Recent studies have elucidated a molecular framework for the attraction of the pollen tube by synergid cells and the control of attraction by female gametes and sensing by the pollen tube.
Abstract
Pollen tube guidance in flowering plants is a unique and critical process for successful sexual reproduction. The pollen tube that grows from pollen, which is the male gametophyte, precisely navigates to the embryo sac, which is the female gametophyte, within the pistil. Recent advances have clarified the molecular framework of gametophytic pollen tube guidance. Multiple species-specific attractant peptides are secreted from synergid cells, the proper development and function of which are regulated by female gametes. Multiple receptor-like kinases on the pollen tube tip are involved in sensing species-specific attractant peptides. In this Update article, recent progress in our understanding of the mechanism of gametophytic pollen tube guidance is reviewed, including attraction by synergid cells, control of pollen tube guidance by female gametes, and directional growth of the pollen tube by directional cue sensing. Future directions in the study of pollen tube guidance also are discussed.
One of the big differences between the development of plants and animals is the formation of a multicellular haploid generation called gametophytes in plants. In flowering plants, both male and female gametophytes are reduced to simple structures consisting of several cells (Borg et al., 2009; Sprunck and Gross-Hardt, 2011; Schmid et al., 2015; Schmidt et al., 2015; Dresselhaus et al., 2016; Hafidh et al., 2016a). Gametophyte development in flowering plants is strictly controlled to allow proper pattern formation, cell number, cell polarity, and nuclear localization. Individual cells of male and female gametophytes have unique and significant functions in sexual reproduction in plants.
The Polygonum-type female gametophyte, which is the most typical type of gametophyte in flowering plants, possesses seven cells with four cell types: one egg cell and two synergid cells at the micropylar pole, one big central cell with two polar nuclei in the middle, and three antipodal cells at the calazal pole (Sprunck and Gross-Hardt, 2011). The male gametophyte consists of three cells with two cell types: two small sperm cells and one vegetative cell (tube cell) that encloses the two sperm cells within its endocytic membrane (Borg et al., 2009; McCue et al., 2011; Hafidh et al., 2016b). In flowering plants, nonmotile sperm cells are delivered by the pollen tube, a tubular cell structure that grows from the vegetative cell of pollen and navigates to the female gametophyte, where the egg cell resides (Fig. 1). These gametophytic cells, with support from diploid sporophytic cells, systemically work together to achieve a unique form of sexual reproduction called double fertilization, which enables rapid seed formation (Dresselhaus et al., 2016).
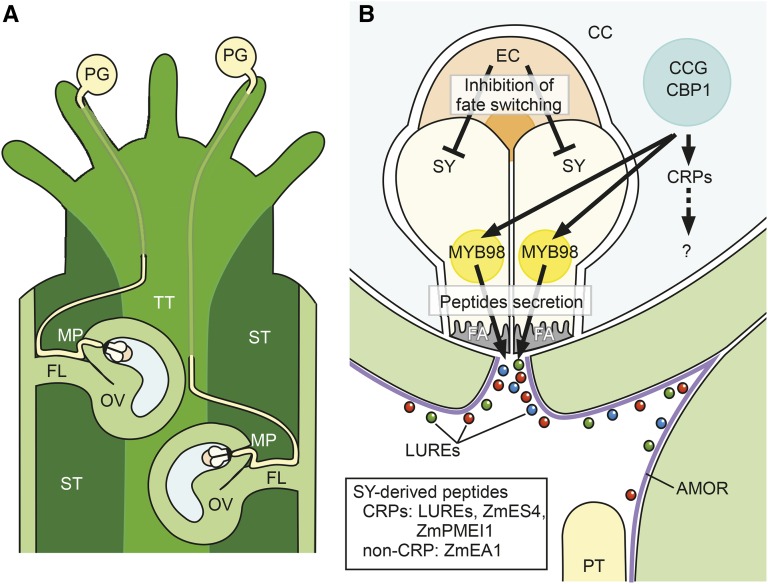
Figure 1.
Pollen tube guidance by the female gametophyte. A, Pollen tubes emerged from the pollen grain (PG) grow through the transmitting tract (TT) and emerge on the surface of the septum (ST) separating two ovary locules. Each pollen tube targets the ovule (OV) via the surface of the funiculus (FL) and the micropyle (MP) of the ovule. B, Two synergid cells (SYs) secrete many peptides, including attractant LUREs and ZmEA1, through the filiform apparatus (FA). The tip-growing pollen tube (PT) is activated by ovular arabinogalactan AMOR to respond to LURE attractant peptides in Torenia. The egg cell (EC) inhibits synergid cells from cell fate switching to the egg cell. CCG and CBP1 in the central cell (CC) control MYB98 expression in synergid cells and some Cys-rich peptide (CRP) expression in the central cell, the function of which remains unknown.
After more than a century of studies on pollen tube guidance, the female gametophyte has been shown to be the source of a diffusible attraction signal (Hülskamp et al., 1995; Ray et al., 1997; Higashiyama et al., 1998). Gametophytic guidance occurs subsequent to sporophytic guidance as a part of ovular guidance in the ovary (Higashiyama and Takeuchi, 2015). Substantial progress in our understanding of the gametophytic pollen tube has been made in the last two decades using molecular genetic techniques and other novel approaches, including microgenomics of individual cell types and imaging and manipulation analyses of live cells. Semi-in vitro (or semi-in vivo) systems in Torenia (Torenia fournieri; a unique plant with a protruding female gametophyte; Higashiyama et al., 1998) and Arabidopsis (Arabidopsis thaliana; Palanivelu and Preuss, 2006), wherein pollen tubes growing through a cut pistil are attracted to isolated ovules on the medium, also have contributed significantly. The molecular mechanisms of gametophytic pollen tube guidance have been clarified in both male and female individual cells.
In this article, we review recent progress in pollen tube guidance by focusing on gametophytic guidance. More comprehensive reviews and other aspects of pollen tube guidance are available elsewhere (Dresselhaus and Franklin-Tong, 2013; Higashiyama and Takeuchi, 2015; Kanaoka and Higashiyama, 2015; Qu et al., 2015).
ATTRACTION OF THE POLLEN TUBE BY SYNERGID CELLS
Synergid cells are specialized cells that play an active role in secretion (Fig. 1B). They have critical functions in pollen tube attraction and reception (Higashiyama, 2002; Dresselhaus et al., 2016). Laser ablation of these cells in mature female gametophytes of Torenia has shown that they are necessary and sufficient for pollen tube attraction to the female gametophyte (Higashiyama et al., 2001). On the other hand, laser ablation of immature, developing female gametophytes of Torenia has led to different results, indicating that cell-to-cell communication is critical to the function of synergid cells, as discussed below (Susaki et al., 2015). Both synergid cells are the sources of pollen tube attraction signals (Higashiyama et al., 2001), and each such cell of Arabidopsis can attract and receive a second pollen tube to recover fertilization if the first tube fails to achieve double fertilization (Kasahara et al., 2012; Nagahara et al., 2015). Genetic evidence in Arabidopsis (Kasahara et al., 2005) and maize (Zea mays; Márton et al., 2005) also has suggested that synergid cells are involved in ovular guidance of pollen tubes at the micropyle (Fig. 1A). However, the distance of pollen tube attraction by synergid cells is a matter of debate in Arabidopsis (Higashiyama and Takeuchi, 2015). Pollen tube guidance at the point just after emergence from the transmitting tract (Fig. 1A) is likely to be governed by synergid cells in Arabidopsis (both funicular and micropylar guidance).
In the typical Polygonum-type embryo sac (e.g. Arabidopsis, maize, Torenia, tobacco [Nicotiana tabacum], and rice [Oryza sativa]), two synergid cells are formed from a single precursor nucleus at the four-nucleate stage (Sprunck and Gross-Hardt, 2011). According to studies that have used live-cell imaging of the female gametophyte of Torenia, cellularization of the eight-nucleate female gametophyte is completed within 54 ± 20 min after the third mitosis of the four-nucleate female gametophyte (Susaki et al., 2015). Cell type-specific gene expression likely begins after cellularization of the female gametophyte (Lawit et al., 2013; Susaki et al., 2015). The MYB98 transcription factor in Arabidopsis, which is expressed specifically in synergid cells, is important for guidance of the pollen tube and the formation of the filiform apparatus (Kasahara et al., 2005; Punwani et al., 2007, 2008). The filiform apparatus is thickened and/or undergoes ingrowing of the cell wall in synergid cells at the micropylar end (Fig. 1B). Pollen tube attractants are secreted through the filiform apparatus (Higashiyama and Hamamura, 2008; Higashiyama and Takeuchi, 2015; Dresselhaus et al., 2016), and the apparatus defines the path of the pollen tube into the female gametophyte (Higashiyama, 2002; Leshem et al., 2013).
Synergid cells secrete many peptides (small proteins; Jones-Rhoades et al., 2007; Punwani et al., 2007; Fig. 1B), which include attractant molecules (Fig. 1B). In dicot plant species such as Torenia and Arabidopsis, defensin-like CRPs known as LUREs are secreted as pollen tube attractants (Okuda et al., 2009; Kanaoka et al., 2011; Takeuchi and Higashiyama, 2012; putatively 60–70 amino acids). In maize, on the other hand, the EGG APPARATUS1 (ZmEA1) peptide has been reported to be a pollen tube attractant (Márton et al., 2005, 2012; putatively 49 amino acids; non CRP). The Arabidopsis genome encodes more than 900 CRPs (Silverstein et al., 2007; Huang et al., 2015), and these often function as ligands in cell-to-cell communication in plants. For example, other CRPs for pollen-pistil interactions include SCR/SP11, LAT52, tomato (Solanum lycopersicum; formerly Lycopersicon esculentum) STIG1 (LeSTIG1), STIGMA/STYLAR CYS-RICH ADHESIN, EGG CELL1, maize EMBRYO SAC4 (ZmES4), and maize PECTIN METHYLESTERASE INHIBITOR1 (ZmPMEI1; Kanaoka and Higashiyama 2015), and CRPs for plant development include RAPID ALKALINIZATION FACTOR, EPIDERMAL PATTERNING FACTOR (EPF)/EPF-like, STOMAGEN, and EMBRYO SURROUNDING FACTOR1 (ESF1; Matsubayashi, 2014; Breiden and Simon, 2016). ZmEA1-like1 (non CRP), secreted by the egg cell of maize, also is involved in cell-to-cell communication to control antipodal cell fate at the opposite pole of the female gametophyte (Krohn et al., 2012; Uebler et al., 2015). It should be noted that, in Arabidopsis, 53% of female gametophyte-specific genes encode CRPs, suggesting their important roles in ovule development and male-female interactions (Huang et al., 2015).
LURE genes are specifically and abundantly expressed in synergid cells (Okuda et al., 2009; Takeuchi and Higashiyama, 2012). To date, two LURE genes (TfLURE1 and TfLURE2) have been reported in Torenia and six duplicated LURE genes (AtLURE1.1–AtLURE1.6) have been reported in Arabidopsis. There might be more LURE peptides in Torenia and Arabidopsis because more synergid cell-specific defensin-like peptides are expressed in these species (Jones-Rhoades et al., 2007; Okuda et al., 2009). AtLURE1 peptides are members of the CRP810 subfamily of CRPs, and at least four other peptides of this subfamily are expressed downstream of MYB98 regulation in the pistil (Takeuchi and Higashiyama, 2012). In a previous study, knockdown of LURE1.1 to LURE1.6 peptides in Arabidopsis by RNA interference impaired pollen tube guidance in only 10% of ovules, although no LURE1 peptide was detected by immunostaining (Takeuchi and Higashiyama, 2012). LURE1 peptides of Arabidopsis diffuse along the path of the pollen tube to the surface of the septum via the surface of the funiculus of ovules (Takeuchi and Higashiyama, 2012). No active transport after secretion has been reported, and passive diffusion is plausible. However, it is interesting that LURE1 peptides do not diffuse toward other parts of the ovular surface, implying the possibility of positional differences in surface properties.
The identification of pollen tube attractants in flowering plants is still limited because homology-based searches are impossible in distantly related species. As suggested in Arabidopsis and Torenia, many CRPs are expressed in synergid cells (Jones-Rhoades et al., 2007; Okuda et al., 2009), which include other functions of CRPs such as triggering pollen tube discharge (defensin-like ZmES4 [Amien et al., 2010] and ZmPMEI1 [Woriedh et al., 2013]). In Arabidopsis, many CRPs expressed in synergid cells, including defensin-like, thionin-like, and PMEI, are under the control of MYB98 (Jones-Rhoades et al., 2007). Homozygous mutants of myb98 do not completely stop pollen tube attraction (Kasahara et al., 2005), suggesting that other attractants are still working in myb98 mutants. To identify LURE- and ZmEA1-type attractant peptides, candidate peptides that are expressed abundantly and specifically in synergid cells should be examined to determine whether they attract competent pollen tubes of the same species and whether their knockdown or knockout leads to impaired pollen tube attraction.
One of the important aspects of pollen tube attractants derived from synergid cells is strong species preferentiality and specificity (Okuda et al., 2009; Kanaoka et al., 2011; Márton et al., 2012; Takeuchi and Higashiyama, 2012). Micropylar pollen tube attraction in the semi-in vitro system is species preferential (pollen tubes preferentially grow toward ovules of their own species) even in closely related species, such as between Torenia and Torenia conclor/Torenia billonii (Higashiyama et al., 2006). In the in vivo crosses between Torenia and T. conclor/T. billonii (Higashiyama et al., 2006), pollen tube attraction is eventually accomplished but is largely delayed after the tubes enter the ovary. Consistently, LURE peptides of these Torenia and Arabidopsis spp. show some preferentiality in the attraction of their own species (Okuda et al., 2009; Takeuchi and Higashiyama, 2012). Species-specific gene duplication also has been reported for LURE1 genes of Arabidopsis and Arabidopsis lyrata (Takeuchi and Higashiyama, 2012). LURE genes might be involved in speciation. It is of interest that LURE and ZmEA1 peptides are involved in species recognition. The AtLURE1.2 peptide expressed in Torenia synergid cells and the ZmEA1 peptide expressed in Arabidopsis synergid cells are able to overcome large species barriers, allowing pollen tubes of Arabidopsis and maize to be attracted to heterologous and transgenic ovules, respectively (Márton et al., 2012; Takeuchi and Higashiyama, 2012). The tertiary structures of LUREs and ZmEA1 peptides have not been solved, while those of some other CRPs, including Raphanus sativus ANTIFUNGAL PROTEIN1 (Fant et al., 1998), SCR/SP11 (Mishima et al., 2003), STOMAGEN (Ohki et al., 2011), and ESF1 (Costa et al., 2014), have been solved. During the evolution of Torenia (Kanaoka et al., 2011) and Arabidopsis (Takeuchi and Higashiyama, 2012), amino acid substitutions of LUREs occurred throughout the peptides. However, it is possible that specific regions of LURE peptides might be important for species-specific recognition, as is the case for SCR/SP11 (Mishima et al., 2003) and STOMAGEN (Ohki et al., 2011). Further progress is expected to be made based on structural biology as well as genomic and evolutionary approaches
FEMALE GAMETIC CONTROL OF POLLEN TUBE GUIDANCE
Genetic studies in Arabidopsis have suggested that, in addition to the synergid, two female gametes, namely the central cell and the egg cell, also play a critical role in micropylar pollen tube guidance (Fig. 1B). Mutations in central cell-specific genes such as CENTRAL CELL GUIDANCE (CCG; Chen et al., 2007), CCG BINDING PROTEIN1 (CBP1; Li et al., 2015), and MAGATAMA3 (MAA3; Shimizu et al., 2008) and the egg-expressed gene GEX3 (Alandete-Saez et al., 2008) abolish the female attraction of the pollen tube in Arabidopsis. Consistently, the LURE1 signal cannot be detected by the LURE1 antibody in ccg and maa3 mutant ovules (Takeuchi and Higashiyama, 2012), which suggests indirect control of pollen tube attraction by the central cell. Recently, it was shown that CCG and CBP1 function as transcriptional regulators that bring together the RNA PolII machinery and AGL transcription factors via an interaction with the Mediator complex (a conserved central coactivator of transcription; Li et al., 2015). Interestingly, CRPs expressed by both central cells and synergid cells, including LURE1, are significantly down-regulated in ccg and cbp1 mutant ovules (Li et al., 2015). This is likely due to the down-regulation of MYB98 in synergid cells via cell-to-cell communication with the central cell (Li et al., 2015). It is of interest that CCG and CBP1 of the central cell control MYB98 expression in synergid cells. On the other hand, the role of central cell-secreted CRPs in pollen tube guidance needs to be investigated. Among six CRPs expressed specifically in central cells (DOWN-REGULATED IN dif1 22 [DD22], DD36, DD66, LOW-MOLECULAR-WEIGHT CYS-RICH24 [LCR24], LCR59, and AT3G04540; Wuest et al., 2010), DD22, DD36, DD66, and AT3G04540 are down-regulated in ccg mutants. Whether these CRPs function directly as pollen tube attractants or indirectly by modulating intercellular communication between central cells and synergid cells remains to be determined. Alternatively, they may play a role in regulating the function of the surrounding integument, which in turn impacts pollen tube guidance (Villanueva et al., 1999; Palanivelu et al., 2003).
The control of synergid cells by female gametes is more complicated than simply triggering the production and secretion of pollen tube attractant peptides. For example, the cell fate of synergid cells is controlled by the egg cell (Fig. 1B). Despite significant functional and morphological differences between synergid and egg cells, fate conversion into the egg cell is likely to readily occur in developing synergid cells. Genetic ablation of egg cells by a barnase toxin has been used to induce the switching of cell fate from synergid cells to an egg-like cell that can be fertilized (Lawit et al., 2013). Laser ablation of a developing egg cell in wild-type Torenia leads to the down-regulation of LURE expression in one of the two synergid cells and to changes in nuclear localization that resemble the egg cell (Susaki et al., 2015). Consistently, in eostre (Pagnussat et al., 2007), rbr1 (Ingouff et al., 2009), and amp1 (Kong et al., 2015) mutants, one of the two synergid cells changes to an egg-like cell. The egg-like cell of rbr1 and amp1 can be fertilized to produce twin embryos in a single female gametophyte. There may be lateral inhibitory signals originating from the egg cell that prevent synergid cells from changing fate to an egg cell. Analyses of amp1 have suggested that ovular sporophytic cells are involved in this signaling. This lateral inhibition must be critical for proper double fertilization by the egg and central cells, as shown in amp1 (Kong et al., 2015). An alternative possibility is that the Polygonum-type egg cell governs the formation of two synergid cells, which are critical for the recovery of fertilization (Kasahara et al., 2012).
In addition, fertilization of the two female gametes controls the cessation of pollen tube attraction by synergid cells (Beale et al., 2012; Kasahara et al., 2012; Maruyama et al., 2013, 2015; Völz et al., 2013). Generally, only one pollen tube is received by the ovule. Blocking polytubey in Arabidopsis involves independent and synergistic control by fertilization of the two female gametes (Maruyama et al., 2013). The fertilized egg cell (zygote) controls the disorganization of the persistent synergid cell nucleus via ethylene signaling (Völz et al., 2013; Maruyama et al., 2015). The fertilized central cell (endosperm), on the other hand, undergoes cell fusion with the persistent synergid cell. This causes diffusion of the contents of synergid cells and enforces the progression of the cell cycle in the fused synergid cell (Maruyama et al., 2015). POLYCOMB REPRESSIVE COMPLEX2 (PRC2) in the central cell is involved in nuclear disorganization of the fused synergid cell (Maruyama et al., 2015). Molecules required for this somatic cell fusion are largely unknown. PRC2 prevents autonomous endosperm formation and cell fusion with two synergid cells in unfertilized female gametophytes (Motomura et al., 2016). This suggests that cell fusion of the endosperm and synergid cells does not require the male-derived fusion protein GENERATIVE CELL SPECIFIC1/HAPLESS2 (Dresselhaus et al., 2016).
DIRECTIONAL GROWTH OF THE POLLEN TUBE TOWARD ATTRACTANT PEPTIDES
Before gametophytic guidance begins, pollen tubes receive many molecular signals from female sporophytic tissues of the pistil that are responsible for directional control, growth stimulation, adhesion to female tissues, self/nonself recognition, and competency control (Higashiyama, 2010; Higashiyama and Takeuchi, 2015). Competency control is important for pollen tubes to respond to the attraction signal from the female gametophyte, although the dependency of pollen tubes on female sporophytic tissues is likely to be different among species (Higashiyama et al., 1998; Palanivelu and Preuss, 2006; Márton et al., 2012; Okuda et al., 2013). In Torenia, the ovular arabinogalactan sugar chain called AMOR has been found to make pollen tubes fully competent for LURE peptides (LURE1 and LURE2) after passing through the style tissue (Mizukami et al., 2016; Fig. 1B). Arabinogalactan proteins and their sugar moieties have been found to be critical for sexual reproduction, including pollen and ovule development, pollen germination, pollen tube growth, delivery of female self-incompatibility factors, synergid cell degeneration, and blocking polytubey (Pereira et al., 2015, 2016; Dresselhaus and Coimbra, 2016; Hou et al., 2016). However, no specific sugar chain structure has been reported to be responsible for bioactivities. Interestingly, the terminal disaccharide 4-Me-GlcA-β-(1→6)-Gal of the arabinogalactan sugar chain is sufficient for AMOR activity. The gametophytic pollen tube guidance of Torenia has been demonstrated using only chemically synthesized disaccharide 4-Me-GlcA-β-(1→6)-Gal and recombinant LURE peptides (LURE1 or LURE2) without other ovular molecules. The use of chemical synthesis is a powerful approach for studying structures that are important to the bioactivity of AMOR. The terminal sugar structure including the 4-O-methyl residue and gluconic acid with a β-glycosidic bond are critical, whereas the structure of the second sugar is not (Jiao et al., 2017). This suggests that chemical labeling of the second sugar in the active disaccharide AMOR for visualization and target identification is possible. The combination of a chemical synthesis approach with a genetic approach to study enzymes of arabinogalactan sugar biosynthesis (Knoch et al., 2014) would clarify the sugar signaling pathway in plants, which is generally critical for plant development and growth (Ogawa-Ohnishi and Matsubayashi, 2015). Partner molecules and signaling pathways of AMOR in the pollen tube remain elusive. Whether the AMOR sugar chain functions similarly in other plants such as Arabidopsis also remains unknown. However, the pollen tube is known to change properties, including its gene expression profile, during its growth in the pistil (Qin et al., 2009; Okuda et al., 2013; Lin et al., 2014).
Competent pollen tubes in the ovary encounter attractant peptides derived from synergid cells and ultimately arrive at the target female gametophyte. It has been predicted that a tip-localized receptor(s) senses species-specific LUREs and ZmEA1 peptides for directional growth (Higashiyama and Takeuchi, 2015). In Arabidopsis, receptors of the pollen tube for LURE sensing were reported recently by two independent studies (Cheung and Wu, 2016; Takeuchi and Higashiyama, 2016; Wang et al., 2016).
A receptor heteromer, MALE DISCOVERER1 (MDIS1)-MDIS1-INTERACTING RECEPTOR LIKE KINASE (MIK), has been identified using a screen based on the expression of the kinase-dead dominant-negative forms of candidate receptors (Wang et al., 2016; Fig. 2). In that study, 24 receptor-like kinases preferentially expressed in the pollen and pollen tube (including POLLEN RECEPTOR-LIKE KINASE6 [PRK6], as noted below) were selected as candidates. Expression of the dominant-negative MDIS1, in which the kinase domain was replaced by the dead kinase domain of the brassinosteroid receptor BRASSINOSTEROID-INSENSITIVE1, caused the mistargeting of pollen tubes at the micropyle of the ovule and, thus, reduced fertility. Using a yeast two-hybrid analysis and knockout mutant analysis, four LRR receptor-like kinases, MDIS1, MDIS2 (LRR-VI subfamily), MIK1, and MIK2 (LRR-XI and LRR-XII subfamilies, respectively), were identified. Knockout mutants of these four genes showed partial defects in pollen tube guidance but no pollen tube growth defects in vivo. Various biochemical analyses, including pull-down assays, coimmunoprecipitation, and microscale thermophoresis (MST), showed direct binding of His-tagged AtLURE1.2 (His-LURE1.2) with MDIS1, MIK1, and MIK2 (dissociation constant values were 1.76 ± 0.09 μm, 672 ± 42.4 nm, and 464 ± 13.4 nm, respectively, by MST). MDIS2 did not interact with His-LURE1.2 in MST analysis. Considering the defect in pollen tube guidance in vivo, MDIS2 might receive other ovular pollen tube attractants. On the other hand, a MIK1 homolog, PXY (the receptor of a CLAVATA3/ESR [CLE] peptide TDIF in vascular development), was shown to bind to His-LURE1.2 with a dissociation constant value of 704 ± 49.2 nm by MST. Expression of MDIS1, MDIS2, MIK1, and MIK2 was shown in the pollen tube and seedlings, while the expression levels of MDIS1 and MDIS2 were predominant in the pollen tube. Expression of PXY was detected in the pollen tube, but its level was low. Together, these results suggest that MDIS1, MIK1, and MIK2 are receptors for LURE1. Consistently, heterologous expression of AtMDIS1 in Capsella (Capsella rubella) pollen tubes increased the targeting frequencies of Arabidopsis ovules. Only AtMDIS1 was tested, because MIK1 appeared not to be expressed in Capsella pollen, suggesting the possibility that the combination of receptors can change readily during evolution. On the other hand, knockout mutations of these genes partially reduced pollen tube attraction to LURE1-containing gelatin beads in a semi-in vivo assay, suggesting the existence of other components responsible for LURE1 sensing.
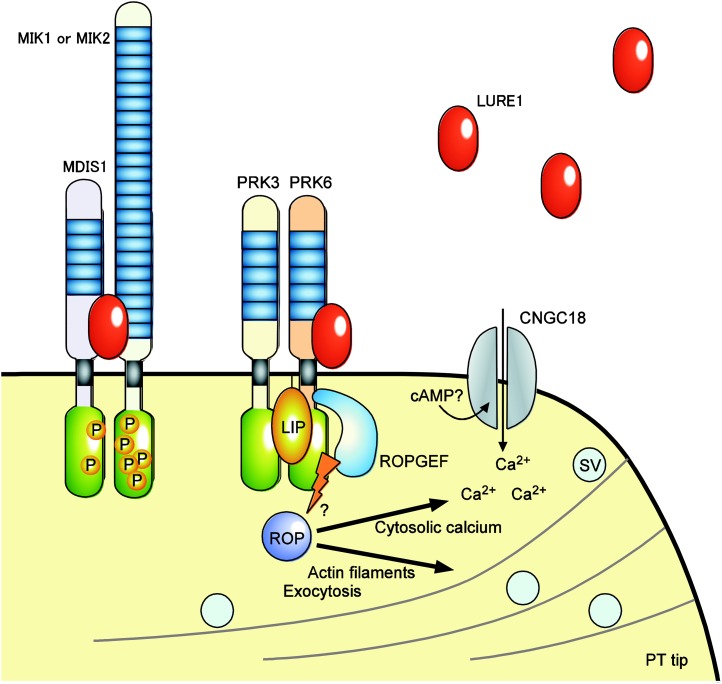
Figure 2.
Directional growth of the pollen tube toward LURE attractant peptide. The LURE1 attractant peptide of Arabidopsis is sensed by the receptor-like kinases MDIS1-MIK and PRK6 with Leu-rich repeats (LRRs; blue boxes) in their ectodomains. LURE1 enhances the dimerization of MDIS1-MIK receptors and the autophosphorylation and transphosphorylation (P) of kinase domains (green boxes) by MIK1. PRK6 interacts with PRK3, LIP1, and LIP2 in the LURE signaling cascade and with ROPGEFs, which might transduce LURE signal to ROPs that regulate the cytosolic calcium gradient, the dynamics of actin filaments (gray lines), and the exocytosis of secretory vesicles (SV) in pollen tube tip (PT tip) growth. The direct interaction of PRK6 with LURE1 has not been shown biochemically. The cyclic nucleotide-gated channel CNGC18 is a Ca2+-permeable channel essential for micropylar pollen tube guidance.
Interestingly, heterodimerization of MDIS1 and MIK1 or MIK2 is enhanced in the presence of LURE1 (Wang et al., 2016; Fig. 2). An in planta coimmunoprecipitation assay with self-pollinated flowers of MDIS1-GFP plants also suggested an interaction between the MDIS1-MIK complex and LURE1. Moreover, LURE1 enhances the autophosphorylation of MIK1 and induces the transphosphorylation of MDIS1 by MIK1, as has been suggested in pathogen and brassinosteroid perception (Santiago et al., 2013; Sun et al., 2013). LURE1-induced heterodimerization of MDIS1-MIK and autophosphorylation and transphosphorylation by MIK1 might play a role in signal transduction to the cytoplasm of the pollen tube (Li and Yang, 2016).
PRK6 of Arabidopsis is another LRR receptor-like kinase that is essential for sensing LURE1 peptide (Takeuchi and Higashiyama, 2016; LRR-III subfamily; Fig. 2). The PRK family was originally found in Petunia spp. (Mu et al., 1994) and has been studied in tomato and Arabidopsis; such studies have shown that PRKs are involved in pollen germination and growth (for review, see Miyawaki and Yang, 2014). Interestingly, LePRK2 in tomato interacts with some CRPs, namely LAT52 in the pollen coat and LeSTIG1 in the stigma exudate, to control pollen germination and growth (Tang et al., 2002, 2004). In a study on Arabidopsis, PRK6 was identified as an essential receptor-like kinase for LURE sensing using a semi-in vivo assay with recombinant AtLURE1.2 peptide embedded in gelatin beads (Takeuchi and Higashiyama, 2016). The beads were placed in front of pollen tubes by micromanipulation, as reported previously (Okuda et al., 2009; Takeuchi and Higashiyama, 2012). A screen using only the LURE1 peptide was performed considering the redundancy of attractant peptides in vivo, as discussed above. The pollen tubes produced by knockout lines for 23 receptor-like kinases, of which phylogenetically related genes are expressed specifically in the pollen (including MDIS1 and MDIS2), were examined. Among single mutants of the 23 genes, prk6 mutant pollen tubes grew normally and were not at all attracted to the AtLURE1.2 peptide. In the pistil, prk6 single-mutant pollen tubes show a moderate ovule-targeting phenotype, which is consistent with a previous AtLURE1 knockdown experiment (Takeuchi and Higashiyama, 2012).
PRK6 localized at the plasma membrane of pollen tube tips (Takeuchi and Higashiyama, 2016; Fig. 2). Its expression level increases in pollen tubes that are growing semi-in vivo (Qin et al., 2009). Among eight PRKs of Arabidopsis, seven are expressed specifically in the pollen and pollen tube (Qin et al., 2009), but PRK7 is not. Genetic analyses have shown that PRK6 also is involved in pollen tube growth, in addition to other PRKs such as PRK1, PRK3, and PRK8 (Takeuchi and Higashiyama, 2016). PRK6 interacted with PRK3, ROPGEFs, which are pollen-expressed guanine nucleotide-exchange factors (GEFs) for Rho-like small GTPases of plants (ROP), and LOST IN POLLEN TUBE GUIDANCE1 (LIP1) and LIP2, which are receptor-like cytoplasmic kinases involved in the LURE signaling cascade (Liu et al., 2013). In that study, specific binding to LURE1 was not shown due to the sticky properties of AtLURE1.2, which is mediated by a basic amino acid patch that is necessary for attraction activity. However, introducing Arabidopsis PRK6 to Capsella enabled Capsella pollen tubes to grow toward AtLURE1.2-containing gelatin beads in a semi-in vivo assay at frequencies of more than 80%. Wild-type Capsella pollen tubes scarcely grew toward the beads. Species differences between Arabidopsis PRK6 and Capsella PRK6 also were detected in a complementation test of Arabidopsis prk6 using a semi-in vivo system called the wavy assay, wherein responsive pollen tubes show severe wavy behavior in LURE-containing culture medium by an unknown mechanism. Considering that PRKs potentially interact with CRPs, it might be possible to assume that PRK6 in the PRK receptor complex interacts with LURE1 to transduce signals via cytoplasmic components, including ROPGEFs and LIPs. In tomato pollen, LePRK2 interacts with LAT52 and LePRK1 (Tang et al., 2002; Wengier et al., 2003). Upon pollination, LAT52 is replaced by pistil-derived LeSTIG1, which promotes the dissociation of LePRK1 from LePRK2 and pollen tube growth (Tang et al., 2004). Pollen tube growth and the guidance of dicot flowering plants might be controlled successively by different tissue-specific CRP ligands for PRKs.
PRK6 was likely to interact with ROPGEF12 via the juxtamembrane domain between the transmembrane and kinase domains to relay the LURE signal to the pollen tube cytoplasm (Takeuchi and Higashiyama, 2016; Fig. 2). The phenotype of LURE sensing in prk6 pollen tubes was complemented by a kinase-deleted PRK6 as long as the juxtamembrane domain remained. The noncatalytic juxtamembrane and C-terminal domains of PRK2 also play a critical role in ROP-induced pollen tube growth through ROPGEF12 at the membrane (Zhao et al., 2013). The kinase domain of PRK6, however, was necessary in pollen tube growth and LURE1 sensing in the absence of PRK3 (Takeuchi and Higashiyama, 2016). Consistently, the kinase domain of PRK2 interacts with and phosphorylates ROPGEF1 in vitro (Chang et al., 2013). PRK2 promotes ROP1 activation via ROPGEFs, including ROPGEF1, to control polarized pollen tube growth with other PRKs (Chang et al., 2013), but it is not involved in LURE1 sensing. Taken together, these results support the model that heteromer PRK receptor complexes containing PRK6 relay the LURE signal to ROPs (via ROPGEFs) and LIPs. Interestingly, live-cell imaging of semi-in vivo assays have shown that PRK6 changes its localization toward LURE1 in 30 to 60 s and changes its direction of growth in 90 s upon exposure to a bead containing LURE1. This behavior might be involved in the asymmetric activation of ROPs that act as core regulators of the dynamics of actin filaments, the cytosolic calcium gradient, and exocytosis in pollen tube tip growth (Miyawaki and Yang, 2014; Michard et al, 2017; Fig. 2).
The relationships between MDIS1-MIK and PRK6 remain to be elucidated. It is possible that multiple attractant peptides and multiple receptors are involved in precise and species-specific pollen tube guidance. It is now of interest whether signals perceived via MDIS1-MIK and PRK6 complexes are integrated in the same or different downstream signaling pathways. Analysis of the interaction between MDIS1-MIK with ROPGEFs is already in progress (Li and Yang, 2016). Mutants of MDIS1-MIK and PRK6 showed slightly different properties: for example, in vivo ovule targeting defects appeared more severe in mutants of MDIS1-MIK, but defects in the bead assay were more severe in mutants of PRK6. Thus, MDIS1-MIK and PRK6 might have different preferences for multiple attractant peptides, which might contribute to robust pollen tube guidance.
MDIS1, MIK, and PRK6 are in different subfamilies of LRR receptor-like kinases, and a specific structural homology in their ectodomains except for LRRs could not be identified. It is also possible that different parts of AtLURE1 are involved in the interaction with MDIS1-MIK and PRK6, consistent with the hypothesis that these receptors might have different preferences for multiple attractant peptides. Netrins, for example, which were first identified as chemotropic guidance cues for neuronal axons, have a variety of functions, including the regulation of cell migration, cell-cell interactions, and extracellular matrix adhesion in neural and nonneural cells (Lai Wing Sun et al., 2011). These diverse functions are mediated by different receptors, including the UNC-5 family, Deleted in colorectal cancer/Neogenin, Down syndrome cell adhesion molecule, and Netrin G ligands (Lai Wing Sun et al., 2011). As in the case of netrins and their receptors, cocrystal structures of ligand-receptor pairs will now be essential to provide further insights into the action mechanism of how a ligand works and interacts with different receptors (Xu et al., 2014).
Moreover, combinations of receptor complexes and ligand-receptor pairs might dynamically change during long-distance growth of the pollen tube cell (single cell) in the pistil. In addition to a deeper understanding of the identified attractant receptor pairs, further identification of the ligands and receptors involved in pollen-pistil interactions is important to clarify such a unique system in flowering plants. Secretome analyses of pollen tubes and female tissues, including ovules (Liu et al., 2015; Hafidh et al., 2016b), as well as translatome (Lin et al., 2014) would contribute to the identification of novel ligands, including attractants for pollen tube growth and guidance.
Recently, CNGC18 was reported to be a calcium channel essential for micropylar pollen tube guidance (Gao et al., 2016; Fig. 2). Among eight Ca2+-permeable channels present in the Arabidopsis pollen tube (six CNGCs and two Glu receptor-like channels [Michard et al., 2011]), only CNGC18 was essential for pollen tube growth, and two point mutations of CNGC18 that impair the activation by cyclic nucleotides showed micropylar pollen tube guidance defects. The relationships between CNGC18 and MDIS1-MIK/PRK6 are of interest. Other pollen tube molecules for ovular guidance include glycosylphosphatidylinositol anchor and its biosynthesis proteins (Li et al., 2013; Dai et al., 2014), mitogen-activated protein kinases (Guan et al., 2014), AGC kinases for eukaryotic polarized cell growth (Zhang et al., 2009), endoplasmic reticulum-localized K+ channel (Lu et al., 2011) and luminal proteins (Li et al., 2011), small secreted peptide phytosulfokine (Stührwohldt et al., 2015), and inositol polyphosphate kinases (Zhan et al., 2015). It remains to be elucidated whether these molecules are involved in sensing and competency control for LURE or other gametophytic attractant pathways (Higashiyama and Takeuchi, 2015). The identification MDIS1-MIK and PRK6 will significantly contribute to progress in our understanding of the gametophytic pollen tube guidance pathways.
CONCLUSION AND PERSPECTIVE
Many interesting issues remain to be addressed in future studies of gametophytic pollen tube guidance (see Outstanding Questions). For future studies, at least two approaches would be very powerful. First, live-cell imaging including single-molecule analysis would reveal how molecules of ligands and receptors, including LUREs, MDIS1-MIK, and PRK6, interact in real time to transduce signals. The rapid response of pollen tubes to attractant peptides is advantageous for real-time analysis (Takeuchi and Higashiyama, 2016). It should be noted that a very small number of LURE peptides, approximately 1,000 molecules in a bead, can attract pollen tubes in Torenia, which is also a considerable advantage for single-molecule analysis (Okuda et al., 2009; Goto et al., 2011). Microfluidics allow precise control of pollen tube guidance in real time using the microscope (Horade et al., 2013; Sanati Nezhad et al., 2014; Sato et al., 2015). In vivo live-cell imaging by two-photon microscopy (Cheung et al., 2010; Mizuta et al., 2015), on the other hand, also would help elucidate complex pollen-pistil interactions that establish the one-on-one relationship between multiple pollen tubes and ovules in real time.
Second, a tertiary structure-based approach is critical to understand ligand receptors as molecular lock-and-key systems of plant reproduction. As discussed above, gametophytic pollen tube guidance is important not only for precise navigation of the male gametophyte to the female gametophyte but also for precise species recognition. Studies in structural biology as well as synthetic chemistry would provide structural insights. Crystal structures for LRR receptor-like kinases and CLE peptides, including TDIF-PXY/TDR (Morita et al., 2016; Zhang et al., 2016) and INFLORESCENCE DEFICIENT IN ABSCISSION-HAESA-SOMATIC EMBRYOGENESIS RECEPTOR KINASE1 (Santiago et al., 2016), have been solved, but the crystal structure of a CRP in complex with a receptor has not. Such a crystal structure would significantly progress pollen tube guidance studies.
Glossary
- CRP
Cys-rich peptide
- LRR
Leu-rich repeat
- MST
microscale thermophoresis
Footnotes
This work was supported by the Japan Society for the Promotion of Science (Grant-in-Aid for Scientific Research on Innovative Areas nos. JP16H06465, JP16H06464, and JP16K21727 to T.H.) and the National Science Foundation of China (grant no. 31330053 to W.Y.).
Articles can be viewed without a subscription.
References
- Alandete-Saez M, Ron M, McCormick S (2008) GEX3, expressed in the male gametophyte and in the egg cell of Arabidopsis thaliana, is essential for micropylar pollen tube guidance and plays a role during early embryogenesis. Mol Plant 1: 586–598 [DOI] [PubMed] [Google Scholar]
- Amien S, Kliwer I, Márton ML, Debener T, Geiger D, Becker D, Dresselhaus T (2010) Defensin-like ZmES4 mediates pollen tube burst in maize via opening of the potassium channel KZM1. PLoS Biol 8: e1000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale KM, Leydon AR, Johnson MA (2012) Gamete fusion is required to block multiple pollen tubes from entering an Arabidopsis ovule. Curr Biol 22: 1090–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg M, Brownfield L, Twell D (2009) Male gametophyte development: a molecular perspective. J Exp Bot 60: 1465–1478 [DOI] [PubMed] [Google Scholar]
- Breiden M, Simon R (2016) Q&A: how does peptide signaling direct plant development? BMC Biol 14: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Gu Y, Ma H, Yang Z (2013) AtPRK2 promotes ROP1 activation via RopGEFs in the control of polarized pollen tube growth. Mol Plant 6: 1187–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Li HJ, Shi DQ, Yuan L, Liu J, Sreenivasan R, Baskar R, Grossniklaus U, Yang WC (2007) The central cell plays a critical role in pollen tube guidance in Arabidopsis. Plant Cell 19: 3563–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Boavida LC, Aggarwal M, Wu HM, Feijó JA (2010) The pollen tube journey in the pistil and imaging the in vivo process by two-photon microscopy. J Exp Bot 61: 1907–1915 [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wu HM (2016) LURE is bait for multiple receptors. Nature 531: 178–180 [DOI] [PubMed] [Google Scholar]
- Costa LM, Marshall E, Tesfaye M, Silverstein KA, Mori M, Umetsu Y, Otterbach SL, Papareddy R, Dickinson HG, Boutiller K, et al. (2014) Central cell-derived peptides regulate early embryo patterning in flowering plants. Science 344: 168–172 [DOI] [PubMed] [Google Scholar]
- Dai XR, Gao XQ, Chen GH, Tang LL, Wang H, Zhang XS (2014) ABNORMAL POLLEN TUBE GUIDANCE1, an endoplasmic reticulum-localized mannosyltransferase homolog of GLYCOSYLPHOSPHATIDYLINOSITOL10 in yeast and PHOSPHATIDYLINOSITOL GLYCAN ANCHOR BIOSYNTHESIS B in human, is required for Arabidopsis pollen tube micropylar guidance and embryo development. Plant Physiol 165: 1544–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresselhaus T, Coimbra S (2016) Plant reproduction: AMOR enables males to respond to female signals. Curr Biol 26: R321–R323 [DOI] [PubMed] [Google Scholar]
- Dresselhaus T, Franklin-Tong N (2013) Male-female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol Plant 6: 1018–1036 [DOI] [PubMed] [Google Scholar]
- Dresselhaus T, Sprunck S, Wessel GM (2016) Fertilization mechanisms in flowering plants. Curr Biol 26: R125–R139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fant F, Vranken W, Broekaert W, Borremans F (1998) Determination of the three-dimensional solution structure of Raphanus sativus antifungal protein 1 by 1H NMR. J Mol Biol 279: 257–270 [DOI] [PubMed] [Google Scholar]
- Gao QF, Gu LL, Wang HQ, Fei CF, Fang X, Hussain J, Sun SJ, Dong JY, Liu H, Wang YF (2016) Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis. Proc Natl Acad Sci USA 113: 3096–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto H, Okuda S, Mizukami A, Mori H, Sasaki N, Kurihara D, Higashiyama T (2011) Chemical visualization of an attractant peptide, LURE. Plant Cell Physiol 52: 49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Lu J, Xu J, McClure B, Zhang S (2014) Two mitogen-activated protein kinases, MPK3 and MPK6, are required for funicular guidance of pollen tubes in Arabidopsis. Plant Physiol 165: 528–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafidh S, Fíla J, Honys D (2016a) Male gametophyte development and function in angiosperms: a general concept. Plant Reprod 29: 31–51 [DOI] [PubMed] [Google Scholar]
- Hafidh S, Potěšil D, Fíla J, Čapková V, Zdráhal Z, Honys D (2016b) Quantitative proteomics of the tobacco pollen tube secretome identifies novel pollen tube guidance proteins important for fertilization. Genome Biol 17: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama T. (2002) The synergid cell: attractor and acceptor of the pollen tube for double fertilization. J Plant Res 115: 149–160 [DOI] [PubMed] [Google Scholar]
- Higashiyama T. (2010) Peptide signaling in pollen-pistil interactions. Plant Cell Physiol 51: 177–189 [DOI] [PubMed] [Google Scholar]
- Higashiyama T, Hamamura Y (2008) Gametophytic pollen tube guidance. Sex Plant Reprod 21: 17–26 [Google Scholar]
- Higashiyama T, Inatsugi R, Sakamoto S, Sasaki N, Mori T, Kuroiwa H, Nakada T, Nozaki H, Kuroiwa T, Nakano A (2006) Species preferentiality of the pollen tube attractant derived from the synergid cell of Torenia fournieri. Plant Physiol 142: 481–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama T, Kuroiwa H, Kawano S, Kuroiwa T (1998) Guidance in vitro of the pollen tube to the naked embryo sac of Torenia fournieri. Plant Cell 10: 2019–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama T, Takeuchi H (2015) The mechanism and key molecules involved in pollen tube guidance. Annu Rev Plant Biol 66: 393–413 [DOI] [PubMed] [Google Scholar]
- Higashiyama T, Yabe S, Sasaki N, Nishimura Y, Miyagishima S, Kuroiwa H, Kuroiwa T (2001) Pollen tube attraction by the synergid cell. Science 293: 1480–1483 [DOI] [PubMed] [Google Scholar]
- Horade M, Kanaoka MM, Kuzuya M, Higashiyama T, Kaji N (2013) A microfluidic device for quantitative analysis of chemoattraction in plants. RSC Advances 3: 22301–22307 [Google Scholar]
- Hou Y, Guo X, Cyprys P, Zhang Y, Bleckmann A, Cai L, Huang Q, Luo Y, Gu H, Dresselhaus T, et al. (2016) Maternal ENODLs are required for pollen tube reception in Arabidopsis. Curr Biol 26: 2343–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Dresselhaus T, Gu H, Qu LJ (2015) Active role of small peptides in Arabidopsis reproduction: expression evidence. J Integr Plant Biol 57: 518–521 [DOI] [PubMed] [Google Scholar]
- Hülskamp M, Schneitz K, Pruitt RE (1995) Genetic evidence for a long-range activity that directs pollen tube guidance in Arabidopsis. Plant Cell 7: 57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingouff M, Sakata T, Li J, Sprunck S, Dresselhaus T, Berger F (2009) The two male gametes share equal ability to fertilize the egg cell in Arabidopsis thaliana. Curr Biol 19: R19–R20 [DOI] [PubMed] [Google Scholar]
- Jiao J, Mizukami AG, Sankaranarayanan S, Yamguchi J, Itami K, Higashiyama T (2017) Structure-activity relation of AMOR sugar molecule that activates pollen tubes for ovular guidance. Plant Physiol 173: 354–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Rhoades MW, Borevitz JO, Preuss D (2007) Genome-wide expression profiling of the Arabidopsis female gametophyte identifies families of small, secreted proteins. PLoS Genet 3: 1848–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaoka MM, Higashiyama T (2015) Peptide signaling in pollen tube guidance. Curr Opin Plant Biol 28: 127–136 [DOI] [PubMed] [Google Scholar]
- Kanaoka MM, Kawano N, Matsubara Y, Susaki D, Okuda S, Sasaki N, Higashiyama T (2011) Identification and characterization of TcCRP1, a pollen tube attractant from Torenia concolor. Ann Bot (Lond) 108: 739–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara RD, Maruyama D, Hamamura Y, Sakakibara T, Twell D, Higashiyama T (2012) Fertilization recovery after defective sperm cell release in Arabidopsis. Curr Biol 22: 1084–1089 [DOI] [PubMed] [Google Scholar]
- Kasahara RD, Portereiko MF, Sandaklie-Nikolova L, Rabiger DS, Drews GN (2005) MYB98 is required for pollen tube guidance and synergid cell differentiation in Arabidopsis. Plant Cell 17: 2981–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch E, Dilokpimol A, Geshi N (2014) Arabinogalactan proteins: focus on carbohydrate active enzymes. Front Plant Sci 5: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Lau S, Jürgens G (2015) Twin plants from supernumerary egg cells in Arabidopsis. Curr Biol 25: 225–230 [DOI] [PubMed] [Google Scholar]
- Krohn NG, Lausser A, Juranić M, Dresselhaus T (2012) Egg cell signaling by the secreted peptide ZmEAL1 controls antipodal cell fate. Dev Cell 23: 219–225 [DOI] [PubMed] [Google Scholar]
- Lai Wing Sun K, Correia JP, Kennedy TE (2011) Netrins: versatile extracellular cues with diverse functions. Development 138: 2153–2169 [DOI] [PubMed] [Google Scholar]
- Lawit SJ, Chamberlin MA, Agee A, Caswell ES, Albertsen MC (2013) Transgenic manipulation of plant embryo sacs tracked through cell-type-specific fluorescent markers: cell labeling, cell ablation, and adventitious embryos. Plant Reprod 26: 125–137 [DOI] [PubMed] [Google Scholar]
- Leshem Y, Johnson C, Sundaresan V (2013) Pollen tube entry into the synergid cell of Arabidopsis is observed at a site distinct from the filiform apparatus. Plant Reprod 26: 93–99 [DOI] [PubMed] [Google Scholar]
- Li H, Yang WC (2016) RLKs orchestrate the signaling in plant male-female interaction. Sci China Life Sci 59: 867–877 [DOI] [PubMed] [Google Scholar]
- Li HJ, Xue Y, Jia DJ, Wang T, Hi DQ, Liu J, Cui F, Xie Q, Ye D, Yang WC (2011) POD1 regulates pollen tube guidance in response to micropylar female signaling and acts in early embryo patterning in Arabidopsis. Plant Cell 23: 3288–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Zhu SS, Zhang MX, Wang T, Liang L, Xue Y, Shi DQ, Liu J, Yang WC (2015) Arabidopsis CBP1 is a novel regulator of transcription initiation in central cell-mediated pollen tube guidance. Plant Cell 27: 2880–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Ge FR, Xu M, Zhao XY, Huang GQ, Zhou LZ, Wang JG, Kombrink A, McCormick S, Zhang XS, et al. (2013) Arabidopsis COBRA-LIKE 10, a GPI-anchored protein, mediates directional growth of pollen tubes. Plant J 74: 486–497 [DOI] [PubMed] [Google Scholar]
- Lin SY, Chen PW, Chuang MH, Juntawong P, Bailey-Serres J, Jauh GY (2014) Profiling of translatomes of in vivo-grown pollen tubes reveals genes with roles in micropylar guidance during pollination in Arabidopsis. Plant Cell 26: 602–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhong S, Guo X, Hao L, Wei X, Huang Q, Hou Y, Shi J, Wang C, Gu H, et al. (2013) Membrane-bound RLCKs LIP1 and LIP2 are essential male factors controlling male-female attraction in Arabidopsis. Curr Biol 23: 993–998 [DOI] [PubMed] [Google Scholar]
- Liu Y, Joly V, Dorion S, Rivoal J, Matton DP (2015) The plant ovule secretome: a different view toward pollen-pistil interactions. J Proteome Res 14: 4763–4775 [DOI] [PubMed] [Google Scholar]
- Lu Y, Chanroj S, Zulkifli L, Johnson MA, Uozumi N, Cheung A, Sze H (2011) Pollen tubes lacking a pair of K+ transporters fail to target ovules in Arabidopsis. Plant Cell 23: 81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márton ML, Cordts S, Broadhvest J, Dresselhaus T (2005) Micropylar pollen tube guidance by egg apparatus 1 of maize. Science 307: 573–576 [DOI] [PubMed] [Google Scholar]
- Márton ML, Fastner A, Uebler S, Dresselhaus T (2012) Overcoming hybridization barriers by the secretion of the maize pollen tube attractant ZmEA1 from Arabidopsis ovules. Curr Biol 22: 1194–1198 [DOI] [PubMed] [Google Scholar]
- Maruyama D, Hamamura Y, Takeuchi H, Susaki D, Nishimaki M, Kurihara D, Kasahara RD, Higashiyama T (2013) Independent control by each female gamete prevents the attraction of multiple pollen tubes. Dev Cell 25: 317–323 [DOI] [PubMed] [Google Scholar]
- Maruyama D, Völz R, Takeuchi H, Mori T, Igawa T, Kurihara D, Kawashima T, Ueda M, Ito M, Umeda M, et al. (2015) Rapid elimination of the persistent synergid through a cell fusion mechanism. Cell 161: 907–918 [DOI] [PubMed] [Google Scholar]
- Matsubayashi Y. (2014) Posttranslationally modified small-peptide signals in plants. Annu Rev Plant Biol 65: 385–413 [DOI] [PubMed] [Google Scholar]
- McCue AD, Cresti M, Feijó JA, Slotkin RK (2011) Cytoplasmic connection of sperm cells to the pollen vegetative cell nucleus: potential roles of the male germ unit revisited. J Exp Bot 62: 1621–1631 [DOI] [PubMed] [Google Scholar]
- Michard E, Lima PT, Borges F, Silva AC, Portes MT, Carvalho JE, Gilliham M, Liu LH, Obermeyer G, Feijó JA (2011) Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 332: 434–437 [DOI] [PubMed] [Google Scholar]
- Michard E, Simon AA, Tavares B, Wudick MM, Feijo AA (2017) Signaling with ions: the keystone for apical cell growth and morphogenesis in pollen tubes. Plant Physiol 173: 91–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima M, Takayama S, Sasaki K, Jee JG, Kojima C, Isogai A, Shirakawa M (2003) Structure of the male determinant factor for Brassica self-incompatibility. J Biol Chem 278: 36389–36395 [DOI] [PubMed] [Google Scholar]
- Miyawaki KN, Yang Z (2014) Extracellular signals and receptor-like kinases regulating ROP GTPases in plants. Front Plant Sci 5: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami AG, Inatsugi R, Jiao J, Kotake T, Kuwata K, Ootani K, Okuda S, Sankaranarayanan S, Sato Y, Maruyama D, et al. (2016) The AMOR arabinogalactan sugar chain induces pollen-tube competency to respond to ovular guidance. Curr Biol 26: 1091–1097 [DOI] [PubMed] [Google Scholar]
- Mizuta Y, Kurihara D, Higashiyama T (2015) Two-photon imaging with longer wavelength excitation in intact Arabidopsis tissues. Protoplasma 252: 1231–1240 [DOI] [PubMed] [Google Scholar]
- Morita J, Kato K, Nakane T, Kondo Y, Fukuda H, Nishimasu H, Ishitani R, Nureki O (2016) Crystal structure of the plant receptor-like kinase TDR in complex with the TDIF peptide. Nat Commun 7: 12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motomura K, Berger F, Kawashima T, Kinoshita T, Higashiyama T, Maruyama D (2016) Fertilization-independent cell-fusion between the synergid and central cell in the polycomb mutant. Cell Struct Funct 41: 121–125 [DOI] [PubMed] [Google Scholar]
- Mu JH, Lee HS, Kao TH (1994) Characterization of a pollen-expressed receptor-like kinase gene of Petunia inflata and the activity of its encoded kinase. Plant Cell 6: 709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara S, Takeuchi H, Higashiyama T (2015) Generation of a homozygous fertilization-defective gcs1 mutant by heat-inducible removal of a rescue gene. Plant Reprod 28: 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa-Ohnishi M, Matsubayashi Y (2015) Identification of three potent hydroxyproline O-galactosyltransferases in Arabidopsis. Plant J 81: 736–746 [DOI] [PubMed] [Google Scholar]
- Ohki S, Takeuchi M, Mori M (2011) The NMR structure of stomagen reveals the basis of stomatal density regulation by plant peptide hormones. Nat Commun 2: 512. [DOI] [PubMed] [Google Scholar]
- Okuda S, Suzuki T, Kanaoka MM, Mori H, Sasaki N, Higashiyama T (2013) Acquisition of LURE-binding activity at the pollen tube tip of Torenia fournieri. Mol Plant 6: 1074–1090 [DOI] [PubMed] [Google Scholar]
- Okuda S, Tsutsui H, Shiina K, Sprunck S, Takeuchi H, Yui R, Kasahara RD, Hamamura Y, Mizukami A, Susaki D, et al. (2009) Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 458: 357–361 [DOI] [PubMed] [Google Scholar]
- Pagnussat GC, Yu HJ, Sundaresan V (2007) Cell-fate switch of synergid to egg cell in Arabidopsis eostre mutant embryo sacs arises from misexpression of the BEL1-like homeodomain gene BLH1. Plant Cell 19: 3578–3592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanivelu R, Brass L, Edlund AF, Preuss D (2003) Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 114: 47–59 [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Preuss D (2006) Distinct short-range ovule signals attract or repel Arabidopsis thaliana pollen tubes in vitro. BMC Plant Biol 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AM, Nobre MS, Pinto SC, Lopes AL, Costa ML, Masiero S, Coimbra S (2016) “Love is strong, and you’re so sweet”: JAGGER is essential for persistent synergid degeneration and polytubey block in Arabidopsis thaliana. Mol Plant 9: 601–614 [DOI] [PubMed] [Google Scholar]
- Pereira AM, Pereira LG, Coimbra S (2015) Arabinogalactan proteins: rising attention from plant biologists. Plant Reprod 28: 1–15 [DOI] [PubMed] [Google Scholar]
- Punwani JA, Rabiger DS, Drews GN (2007) MYB98 positively regulates a battery of synergid-expressed genes encoding filiform apparatus localized proteins. Plant Cell 19: 2557–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punwani JA, Rabiger DS, Lloyd A, Drews GN (2008) The MYB98 subcircuit of the synergid gene regulatory network includes genes directly and indirectly regulated by MYB98. Plant J 55: 406–414 [DOI] [PubMed] [Google Scholar]
- Qin Y, Leydon AR, Manziello A, Pandey R, Mount D, Denic S, Vasic B, Johnson MA, Palanivelu R (2009) Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet 5: e1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu LJ, Li L, Lan Z, Dresselhaus T (2015) Peptide signalling during the pollen tube journey and double fertilization. J Exp Bot 66: 5139–5150 [DOI] [PubMed] [Google Scholar]
- Ray SM, Park SS, Ray A (1997) Pollen tube guidance by the female gametophyte. Development 124: 2489–2498 [DOI] [PubMed] [Google Scholar]
- Sanati Nezhad A, Packirisamy M, Geitmann A (2014) Dynamic, high precision targeting of growth modulating agents is able to trigger pollen tube growth reorientation. Plant J 80: 185–195 [DOI] [PubMed] [Google Scholar]
- Santiago J, Brandt B, Wildhagen M, Hohmann U, Hothorn LA, Butenko MA, Hothorn M (2016) Mechanistic insight into a peptide hormone signaling complex mediating floral organ abscission. eLife 5: e15075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J, Henzler C, Hothorn M (2013) Molecular mechanism for plant steroid receptor activation by somatic embryogenesis co-receptor kinases. Science 341: 889–892 [DOI] [PubMed] [Google Scholar]
- Sato Y, Sugimoto N, Higashiyama T, Arata H (2015) Quantification of pollen tube attraction in response to guidance by female gametophyte tissue using artificial microscale pathway. J Biosci Bioeng 120: 697–700 [DOI] [PubMed] [Google Scholar]
- Schmid MW, Schmidt A, Grossniklaus U (2015) The female gametophyte: an emerging model for cell type-specific systems biology in plant development. Front Plant Sci 6: 907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Schmid MW, Grossniklaus U (2015) Plant germline formation: common concepts and developmental flexibility in sexual and asexual reproduction. Development 142: 229–241 [DOI] [PubMed] [Google Scholar]
- Shimizu KK, Ito T, Ishiguro S, Okada K (2008) MAA3 (MAGATAMA3) helicase gene is required for female gametophyte development and pollen tube guidance in Arabidopsis thaliana. Plant Cell Physiol 49: 1478–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein KA, Moskal WA Jr, Wu HC, Underwood BA, Graham MA, Town CD, VandenBosch KA (2007) Small cysteine-rich peptides resembling antimicrobial peptides have been under-predicted in plants. Plant J 51: 262–280 [DOI] [PubMed] [Google Scholar]
- Sprunck S, Gross-Hardt R (2011) Nuclear behavior, cell polarity, and cell specification in the female gametophyte. Sex Plant Reprod 24: 123–136 [DOI] [PubMed] [Google Scholar]
- Stührwohldt N, Dahlke RI, Kutschmar A, Peng X, Sun MX, Sauter M (2015) Phytosulfokine peptide signaling controls pollen tube growth and funicular pollen tube guidance in Arabidopsis thaliana. Physiol Plant 153: 643–653 [DOI] [PubMed] [Google Scholar]
- Sun Y, Li L, Macho AP, Han Z, Hu Z, Zipfel C, Zhou JM, Chai J (2013) Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science 342: 624–628 [DOI] [PubMed] [Google Scholar]
- Susaki D, Takeuchi H, Tsutsui H, Kurihara D, Higashiyama T (2015) Live imaging and laser disruption reveal the dynamics and cell-cell communication during Torenia fournieri female gametophyte development. Plant Cell Physiol 56: 1031–1041 [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Higashiyama T (2012) A species-specific cluster of defensin-like genes encodes diffusible pollen tube attractants in Arabidopsis. PLoS Biol 10: e1001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Higashiyama T (2016) Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 531: 245–248 [DOI] [PubMed] [Google Scholar]
- Tang W, Ezcurra I, Muschietti J, McCormick S (2002) A cysteine-rich extracellular protein, LAT52, interacts with the extracellular domain of the pollen receptor kinase LePRK2. Plant Cell 14: 2277–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Kelley D, Ezcurra I, Cotter R, McCormick S (2004) LeSTIG1, an extracellular binding partner for the pollen receptor kinases LePRK1 and LePRK2, promotes pollen tube growth in vitro. Plant J 39: 343–353 [DOI] [PubMed] [Google Scholar]
- Uebler S, Márton ML, Dresselhaus T (2015) Classification of EA1-box proteins and new insights into their role during reproduction in grasses. Plant Reprod 28: 183–197 [DOI] [PubMed] [Google Scholar]
- Villanueva JM, Broadhvest J, Hauser BA, Meister RJ, Schneitz K, Gasser CS (1999) INNER NO OUTER regulates abaxial-adaxial patterning in Arabidopsis ovules. Genes Dev 13: 3160–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völz R, Heydlauff J, Ripper D, von Lyncker L, Groß-Hardt R (2013) Ethylene signaling is required for synergid degeneration and the establishment of a pollen tube block. Dev Cell 25: 310–316 [DOI] [PubMed] [Google Scholar]
- Wang T, Liang L, Xue Y, Jia PF, Chen W, Zhang MX, Wang YC, Li HJ, Yang WC (2016) A receptor heteromer mediates the male perception of female attractants in plants. Nature 531: 241–244 [DOI] [PubMed] [Google Scholar]
- Wengier D, Valsecchi I, Cabanas ML, Tang WH, McCormick S, Muschietti J (2003) The receptor kinases LePRK1 and LePRK2 associate in pollen and when expressed in yeast, but dissociate in the presence of style extract. Proc Natl Acad Sci USA 100: 6860–6865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woriedh M, Wolf S, Márton ML, Hinze A, Gahrtz M, Becker D, Dresselhaus T (2013) External application of gametophyte-specific ZmPMEI1 induces pollen tube burst in maize. Plant Reprod 26: 255–266 [DOI] [PubMed] [Google Scholar]
- Wuest SE, Vijverberg K, Schmidt A, Weiss M, Gheyselinck J, Lohr M, Wellmer F, Rahnenführer J, von Mering C, Grossniklaus U (2010) Arabidopsis female gametophyte gene expression map reveals similarities between plant and animal gametes. Curr Biol 20: 506–512 [DOI] [PubMed] [Google Scholar]
- Xu K, Wu Z, Renier N, Antipenko A, Tzvetkova-Robev D, Xu Y, Minchenko M, Nardi-Dei V, Rajashankar KR, Himanen J, et al. (2014) Neural migration: structures of netrin-1 bound to two receptors provide insight into its axon guidance mechanism. Science 344: 1275–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan H, Zhong Y, Yang Z, Xia H (2015) Enzyme activities of Arabidopsis inositol polyphosphate kinases AtIPK2α and AtIPK2β are involved in pollen development, pollen tube guidance and embryogenesis. Plant J 82: 758–771 [DOI] [PubMed] [Google Scholar]
- Zhang H, Lin X, Han Z, Qu LJ, Chai J (2016) Crystal structure of PXY-TDIF complex reveals a conserved recognition mechanism among CLE peptide-receptor pairs. Cell Res 26: 543–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, He J, McCormick S (2009) Two Arabidopsis AGC kinases are critical for the polarized growth of pollen tubes. Plant J 58: 474–484 [DOI] [PubMed] [Google Scholar]
- Zhao XY, Wang Q, Li S, Ge FR, Zhou LZ, McCormick S, Zhang Y (2013) The juxtamembrane and carboxy-terminal domains of Arabidopsis PRK2 are critical for ROP-induced growth in pollen tubes. J Exp Bot 64: 5599–5610 [DOI] [PMC free article] [PubMed] [Google Scholar]