Diurnal photoprotection in Oryza sativa reduces the curvature factor of the relationship between electron transport rate and irradiance, reducing diurnal radiation use efficiency in comparison with steady-state measurements in the same leaves.
Abstract
Genetic improvement of photosynthetic performance of cereal crops and increasing the efficiency with which solar radiation is converted into biomass has recently become a major focus for crop physiologists and breeders. The pulse amplitude modulated chlorophyll fluorescence technique (PAM) allows quantitative leaf level monitoring of the utilization of energy for photochemical light conversion and photoprotection in natural environments, potentially over the entire crop lifecycle. Here, the diurnal relationship between electron transport rate (ETR) and irradiance was measured in five cultivars of rice (Oryza sativa) in canopy conditions with PAM fluorescence under natural solar radiation. This relationship differed substantially from that observed for conventional short term light response curves measured under controlled actinic light with the same leaves. This difference was characterized by a reduced curvature factor when curve fitting was used to model this diurnal response. The engagement of photoprotective processes in chloroplast electron transport in leaves under canopy solar radiation was shown to be a major contributor to this difference. Genotypic variation in the irradiance at which energy flux into photoprotective dissipation became greater than ETR was observed. Cultivars capable of higher ETR at midrange light intensities were shown to produce greater leaf area over time, estimated by noninvasive imaging.
Improvement in rice (Oryza sativa) yields is required to match projected global population growth and forecasted future demand for food (Mitchell et al., 1998; Long et al., 2006; Evans, 2013). Rice is the most important crop for global food security, given that 3.4 billion people are dependent on it as their staple calorie source worldwide (Seck et al., 2012). There has been little improvement in rice yield potential over the past 40 years, likely as a result of post-green-revolution breeding efforts exhausting advances in grain number and harvest index (partitioning of biomass into grain), now considered to have reached a maximum (see Parry et al., 2011, and refs. therein). Increasing the efficiency with which solar energy is converted to biomass through improving the efficiency of photosynthetic pathways has been highlighted as an area with great unexploited potential (Sheehy, 2001; Long et al., 2006; Murchie et al., 2009). The theoretical upper limit of C3 plant photosynthetic efficiency is approximately 4.6% of incoming solar radiation under atmospheric CO2 (Zhu et al., 2008, 2010). In reality, photosynthetic efficiency in the field falls well short of this ceiling, at around 3.6% (Ort et al., 2011). In support of the likely positive impact of a strategy to improve photosynthetic efficiency on yield, increases in CO2 assimilation rate under elevated CO2 have indeed resulted in large increases in plant biomass in rice (Ainsworth, 2008; Liu et al., 2008; Hasegawa et al., 2013) and soybean (Bernacchi et al., 2005; Leakey et al., 2009).
Prior to the recent focus on radiation use efficiency, traditional breeding for photosynthetic traits has not been a common strategy in any major cereal crop, in part due to the difficulty in measuring photosynthesis in high throughput (Parry et al., 2011; Furbank and Tester, 2011). Recent attempts in wheat (Driever et al., 2014) to screen germplasm using modeled parameters derived from the response of assimilation to CO2 concentration (Farquhar et al., 1980; von Caemmerer, 2000) indicate substantial genetic variation in photosynthetic capacity and efficiency. However, as approximately 30 min is required to generate a gas analysis data set to derive these modeled parameters, the size of germplasm diversity panels and mapping populations that can be practically measured is limited. Other limitations of gas exchange techniques include the sensitivity of assimilation measurements to stomatal conductance, the difficulty in measuring a number of leaves over development of the crop, and the challenges of interpreting such gas analysis data in terms of the canopy light environment, given that leaves are commonly clamped into a cuvette and artificially illuminated. All of these factors may have also contributed to the long-held view that the photosynthetic rate of leaves did not limit or in fact correlate with yield potential in cereals (Evans, 1975).
An alternative to gas exchange as an estimate of photosynthetic performance is to use pulse amplified modulated (PAM) chlorophyll fluorescence to calculate chloroplast electron transport rate (ETR; Baker et al., 2007). This technique can be utilized under ambient illumination, in air without an enclosed cuvette, and offers quantitative insight into the fate of light harvested by photosystem 2. As it captures both energy use associated with the Calvin cycle and the photo respiratory cycle rather than just measuring fixation of CO2 via Rubisco, it is less dependent on stomatal behavior. By using quenching analysis (Genty et al., 1989) calculation of the partitioning of energy into photoprotective pathways also enables longer term measurements that consider changing crop canopy light dynamics. This is particularly important in a canopy environment, as fluctuating light conditions also trigger the transient photoprotective responses of nonphotochemical quenching (NPQ) When sunlight is absorbed in excess of a plant’s ability to fix CO2, conformational changes in Psbs, and a decrease in lumen pH activates the xanthophyll cycle, whereby violaxanthin is converted to zeaxanthin, via antheraxanthin, by the catalyst violaxanthin de-epoxidase. Through these process, damaging singlet-excited chlorophylls are dissipated as heat to prevent irreversible degradation of the D1 protein and maintain the integrity of photosystem 2 (Müller et al., 2001). However, this can further widen the gap between actual photosynthetic efficiency and crop theoretical solar conversion efficiency if photoprotection is “inappropriately” engaged under fluctuating irradiance due to the long relaxation time for such processes (Horton et al., 2001; Zhu et al., 2004; Murchie and Niyogi, 2011). It has also been proposed that overengagement of photoprotection could be due to evolutionary pressure for survival under stress, resulting in conservatism in photoprotection not appropriate to an agronomic environment (Horton, 1994). Evidence of the need for a “tradeoff” between resistance to photoinhibition and the ability to respond rapidly to fluctuating light has also been provided by the study of transgenic rice in which levels of the PsbS protein in photosystem 2 had been altered by RNAi and overexpression, resulting in large effects on the ability of these plants to establish NPQ (Hubbart et al., 2012). In the field, environmental conditions such as temperature and humidity also vary both diurnally and seasonally so that the leaf level response to these parameters prevents photosynthesis in the field from operating under the steady-state conditions commonly used for photosynthetic measurements in the glasshouse and laboratory. Thus, while gas exchange measurements of the response of CO2 assimilation to CO2 or light under laboratory conditions may allow estimation of photosynthetic capacity, they may not reflect the realization of this capacity under field conditions. In fact, it has been established that in a rice canopy environment, leaves are rarely performing photosynthesis at their maximum capacity (Sheehy and Mitchell, 2013), more commonly experiencing short periods where light is either limiting for or in excess of photosynthetic demand from carbon metabolism. If increases in crop biomass are to be achieved through breeding for crop radiation use efficiency, methods of measuring photosynthetic performance in high throughput and as a function of changing environmental conditions in a canopy are required (Furbank and Tester, 2011).
Here, we assess leaf level photosynthetic performance in rice in a canopy environment over a number of days of the diurnal time course, under natural illumination and atmospheric conditions, in a glasshouse. We measured chlorophyll fluorescence of rice leaves in a canopy every 30 min throughout the photoperiod using a PAM fluorescence sensor attached to the leaf over a period of days. The data were then used to calculate diurnal changes in photosynthetic energy conversion efficiency. This technique is based upon the well-documented correlation between the quantum yield of PSII (φPSII), electron transport rate, and CO2 assimilation (Edwards and Baker, 1993; Fryer et al., 1998; Cheng et al., 2001; see Supplemental Fig. S1). Light response curves for ETR were generated for each leaf either by (1) plotting ETR against irradiance reaching the leaf surface throughout the day at each time point or (2) by plotting ETR against irradiance when light was applied in a number of incremental steps by an exogenous light source over a 30 to 40 min period without solar radiation, as is commonly performed in laboratory-based measurements of gas exchange. These measurements were used to probe leaf-level radiation use efficiency, genotypic differences in photochemical efficiency in the light, maximum photosynthetic efficiency of light harvesting and leaf photosynthetic capacity, and to explore genotypic variation in photosynthetic and photoprotective responses to irradiance.
RESULTS
The Diurnal Response of ETR to Irradiance in cv Nipponbare
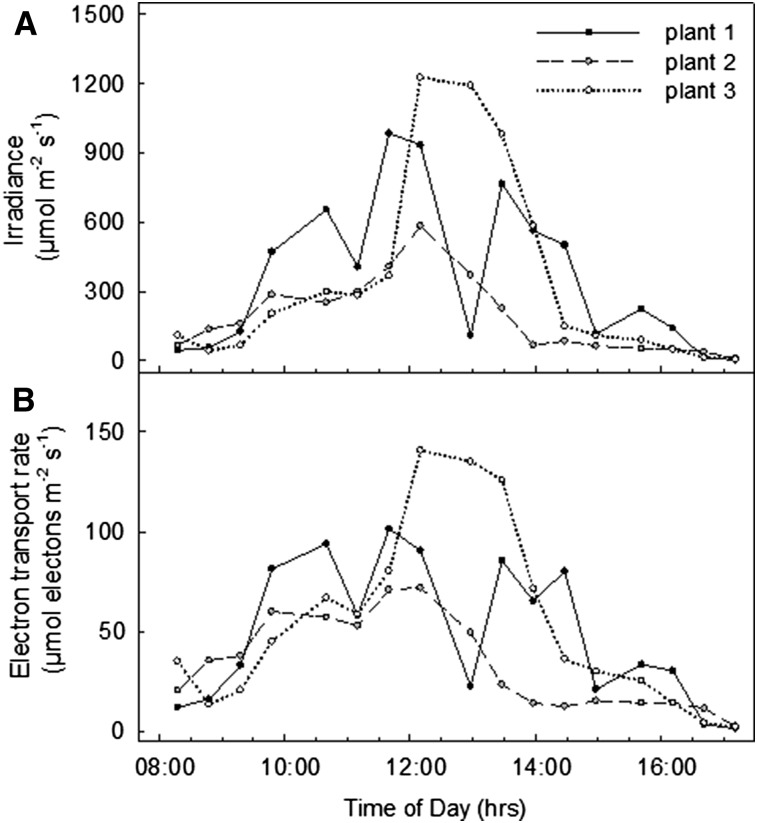
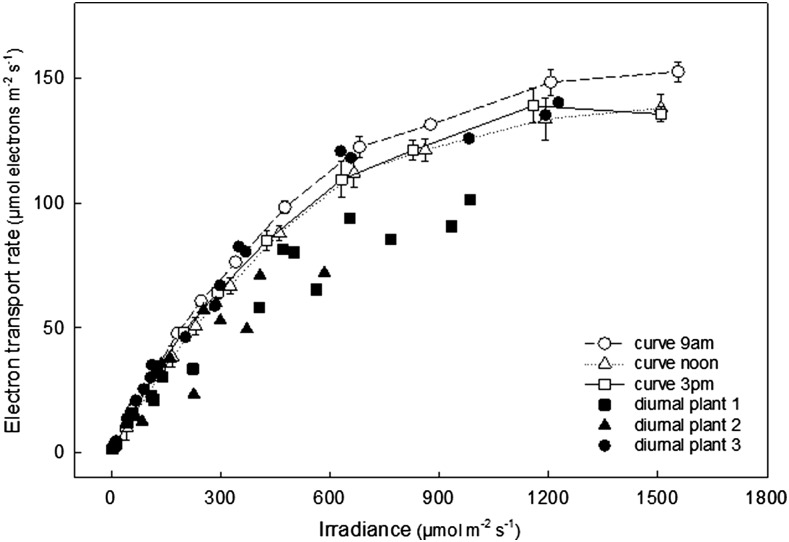
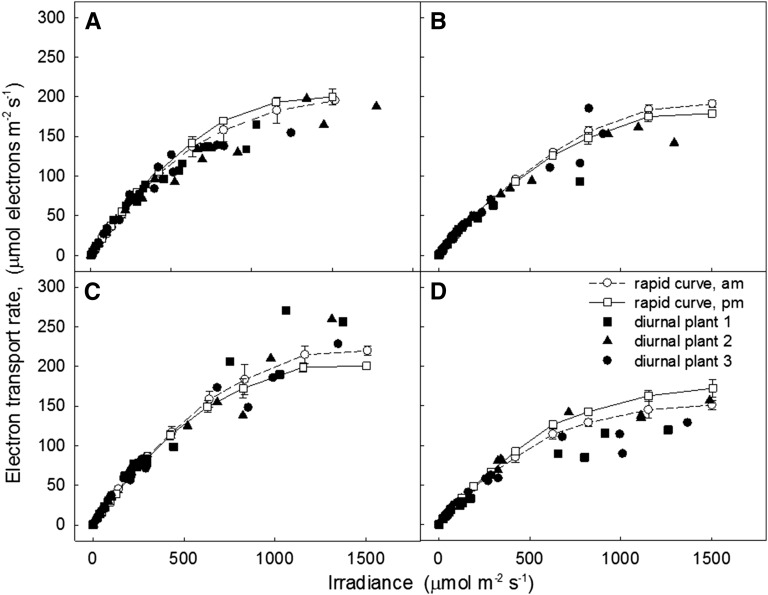
Figure 1 shows a typical plot of light intercepted by leaf 13 of the rice cultivar Nipponbare (PAR measured at and in the plane of the leaf surface) and ETR calculated from PAM chlorophyll fluorescence measured at 30 min intervals over the course of a day. As expected, as intercepted irradiance increased toward midday with increased sunlight, ETR broadly increased and then subsequently declined as sunlight decreased through the late afternoon. However, depending on the orientation of the individual leaf in the canopy, the contribution of direct solar radiation varied from leaf to leaf throughout the day. In Figure 2, these data are replotted as ETR against each irradiance for the three leaves at each point of measurement over the diurnal time course. This diurnal response of ETR to solar irradiance is then compared to a more conventional light response curve with solar irradiation excluded by covering the leaf and actinic light provided by the measuring head of the Walz Monitoring PAM device (total curve time approximately 42 min; Fig. 2). In general, the shape of the diurnal solar light response curves differed considerably from the conventional curves (Fig. 2). The conventional light response curves were similar to those commonly reported in the literature for rice, regardless of the time of day and similar for all leaves of this cultivar. However, for the diurnal solar response curves, ETR of most leaves measured fell below that achieved for a given irradiance in the conventional response curves and displayed reduced curvature of the response to irradiance.
Figure 1.
Intercepted irradiance (A) and ETR (B) measured at 30 min intervals throughout a day in leaf 13 of three plants of rice cv Nipponbare at 122 DAS, with Walz Monitoring PAM.
Figure 2.
The diurnal relationship between ETR and irradiance in leaf 13 of rice cv Nipponbare at 122 DAS as a function of natural intercepted irradiance (as shown in Fig. 1) in black symbols. Conventional light response curves were made on the same leaves, with the same measuring heads on the same day, at 9 a.m., noon, and 3 p.m., but with light intensity incrementing from 0 saturating intensity from the actinic light source inside the measuring head. Natural irradiance was blocked out for conventional light response curve duration, with 3.5 min acclimation time at a given light intensity (total curve time ∼42 min).
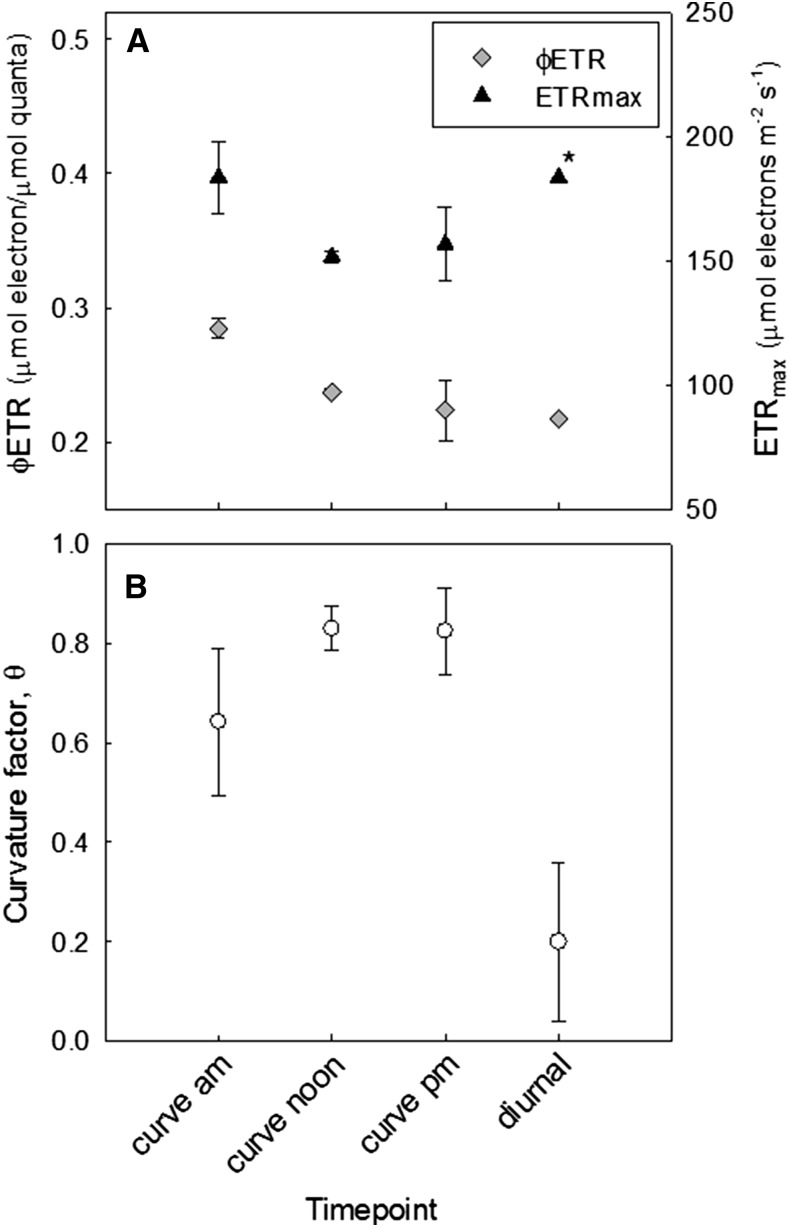
In order to quantify the shape of these curves, the data were fitted using the electron transport (J) model as described in von Caemmerer (2000), using a least sum of squares function to iteratively reduce the error between collected data and the model by adjusting outputs φETR, θ, and ETRmax (see “Materials and Methods”; Supplemental Fig. S3 and supplementary ETR curve fitting utility). When comparing diurnal data to conventional light response, ETRmax was constrained in the curve fitting utility for the diurnal data set to the maximum ETR value estimated from fitting the conventional light response curves in the same leaves on the same day (e.g. 183.6 µmol electrons m−2 s−1 in cv Nipponbare leaf 13 at 122 DAS [days after sowing]; Fig. 5 legend). The parameters calculated from the outputs of this model are shown in Figure 3. No significant difference was observed between the slopes of the diurnal and conventional response curves at low irradiance, i.e. in the quantum yield of ETR (Fig. 3A). The major difference between the diurnal and rapid curves was a reduction in curvature factor (θ) in the diurnal curves by up to 4 fold (Fig. 3B).
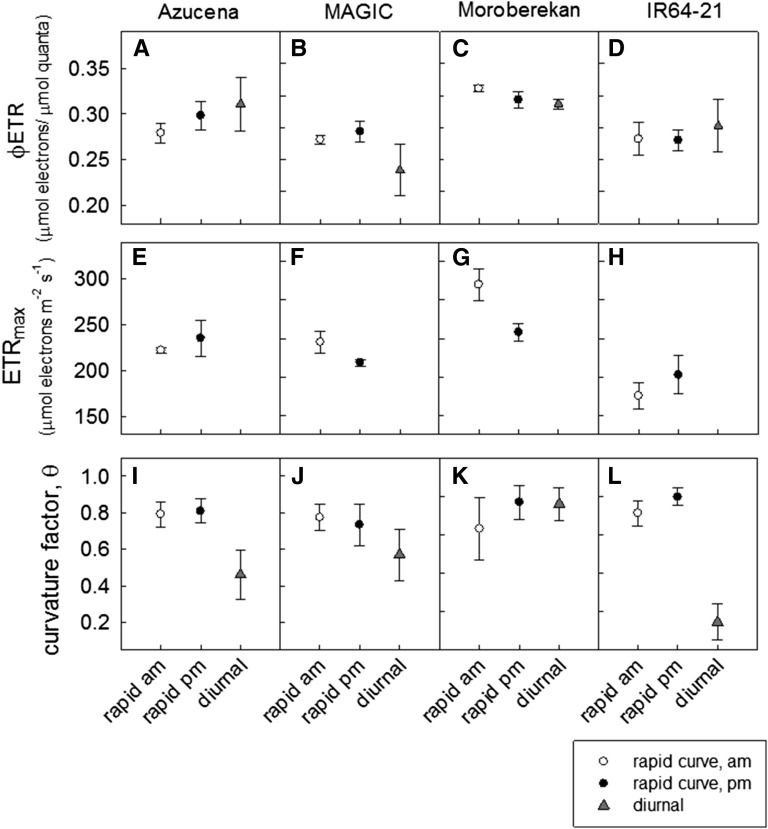
Figure 5.
Quantum yield of ETR (A–D), ETRmax (E–H), and the curvature factor θ (I–L) for the diurnal and conventional (rapid) curves response of ETR to irradiance in leaf 8 of four cultivars of rice. All parameters are obtained from using Equation 3 using the Microsoft Excel curve fitting utility attached as Supplemental Data S1. For calculation of diurnal model parameters φETR and θ, ETRmax was constrained at the highest value predicted from fitting the conventional response curves for each cultivar as Azucena 235.1 ± 20.6, MAGIC 236.2 ± 11.32, Moroberekan 296.2 ± 16.4, and IR64-21 202 ± 19.8.
Figure 3.
Quantum yield of ETR and ETRmax (A) and the curvature factor θ (B) of the modeled response of ETR to irradiance is compared for the conventional response measurements and the diurnal response of ETR to irradiance in leaf 13 of cv Nipponbare at 122 DAS. Parameters were generated from Equation 3 using the Microsoft Excel curve fitting utility attached as Supplemental Data S1. For calculation of diurnal model parameters, ETRmax* was constrained at the highest value predicted from fitting the conventional response curves, from the am curve, at the value 183.6 µmol electrons m−2 s−1.
The Diurnal Response of ETR to Irradiance in Four Rice Cultivars
The possibility that there may be genetic variation in the response of photosynthetic electron transport to diurnal irradiance was examined by comparing diurnal and conventional ETR versus irradiance curves in four rice cultivars. Again, the diurnal response differed from the rapid curves in all cases by varying degrees (Fig. 4). In cvs Azucena, MAGIC, and IR64-21, the diurnal rates of electron transport fell below that observed for a given irradiance in the conventional response curves at irradiances higher than the linear quantum yield region. In contrast, in cv Moroberekan, diurnal rates of electron transport at higher light intensities were substantially higher than observed in the conventional response curves.
Figure 4.
The relationship between electron transport and irradiance, measured with Walz Monitoring PAM, diurnally (black symbols) and with conventional light response curves (white symbols), on leaf 8 for four genotypes: Azucena (A), MAGIC, IR77298-14-1-2-10 (B), Moroberekan (C), and IR64-21 (D). Measurements in Azucena and MAGIC were made at 70 DAS on November 3, 2014 (A and B), and in Moroberekan and IR64-21 at 72 DAS on November 5, 2014 (C and D). Conventional and diurnal response is collected with the method described in Figure 2.
In all cases, there was a less obvious saturating hyperbolic relationship between ETR and irradiance under high natural intercepted solar irradiance for the diurnal measurements compared to the conventional response curves. However, similar to the results shown for leaf 13 of cv Nipponbare (Fig. 2), there are some individual replicates of the diurnal response in leaf 8 from the three individual plants in the four genotypes studied that follow the rapid curve response more closely than others.
As with Nipponbare, the diurnal solar irradiance response curves of ETR for these four cultivars could be fitted with a curvature factor between 0 and 1 with the curve-fitting utility if ETRmax was constrained at an estimated value from fitting the conventional light response curves (Fig. 5). From the modeled parameters, no significant differences were seen between φETR from conventional light response curves and the diurnal solar time course-derived curves for each cultivar (Fig. 5, A–D). As in cv Nipponbare, where diurnal values of ETR for a given irradiance fell below that observed from conventional light response curves, the curvature factor was significantly lower, up to 4-fold in the case of IR64 (Fig. 5L). In contrast, for cv Moroberekan, there was no difference between diurnal and rapid response curvature factor values (Fig. 5C). In all cases, leaf temperature followed the diurnal pattern, and the application of aluminum foil did not affect leaf temperature significantly during the conventional response curve measuring period.
ETR and Photoprotection in cv Nipponbare
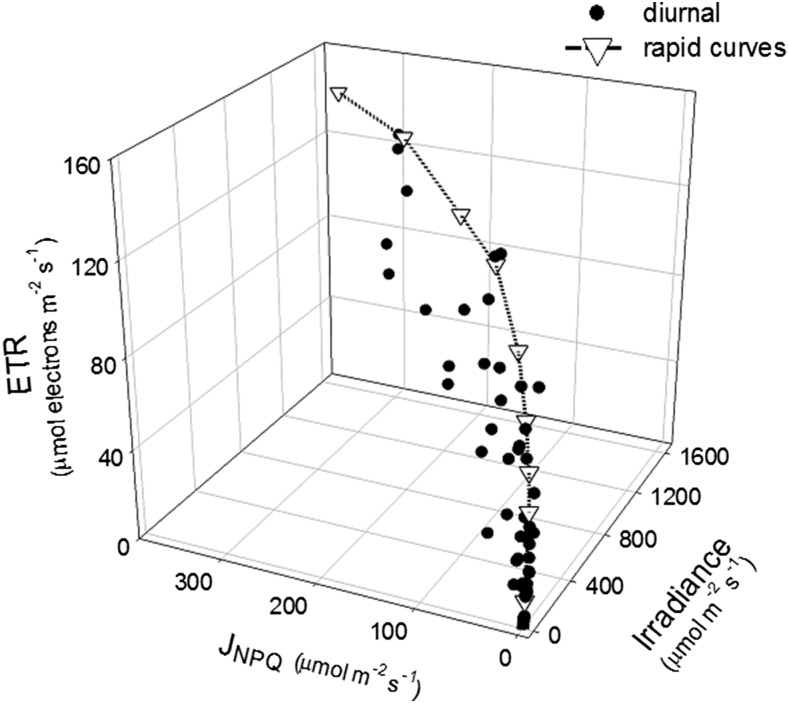
It is possible that the differences between conventional light response curves of ETR and those derived from diurnal solar irradiance shown above could result from preconditioning of NPQ engagement in leaves to the variable light environment of the glasshouse. To examine this, NPQ was expressed as a flux in the same units as ETR (JNPQ; Hendrickson et al., 2004). The relationship between ETR, JNPQ, and irradiance for leaf 13 of cv Nipponbare showed variation in the value of ETR for any given JNPQ between the diurnal and rapid response curve measurements, apart from at very low irradiance (Fig. 6). Notably, JNPQ estimated in diurnally derived ETR versus irradiance curves was significantly higher per unit of irradiance than in rapid response measurements P ≤ 0.05, (Fig. 6).
Figure 6.
The relationship between ETR and JNPQ to irradiance in cv Nipponbare leaf 13 at 122 DAS, calculated from diurnal fluorescence measurements and conventional response curves. ETR and JNPQ from conventional curves at three time points throughout the day are combined to form a single conventional response trend. The diurnal data represent leaf 13 of three plants all plotted individually.
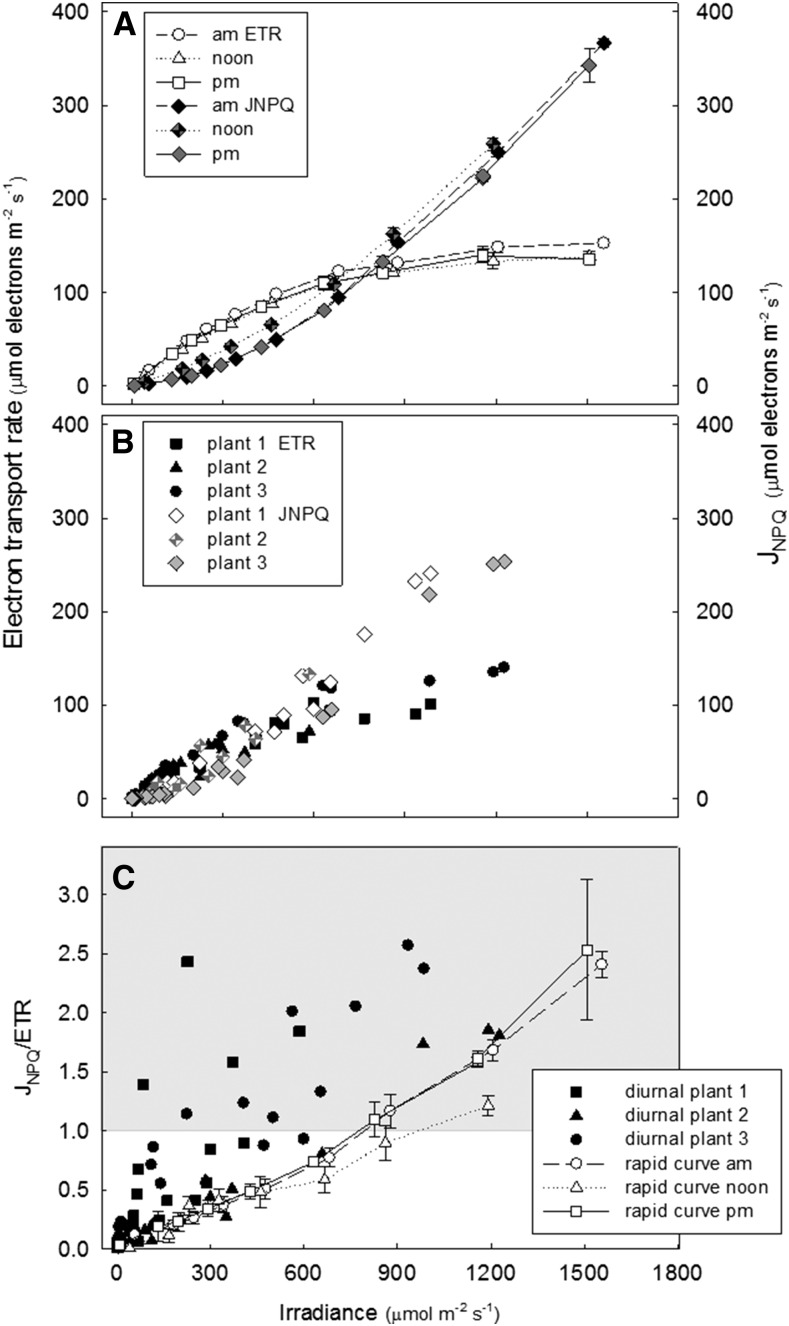
The above data suggests that for a given ETR in a diurnal solar response, NPQ is more actively engaged than in a conventional light response curve. This was examined more quantitatively in Figure 7. For cv Nipponbare, JNPQ calculated from a set of conventional ETR light response curves either in the morning, noon, or midafternoon followed a similar concave upwards pattern, and ETR followed a saturating nonrectangular hyperbola, as expected. JNPQ intercepted the ETR/irradiance response at 120 µmol electrons m−2 s−1 ± 5, at irradiance 780 µmol m−2 s−1 ± 80 (Fig. 7A). This intercept indicates the point at which heat dissipation (estimated as JNPQ) became greater than photochemistry (ETR). In the diurnal ETR versus solar irradiation response for the same leaves (Fig. 7B), a similar response was seen, but the intersection of ETR and JNPQ was more difficult to define due to more variation between replicate leaves but appeared to occur at a lower irradiance in the diurnal measurements when compared to conventional curves.
Figure 7.
A and B, ETR and JNPQ response to irradiance, calculated with Equations 2 and 5, respectively, from conventional light response curves in the morning, noon, and afternoon (A) and diurnally with saturating flashes made at 30 min intervals as a function of solar irradiance with Walz Monitoring PAM (B). Measurements were made on leaf 13 of cv Nipponbare at 122 DAS (n = 3). C, The ratio of JNPQ/ETR at a given irradiance calculated for the diurnal and conventional response curves in the same leaves. The shaded area begins where JNPQ/ETR = 1 and signifies where JNPQ becomes greater than ETR.
JNPQ per unit ETR in leaf 13 of cv Nipponbare, is shown in Figure 7C for each plant analyzed diurnally, and for the rapid response curves. Typically, there was variation between the responses of the three replicate leaves, but the light intensity at which JNPQ equaled flux through photochemistry was significantly higher in conventional light response curves than in the diurnal measurements. This plot clearly indicates that there was much more variation in JNPQ in the diurnal dataset for a given light intensity, both between individual leaves of a plant throughout the day and between the individual plants analyzed. This clearly shows there is no strict relationship between ETR and JNPQ in the diurnal response, unlike in the conventional light response data, possibly due to the variability in local light environment in which each leaf is exposed to prior to measurement over the diurnal time course.
ETR and Photoprotection in Four Cultivars
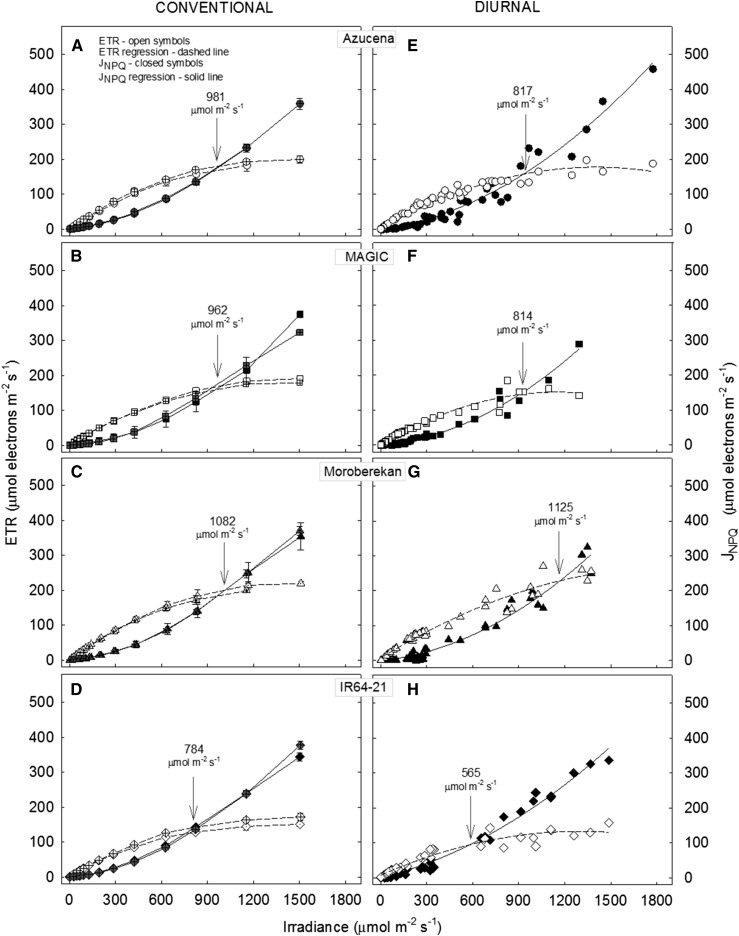
Figure 8 explores the possibility for genetic variation in the relationship between JNPQ, ETR, and irradiance shown above for cv Nipponbare. The response of JNPQ to irradiance showed the same concave upwards shape for all four cultivars examined (Fig. 8, A–H) as seen in cv Nipponbare (Fig. 7, A and B) and the intersect of the ETR and JNPQ light response curves was again more clear from conventional response curves. However, in all cases except for Moroberekan, the diurnal solar radiation response curves resulted in JNPQ exceeding ETR at lower irradiance than under conventional ETR versus light response curve conditions.
Figure 8.
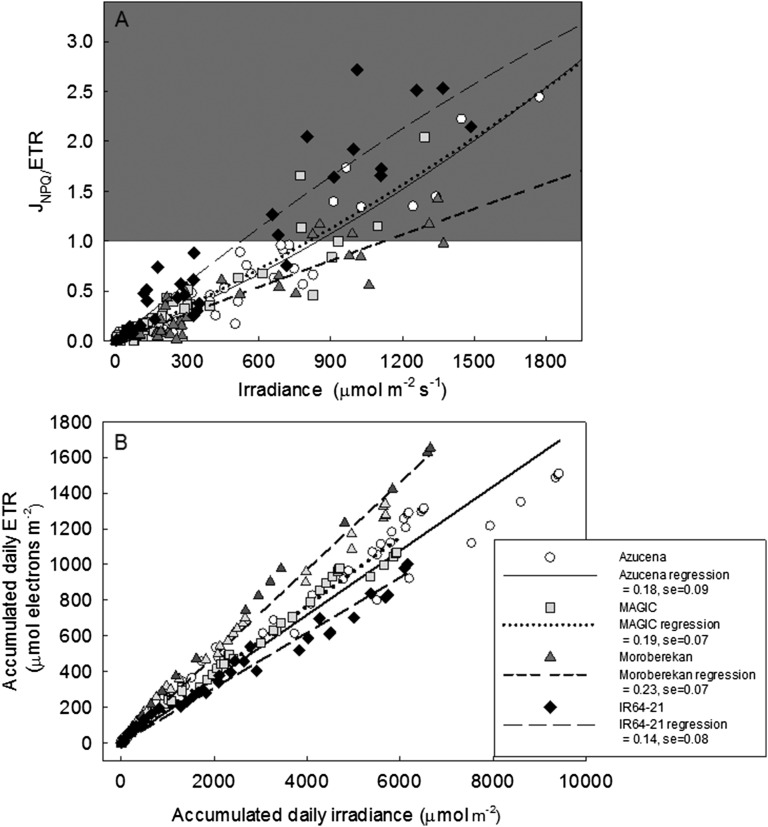
The response of ETR and JNPQ to irradiance in leaf 8 of four cultivars of rice: Azucena, MAGIC, IR64-21, and Moroberekan. A to D show the response from conventional light response curves at 10.30 a.m. and 2.30 p.m., with Walz Monitoring PAM in each cultivar. For each conventional light response curve, ETR and JNPQ were calculated from a saturating pulse incrementing from 0 to 1800 µmol m−2 s−1 in 3.5 min intervals (total curve time ∼5 min), using actinic light source in the measuring heads. The diurnal response of ETR and JNPQ to irradiance was measured in the same leaves, on the same day with the same device, but at 30 min intervals as a function of naturally intercepted irradiance in each cultivar (E–H). Irradiance at JNPQ/ETR = 1 for four cultivars for conventional and diurnal measurements made is shown with arrows as calculated by solving best fit second-order polynomial regression (y = ax2 + bx + 0) for both conventional curves and the diurnal response.
From the data in Figure 8, a second-order polynomial fit for the ratio JNPQ/ETR plotted against irradiance was carried out for each cultivar, and the intersection threshold was calculated (i.e. JNPQ = 1; Supplemental Table S1). This showed that there was variation in the threshold irradiance at which JNPQ exceeded ETR between cultivars when calculated from the conventional response curves, but more markedly in the diurnal response (arrows show the point of intersection of the fitted JNPQ curve with ETR and irradiance values for this point are shown on Fig. 8). There were also differences between the conventional and diurnal measurements in the threshold irradiance for JNPQ/ETR = 1 within the same cultivar, the largest difference being for IR64-21 (Fig. 8, D and H). This suggests there is genotypic variation in photoprotective capacity or engagement and that the threshold irradiance under which photoprotection is substantively engaged diurnally under natural solar irradiance is not reflected in conventional light response curves.
Genetic variation in the relationship between JNPQ/ETR and diurnal irradiance was further explored in Figure 9A by extending the study of the same four cultivars over two photoperiods (70 and 72 DAS). Again, cv IR64-21 displayed a higher JNPQ/ETR over the course of the day, while cv Moroberekan had a lower diurnal ratio of JNPQ/ETR, and JNPQ exceeded ETR at substantially higher light intensities.
Figure 9.
A, The fraction of JNPQ/ETR calculated for four cultivars for the diurnal conventional light response in leaf 8 of four cultivars at 70 and 72 DAS from measurements of ETR and irradiance at 30 min intervals with Walz Monitoring PAM. The shaded area begins at JNPQ/ETR = 1 and signifies where JNPQ exceeds ETR. Best fit second-order polynomial regression is applied to the dataset for each cultivar (y = ax2 + bx + c, c = 0), with coefficients, R2 value, and the irradiance at which JNPQ/ETR = 1 presented in Supplemental Table S1. B, The relationship between daily accumulated ETR and daily accumulated irradiance in leaf 8 of four cultivars of rice, for the same dataset. All leaves for a single genotype are plotted as a single series with linear regression for each cultivar. The value of the linear regression slope (in legend) gives daily ETe for each genotype.
When daily electron transport efficiency (ETe) was calculated as the slope of the relationship between accumulated daily ETR and irradiance (Fig. 9B), there were also differences in this value between genotypes (Fig. 9B). This variation appeared to be correlated with the irradiance at which JNPQ exceeded ETR for each cultivar. Cultivars where photoprotective flux exceeded ETR at lower light intensities (such as IR64-21) exhibited lower slopes, termed here “diurnal ETe.”
ETe and Biomass Accumulation
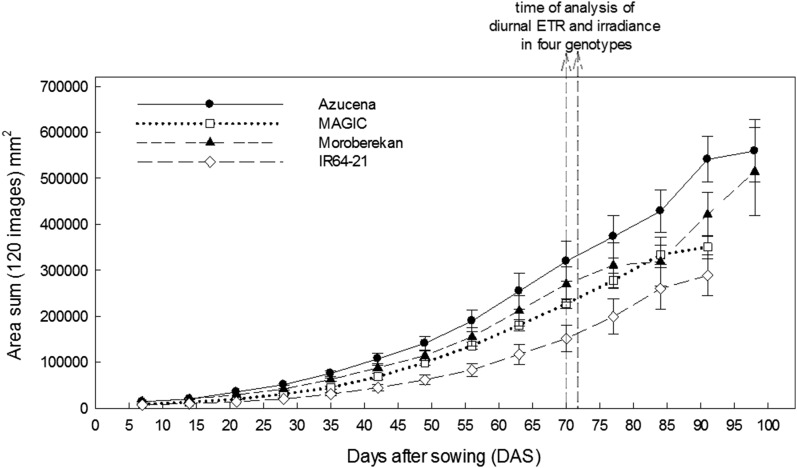
Although destructive harvest for analysis of differences in growth between rice cultivars was not feasible due to physical limitations, plants grown in pots within the canopy were removed once per week and scanned to obtain “digital biomass,” a proxy based on the area of green pixels from 120 side views of each plant (Fig. 10). Such biomass proxies using projected area are routinely used in commercial phenotyping platforms, and high correlations with destructive determination of shoot biomass and leaf area have been obtained for cereals with just three camera views (e.g. Rajendran et al., 2009). On the days when the diurnal and conventional responses of ETR and JNPQ to irradiance were compared in the four cultivars, cv Azucena had accumulated more digital biomass than any of the other cultivars, and IR64-21 the lowest (Fig. 10). Accumulated digital biomass for each cultivar did not always correlate with the irradiance at which photoprotection exceeded ETR for that cultivar or with diurnal ETe, for example cv Moroberekan showed the highest values for all parameters but ranked second in digital biomass production. Notably, 72 DAS, cv IR64-61 showed the lowest accumulated daily ETR, lowest daily ETe, and the lowest accumulated biomass (Fig. 10). Correlations between diurnal ETR efficiency, photoprotective engagement, and digital biomass accumulation was also seen in cv MAGIC.
Figure 10.
Projected 2D area sum over time in four cultivars. Area sum is indicative of “digital biomass,” calculated as the summed area of green pixels from 120 2D images, from periodical weekly scanning in the Plantscan system. Each data point represents the mean area sum from a daily scan of four plants of each cultivar. Dashed arrows indicate the DAS at which diurnal photosynthetic data presented in Figure 4 were collected for each genotype.
DISCUSSION
Selecting cereal crop germplasm for lines with superior photosynthetic performance requires the development of rapid screening tools that can be used on diversity panels of many hundred, if not thousands, of lines or mapping populations that have been genotyped or sequenced (see Parry et al., 2011, Furbank and Tester, 2011; Flood et al., 2011; Gu et al., 2012). Thus far, medium to large-scale screening for photosynthetic traits in rice and wheat to explore genetic variation has been largely limited to CO2 assimilation rate derived from single-leaf gas exchange measurements and/or ETR derived from PAM at one or perhaps two developmental stages (Gu et al., 2012, Driever et al., 2014). It has been difficult to correlate these measurements with yield and biomass, possibly due to the lack of temporal resolution of the measurements over development and lack of extrapolation to multiple leaves in the canopy environment (Driever et al., 2014). Modeling the response of CO2 assimilation to CO2 concentration in leaf intercellular spaces (A versus Ci) is well developed (see Farquhar et al., 1980; von Caemmerer, 2000) and may provide more robust parameters for genetic mapping than single point measurements of assimilation. For example Vcmax, a parameter derived from the initial slope of the A versus Ci curve reflects the amount and kinetic properties of active Rubisco in a leaf and is a widely used metric for photosynthetic capacity (Gu et al., 2012, Driever et al., 2014). However, each A versus Ci curve requires 20 to 30 min to complete, reducing the utility of this method for high-throughput screening across plant development or even at multiple times during the photoperiod.
In field canopy conditions, light levels will vary greatly from the top to the bottom of the canopy diurnally, and light intensity is likely to be the greatest driver of variation in the rate of photosynthesis under nonstressed conditions in all but the leaves highest in the canopy, which will most likely be colimited by electron transport capacity and Rubisco levels (Monteith, 1972; Reynolds et al., 2000; Zhu et al., 2010). Fitting of conventional instantaneous light response curves provides a quantitative estimation of this maximum capacity of chloroplast electron transport in measured leaves (presented usually as Jmax) and also quantum efficiency of light capture (φ) from the initial slope (von Caemmerer, 2000). However, rice leaves in canopy conditions are rarely operating at their maximum capacity or at the very low irradiance levels pertinent to the linear portion of the light response curve (Murchie et al., 1999). Most leaves in a canopy will be harvesting light at intermediate light intensities where the modeled response of photosynthesis is described only by the empirical curvature value θ (see Fig. 1). Complicating attempts to develop strategies for improvement of photosynthetic performance in the field is also the fact that leaves in a canopy encounter frequent transients in irradiance (Pearcy, 1990; Pearcy et al., 1996) due to the changing diurnal azimuth of the sun, movement in cloud cover, winds, and shading from upper leaves and surrounding plants. These transient conditions trigger a suite of photoprotective processes that balance the utilization of light with the need to dissipate damaging excess light (Demmig-Adams and Adams, 1992; Müller et al., 2001).
In this study, we explored diurnal changes in light use for photosynthetic electron transport under physiologically relevant irradiance in a canopy environment by using pulse modulated chlorophyll fluorescence with an open leaf clip incorporating a light sensor, attached to leaves at midcanopy height for a number of days. This approach allowed us to determine if time of day and the canopy environment influenced the apportioning of solar radiation absorbed between photochemistry and photoprotection by calculating ETR and JNPQ from saturating light flashes provided at 30 min intervals. Somewhat surprisingly, when solar PAR at each of these time points was used to calculate ETR for each light intensity for three leaves of a given genotype, robust relationships could be observed where these response curves fell clearly on the initial slope of the more conventional light response curve carried out over a period of ∼40 min (Fig. 2; Supplemental Fig. S3). In general, however, ETR fell below that derived from conventional light response curves at intermediate irradiance. We interpret this behavior in terms of engagement of photoprotective nonphotochemical dissipatory processes to prevent irreparable photodamage to the PSII reaction centers when excitation exceeds utilization by carbon metabolism (Schreiber et al., 1986; Foyer et al., 1990; Müller et al., 2001; Murchie and Niyogi, 2011). This behavior removes the clear transition to saturation of ETR in the midrange light intensities (400–1200 µmol) seen in conventional light response curves and reduces curvature factor from between 0.6 and 0.8 to less than 0.2 in cv Nipponbare (Fig. 3). Similar behavior was observed for 3 out of 4 other genotypes examined (Fig. 5).
The data discussed above presents an apparent paradox. Depression of curvature in the ETR versus radiance response is presumably due to increased engagement of photoprotection at moderate light intensities in the canopy compared to the conventional, more rapid curves. If this were progressive throughout the day and slowly relaxing (as one would expect with xanthophyll cycle-dependent quenching or qi; Demmig-Adams and Adams., 1992), a hysteresis in the diurnal response of ETR to irradiance would occur, and an effect would be observed on conventional curves depending on whether they were measured a.m., noon, or p.m. Neither of these phenomena are observed in the data obtained here. These observations suggest that “preconditioning” of NPQ of leaves in the canopy environment fully relaxes within the timeframe of adapting leaves in preparation for a conventional light response curve and is not reestablished during the subsequent measurement.
The Balance of Dissipation and Photochemical Energy Utilization
The three competing fates of intercepted light allow estimation of the amount of energy available for photochemistry (ETR), photoprotective heat dissipation (NPQ), and fluorescence (Müller et al., 2001). Determining the amount of heat dissipated as an energy flux, (represented here as JNPQ; Eq. 4) with the flux of energy used for photochemistry (ETR; Eq. 1) allows an empirical but semiquantitative comparison of the point at which the energy dissipated exceeds the energy available for photochemistry over the day. Using this approach, we examined the diurnal fate of absorbed light in rice leaves to explore the hypothesis that engagement of photoprotective dissipation was responsible for the differences between diurnal responses to solar energy and conventional light response curves seen here. For cv Nipponbare (Figs. 6 and 7), as irradiance increased over the diurnal period, the amount of energy dissipated as heat (JNPQ) increased, but for a given ETR, higher values of JNPQ were observed than seen in conventional light response curves. This is more obvious for the three cv Nipponbare plants examined in Figure 7, where it is evident that the ratio of JNPQ/ETR is higher at all but very low irradiances when calculated from diurnally derived data compared to conventional light response curves.
NPQ has previously been shown to decrease the convexity of the nonrectangular hyperbolic response of CO2 assimilation to irradiance, when calculated from traditional gas exchange light response curve measurements (Leverenz et al., 1990; Zhu et al., 2004). Thus, it follows that if JNPQ is higher at midrange light intensities (400–1200 µmol) over the diurnal time course, the convexity of the diurnal response of ETR to irradiance will be commensurately reduced. What is also interesting in the data of Figure 7C is that while the ratio JNPQ/ETR increases exponentially with irradiance and replicates are tightly grouped in conventional light response curves, the same leaves behave more erratically, showing little evidence of an exponential relationship between JNPQ/ETR and irradiance but clearly have the same capacity for photoprotective dissipation of energy in both circumstances. This again leads to the hypothesis that preconditioning of leaves in the canopy is occurring through a rapidly relaxing nonphotochemical dissipatory process, but the relationship between this process and utilization of energy for photochemistry at any given irradiance is very different to leaves measured in a conventional manner. Whether this is due to the leaves becoming “primed” in the canopy for sunflecks (Pearcy, 1996, 2007) and fluctuating environments through activation and deactivation of Calvin cycle enzymes and metabolite pools (Lawson et al., 2012) or whether short periods of high irradiance trigger short-term “inappropriate” photoprotective mechanisms causing a reduction in photosynthesis at intermediate light intensities (Murchie and Niyogi, 2011) remains to be determined.
Comparisons between Rice Cultivars
Where four cultivars were compared, the irradiance at which ETR equaled JNPQ in magnitude differed in conventional light response curves but more so in diurnal solar response curves (Fig. 8). It has long been recognized that there may be “lost photosynthesis” during the diurnal time course of photosynthesis in the canopy due to inappropriate engagement of NPQ (Horton, 1994; Horton, 2000, 2012; Zhu et al., 2004; Murchie and Niyogi, 2011). The results shown here support this possibility and the idea that “optimizing” NPQ to better adapt to changing canopy environmental conditions could increase biomass and crop yields. Overengagement of photoprotective mechanisms could be the result of selective pressure during evolution to produce plants that are conservative in their response as a survival mechanism that is no longer appropriate to a high-input cropping environment (Horton, 1994). Alternatively, there may be genetic variation in the dynamics of engagement of photoprotection under fluctuating solar irradiance with some cultivars operating more closely to the appropriate level of photoprotection as not to “limit” photochemistry (Horton, 2012). As a major proportion of NPQ is triggered by high transthylakoid ΔpH, nonphotochemical dissipation engagement will also be dependent on the capacity of photosynthetic carbon metabolism enzymes, including Rubisco, the regenerative phase of the photosynthetic carbon reduction cycle and downstream processes to utilize incoming energy from photochemistry, particularly at intermediate light intensities. Coordination of ATP and NADPH utilization by photosynthetic carbon metabolism with ETR, often called “photosynthetic control” has long been thought to involve a close connection between photoprotection and ATP utilization via the transthylakoid delta pH and qE quenching (Foyer et al., 1990; Baker et al., 2007; Foyer et al., 2012).
Most importantly, it seems from the data presented here that leaves of some cultivars are able to use higher light intensities for photochemistry before photoprotection exceeds ETR and thus have a higher diurnal ETe. When leaves of four cultivars were compared, the irradiance at which JNPQ becomes greater than ETR (JNPQ/ETR = 1; Fig. 9A) correlates with the convexity of the diurnal response of ETR to irradiance (Fig. 5, I–L). Cultivars with lower convexity in the diurnal response of ETR to irradiance also utilize less diurnally available light for photochemistry and dissipate more. This results in a reduction in diurnal ETe (Fig. 9B). This could be interpreted as “overinduction” of photoprotective mechanisms in the diurnal measurements, restricting ETR (Murchie and Niyogi, 2011). This is significant as the quantum efficiency of ETR at low light was relatively constant across genotypes (Fig. 5, A–D), consistent with the view that the maximum quantum efficiency of C3 photosynthesis (as defined by the initial slope of the photosynthetic response to irradiance), in the absence of variation in photorespiration, is relatively invariant (Evans, 1987). Comparisons of electron transport at moderate light intensities potentially offers an avenue to quantify variation in leaf level radiation use efficiency, facilitating manipulation of photoprotection to increase yield.
Cv Moroberekan seems to exhibit superior photoprotective characteristics as determined from the high irradiance at which photoprotection becomes greater than ETR (Figs. 8, C and G, and 9A). Consistent with this, the diurnal solar irradiance response curves of ETR and JNPQ are more similar in Moroberekan than in other genotypes examined. Mechanistically, this could be due to differences in the capacity of this variety for photoprotection, a difference in sensitivity of NPQ to thylakoid ΔpH, differences in the relaxation kinetics of NPQ or the capacity of the Calvin cycle to utilize the products of photochemistry. Further study of NPQ regulation in Moroberekan and cultivars behaving similarly will be required to gain insight into these mechanisms and possible avenues for improvement of this response for plant breeding.
Diurnal Heat Dissipation and Biomass Accumulation
Although not a direct indicator of net carbon fixation, ETR offers a higher throughput measure of the primary energy partitioned for utilization in photosynthesis, and the relationship between ETR and CO2 assimilation at a given CO2 concentration is robust (see Supplemental Fig. S1). Thus, one would predict that leaves with higher diurnal ETe;(Fig. 9B) should productively harvest more light and hence CO2 during a diurnal time course, resulting in plants with higher biomass. As large-scale replication and destructive harvest was not logistically possible in this experiment, a “digital biomass index” was used based on projected leaf area measured nondestructively (see “Materials and Methods”). Given the caveat that chlorophyll fluorescence traits shown here were acquired on only three replicates of leaves on a single day, it is interesting that in the case of cv IR64-21, all parameters measured are consistent with this hypothesis: lowest convexity of the diurnal response of ETR to irradiance (Fig. 8, I–L), lowest irradiance at which dissipation is greater than ETR (Figs. 8 and 9A), lowest diurnal ETe (Fig. 9B), and lowest overall plant digital biomass (Fig. 10). In the case of cultivars Azucena and Moroberekan, all parameters correlate with the exception of whole-plant biomass, which could be due to differences in partitioning of fixed carbon between plant organs (root, shoot, and stem, given the diverse plant architecture of these rice cultivars), leaf thickness, or downstream biochemical inefficiencies.
Implication for Modeling Photosynthesis
Many attempts have been made to link leaf-level photosynthetic parameters to canopy photosynthesis and canopy light use efficiency. In these models, canopy photosynthetic rates are mostly quantified through extrapolations of individual leaf light response curves to a given canopy irradiance often based on the maximum light saturated rate of photosynthesis (Pmax; Monsi and Saeki, 1953; Hirose and Werger, 1987; Anten et al., 1995; Hirose and Terashima, 2005). Attempts to scale from the leaf response to the canopy also need to make large assumptions about the canopy light environment, which is done most frequently by assuming canopy light distribution according to Beer’s Law (de Wit, 1965; Cowan, 1968; Goudriaan, 1977; Norman, 1979). Even the more complex models that separate light attenuation into canopy layers (Goudriaan., 1977; Norman, 1979), attempt to account for sunflecks (De Pury and Farquhar, 1997; Leuning et al., 1998), or ray-tracing models of light distribution (Song et al., 2013) would likely overestimate photosynthetic performance at moderate irradiance if using a simple nonrectangular hyperbola for leaf level response of assimilation to light. Where three representative examples of a given leaf have been measured in a single cultivar in this study, the shape of the response of ETR to irradiance varied even between repetitions. This is almost certainly a function of the variation in the preconditioning of the leaf to intercepted irradiance before a saturating flash is applied, and ETR is calculated using Equation 1. If, as proposed in this study, local canopy light environments cause a reduction in diurnal photosynthetic efficiency not reflected in traditional light response curves, applying such response curves to canopy modeling is certainly a large approximation. While the use of PAM fluorescence with an open leaf clip allows a measurement at a given time interval as a function of intercepted irradiance at that given time point, sophisticated monitoring of light conditions at the leaf level between the measurements made at 30 min intervals and validation of models against whole-canopy gas exchange would be required in order to further explore this issue.
CONCLUSION
In this study, an overestimation of ETR in the midrange light intensities (400–1200 µmol) is shown from conventional light response curves when compared with data collected under ambient solar irradiation over the diurnal in the same leaves. The overestimation is proposed to be a function of the photoprotective response to light gradients and fluctuations in a canopy environment, and resultant regulation of the partitioning of light energy to photochemistry. Genetic variation in this photoprotective response is shown to affect daily ETe, and a parameter based on the irradiance where photoprotective dissipation exceeds photochemical energy utilization is proposed as a metric of efficiency of photoprotective engagement for genotypic screening. The data shown here support suggestions in the literature that there is potential to manipulate photoprotection to increase plant biomass accumulation and select photosynthetically “superior” genotypes of rice on this basis. Furthermore, given that leaves in a crop canopy operate for the majority of the time in these midrange light intensities, the impact of improvements in performance at these light levels is likely to be very significant at the canopy level in the field. Data of the kind collected here can be used to populate models that upscale from the leaf to the canopy level to test this hypothesis.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Studies were conducted on five cultivars of rice (Oryza sativa): Azucena, Morberekan, and Nipponbare (japonica), IR64 (indica), a multiparent advanced generation intercross line, and IR77298-14-1-2-10 (abbreviated as MAGIC), see Cavanagh et al. (2008). Cultivars were selected from the Oryza SNP panel (McNally et al., 2009), supplied by the International Rice Research Institute (Los Baños, Philippines), to represent morphological and plant architectural diversity in well-studied, high-yielding lines reported to achieve similar harvest index (see Supplemental Table S2; Jahn et al., 2011).
Canopies were constructed in large bins 1162 × 1162 mm, depth 650 mm. Seedlings were transplanted into nonlimiting anaerobically prepared soil conditions at the three-leaf stage, with 25 × 25 cm spacing. To allow periodic removal of plants from the mini canopy for characterization of digital plant biomass, four plants were sown in smaller pots with the same prepared soil and spacing maintained and inserted into the larger bins in a central canopy position (Supplemental Fig. S2, A and B). Plants were grown in controlled environment glasshouse facilities at CSIRO Black Mountain, Canberra, Australia. The glasshouse is equipped with Plexiglass Alltop SDP 16 (Envonik Industries), which allows light transmission of 91% (compared with 75%–86% in glass) across the full PAR spectrum and passes UV light. Natural solar irradiance conditions were used for plant growth; CO2 levels were ambient (380–400 μmol m−2 s−1). Day and night temperatures were set to 30°C/27°C.
PAM Fluorescence Measurements
The diurnal response of ETR to natural solar irradiance was compared to instantaneous light response curves under controlled actinic light in the same leaves with the same device, a Walz Monitoring PAM (Heinz Walz GmbH). First, in mini canopies of single cultivar cv Nipponbare, measurements were made on leaf 13 at 122 DAS on July 11, 2013. In a second experiment including four additional cultivars, measurements were made on leaf 8 at 70 DAS on November 3, 2014 (Azucena and MAGIC) and at 72 DAS on November 5, 2014 (IR64-21 and Moroberekan).
The monitoring PAM allowed simultaneous and continuous measurements of up to seven leaves under natural solar irradiance, with measuring heads that act as independent chlorophyll fluorometers attached to a central interface. Measurements of diurnal ETR as a function of natural intercepted solar irradiance were calculated from PAM saturating pulses at 30 min intervals over the full course of a day. Measuring heads were placed following natural leaf orientation in the leaf center, on the newest fully expanded leaf. Instantaneous light response curves were carried out on the same leaf, with the same device without moving the measuring head, while natural solar irradiance was blocked out with aluminum foil (Supplemental Fig. S2C). The blue actinic light source within the measuring heads was used to increase irradiance from 0 saturating (0, 70, 100, 130, 200, 300, 420, 620, 820, 1150, 1500 µmol m−2 s−1). A saturating pulse (∼6000 µmol m−2 s−1) was made after 3.5 min acclimation time to a given irradiance to create an instantaneous response curve in approximately 40 min total. Instantaneous curves were made at three time points in cv Nipponbare (9 a.m., noon, 3 p.m.) and twice in the further cultivars (10:30 a.m. and 2:30 p.m.). Between instantaneous curves, aluminum foil was removed to allow continued measurement of diurnal ETR as a function of natural light.
WIN software (Heinz Walz GmbH) allowed easy analysis of the flash trace to ensure full saturation of PSII at the pulse intensity provided. Any applied pulses that did not fully saturate were not included in analysis. The monitoring PAM measuring head quantum sensors were calibrated with integrated cosine corrected values from a LI-COR LI-190R quantum (PAR) sensor.
ETR was calculated from fluorescence kinetic parameters and irradiance measured with Walz Monitoring PAM as:
| (1) |
where PARi is intercepted irradiance and abs is leaf absorptance value as calculated with integrating sphere and Fieldspec4 (Analytical Spectral Devices). PSI/PSII is the division of electrons shared between each of the photosystems, taken to be 0.5, and ΦPSII is the quantum efficiency of PSII calculated as:
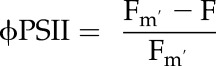 |
(2) |
where Fm′ is the maximal fluorescence after a saturating flash is applied under natural light and F is steady-state fluorescence.
ETR calculations include measured leaf absorptance values for each cultivar across the full spectrum (400–2500 nm), with an integrating sphere and Fieldspec4 (Analytical Spectral Devices). Cultivar-specific absorption values are an average across the PAR spectrum (400–700 nm). Equations for calculation and values are given in Supplemental Appendix S1 and Supplemental Table S3, respectively.
Curve Fitting
The electron transport (J) model as described in von Caemmerer (2000) was used to create a curve fitting utility in Microsoft Excel as:
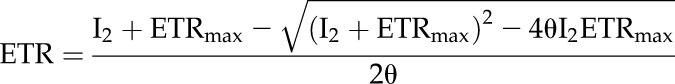 |
(3) |
where ETRmax is maximal ETR, θ is the curvature factor, and I2 the useful light absorbed by PSII and in this work the quantum yield of PSII (φPSII) calculated from PAM fluorescence multiplied by intercepted irradiance (PARi) as measured at the leaf level with reflectance at the monitoring PAM measuring head.
Data pairs of light intensity and ETR from collected fluorescence light response curves were entered into the utility. The sum of squares function in Microsoft Excel was used to reduce the error between collected data and the model by adjusting outputs φETR, θ, and ETRmax. Notations are called ETR rather than J to distinguish from CO2 response modeling and signify ETR as calculated directly or modeled using Equation 1 from PAM fluorescence measurements.
Where curve fitting is used to analyze the diurnal ETR irradiance response, ETRmax was constrained to the maximal value estimated from instantaneous light response curves in the same cultivar on the same day. See Supplemental Table S1 for values where four cultivars are compared. For cv Nipponbare, 183.6 µmol electrons m−2 s−1 was the predicted maximal ETR value on the day of analysis (see Fig. 4).
The working ETR curve fitting utility is provided as Supplemental Data S1.
Calculation of Photoprotection
NPQ is presented as an energy flux via PSII according to Hendrickson et al. (2004) and is directly relatable to ETR using:
| (4) |
where φNPQ = F/Fm’ − F/Fm, IA is intercepted irradiance, and 0.5 is the proportion of intercepted PAR used by PSII.
Diurnal ETe
Diurnal ETe was calculated as the slope of the relationship between daily cumulative ETR and daily cumulative irradiance (µmol m−2 s−1). Cumulative daily ETR and irradiance were estimated by taking the ETR calculated at the time of a saturating flash from the monitoring PAM and the intercepted irradiance at the same flash as the average for a 30 min time period and summing these values over the photoperiod.
Digital Biomass
Plants in 10 L pots grown in the center of mini canopies were removed weekly during vegetative growth and scanned in the Plantscan system designed and developed at the High Resolution Plant Phenomics Centre, Canberra, Australia; see Sirault et al. (2013) for design specifications. Plantscan system was designed to allow creation of a 3D plant reconstruction at every scanning event. However quantification of “2D projected area sum” is used as a proxy for “digital plant biomass,” rather than the volume of 3D plant reconstructions given difficulty in separating tillers using the latter method of analysis. From 120 images taken during a 360° scan of a single plant from the horizontal camera, for each image green pixels were filtered to identify plant area. Plant area was then averaged across the 120 images to give the 2D projected area sum for each scanning event. Published validation of the area sum from the Plantscan system with manual plant measurements are presented in Paproki et al. (2012).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. The response of CO2 assimilation and ETR to irradiance in leaf 8 on four plants of cv Nipponbare.
Supplemental Figure S2. Mini canopies of rice, sown in a controlled environment glasshouse at CSIRO, Black Mountain, Canberra, Australia.
Supplemental Figure S3. The measured response of ETR to irradiance compared with model predictions in leaf 13 of cv Nipponbare.
Supplemental Table S1. Coefficients, R2 value, and the irradiance at which JNPQ/ETR = 1 presented from polynomial regression (y = ax2 + bx + c, c = 0) applied to the dataset for each cultivar in Figure 8.
Supplemental Table S2. Plant architectural measurements made in five cultivars of rice grown and analyzed in quarantine glasshouse facilities at CSIRO, Black Mountain, Canberra, Australia.
Supplemental Table S3. Absorption values for five cultivars of rice.
Supplemental Appendix S1. Calculation of leaf absorption values with an integrating sphere and Fieldspec4 (Analytical Spectral Devices).
Supplemental Data S1. ETR irradiance curve fitting utility.
Supplementary Material
Acknowledgments
We thank the International Rice Research Institute for supplying seed material and agronomic support. Experimental work was carried out at and supported by the High Resolution Plant Phenomics Centre, part of CSIRO in Canberra, Australia. Dr. Helen Daily helped with experimental setup. Dr. Peter Ansell, Dr. Julio Hernandez Zaragoza, Jianming Guo, and Dr. Chuong Nguyen carried out image analysis from Plantscan images. Dr. Robert Coe offered experimental design and advice on data analysis. Peter Kuffner, Michael Salim, and Dac Nguyen gave engineering support.
Glossary
- PAM
pulse amplified modulated
- ETR
electron transport rate
- NPQ
nonphotochemical quenching
Footnotes
The work was supported by a PhD Global Rice Science Scholarship and the Centre of Excellence for Translational Photosynthesis at the Australian National University, Canberra.
Articles can be viewed without a subscription.
References
- Ainsworth EA. (2008) Rice production in a changing climate: a meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Glob Change Biol 14: 1642–1650 [Google Scholar]
- Anten NPR, Schieving F, Werger MJA (1995) Patterns of light and nitrogen distribution in relation to whole canopy carbon gain in C3 and C4 mono- and dicotyledonous species. Oecologia 101: 504–513 [DOI] [PubMed] [Google Scholar]
- Baker NR, Harbinson J, Kramer DM (2007) Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ 30: 1107–1125 [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Morgan PB, Ort DR, Long SP (2005) The growth of soybean under free air [CO(2)] enrichment (FACE) stimulates photosynthesis while decreasing in vivo Rubisco capacity. Planta 220: 434–446 [DOI] [PubMed] [Google Scholar]
- Cavanagh C, Morell M, Mackay I, Powell W (2008) From mutations to MAGIC: resources for gene discovery, validation and delivery in crop plants. Curr Opin Plant Biol 11: 215–221 [DOI] [PubMed] [Google Scholar]
- Cheng L, Fuchigami LH, Breen PJ (2001) The relationship between photosystem II efficiency and quantum yield for CO(2) assimilation is not affected by nitrogen content in apple leaves. J Exp Bot 52: 1865–1872 [DOI] [PubMed] [Google Scholar]
- Cowan IR. (1968) The interception and absorption of radiation in plant stands. J Appl Ecol 5: 367–379 [Google Scholar]
- De Pury DGG, Farquhar GD (1997) Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant Cell Environ 20: 537–557 [Google Scholar]
- de Wit CT. (1965) Photosynthesis of leaf canopies. Agric Res Rep 663: 57 [Google Scholar]
- Demmig-Adams B, Adams WW III (1992) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43: 599–626 [Google Scholar]
- Driever SM, Lawson T, Andralojc PJ, Raines CA, Parry MA (2014) Natural variation in photosynthetic capacity, growth, and yield in 64 field-grown wheat genotypes. J Exp Bot 65: 4959–4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards GE, Baker NR (1993) Can CO2 assimilation in maize leaves be predicted accurately from chlorophyll fluorescence analysis? Photosynth Res 37: 89–102 [DOI] [PubMed] [Google Scholar]
- Evans J. (1987) The relationship between electron transport components and photosynthetic capacity in pea leaves grown at different irradiances. Funct Plant Biol 14: 157–170 [Google Scholar]
- Evans JR. (2013) Improving photosynthesis. Plant Physiol 162: 1780–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LT. (1975) The physiological basis of crop yield. In Evans LT, ed, Crop Physiology. Cambridge University Press, Cambridge, UK, pp 327–335 [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C 3 species. Planta 149: 78–90 [DOI] [PubMed] [Google Scholar]
- Flood PJ, Harbinson J, Aarts MGM (2011) Natural genetic variation in plant photosynthesis. Trends Plant Sci 16: 327–335 [DOI] [PubMed] [Google Scholar]
- Foyer C, Furbank R, Harbinson J, Horton P (1990) The mechanisms contributing to photosynthetic control of electron transport by carbon assimilation in leaves. Photosynth Res 25: 83–100 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J (2012) Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot 63: 1637–1661 [DOI] [PubMed] [Google Scholar]
- Fryer MJ, Andrews JR, Oxborough K, Blowers DA, Baker NR (1998) Relationship between CO2 assimilation, photosynthetic electron transport, and active O2 metabolism in leaves of maize in the field during periods of low temperature. Plant Physiol 116: 571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbank RT, Tester M (2011) Phenomics--technologies to relieve the phenotyping bottleneck. Trends Plant Sci 16: 635–644 [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92 [Google Scholar]
- Goudriaan J. (1977) Crop micrometeorology: a simulation study. PhD thesis. Wageningen University, Wageningen, The Netherlands
- Gu J, Yin X, Struik PC, Stomph TJ, Wang H (2012) Using chromosome introgression lines to map quantitative trait loci for photosynthesis parameters in rice (Oryza sativa L.) leaves under drought and well-watered field conditions. J Exp Bot 63: 455–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Sakai H, Tokida T, Nakamura H, Zhu C, Usui Y, Yoshimoto M, Fukuoka M, Wakatsuki H, Katayanagi N, et al. (2013) Rice cultivar responses to elevated CO2 at two free-air CO2 enrichment (FACE) sites in Japan. Funct Plant Biol 40: 148–159 [DOI] [PubMed] [Google Scholar]
- Hendrickson L, Furbank RT, Chow WS (2004) A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth Res 82: 73–81 [DOI] [PubMed] [Google Scholar]
- Hirose T, Terashima I (2005) Structure and function of plant canopies. Ann Bot (Lond) 95: 481–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T, Werger MJA (1987) Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy. Oecologia 72: 520–526 [DOI] [PubMed] [Google Scholar]
- Horton P. (2000) Prospects for crop improvement through the genetic manipulation of photosynthesis: morphological and biochemical aspects of light capture. J Exp Bot 51: 475–485 [DOI] [PubMed] [Google Scholar]
- Horton P. (2012) Optimization of light harvesting and photoprotection: molecular mechanisms and physiological consequences. Philos Trans R Soc Lond B Biol Sci 367: 3455–3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P, Murchie EH, Ruban AV, Walters RG (2001) Increasing rice photosynthesis by manipulation of the acclimation and adaptation to light. In Goode JA, Chadwick D, eds, Novartis Foundation Symposium: Rice Biotechnology: Improving Yield, Stress Tolerance and Grain Quality, Vol 236 John Wiley & Sons, Hoboken, NJ, pp 117–134 [DOI] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Walters RG (1994) Regulation of Light Harvesting in Green Plants (Indication by Nonphotochemical Quenching of Chlorophyll Fluorescence). Plant Physiol, 106: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbart S, Ajigboye OO, Horton P, Murchie EH (2012) The photoprotective protein PsbS exerts control over CO2 assimilation rate in fluctuating light in rice. Plant J 71: 402–412 [DOI] [PubMed] [Google Scholar]
- Jahn CE, Mckay JK, Mauleon R, Stephens J, McNally KL, Bush DR, Leung H, Leach JE (2011) Genetic variation in biomass traits among 20 diverse rice varieties. Plant Physiol 155: 157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Kramer DM, Raines CA (2012) Improving yield by exploiting mechanisms underlying natural variation of photosynthesis. Curr Opin Biotechnol 23: 215–220 [DOI] [PubMed] [Google Scholar]
- Leakey ADB, Xu F, Gillespie KM, McGrath JM, Ainsworth EA, Ort DR (2009) Genomic basis for stimulated respiration by plants growing under elevated carbon dioxide. Proc Natl Acad Sci USA 106: 3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuning R, Dunin FX, Wang YP (1998) A two-leaf model for canopy conductance, photosynthesis and partitioning of available energy. II. Comparison with measurements. Agric For Meteorol 91: 113–125 [Google Scholar]
- Leverenz JW, Falk S, Pilström C-M, Samuelsson G (1990) The effects of photoinhibition on the photosynthetic light-response curve of green plant cells (Chlamydomonas reinhardtii). Planta 182: 161–168 [DOI] [PubMed] [Google Scholar]
- Liu H, Yang L, Wang Y, Huang J, Zhu J, Yunxia W, Dong G, Liu G (2008) Yield formation of CO2-enriched hybrid rice cultivar Shanyou 63 under fully open-air field conditions. Field Crops Res 108: 93–100 [Google Scholar]
- Long SP, Zhu X-G, Naidu SL, Ort DR (2006) Can improvement in photosynthesis increase crop yields? Plant Cell Environ 29: 315–330 [DOI] [PubMed] [Google Scholar]
- McNally KL, Childs KL, Bohnert R, Davidson RM, Zhao K, Ulat VJ, Zeller G, Clark RM, Hoen DR, Bureau TE, et al. (2009) Genomewide SNP variation reveals relationships among landraces and modern varieties of rice. Proc Natl Acad Sci USA 106: 12273–12278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P, Sheehy J, Woodward F (1998) Potential Yields and the Efficiency of Radiation Use in Rice. International Rice Research Institute, Manila, Philippines [Google Scholar]
- Monsi M, Saeki T (1953) Uber den Lichtfaktor in den Pflanzengesellschaften und seine Bedeutung fur die Stoffproduktion. Jpn J Bot 14: 22–52 [Google Scholar]
- Monteith J. (1972) Solar radiation and productivity in tropical ecosystems. J Appl Ecol 9: 747–766 [Google Scholar]
- Müller P, Li X-P, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125: 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie EH, Chen Y, Hubbart S, Peng S, Horton P (1999) Interactions between senescence and leaf orientation determine in situ patterns of photosynthesis and photoinhibition in field-grown rice. Plant Physiol 119: 553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie EH, Niyogi KK (2011) Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol 155: 86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie EH, Pinto M, Horton P (2009) Agriculture and the new challenges for photosynthesis research. New Phytol 181: 532–552 [DOI] [PubMed] [Google Scholar]
- Norman J. (1979) Modeling the complete crop canopy. In Barfield BJ, Gerber JF, eds, Modification of the Aerial Environment of Crops. American Society Agricultural Engineers, St. Joseph, MI, pp 249–280 [Google Scholar]
- Ort DR, Zhu Xinguang, Melis A (2011) Optimizing antenna size to maximize photosynthetic efficiency. Plant Physiol 155: 79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paproki A, Sirault X, Berry S, Furbank R, Fripp J (2012) A novel mesh processing based technique for 3D plant analysis. BMC Plant Biol 12: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry MAJ, Reynolds M, Salvucci ME, Raines C, Andralojc PJ, Zhu X-G, Price GD, Condon AG, Furbank RT (2011) Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J Exp Bot 62: 453–467 [DOI] [PubMed] [Google Scholar]
- Pearcy RW. (1990) Sunflecks and photosynthesis in plant canopies. Annu Rev Plant Physiol Plant Mol Biol 41: 421–453 [Google Scholar]
- Pearcy RW, Krall JP, Sassenrath-Cole GF (1996) Photosynthesis in fluctuating light environments. In Baker NR, ed, Photosynthesis and the Environment. Springer, Dordrecht, The Netherlands, pp 321–346 [Google Scholar]
- Rajendran K, Tester M, Roy SJ (2009) Quantifying the three main components of salinity tolerance in cereals. Plant Cell Environ 32: 237–249 [DOI] [PubMed] [Google Scholar]
- Reynolds MP, van Ginkel M, Ribaut JM (2000) Avenues for genetic modification of radiation use efficiency in wheat. J Exp Bot 51: 459–473 [DOI] [PubMed] [Google Scholar]
- Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10: 51–62 [DOI] [PubMed] [Google Scholar]
- Seck PA, Diagne A, Mohanty S, Wopereis MC (2012) Crops that feed the world 7: rice. Food Security 4: 7–24 [Google Scholar]
- Sheehy J. (2001) Future food requirements: are improvements in photosynthesis required? Science Access 3: 10.1071/SA0403641 [Google Scholar]
- Sheehy JE, Mitchell PL (2013) Designing Rice for the 21st Century: Three Laws of Maximal Yield. International Rice Research Institute, Los Banos, Philippines [Google Scholar]
- Sirault X, Fripp J, Paproki A, Kuffner P, Nguyen C, Li R, Daily H, Guo J, Furbank R (2013) PlantScan: a three-dimensional phenotyping platform for capturing the structural dynamic of plant development and growth. In Proceedings of the 7th International Conference on Functional-Structural Plant Models. Saariselkä, Finland, pp 45–48 [Google Scholar]
- Song Q, Zhang G, Zhu XG (2013) Optimal crop canopy architecture to maximise canopy photosynthetic CO2 uptake under elevated CO2—a theoretical study using a mechanistic model of canopy photosynthesis. Funct Plant Biol 40: 109–124 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S. (2000) Biochemical Models of Leaf Photosynthesis. CSIRO Publishing, Colingwood, Australia [Google Scholar]
- Zhu X-G, Long SP, Ort DR (2008) What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr Opin Biotechnol 19: 153–159 [DOI] [PubMed] [Google Scholar]
- Zhu X-G, Long SP, Ort DR (2010) Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol 61: 235–261 [DOI] [PubMed] [Google Scholar]
- Zhu XG, Ort DR, Whitmarsh J, Long SP (2004) The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: a theoretical analysis. J Exp Bot 55: 1167–1175 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.