1 mutant is male sterile due to defective tapetal PCD (Zhang et al., 2014). Previous studies showed two different localization patterns of CEP1 in different tissues. CEP1 showed ER pattern in transgenic Arabidopsis roots (Höwing et al., 2014) but vacuole pattern in tapetal cells (Zhang et al., 2014). Due to the tissue-specific localization pattern of CEP1, the possible effects of mon1 on the vacuolar transport of CEP1 in tapetum can be done in future research using transgenic Arabidopsis plants expressing GFP-tagged CEP1 driven by tapetum-specific promoter in the wild type and mon1. Our study herein suggests a general cellular mechanism that PCD requires successful vacuolar transport system. During tracheary element differentiation, Rab7 is essential for this developmentally regulated PCD process (Kwon et al., 2010). Autophagic cell death has been implicated as a conserved mechanism for both animal and plant PCD (Avila-Ospina et al., 2014). As MON1/CCZ1-mediated Rab7 activation is conserved among eukaryotes, this study will extend our knowledge about the functional roles of Rab7-mediated vacuolar transport in regulating PCD in different species in an evolutionary perspective.
In addition to tapetal cell layers, MON1 also showed preferential expression in pollen (Fig. 3), indicating that MON1 may function in pollen development, germination, and tube growth. Regarding Alexander and DAPI staining results, no significant difference between the wild type and mon1 was observed, indicating the normal pollen viability of mon1 mutants (Supplemental Fig. S3, E–H). On the other hand, in vitro and in vivo pollen germination assay using mon1 pollen with abnormal pollen coats from mon1 homozygous mutants showed reduced pollen germination and tube growth (Supplemental Figs. S7 and S8). The results from the manual pollination and germination assay (Supplemental Fig. S8) did not fully phenocopy the reduced mon1 pollen release and pollen attachment as observed in the natural condition (Fig. 2, A–D), which may be due to the following reasons: (1) As shown in Figure 2B, fewer pollen grains were observed in mon1 mutant. This can be caused by reduced pollen release, reduced pollen attachment, or both. Indeed, when manually pollinated onto wild-type stigma in a limited fashion, fewer mon1 pollen grains were attached (Supplemental Fig. S1F), supporting that MON1 mutation affects the pollen grain release and attachment process. (2) For the in vivo germination assay in Supplemental Figure S8, to visualize the pollen tube growth, excessive amounts of mon1 pollen grains were pollinated onto the wild-type stigmas, which increased the possibility of pollen capture and germination on these stigmas. Since not many pollen grains exist in the mutant plant without manual pollination, a reduced pollen attachment phenotype was thus visible in the mutant.
In mon1 pollen tubes (Supplemental Fig. S9, G and H), Rab7 showed cytosolic pattern and separated with Rab5, indicating that Rab5- and Rab7-mediated vacuolar transport was inhibited in mon1 pollen tube. However, homozygous mon1 mutants (46% ± 9%) showed decreased germination rate compared with the wild type (85% ± 2%), heterozygous mon1 mutants (75% ± 6%), and GFP-MON1/mon1-2−/− (82% ± 3%) in vitro (Supplemental Fig. S7), suggesting the possible gametophytic effect of MON1 mutation, which may contribute to the mon1 phenotype as a minor reason. In plant cells, multiple vacuolar trafficking pathways, including the MON1-mediated pathway, exist (Cui et al., 2014; Ebine et al., 2014; Singh et al., 2014). Thus, other vacuolar trafficking pathways may also compensate the inhibitory effect of MON1 mutation in mediating pollen germination and pollen tube growth.
To distinguish as much as possible tapetum/sporophyte versus gametophyte MON1 effect, we have tried our best in this study to provide strong supporting evidence using different methods. We found that (1) mon1 homozygous mutant showed reduced ability in pollen grain release, attachment, rehydration, germination, and pollen tube growth. The above phenotypes are from combined defects in tapetum and gametophyte development. (2) mon1 heterozygous mutants, containing normal pollen coat, only show a slightly reduced ability in pollen germination in vitro. This indicates the possible gametophytic effect of MON1 mutation, which may contribute to the mon1 phenotype as a minor reason. Tissue-specific complementation of MON1 function in tapetum versus pollen will provide more precise information about MON1 function in tapetum and pollen in future study.
MON1 may function in other types of PCD. To find out whether MON1-mediated PCD is general or specific to tapetum, we performed the root cap PCD experiments (Supplemental Fig. S6) as described previously (Fendrych et al., 2014). The results showed no significant difference between the wild type and mon1 in root cap PCD process, indicating that MON1-mediated PCD is not involved in the root cap PCD process. More experiments need to be carried out in the future to learn whether MON1 participates in other types of PCD.
In conclusion, through the mechanistic study of the phenotypic change in mon1 mutants, we demonstrated that MON1-mediated timely vacuolar transport is essential for tapetal PCD and pollen development (Fig. 10). Our study sheds new light on the underlying mechanism of how Rab7-mediated vacuolar transport contributes to tapetal PCD and pollen development in plants.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The T-DNA insertion line (SALK_075382) of mon1-1 was obtained from the Arabidopsis Biological Resource Center (Alonso et al., 2003). The Ds transposon line (54-4894-1) of mon1-2 was obtained from RIKEN (Sakurai et al., 2005). The Arabidopsis (Arabidopsis thaliana) WAVE lines expressing YFP (WAVE 1), mCherry-RHA1 (WAVE 7), and YFP-VAMP711 (WAVE 9) were obtained from the Nottingham Arabidopsis Stock Centre (Geldner et al., 2009). To generate GFP-RABG3f, GFP-RABG3fQ67L, and GFP-RABG3fT22N transgenic plants, the corresponding constructs were introduced into Agrobacterium tumefaciens and transformed into wild-type Arabidopsis plants by the floral dip method (X. Zhang et al., 2006). Seedlings were grown on standard Murashige and Skoog growth medium supplemented with 1% Suc at 22°C under a long-day (16 h light/8 h dark) photoperiod.
Plasmid Construction
Detailed primer sequences are listed in Supplemental Table S2. For the GFP-RABG3f, GFP-RABG3fQ67L, and GFP-RABG3fT22N constructs, GFP fusions of Rab7 mutants were cloned into the pBI121 backbone (under the UBQ10 promoter). For the following transient expression constructs, RD21-YFP, RD19-YFP, RDL1-YFP, AT4G32940-YFP, and γVPE-YFP, the individual gene was amplified and cloned into the pBI221 backbone for construction of the YFP fusion. All constructs were confirmed by restriction mapping and DNA sequencing.
Pollen Analysis
To detect pollen viability, mature pollen grains were collected from fully opened flowers and then stained with Alexander or DAPI dye before capturing images with a Nikon E80i fluorescent microscope. Pollen in vitro germination and in vivo analysis were performed as described previously (Samuel et al., 2009; Xie et al., 2014; H. Wang et al., 2016). For pollen hydration analysis, pistils were cut and attached on a slide. Individual pollen from the wild type or mon1-2 mutants was put on a wild-type stigmatic papilla using an Eppendorf micromanipulator system. The images were captured by a Leica confocal microscope with a CCD camera. The experiments were repeated more than three times. For limited pollination assay, less than 30 pollen grains from the wild type or mon1-1 were put onto the emasculated wild-type pistil. At 54 HAP, the number of fertilized ovules was calculated under a fluorescent microscope.
Scanning Electron Microscopy
Pollen grains were collected from freshly dehisced anthers and then mounted on scanning electron microscopy (SEM) stubs. After 3 h of air-drying, the pollen grains were coated with palladium-gold in a sputter coater (S150B; Edwards) and examined by SEM (S-3400N; Hitachi) at an acceleration voltage of 10 kV.
Semithin Sections
For semithin sections, anthers at various developmental stages were prefixed and embedded as described previously (Xie et al., 2014). Semithin sections of 2 mm were cut using a UC6 ultramicrotome (Leica) and stained with 1% toluidine blue O (Sigma-Aldrich). The images were captured by a Leica DM 6000 B microscope.
RNA in Situ Hybridization
In situ hybridization was carried out as described previously (Xie et al., 2014). In brief, inflorescences of wild-type plants were fixed in formaldehyde solution overnight at 4°C. After dehydration, the samples were embedded in Paraplast (Sigma-Aldrich). The 305 bp MON1 fragment was amplified with the primer pair MON1-Forward/MON1-Reverse. Sense and antisense probes were transcribed in vitro with digoxigenin-UTP by SP6 and T7 RNA polymerases, respectively. The 8 μm tissue sections were hybridized with 1.0 ng/mL probes at 42°C overnight in a hybridization solution. Digoxigenin antibodies were applied to detect hybridization signals. The images were captured by an Olympus BX51 microscope.
TUNEL Assay
The general TUNEL assay was performed as described previously (Fendrych et al., 2014; Xie et al., 2014; Zhang et al., 2014). In situ nick-end labeling of nuclear DNA fragmentation was carried out with a TUNEL apoptosis detection kit (DeadEnd Fluorometric TUNEL system; Promega). Images were captured by a confocal laser scanning microscope (Zeiss).
Protein Preparation and Immunoblot Analysis
To prepare total proteins, buds at various developmental stages were collected and ground in liquid nitrogen and extracted with the lysis buffer containing 25 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1% SDS, and 1× Complete Protease Inhibitor Cocktail (Roche). For immunoblot analysis, RD21 antibody was used at a dilution of 1:1000. The percentage of pRD21 (pRD21/(pRD21 + iRD21 + mRD21)) was calculated according to the intensity of immunoblot bands, which was measured by ImageJ program as described previously (Lin et al., 2015).
Transient Expression and Confocal Microscopy Images
Maintenance of Arabidopsis suspension-cultured cells and transient expression methods were described previously (Miao and Jiang, 2007; Woo et al., 2015; Zeng et al., 2015; Shen et al., 2016; X. Wang et al., 2016). The general procedures for transient gene expression analysis in leaf protoplast were performed essentially as described previously (Yoo et al., 2007). Confocal images were collected at 12 to 18 h after transformation using a Leica TCS SP8 confocal microscope. Images were processed using Adobe Photoshop as described previously (Miao and Jiang, 2007; Zhao et al., 2015; Zhuang et al., 2015; J. Wang et al., 2016).
Transmission Electron Microscopy Study
The general procedures for TEM sample preparation, thin sectioning, and immunogold labeling were performed essentially as described previously (Tse et al., 2004; Gao et al., 2014, 2015). For high-pressure freezing, anthers at various developmental stages were frozen in a high-pressure freezing machine (EM HPM100; Leica). Ultrathin sections (70 nm) were obtained with a UC7 ultramicrotome (Leica), and immunogold labeling was performed with antibodies against VSR (40 mg/mL) and RD21 (dilution 1:50) and gold-coupled secondary antibody at a 1:50 dilution (Hayashi et al., 2001; Cui et al., 2014). The sections were poststained with aqueous uranyl acetate/lead citrate, and images were captured with a Hitachi H7650 transmission electron microscope (Hitachi High-Technologies) operating at 80 kV.
Accession Numbers
The Arabidopsis Genome Initiative locus identifiers for the genes mentioned in this article are MON1 (At2g28390), RABG3f (At3g18820), RD21 (AT1G47128), RD19 (AT4G39090), RDL1 (AT4G36880), and γVPE (AT4G32940).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. MON1 is essential for normal plant growth and reproduction.
Supplemental Figure S2. MON1 mutation causes defective pollen coat formation.
Supplemental Figure S3. The mon1 mutants show normal floral organs and pollen viability.
Supplemental Figure S4. Arabidopsis eFP Browser report shows MON1 gene expression in different plant organs and tissues.
Supplemental Figure S5. The mon1 tapetum shows normal tapetosomes and elaioplasts, but enlarged PVCs, in TEM analysis.
Supplemental Figure S6. MON1 mutation does not significantly alter the PCD in root cap.
Supplemental Figure S7. MON1 mutation causes defective pollen germination in vitro.
Supplemental Figure S8. The mon1-2 mutants show retarded pollen tube growth in vivo.
Supplemental Figure S9. Loss of function of MON1 impairs the Rab5-to-Rab7 transition on PVCs in germinated pollen tubes, resulting in their retarded growth.
Supplemental Table S1. MON1 mutation results in defective male transmission.
Supplemental Table S2. Primers used in this study.
Supplementary Material
Acknowledgments
We thank Professor Ikuko Hara-Nishimura (Kyoto University, Kyoto, Japan) for providing us with RD21 antibody.
Glossary
- PCD
programmed cell death
- PVC
prevacuolar compartment
Footnotes
This work was supported by grants from the Research Grants Council of Hong Kong (CUHK465112, 466613, CUHK2/CRF/11G, C4011-14R, HKUST10/CRF/12R, HKUST12/CRF/13G, and AoE/M-05/12), NSFC/RGC (N_CUHK406/12), the National Natural Science Foundation of China (31270226 and 31470294), the Chinese Academy of Sciences-Croucher Funding Scheme for Joint Laboratories, and Shenzhen Peacock Project (KQTD201101) to L.J., and National Natural Science Foundation of China (31471304) to Y.Z.
Articles can be viewed without a subscription.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Avila-Ospina L, Moison M, Yoshimoto K, Masclaux-Daubresse C (2014) Autophagy, plant senescence, and nutrient recycling. J Exp Bot 65: 3799–3811 [DOI] [PubMed] [Google Scholar]
- Bozhkov PV, Lam E (2011) Green death: revealing programmed cell death in plants. Cell Death Differ 18: 1239–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Wang Y, Wang S, Ma H (2011) Molecular control of microsporogenesis in Arabidopsis. Curr Opin Plant Biol 14: 66–73 [DOI] [PubMed] [Google Scholar]
- Chung KP, Zeng Y, Jiang L (2016) COPII paralogs in plants: functional redundancy or diversity? Trends Plant Sci 21: 758–769 [DOI] [PubMed] [Google Scholar]
- Cui Y, Shen J, Gao C, Zhuang X, Wang J, Jiang L (2016) Biogenesis of plant prevacuolar multivesicular bodies. Mol Plant 9: 774–786 [DOI] [PubMed] [Google Scholar]
- Cui Y, Zhao Q, Gao C, Ding Y, Zeng Y, Ueda T, Nakano A, Jiang L (2014) Activation of the Rab7 GTPase by the MON1-CCZ1 complex is essential for PVC-to-vacuole trafficking and plant growth in Arabidopsis. Plant Cell 26: 2080–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf BH, Cheung AY, Andreyeva T, Levasseur K, Kieliszewski M, Wu HM (2005) Rab11 GTPase-regulated membrane trafficking is crucial for tip-focused pollen tube growth in tobacco. Plant Cell 17: 2564–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresselhaus T, Franklin-Tong N (2013) Male-female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol Plant 6: 1018–1036 [DOI] [PubMed] [Google Scholar]
- Dresselhaus T, Sprunck S, Wessel GM (2016) Fertilization mechanisms in flowering plants. Curr Biol 26: R125–R139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q, Kita D, Johnson EA, Aggarwal M, Gates L, Wu HM, Cheung AY (2014) Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat Commun 5: 3129. [DOI] [PubMed] [Google Scholar]
- Ebine K, Inoue T, Ito J, Ito E, Uemura T, Goh T, Abe H, Sato K, Nakano A, Ueda T (2014) Plant vacuolar trafficking occurs through distinctly regulated pathways. Curr Biol 24: 1375–1382 [DOI] [PubMed] [Google Scholar]
- Fendrych M, Van Hautegem T, Van Durme M, Olvera-Carrillo Y, Huysmans M, Karimi M, Lippens S, Guérin CJ, Krebs M, Schumacher K, et al. (2014) Programmed cell death controlled by ANAC033/SOMBRERO determines root cap organ size in Arabidopsis. Curr Biol 24: 931–940 [DOI] [PubMed] [Google Scholar]
- Gao C, Luo M, Zhao Q, Yang R, Cui Y, Zeng Y, Xia J, Jiang L (2014) A unique plant ESCRT component, FREE1, regulates multivesicular body protein sorting and plant growth. Curr Biol 24: 2556–2563 [DOI] [PubMed] [Google Scholar]
- Gao C, Zhuang X, Cui Y, Fu X, He Y, Zhao Q, Zeng Y, Shen J, Luo M, Jiang L (2015) Dual roles of an Arabidopsis ESCRT component FREE1 in regulating vacuolar protein transport and autophagic degradation. Proc Natl Acad Sci USA 112: 1886–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N, Dénervaud-Tendon V, Hyman DL, Mayer U, Stierhof YD, Chory J (2009) Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J 59: 169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Shabab M, Strasser R, Wolters PJ, Shindo T, Niemer M, Kaschani F, Mach L, van der Hoorn RA (2012) Post-translational regulation and trafficking of the granulin-containing protease RD21 of Arabidopsis thaliana. PLoS One 7: e32422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsugai N, Hara-Nishimura I (2010) Two vacuole-mediated defense strategies in plants. Plant Signal Behav 5: 1568–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsugai N, Iwasaki S, Tamura K, Kondo M, Fuji K, Ogasawara K, Nishimura M, Hara-Nishimura I (2009) A novel membrane fusion-mediated plant immunity against bacterial pathogens. Genes Dev 23: 2496–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsugai N, Kuroyanagi M, Yamada K, Meshi T, Tsuda S, Kondo M, Nishimura M, Hara-Nishimura I (2004) A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 305: 855–858 [DOI] [PubMed] [Google Scholar]
- Hatsugai N, Yamada K, Goto-Yamada S, Hara-Nishimura I (2015) Vacuolar processing enzyme in plant programmed cell death. Front Plant Sci 6: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Yamada K, Shimada T, Matsushima R, Nishizawa NK, Nishimura M, Hara-Nishimura I (2001) A proteinase-storing body that prepares for cell death or stresses in the epidermal cells of Arabidopsis. Plant Cell Physiol 42: 894–899 [DOI] [PubMed] [Google Scholar]
- Höwing T, Huesmann C, Hoefle C, Nagel MK, Isono E, Hückelhoven R, Gietl C (2014) Endoplasmic reticulum KDEL-tailed cysteine endopeptidase 1 of Arabidopsis (AtCEP1) is involved in pathogen defense. Front Plant Sci 5: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Nagata N, Yoshiba Y, Ohme-Takagi M, Ma H, Shinozaki K (2007) Arabidopsis MALE STERILITY1 encodes a PHD-type transcription factor and regulates pollen and tapetum development. Plant Cell 19: 3549–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanabe T, Ariizumi T, Kawai-Yamada M, Uchimiya H, Toriyama K (2006) Abolition of the tapetum suicide program ruins microsporogenesis. Plant Cell Physiol 47: 784–787 [DOI] [PubMed] [Google Scholar]
- Ku S, Yoon H, Suh HS, Chung YY (2003) Male-sterility of thermosensitive genic male-sterile rice is associated with premature programmed cell death of the tapetum. Planta 217: 559–565 [DOI] [PubMed] [Google Scholar]
- Kuroyanagi M, Nishimura M, Hara-Nishimura I (2002) Activation of Arabidopsis vacuolar processing enzyme by self-catalytic removal of an auto-inhibitory domain of the C-terminal propeptide. Plant Cell Physiol 43: 143–151 [DOI] [PubMed] [Google Scholar]
- Kwon SI, Cho HJ, Jung JH, Yoshimoto K, Shirasu K, Park OK (2010) The Rab GTPase RabG3b functions in autophagy and contributes to tracheary element differentiation in Arabidopsis. Plant J 64: 151–164 [DOI] [PubMed] [Google Scholar]
- Lam E, del Pozo O (2000) Caspase-like protease involvement in the control of plant cell death. Plant Mol Biol 44: 417–428 [DOI] [PubMed] [Google Scholar]
- Li N, Zhang DS, Liu HS, Yin CS, Li XX, Liang WQ, Yuan Z, Xu B, Chu HW, Wang J, et al. (2006) The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell 18: 2999–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhou LZ, Feng QN, McCormick S, Zhang Y (2013) The C-terminal hypervariable domain targets Arabidopsis ROP9 to the invaginated pollen tube plasma membrane. Mol Plant 6: 1362–1364 [DOI] [PubMed] [Google Scholar]
- Lin Y, Ding Y, Wang J, Shen J, Kung CH, Zhuang X, Cui Y, Yin Z, Xia Y, Lin H, et al. (2015) Exocyst-Positive Organelles and Autophagosomes Are Distinct Organelles in Plants. Plant Physiol 169: 1917–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Chanroj S, Zulkifli L, Johnson MA, Uozumi N, Cheung A, Sze H (2011) Pollen tubes lacking a pair of K+ transporters fail to target ovules in Arabidopsis. Plant Cell 23: 81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S. (2013) Pollen. Curr Biol 23: R988–R990 [DOI] [PubMed] [Google Scholar]
- Miao Y, Jiang L (2007) Transient expression of fluorescent fusion proteins in protoplasts of suspension cultured cells. Nat Protoc 2: 2348–2353 [DOI] [PubMed] [Google Scholar]
- Millar AA, Gubler F (2005) The Arabidopsis GAMYB-like genes, MYB33 and MYB65, are microRNA-regulated genes that redundantly facilitate anther development. Plant Cell 17: 705–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu N, Liang W, Yang X, Jin W, Wilson ZA, Hu J, Zhang D (2013) EAT1 promotes tapetal cell death by regulating aspartic proteases during male reproductive development in rice. Nat Commun 4: 1445. [DOI] [PubMed] [Google Scholar]
- Olvera-Carrillo Y, Van Bel M, Van Hautegem T, Fendrych M, Huysmans M, Simaskova M, van Durme M, Buscaill P, Rivas S, S Coll N, et al. (2015) A conserved core of programmed cell death indicator genes discriminates developmentally and environmentally induced programmed cell death in plants. Plant Physiol 169: 2684–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan HA, Iacuone S, Li SF, Parish RW (2011) The MYB80 transcription factor is required for pollen development and the regulation of tapetal programmed cell death in Arabidopsis thaliana. Plant Cell 23: 2209–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilichini TD, Douglas CJ, Samuels AL (2014) New views of tapetum ultrastructure and pollen exine development in Arabidopsis thaliana. Ann Bot (Lond) 114: 1189–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richau KH, Kaschani F, Verdoes M, Pansuriya TC, Niessen S, Stüber K, Colby T, Overkleeft HS, Bogyo M, Van der Hoorn RA (2012) Subclassification and biochemical analysis of plant papain-like cysteine proteases displays subfamily-specific characteristics. Plant Physiol 158: 1583–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo E, Gillmor CS, Kovaleva V, Somerville CR, Raikhel NV (2001) VACUOLELESS1 is an essential gene required for vacuole formation and morphogenesis in Arabidopsis. Dev Cell 1: 303–310 [DOI] [PubMed] [Google Scholar]
- Sakurai T, Satou M, Akiyama K, Iida K, Seki M, Kuromori T, Ito T, Konagaya A, Toyoda T, Shinozaki K (2005) RARGE: a large-scale database of RIKEN Arabidopsis resources ranging from transcriptome to phenome. Nucleic Acids Res 33: D647–D650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Chong YT, Haasen KE, Aldea-Brydges MG, Stone SL, Goring DR (2009) Cellular pathways regulating responses to compatible and self-incompatible pollen in Brassica and Arabidopsis stigmas intersect at Exo70A1, a putative component of the exocyst complex. Plant Cell 21: 2655–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Gao C, Zhao Q, Lin Y, Wang X, Zhuang X, Jiang L (2016) AtBRO1 functions in ESCRT-I complex to regulate multivesicular body protein sorting. Mol Plant 9: 760–763 [DOI] [PubMed] [Google Scholar]
- Shindo T, Misas-Villamil JC, Hörger AC, Song J, van der Hoorn RA (2012) A role in immunity for Arabidopsis cysteine protease RD21, the ortholog of the tomato immune protease C14. PLoS One 7: e29317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Krüger F, Beckmann H, Brumm S, Vermeer JE, Munnik T, Mayer U, Stierhof YD, Grefen C, Schumacher K, et al. (2014) Protein delivery to vacuole requires SAND protein-dependent Rab GTPase conversion for MVB-vacuole fusion. Curr Biol 24: 1383–1389 [DOI] [PubMed] [Google Scholar]
- Solomon M, Belenghi B, Delledonne M, Menachem E, Levine A (1999) The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell 11: 431–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, Jiang L (2004) Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16: 672–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Durme M, Nowack MK (2016) Mechanisms of developmentally controlled cell death in plants. Curr Opin Plant Biol 29: 29–37 [DOI] [PubMed] [Google Scholar]
- Van Hautegem T, Waters AJ, Goodrich J, Nowack MK (2015) Only in dying, life: programmed cell death during plant development. Trends Plant Sci 20: 102–113 [DOI] [PubMed] [Google Scholar]
- Varnier AL, Mazeyrat-Gourbeyre F, Sangwan RS, Clément C (2005) Programmed cell death progressively models the development of anther sporophytic tissues from the tapetum and is triggered in pollen grains during maturation. J Struct Biol 152: 118–128 [DOI] [PubMed] [Google Scholar]
- Vizcay-Barrena G, Wilson ZA (2006) Altered tapetal PCD and pollen wall development in the Arabidopsis ms1 mutant. J Exp Bot 57: 2709–2717 [DOI] [PubMed] [Google Scholar]
- Wang H, Zhuang X, Wang X, Law AH, Zhao T, Du S, Loy MM, Jiang L (2016) A distinct pathway for polar exocytosis in plant cell wall formation. Plant Physiol 172: 1003–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ding Y, Zhuang X, Hu S, Jiang L (2016) Protein co-localization studies: issues and considerations. Mol Plant 9: 1221–1223 [DOI] [PubMed] [Google Scholar]
- Wang X, Zhong F, Woo CH, Miao Y, Grusak MA, Zhang X, Tu J, Wong YS, Jiang L (2016) A rapid and efficient method to study the function of crop plant transporters in Arabidopsis. Protoplasma http://dx.doi.org/10.1007/s00709-016-0987-6 [DOI] [PubMed] [Google Scholar]
- Wilson ZA, Zhang DB (2009) From Arabidopsis to rice: pathways in pollen development. J Exp Bot 60: 1479–1492 [DOI] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ (2007) An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS One 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CH, Gao C, Yu P, Tu L, Meng Z, Banfield DK, Yao X, Jiang L (2015) Conserved function of the lysine-based KXD/E motif in Golgi retention for endomembrane proteins among different organisms. Mol Biol Cell 26: 4280–4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie HT, Wan ZY, Li S, Zhang Y (2014) Spatiotemporal production of reactive oxygen species by NADPH oxidase is critical for tapetal programmed cell death and pollen development in Arabidopsis. Plant Cell 26: 2007–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yang C, Yuan Z, Zhang D, Gondwe MY, Ding Z, Liang W, Zhang D, Wilson ZA (2010) The ABORTED MICROSPORES regulatory network is required for postmeiotic male reproductive development in Arabidopsis thaliana. Plant Cell 22: 91–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Vizcay-Barrena G, Conner K, Wilson ZA (2007) MALE STERILITY1 is required for tapetal development and pollen wall biosynthesis. Plant Cell 19: 3530–3548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Chung KP, Li B, Lai CM, Lam SK, Wang X, Cui Y, Gao C, Luo M, Wong KB, et al. (2015) Unique COPII component AtSar1a/AtSec23a pair is required for the distinct function of protein ER export in Arabidopsis thaliana. Proc Natl Acad Sci USA 112: 14360–14365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Liu D, Lv X, Wang Y, Xun Z, Liu Z, Li F, Lu H (2014) The cysteine protease CEP1, a key executor involved in tapetal programmed cell death, regulates pollen development in Arabidopsis. Plant Cell 26: 2939–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Sun Y, Timofejeva L, Chen C, Grossniklaus U, Ma H (2006) Regulation of Arabidopsis tapetum development and function by DYSFUNCTIONAL TAPETUM1 (DYT1) encoding a putative bHLH transcription factor. Development 133: 3085–3095 [DOI] [PubMed] [Google Scholar]
- Zhang X, Henriques R, Lin SS, Niu QW, Chua NH (2006) Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc 1: 641–646 [DOI] [PubMed] [Google Scholar]
- Zhang Y, He J, Lee D, McCormick S (2010) Interdependence of endomembrane trafficking and actin dynamics during polarized growth of Arabidopsis pollen tubes. Plant Physiol 152: 2200–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, McCormick S (2010) The regulation of vesicle trafficking by small GTPases and phospholipids during pollen tube growth. Sex Plant Reprod 23: 87–93 [DOI] [PubMed] [Google Scholar]
- Zhao Q, Gao C, Lee P, Liu L, Li S, Hu T, Shen J, Pan S, Ye H, Chen Y, et al. (2015) Fast-suppressor screening for new components in protein trafficking, organelle biogenesis and silencing pathway in Arabidopsis thaliana using DEX-inducible FREE1-RNAi plants. J Genet Genomics 42: 319–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XY, Wang Q, Li S, Ge FR, Zhou LZ, McCormick S, Zhang Y (2013) The juxtamembrane and carboxy-terminal domains of Arabidopsis PRK2 are critical for ROP-induced growth in pollen tubes. J Exp Bot 64: 5599–5610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Cui Y, Gao C, Jiang L (2015) Endocytic and autophagic pathways crosstalk in plants. Curr Opin Plant Biol 28: 39–47 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.