Abstract
Loss of function of Bruton's tyrosine kinase (Btk) causes X-linked agammaglobulinemia (XLA) in humans and X-linked immunodeficiency in mice (xid). By using MS analysis and phosphopeptide-specific antibodies, we identified a tyrosine phosphorylation site (Y617) near the carboxyl terminus of the Btk domain from Btk expressed in 293T as well as DT-40 cells. Y617 is conserved in all Tec family kinases except murine Tec. Replacement of Y617 with a negatively charged glutamic acid (E) suppressed Btk-mediated phospholipase Cγ2 activation and calcium response in DT-40 cells, whereas Akt activation was not affected. The Btk Y617E mutant could partially restore conventional B cell development and proliferation in Btk–/Tec– mice but failed to rescue CD5+ B-1 cell development and the TI-II immune response to 2,4,6,-trinitrophenyl-Ficoll. These data suggest that Y617 phosphorylation or a negative charge at this site may down-regulate the function of Btk by selectively suppressing the B cell calcium signaling pathway.
Immunoreceptors, including the B cell receptor (BCR), transduce cell surface stimulations through signaling pathways that regulate proliferation, differentiation, or apoptosis (1, 2). The activation of phospholipase C (PLC) is one of the most commonly used signal-transduction pathways. Surface stimulation activates phosphatidylinositol 3-kinase (PI 3-kinase) and nonreceptor tyrosine kinases including Src, Syk, and Tec family kinases. This activation leads to PLC activation and the hydrolysis of phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] to produce inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) (2). DAG activates protein kinase C (PKC), whereas IP3 triggers calcium release from the endoplasmic reticulum and subsequent extracellular calcium entry (3). The sustained calcium signal is critical for the activation of downstream components and participates in the regulation of immune cell maturation (1).
Tec family kinases play important roles in lymphocyte development and function (4, 5). Bruton's tyrosine kinase (Btk), a member of this family, is required for both the initial calcium release and extracellular calcium entry (6, 7). Btk not only regulates the production of phosphatidylinositol 4,5-bisphosphate (8) but also phosphorylates critical tyrosine residues on PLC, resulting in activation of PLCγ2 (9). B cells lacking Btk show a blunted calcium response after BCR stimulation (7, 10).
Btk is expressed in multiple lineages within the hematopoietic system, however, Btk plays an indispensable role only in B cell development and function (11). Mutations in Btk cause human X-linked agammaglobulinemia (XLA), characterized by a dramatic reduction in peripheral B cells (12, 13). A spontaneous point mutation in murine Btk (R28C) results in a milder condition, termed X-linked immunodeficiency (xid) (14, 15). Btk-deficient mice show a similar phenotype as the xid animals: peripheral B cells are reduced by 30–50%, and these cells proliferate to a lesser extent than normal B cells when stimulated in vitro. Additionally, serum levels of IgM and IgG3 are lower in these mice. Other defects include the absence of CD5+ B-1 cells and the inability to respond to type II T-independent antigens (16–18).
Btk and other Tec kinases share a similar domain structure with Src and Abl family kinases. All have a Src homology 3 (SH3) domain followed by an SH2 domain and a kinase domain. The significance of tyrosine phosphorylation in the regulation of Src and Abl family kinases has been studied extensively (19, 20). Phosphorylation has also been shown to be a critical regulatory mechanism for controlling Btk function. Y551 lies within the activation loop of Btk, and phosphorylation at this site by Lyn activates the kinase. Y223 in the SH3 domain is an autophosphorylation site that potentially down-regulates Btk activity, and S180 in the Tec homology domain (TH domain) negatively regulates Btk function when phosphorylated by protein kinase C-β (14, 21, 22).
We conducted MS analysis on purified murine Btk from 293T cells and DT-40 cells to search for new phosphorylation sites. A number of potential phosphorylation sites were found in the carboxyl terminus of the kinase domain, but most did not affect Btk function when mutated. However, Y617 was discovered to be a phosphorylation site that regulates Btk-mediated calcium signaling. When replaced by glutamic acid (Y617E) to mimic the phosphorylated state, a decreased calcium response but normal Akt activation was observed after BCR stimulation. The Y617E mutant could partially rescue defects in B cell development and function, suggesting that phosphorylation or a negatively charged modification at this site may selectively inhibit Btk's function in B cell development and activation.
Materials and Methods
Cell Culture, Constructs, and Retrovirus Production. Btk-deficient DT-40 cells were maintained as described in ref. 10. HEK 293T cells were cultured with Iscove's modified Dulbecco's medium by using 10% FBS and transfected by using a standard calcium phosphate-mediated method. Helper-free retroviruses were generated as described in ref. 23 with a ψ-amphotropic packaging plasmid. WT murine Btk and the mutants were cloned into the NotI site of retroviral vector murine stem cell virus–internal ribosome entry sequence–GFP. Point mutations were introduced by using the QuickChange Site-Directed Mutagenesis Kit (Stratagene).
Protein Purification and MS Analysis. His-tagged Btk was purified with a Ni-NTA-agarose column (Qiagen). The purified fractions were separated by SDS/PAGE and stained with Coomassie blue. Gel slices were washed and digested, and the resulting tryptic peptides were extracted by using the standard protocols in ref. 24. Trypsin digestion, extraction, and peptide spotting onto a matrix-assisted laser desorption ionization (MALDI) target were accomplished by using a robotic liquid handling workstation (MassPrep, Micromass-Waters, Beverly, MA). Peptide fingerprint mass spectra were acquired with a MALDI time-of-f light instrument (M@LDI-R, Micromass-Waters) and searched against rodent proteins in the SWISS-PROT protein sequence database by using the mascot search program (www.matrixscience.com).
Phosphopeptide-Specific Antibody Generation. Phosphopeptide (residue 610–624 with a phosphate on Y617) was synthesized at Synpep (Dublin, CA), conjugated to keyhole limpet hemocyanin, and used to immunize rabbits (Covance, Denver, PA). Serum was first absorbed with BSA peptide 610–624 without phosphorylation to remove peptide-specific antibodies and then purified through a BSA-phosphopeptide column.
Immunoprecipitation and Western Blot Analysis. The following antibodies were used in this study: Btk N-terminal antibody was produced as described in ref. 13; PLCγ2 antibody, 4G10, Akt, and phospho-Akt antibodies were purchased from Santa Cruz Biotechnology, Upstate Biotechnology (Lake Placid, NY), and New England Biolabs, respectively; mouse anti-chicken IgM M4 was obtained from Southern Biotechnology Associates; and goat anti-mouse IgM F(ab′)2 was purchased from Jackson ImmunoResearch. Cells were lysed in a buffer containing 1% Triton X-100, 50 mM Tris (pH 8.0), 150 mM NaCl, and Complete protease inhibitors (Roche Diagnostics). Immunoprecipitation, Western transfer, and Western blotting were performed by using standard techniques.
Calcium Flux Analysis. DT-40 cells were labeled with 1 μM Indo-1AM (Molecular Probes) for 30–45 min at 37°C, and calcium flux was measured by using a fluorimeter (SLM 8000, OLIS, Bogart, GA).
Reconstitution of Btk–/Tec– Mice. Btk–/Tec– mice were generously provided by Wilfried Ellmeier (Institute of Immunology, Medical University of Vienna, Vienna). For reconstitution, 5-fluorouracil-treated bone marrow cells were collected at day 3 and stimulated with IL-3, IL-6, and SCF for 48 h. The cells were then injected (i.v.) into lethally irradiated Btk–/Tec– mice (1,100 rad) at 1 × 106 cell per mouse after two rounds of spin infection (25, 26).
Fluorescence Activated Cell Sorting (FACS) Analysis. Single-cell suspensions from spleen and peritoneal wash were depleted of red blood cells and stained with the following antibodies: IgM-PE (Pharmingen) and IgD-biotin (Southern Biotechnology Associates), followed by streptavidin-APC (Caltag, South San Francisco, CA) and CD5-APC (Pharmingen). Data were acquired on a FACScan (Becton Dickinson) and analyzed by using winmdi software (Scripps Research Institute, La Jolla, CA).
B Cell Proliferation and ELISA. B220+ splenocytes were purified by using a B cell isolation kit (Miltenyi Biotec, Auburn, CA), and the resulting purity was >93%. The B cell proliferation assay, serum immunoglobulins, and 2,4,6,-trinitrophenyl-specific immunoglobulins were measured as described in ref. 27.
Results
His-Tagged Btk Is Functional and Effectively Purified by Using Chromatography. MS has been demonstrated to be a powerful tool for identifying protein modifications (28). To ensure that enough Btk was isolated for MS analysis, we used a 6× His-tag at the C terminus to facilitate the purification because human Btk has been tagged similarly and retained its kinase activity (29). To confirm functionality, His-tagged Btk was introduced into Btk-deficient DT-40 cells. The tagged protein was stably expressed at a level comparable with WT Btk, suggesting that the tag does not affect protein folding or degradation (Fig. 1A). Btk is essential for the BCR-mediated calcium response in DT-40 cells (10). When these cells were stimulated with anti-IgM, His-tagged and WT Btk mediated calcium flux similarly (Fig. 1 A). Moreover, His-tagged Btk activated PLCγ2 after BCR stimulation with kinetics similar to those seen in the WT Btk response (data not shown).
Fig. 1.
His-tagged Btk is functional and can be purified with a Ni-column. (A) Btk– DT-40 cells were transduced with WT or 6× His-tagged Btk, and the calcium response was measured with anti-IgM (2.5 μg/ml) stimulation. (B)6× His-tagged Btk was coexpressed with activated Lyn (Y508F) in 293T cells and purified through a Ni-column. The purified fraction was separated on SDS/PAGE, stained with Coomassie blue, and confirmed by Western blot with anti-Btk antibody. (C) 10× His-tagged Btk was infected into Btk-deficient DT-40 cells, and purified Btk was stained with Coomassie blue and confirmed by Western blot. (D) Phosphopeptides identified by MS. P, phosphorylation.
Previous studies have demonstrated that phosphorylated Btk represents <5% of the cellular Btk pool in B cells (30), necessitating enhanced phosphorylation of Btk to obtain an acceptable phosphopeptide spectrum. Because coexpression of Lyn with Btk significantly augments the total tyrosine phosphorylation of Btk (31), we coexpressed Btk with activated Lyn (Y508F, LynF) in HEK 293T cells. After purification with Ni-NTA-agarose, the eluate was separated by SDS/PAGE and stained with Coomassie blue. A relatively abundant band, absent in the control sample, was identified as Btk and confirmed by immunoblot with anti-Btk antibody (Fig. 1B).
Although overexpression in 293T cells results in large quantities of Btk, the intracellular environment of these cells does not reflect the in vivo environment of Btk. To confirm the phosphorylation data from 293T cells, we purified His-tagged Btk from DT-40 cells. To eliminate contaminating proteins that are more abundant in DT-40 cells, we engineered a 10× His-tagged Btk. The 10× His-tagged Btk, like the 6× His-tagged Btk, is functional and can be purified from DT-40 cells at a purity sufficient for MS analysis (Fig. 1C and data not shown).
Multiple Phosphorylation Sites Were Identified in the C Terminus of the Btk Domain. Matrix-associated laser desorption ionization MS identified putative phosphorylation sites throughout the Btk protein sequence, including Y551, a known phosphorylation site. Interestingly, a number of potential phosphorylation sites were clustered in the carboxyl terminus of the kinase domain. Most of these potential sites appeared only in Btk of 293T origin, suggesting that they could be artifacts of overexpression and coexpression of Lyn (data not shown). Two putative phosphopeptides were identified in multiple samples of Btk isolated from both 293T cells and DT-40 cells: 616LYRPHLASER625 and 626VYTIMYSCWHEK637 (Fig. 1D). Although more than one tyrosine/serine/threonine can be found in each sequence, both singly and doubly phosphorylated peptides were identified for peptide 616–625 in 293T cells (data not shown), indicating that both Y617 and S623 were subject to phosphorylation. Among these potential phosphorylation sites, only mutation of Y617 caused a major functional defect in Btk signaling as discussed below. Therefore, we focused our study on the regulation of Btk function by means of Y617 phosphorylation.
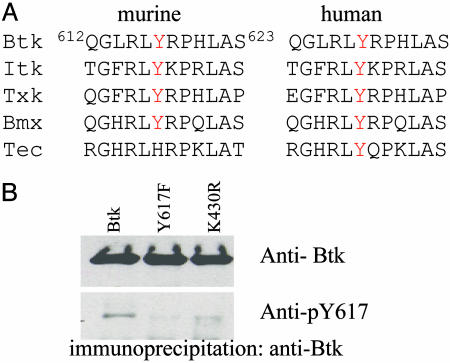
Phosphopeptide-Specific Antibody Confirms Tyrosine Phosphorylation at Y617, Which Is Conserved in Tec Family Kinases. To gain insight into the significance of Y617 phosphorylation, we examined the conservation of this residue in nonreceptor tyrosine kinases. Multiple sequence alignment showed that this tyrosine and the surrounding residues are highly conserved in both murine and human Tec family kinases, with the exception of murine Tec (Fig. 2A). The substitution of histidine for tyrosine in murine Tec is found in various splicing forms (32). Additionally, analogous sites are not found in Syk, Abl, or Src family kinases, suggesting that potential phosphorylation of Y617 might be a regulatory mechanism unique to Tec family kinases.
Fig. 2.
Y617 is conserved in Tec family kinases. Sequence alignment of human and murine Tec kinases around Y617. (A), and phospho-specific antibody confirms the phosphorylation of this site (B). WT Btk and mutants were expressed in 293T cells and immunoprecipitated with anti-Btk antibody and blotted with Btk antibody (B Upper) and pY617-specific antibody (B Lower).
To further verify Y617 phosphorylation of Btk, we produced pY617-specific antibodies. As shown in Fig. 2B, WT Btk and the Y617F mutant were immunoprecipitated from 293T cells and blotted with pY617 antibody or Btk antibody. Although equal amounts of Btk protein were brought down for both samples (lanes 1 and 2), pY617 antibody recognized WT Btk (lane 1) but not the Y617F mutant (lane 2). This result demonstrates that phosphorylated Y617 is part of the epitope(s) that pY617 antibody recognizes and confirms that Y617 is phosphorylated under these conditions. In addition, Y617 phosphorylation was attenuated in the kinase-inactive mutant (K430R) (lane 3), suggesting that Y617 could be an autophosphorylation site. However, the crystal structure of the Btk domain shows that Y617 is located on the surface of the large lobe, away from the active site (33), so Y617 is more likely phosphorylated through transphosphorylation between Btk molecules.
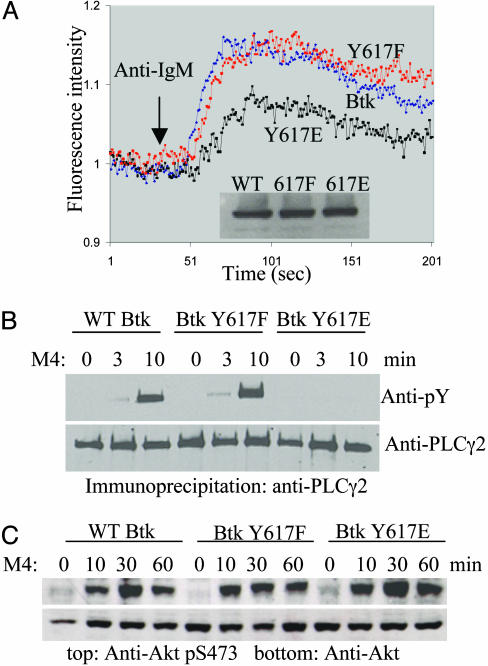
Btk Y617E Is Selectively Defective in Mediating Calcium Response. We decided to alter Y617 to investigate its function as a phosphorylation site. Two common methods used to perturb a phosphorylation site are phenylalanine (F) mutation to block phosphorylation and glutamic acid/aspartic acid (E/D) mutation to mimic the phosphorylated residue (34). Although both Y617F and Y617E mutants showed WT kinase activity in vitro for autophosphorylation and for transphosphorylation of enolase (data not shown), the Y617F mutant exhibited a calcium responses similar to WT Btk after anti-BCR stimulation, whereas the Y617E mutant showed a decreased response (Fig. 3A). This finding indicates that there is a defect in mediating the calcium signal when the Btk Y617 site is negatively charged. To confirm these results, we also checked PLCγ2 activation. Tyrosine phosphorylation of PLCγ2 after BCR stimulation was significantly attenuated in the Y617E mutant, and no change in phosphorylation status was observed in the Y617F mutant (Fig. 3B), consistent with the calcium flux data.
Fig. 3.
The Y617E mutant shows defects in the BCR-mediated calcium response but not in Akt activation. (A) The calcium response of DT-40 cells with anti-IgM stimulation (2.5 μg/ml M4) was measured with a fluorimeter. (B) DT-40 cells were stimulated with 10 μg/ml M4, and PLCγ2 activation was measured by immunoprecipitation followed by Western blot with 4G10 antibody or PLCγ2 antibody. (C) DT-40 cells were stimulated as in B, and Akt activation was measured by Western blot with phosphoserine 473 or Akt antibodies with total cell lysate.
Although Btk is primarily responsible for mediating calcium response, it can also activate a number of other downstream signaling pathways, including the mitogen-activated protein kinase pathway, cyclins, Bcl-xl, and the Akt pathway (4, 5, 35). In DT-40 cells, Akt activation is downstream of Btk and is independent of calcium flux (36), prompting us to investigate the effect of the Y617E mutation on downstream effectors that are not involved in the calcium pathway. DT-40 cells were stimulated with anti-BCR and probed for phosphorylation at S473, a known activation marker for Akt (37). Interestingly, Akt activation was not affected by either the Y617E mutation or the Y617F mutation (Fig. 3C), suggesting that Y617 phosphorylation or a negative charge at this site selectively regulates a subset of the pathways downstream of Btk. This conclusion was confirmed by microarray analysis with a chicken cDNA library (38). Of the 4,000 genes, only a small number showed a significant difference in DT-40 cells expressing WT Btk vs. Y617E mutant after BCR stimulation (see Fig. 6 and Table 1, which are published as supporting information on the PNAS web site).
Y617E Partially Rescues B Cell Development and Function in Btk–/Tec– Mice. Although the BCR signaling pathway in DT-40 cells, murine, and human B cells share many elements, DT-40 cells are different in that they are a transformed cell line, and the cells continuously divide. Although Akt activation is dependent on Btk in DT-40 cells, this is not the case in murine splenocytes (39). Therefore, we used a mouse model to study the effect of the Btk Y617 mutants on B cell development and activation. Yu et al. (40) developed an effective bone marrow reconstitution system in Btk–/Tec– mice by using a retroviral vector to deliver genes of interest. Following this protocol, Btk–/Tec– mice were reconstituted with either a vector control, WT Btk, or the Y617 mutants (Fig. 4A). After reconstitution, comparable levels of GFP-positive cells (30–40% in the spleen), an indication of infection efficiency, were found in the periphery of these mice (data not shown).
Fig. 4.
B cell development is partially rescued by the Btk Y617E mutant. (A) 5-FU bone marrow cells from Btk–/Tec– mice were infected with vector control, Btk, or mutants and put back into lethally irradiated mice. (B) Cell-surface-marker analysis. Splenocytes and peritoneal cells were harvested and red blood cells were lysed before staining with IgM/IgD and IgM/CD5, respectively. All live cells were gated based on forward and size scatter. B-1 cells were gated on CD5lowIgM+ cells. The green panels were gated on GFP+ cells, and the black panels represent all live cells.
Btk–/Tec– mice exhibit a similar but more severe phenotype than Btk-deficient mice (41). After reconstitution, bone marrow and peripheral immune cells were harvested and studied for B cell maturation. In WT mice, surface IgM is down-regulated and IgD is up-regulated in splenic B cells, but this process is blocked in the double knockout mice (41). Both WT Btk and the Y617F mutant rescued this IgM/IgD transition, although the Y617E mutant was less efficient (Fig. 4B Upper). Infection with WT Btk and the Y617F mutant also partially restored the peritoneal CD5+ B-1 cell compartment, but the Y617E mutant was ineffective (Fig. 4B Lower). Furthermore, WT Btk and the Y617F mutant also rescued early B cell development in bone marrow, whereas the Y617E mutant was less effective (data not shown). We were able to follow the fate of infected cells by using the GFP marker in the introduced retrovirus. The green panel in Fig. 4B shows that a high percentage of GFP+ cells enter the B cell lineage, with the exception of the vector control, suggesting that Btk expression helps drive cells into the B cell lineage and facilitates subsequent maturation. GFP– cells showed a similar pattern in all transplanted mice (data not shown).
The ability of the rescued cells to proliferate in response to stimulation was also tested. Splenic B cells were purified and stimulated with anti-IgM or LPS. As shown in Fig. 5A, vector-infected cells did not proliferate in response to anti-IgM stimulation and exhibited a minimal response to LPS stimulation, whereas WT Btk and the Y617F mutant infected cells showed robust proliferation in response to both stimulations. However, the Y617E mutant-infected cells exhibited minimal proliferation to high anti-IgM stimulation and moderate proliferation in response to LPS stimulation. These data demonstrate that the Y617E mutation in Btk partially limits B cell activation.
Fig. 5.
The Y617E mutant partially rescues B cell proliferation and immune response in Btk–/Tec– mice. (A) Splenic B cells were purified by using a B cell isolation kit and seeded in 96-well plates in triplicate with various stimulations. The culture was pulsed with 3H-thymidine at 40 h and harvested at 60 h. Serum IgM (B) and 2,4,6,-trinitrophenyl-Ficoll specific IgM (C) were measured by ELISA.
We also analyzed serum Ig levels in these mice. As shown in Fig. 5B, WT mice have a much higher serum IgM level than Btk–/Tec– mice. IgM levels were restored by WT Btk reconstitution and remained low in the vector control mice, demonstrating that the reconstitution was successful. The Y617F mutant also restored serum IgM to WT levels, but the Y617E mutant only partially restored serum IgM levels. After immunization with TNP-Ficoll, a TI-II antigen, both WT-Btk-reconstituted and the Y617F-mutant-reconstituted mice exhibited responses similar to the WT mice, whereas the Y617E-mutant-reconstituted mice did not respond at all (Fig. 5C). Serum IgG3 and TNP-specific IgG3 levels in these mice were reconstituted in a pattern similar to IgM (data not shown). These data demonstrate that Y617E, which we used to mimic phosphorylated Btk, can mediate the production of IgM and IgG3 to intermediate levels but fails to support a TI-II response. The Y617F mutant, on the other hand, behaved similarly to WT Btk in both the DT-40 system and the Btk–/Tec– reconstitution model, suggesting that the failure to phosphorylate Y617 does not have a detrimental effect on Btk function.
Discussion
Tyrosine phosphorylation is a critical regulatory element that determines the functional state of many kinases. In this study, we identified a tyrosine phosphorylation site (Y617) in the C terminus of the Btk domain, which partially disrupts protein function when mutated to glutamic acid to mimic the phosphorylated state.
Y617 Phosphorylation Selectively Suppresses the Calcium Response Mediated by Btk. Y617 phosphorylation was first identified by MS analysis from 293T and DT-40 cells and then confirmed by phosphopeptide-specific antibody in 293T cells. However, we have not been able to detect Y617 phosphorylation in DT-40 cells and primary murine B cells with this pY617 antibody. Because Btk in 293T cells was highly overexpressed (∼10-fold of DT-40 cells and 100-fold over primary B cells), this failure is possibly due to a low/labile phosphorylation in B cells or low sensitivity/specificity of the antibody. Further studies are needed to validate that this phosphorylation occurs at physiologic conditions and to investigate the mode of regulation of this phosphorylation. Nonetheless, the most striking property of Y617 phosphorylation as mimicked by a negative charge at this site is that it can selectively affect the downstream pathways of Btk. The Btk Y617E mutant fails to activate PLCγ2 and calcium response in DT-40 cells but retains its capacity to mediate Akt activation. These findings suggest that Btk Y617 phosphorylation as mimicked by the Y617E mutation inhibits PLCγ2 phosphorylation, possibly by changing Btk conformation or by altering protein–protein interactions within B cells. Surprisingly, no PLCγ2 phosphorylation was detected with Y617E mutant, whereas residual phosphorylation was present in Btk– DT-40 cells (10), hinting at a possible role of Btk in scaffolding/assembly of the BCR signalosome that is disrupted by Y617 phosphorylation or the acidic mutation.
Y617 Phosphorylation or a Negative Charge at This Site Is Associated with a Reduction in Btk Function. Although Y617 phosphorylation was first identified in cells where Btk was coexpressed with activated Lyn, it is not Lyn-dependent because it was also identified in cells expressing Btk only. The Y617 phosphorylation was also identified by MS in DT-40 cells with and without stimulation (data not shown). These data suggest that Y617 phosphorylation may correlate with an inactive Btk, and mutagenesis studies provide corroborating evidence. The Y617E mutant mimics constitutively phosphorylated Btk and was unable to properly mediate a calcium response in DT-40 cells. In the Btk–/Tec– reconstitution system, the Y617E mutant only partially rescued B cell maturation and proliferation. These data suggest that the Y617E mutant is a potential loss-of-function mutant, supporting the hypothesis that Y617 phosphorylation is associated with a less active Btk. Mutations at residue Y617 have not been reported to cause X-linked agammaglobulinemia (42), possibly because such mutations may be neutral or cause a mild phenotype.
Murine Tec Lacks a Tyrosine at Position 617. A major difference between the murine Btk-deficient model and human X-linked agammaglobulinemia patients is that the murine phenotype is milder (43), and this is partially due to the compensation of murine Tec (41). The severity of the phenotype varies in both human and murine models as a result of genetic background. One of the genetic variations between human and mouse is that Y617 is conserved in human but not murine Tec (Fig. 2 A). We postulate that phosphorylation of Y617 down-regulates Btk function, and it is possible that an allelic variation at this site (Y617H) makes murine Tec more readily activated to compensate for the loss of Btk function.
Our study suggests that Y617 phosphorylation or a negative charge at this site selectively affects Btk function by inhibiting PLCγ2 activation and calcium response. This conclusion provides insight into the mechanism of regulation of Btk and other Tec family kinases. Further studies may contribute to new therapeutic approaches to regulating these kinases.
Supplementary Material
Acknowledgments
We thank Dr. Paul Neiman, Dr. Jeffery Delrow, and Andy Marty at the Fred Hutchinson Cancer Center for their help with the microarray study; Dr. Wilfried Ellmeier for generously providing Btk–/Tec– mice; Shirley Quan, James Johnson, and Donghui Cheng for their excellent technical assistance; Barbara Anderson for the preparation of the manuscript; and Drs. Anne Satterthwaite, John Colicelli, Genhong Cheng, Purnima Dubey, Caius Radu, and Manu Beillard for critically reviewing the manuscript. O.N.W. is an Investigator of the Howard Hughes Medical Institute. This work was supported by National Institutes of Health Tumor Immunology Training Grant 5-T32-CA009120–28 (to G.Z.F.); the W. M. Keck Foundation, which established and equipped the University of California, Los Angeles (UCLA), Mass Spectrometry and Proteomics Technology Center; and the Proteomics Center of the UCLA Jonsson Comprehensive Cancer Center.
Abbreviations: BCR, B cell receptor; Btk, Bruton's tyrosine kinase; PLC, phospholipase C.
References
- 1.Jun, J. E. & Goodnow, C. C. (2003) Nat. Immunol. 4, 1057–1064. [DOI] [PubMed] [Google Scholar]
- 2.Gauld, S. B., Dal Porto, J. M. & Cambier, J. C. (2002) Science 296, 1641–1642. [DOI] [PubMed] [Google Scholar]
- 3.Putney, J. W., Jr., Broad, L. M., Braun, F. J., Lievremont, J. P. & Bird, G. S. (2001) J. Cell Sci. 114, 2223–2229. [DOI] [PubMed] [Google Scholar]
- 4.Miller, A. T. & Berg, L. J. (2002) Curr. Opin. Immunol. 14, 331–340. [DOI] [PubMed] [Google Scholar]
- 5.Takesono, A., Finkelstein, L. D. & Schwartzberg, P. L. (2002) J. Cell Sci. 115, 3039–3048. [DOI] [PubMed] [Google Scholar]
- 6.Bolland, S., Pearse, R. N., Kurosaki, T. & Ravetch, J. V. (1998) Immunity 8, 509–516. [DOI] [PubMed] [Google Scholar]
- 7.Fluckiger, A. C., Li, Z., Kato, R. M., Wahl, M. I., Ochs, H. D., Longnecker, R., Kinet, J. P., Witte, O. N., Scharenberg, A. M. & Rawlings, D. J. (1998) EMBO J. 17, 1973–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito, K., Tolias, K. F., Saci, A., Koon, H. B., Humphries, L. A., Scharenberg, A., Rawlings, D. J., Kinet, J. P. & Carpenter, C. L. (2003) Immunity 19, 669–678. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe, D., Hashimoto, S., Ishiai, M., Matsushita, M., Baba, Y., Kishimoto, T., Kurosaki, T. & Tsukada, S. (2001) J. Biol. Chem. 276, 38595–38601. [DOI] [PubMed] [Google Scholar]
- 10.Takata, M. & Kurosaki, T. (1996) J. Exp. Med. 184, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith, C. I., Islam, T. C., Mattsson, P. T., Mohamed, A. J., Nore, B. F. & Vihinen, M. (2001) BioEssays 23, 436–446. [DOI] [PubMed] [Google Scholar]
- 12.Vetrie, D., Vorechovsky, I., Sideras, P., Holland, J., Davies, A., Flinter, F., Hammarstrom, L., Kinnon, C., Levinsky, R., Bobrow, M., et al. (1993) Nature 361, 226–233. [DOI] [PubMed] [Google Scholar]
- 13.Tsukada, S., Saffran, D. C., Rawlings, D. J., Parolini, O., Allen, R. C., Klisak, I., Sparkes, R. S., Kubagawa, H., Mohandas, T., Quan, S., et al. (1993) Cell 72, 279–290. [DOI] [PubMed] [Google Scholar]
- 14.Rawlings, D. J., Saffran, D. C., Tsukada, S., Largaespada, D. A., Grimaldi, J. C., Cohen, L., Mohr, R. N., Bazan, J. F., Howard, M., Copeland, N. G., et al. (1993) Science 261, 358–361. [DOI] [PubMed] [Google Scholar]
- 15.Thomas, J. D., Sideras, P., Smith, C. I., Vorechovsky, I., Chapman, V. & Paul, W. E. (1993) Science 261, 355–358. [DOI] [PubMed] [Google Scholar]
- 16.Hendriks, R. W., de Bruijn, M. F., Maas, A., Dingjan, G. M., Karis, A. & Grosveld, F. (1996) EMBO J. 15, 4862–4872. [PMC free article] [PubMed] [Google Scholar]
- 17.Kerner, J. D., Appleby, M. W., Mohr, R. N., Chien, S., Rawlings, D. J., Maliszewski, C. R., Witte, O. N. & Perlmutter, R. M. (1995) Immunity 3, 301–312. [DOI] [PubMed] [Google Scholar]
- 18.Khan, W. N., Alt, F. W., Gerstein, R. M., Malynn, B. A., Larsson, I., Rathbun, G., Davidson, L., Muller, S., Kantor, A. B., Herzenberg, L. A., et al. (1995) Immunity 3, 283–299. [DOI] [PubMed] [Google Scholar]
- 19.Harrison, S. C. (2003) Cell 112, 737–740. [DOI] [PubMed] [Google Scholar]
- 20.Hantschel, O. & Superti-Furga, G. (2004) Nat. Rev. Mol. Cell Biol. 5, 33–44. [DOI] [PubMed] [Google Scholar]
- 21.Kang, S. W., Wahl, M. I., Chu, J., Kitaura, J., Kawakami, Y., Kato, R. M., Tabuchi, R., Tarakhovsky, A., Kawakami, T., Turck, C. W., et al. (2001) EMBO J. 20, 5692–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park, H., Wahl, M. I., Afar, D. E., Turck, C. W., Rawlings, D. J., Tam, C., Scharenberg, A. M., Kinet, J. P. & Witte, O. N. (1996) Immunity 4, 515–525. [DOI] [PubMed] [Google Scholar]
- 23.Afar, D. E., Park, H., Howell, B. W., Rawlings, D. J., Cooper, J. & Witte, O. N. (1996) Mol. Cell. Biol. 16, 3465–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shevchenko, A., Wilm, M., Vorm, O. & Mann, M. (1996) Anal. Chem. 68, 850–858. [DOI] [PubMed] [Google Scholar]
- 25.Pear, W. S., Miller, J. P., Xu, L., Pui, J. C., Soffer, B., Quackenbush, R. C., Pendergast, A. M., Bronson, R., Aster, J. C., Scott, M. L. & Baltimore, D. (1998) Blood 92, 3780–3792. [PubMed] [Google Scholar]
- 26.Zhang, X. & Ren, R. (1998) Blood 92, 3829–3840. [PubMed] [Google Scholar]
- 27.Satterthwaite, A. B., Cheroutre, H., Khan, W. N., Sideras, P. & Witte, O. N. (1997) Proc. Natl. Acad. Sci. USA 94, 13152–13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ficarro, S. B., McCleland, M. L., Stukenberg, P. T., Burke, D. J., Ross, M. M., Shabanowitz, J., Hunt, D. F. & White, F. M. (2002) Nat. Biotechnol. 20, 301–305. [DOI] [PubMed] [Google Scholar]
- 29.Bence, K., Ma, W., Kozasa, T. & Huang, X. Y. (1997) Nature 389, 296–299. [DOI] [PubMed] [Google Scholar]
- 30.Wahl, M. I., Fluckiger, A.-C., Kato, R. M., Park, H., Witte, O. N. & Rawlings, D. J. (1997) Proc. Natl. Acad. Sci. USA 94, 11526–11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rawlings, D. J., Scharenberg, A. M., Park, H., Wahl, M. I., Lin, S., Kato, R. M., Fluckiger, A.-C., Witte, O. N. & Kinet, J. P. (1996) Science 271, 822–825. [DOI] [PubMed] [Google Scholar]
- 32.Merkel, A. L., Atmosukarto, I. I., Stevens, K., Rathjen, P. D. & Booker, G. W. (1999) Cytogenet. Cell Genet. 84, 132–139. [DOI] [PubMed] [Google Scholar]
- 33.Mao, C., Zhou, M. & Uckun, F. M. (2001) J. Biol. Chem. 276, 41435–41443. [DOI] [PubMed] [Google Scholar]
- 34.Meyer, R. D., Dayanir, V., Majnoun, F. & Rahimi, N. (2002) J. Biol. Chem. 277, 27081–27087. [DOI] [PubMed] [Google Scholar]
- 35.Satterthwaite, A. B. & Witte, O. N. (2000) Immunol. Rev. 175, 120–127. [PubMed] [Google Scholar]
- 36.Craxton, A., Jiang, A., Kurosaki, T. & Clark, E. A. (1999) J. Biol. Chem. 274, 30644–30650. [DOI] [PubMed] [Google Scholar]
- 37.Delcommenne, M., Tan, C., Gray, V., Rue, L., Woodgett, J. & Dedhar, S. (1998) Proc. Natl. Acad. Sci. USA 95, 11211–11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neiman, P. E., Grbic, J. J., Polony, T. S., Kimmel, R., Bowers, S. J., Delrow, J. & Beemon, K. L. (2003) Oncogene 22, 1073–1086. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki, H., Matsuda, S., Terauchi, Y., Fujiwara, M., Ohteki, T., Asano, T., Behrens, T. W., Kouro, T., Takatsu, K., Kadowaki, T. & Koyasu, S. (2003) Nat. Immunol. 4, 280–286. [DOI] [PubMed] [Google Scholar]
- 40.Yu, P., Tabuchi, R., Kato, R., Chae, K., Ellmeier, W., Witte, O. & Rawlings, D. (2004) Blood 104, 1281–1290. [DOI] [PubMed] [Google Scholar]
- 41.Ellmeier, W., Jung, S., Sunshine, M. J., Hatam, F., Xu, Y., Baltimore, D., Mano, H. & Littman, D. R. (2000) J. Exp. Med. 192, 1611–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vihinen, M., Kwan, S. P., Lester, T., Ochs, H. D., Resnick, I., Valiaho, J., Conley, M. E. & Smith, C. I. (1999) Hum. Mutat. 13, 280–285. [DOI] [PubMed] [Google Scholar]
- 43.Maas, A. & Hendriks, R. W. (2001) Dev. Immunol. 8, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.