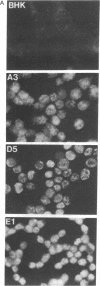
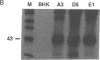
Abstract
The manner in which oncogenes influence tumorigenicity beyond their ability to immortalize cells is uncertain. We tested the hypothesis that, in addition to subverting cellular growth controls, oncogenes can actively determine tumor-inducing capacity by affecting neoplastic cell susceptibility to destruction by the host cellular immune response. The adenovirus type 5 E1A oncogene, which induces susceptibility to lysis by natural killer cells and encodes epitopes recognized by cytotoxic T lymphocytes, was transfected into highly tumorigenic sarcoma cells. E1A expression in these sarcoma cells eliminated their tumorigenicity in recipients with natural killer cell activity that was competent to lyse these E1A-positive targets. Thymus-dependent responses were not required for tumor rejection. These results indicate that oncogene-regulated cellular pathways that affect neoplastic cell susceptibility to natural killer cell lytic mechanisms may influence tumor development in the immunocompetent host.
Full text
PDF

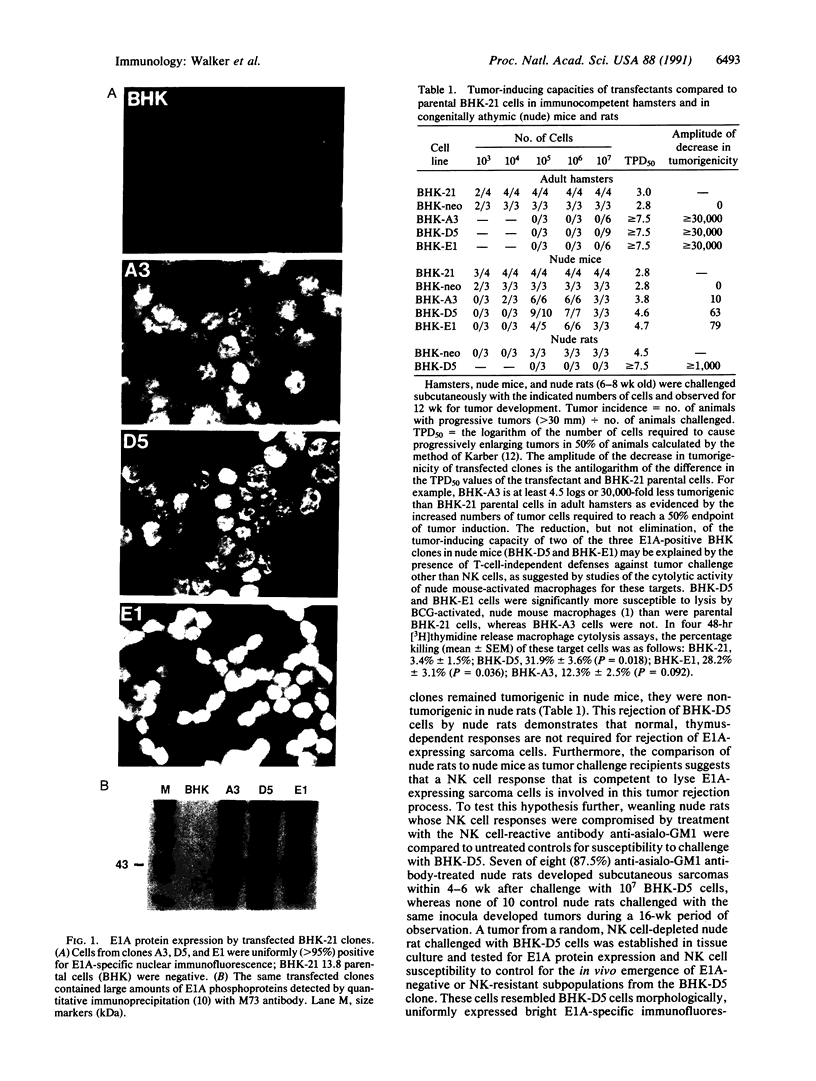
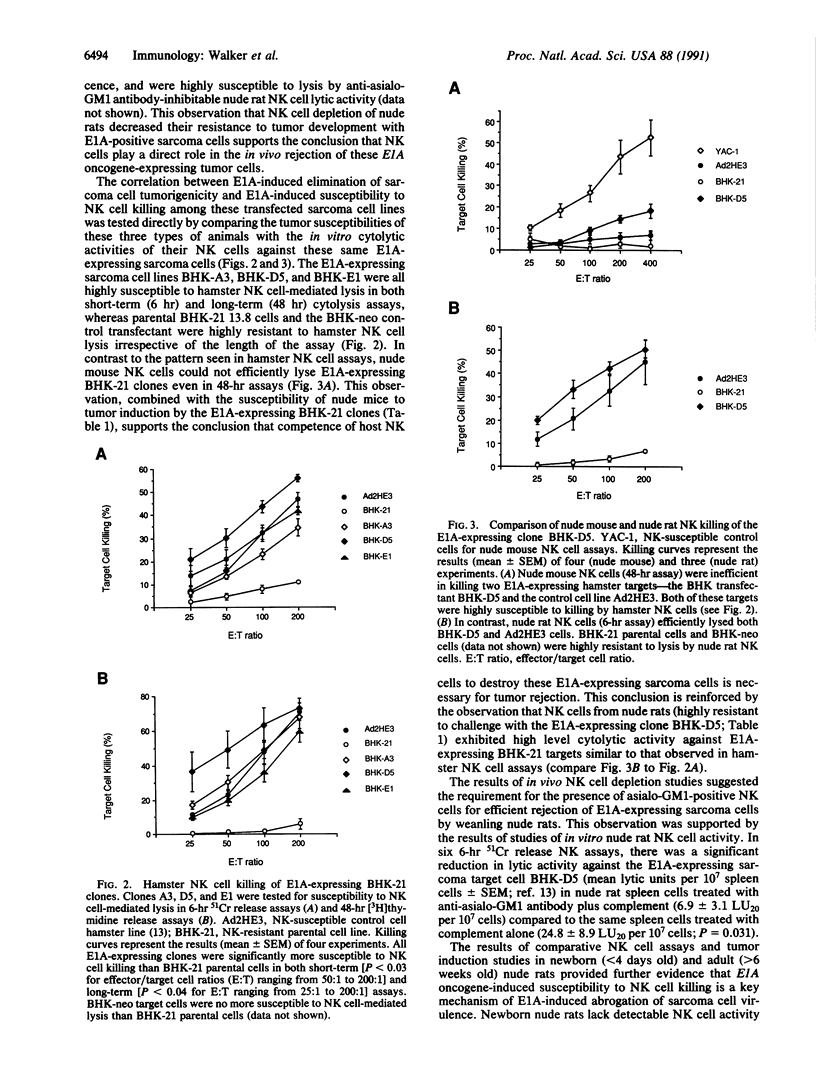

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. K., Stankova J., Roder J. C. Decreased p21 levels in anti-sense ras transfectants augments NK sensitivity. Mol Immunol. 1989 Oct;26(10):985–991. doi: 10.1016/0161-5890(89)90117-x. [DOI] [PubMed] [Google Scholar]
- Bagli D. J., D'Emilia J. C., Summerhayes I. C., Steele G. D., Barlozzari T. c-Ha-ras-I oncogene-induced differentiation and natural killer cell resistance in a human colorectal carcinoma cell line. Cancer Res. 1990 Apr 15;50(8):2518–2523. [PubMed] [Google Scholar]
- Barlozzari T., Leonhardt J., Wiltrout R. H., Herberman R. B., Reynolds C. W. Direct evidence for the role of LGL in the inhibition of experimental tumor metastases. J Immunol. 1985 Apr;134(4):2783–2789. [PubMed] [Google Scholar]
- Bellgrau D., Walker T. A., Cook J. L. Recognition of adenovirus E1A gene products on immortalized cell surfaces by cytotoxic T lymphocytes. J Virol. 1988 May;62(5):1513–1519. doi: 10.1128/jvi.62.5.1513-1519.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards R., Houweling A., Schrier P. I., Bos J. L., Van der Eb A. J. Characterization of cells transformed by Ad5/Ad12 hybrid early region I plasmids. Virology. 1982 Jul 30;120(2):422–432. doi: 10.1016/0042-6822(82)90042-3. [DOI] [PubMed] [Google Scholar]
- Bienz B., Zakut-Houri R., Givol D., Oren M. Analysis of the gene coding for the murine cellular tumour antigen p53. EMBO J. 1984 Sep;3(9):2179–2183. doi: 10.1002/j.1460-2075.1984.tb02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. L., Kirkpatrick C. H., Rabson A. S., Lewis A. M., Jr Rejection of adenovirus 2-transformed cell tumors and immune responsiveness in Syrian hamsters. Cancer Res. 1979 Dec;39(12):4949–4955. [PubMed] [Google Scholar]
- Cook J. L., Lewis A. M., Jr Immunological surveillance against DNA-virus-transformed cells: correlations between natural killer cell cytolytic competence and tumor susceptibility of athymic rodents. J Virol. 1987 Jul;61(7):2155–2161. doi: 10.1128/jvi.61.7.2155-2161.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. L., May D. L., Lewis A. M., Jr, Walker T. A. Adenovirus E1A gene induction of susceptibility to lysis by natural killer cells and activated macrophages in infected rodent cells. J Virol. 1987 Nov;61(11):3510–3520. doi: 10.1128/jvi.61.11.3510-3520.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. L., May D. L., Wilson B. A., Holskin B., Chen M. J., Shalloway D., Walker T. A. Role of tumor necrosis factor-alpha in E1A oncogene-induced susceptibility of neoplastic cells to lysis by natural killer cells and activated macrophages. J Immunol. 1989 Jun 15;142(12):4527–4534. [PubMed] [Google Scholar]
- Cook J. L., May D. L., Wilson B. A., Walker T. A. Differential induction of cytolytic susceptibility by E1A, myc, and ras oncogenes in immortalized cells. J Virol. 1989 Aug;63(8):3408–3415. doi: 10.1128/jvi.63.8.3408-3415.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. L., Walker T. A., Lewis A. M., Jr, Ruley H. E., Graham F. L., Pilder S. H. Expression of the adenovirus E1A oncogene during cell transformation is sufficient to induce susceptibility to lysis by host inflammatory cells. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6965–6969. doi: 10.1073/pnas.83.18.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg A. H., Egan S. E., Jarolim L., Wright J. A. NK sensitivity of H-ras transfected fibroblasts is transformation- independent. Cell Immunol. 1987 Oct 15;109(2):444–450. doi: 10.1016/0008-8749(87)90327-3. [DOI] [PubMed] [Google Scholar]
- Harlow E., Franza B. R., Jr, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985 Sep;55(3):533–546. doi: 10.1128/jvi.55.3.533-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochemsen A. G., Peltenburg L. T., te Pas M. F., de Wit C. M., Bos J. L., van der Eb A. J. Activation of adenovirus 5 E1A transcription by region E1B in transformed primary rat cells. EMBO J. 1987 Nov;6(11):3399–3405. doi: 10.1002/j.1460-2075.1987.tb02663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. W., Trimble W. S., Hozumi N., Roder J. C. Enhanced lytic susceptibility of Ha-ras transformants after oncogene induction is specific to activated NK cells. J Immunol. 1987 Jun 1;138(11):3996–4003. [PubMed] [Google Scholar]
- Kaddurah-Daouk R., Lillie J. W., Daouk G. H., Green M. R., Kingston R., Schimmel P. Induction of a cellular enzyme for energy metabolism by transforming domains of adenovirus E1a. Mol Cell Biol. 1990 Apr;10(4):1476–1483. doi: 10.1128/mcb.10.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast W. M., Offringa R., Peters P. J., Voordouw A. C., Meloen R. H., van der Eb A. J., Melief C. J. Eradication of adenovirus E1-induced tumors by E1A-specific cytotoxic T lymphocytes. Cell. 1989 Nov 17;59(4):603–614. doi: 10.1016/0092-8674(89)90006-8. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Lanza L. A., Wilson D. J., Ikejiri B., Roth J. A., Grimm E. A. Human oncogene-transfected tumor cells display differential susceptibility to lysis by lymphokine-activated killer cells (LAK) and natural killer cells. J Immunol. 1986 Oct 15;137(8):2716–2720. [PubMed] [Google Scholar]
- Lewis A. M., Jr, Cook J. L. Presence of allograft-rejection resistance in simian virus 40-transformed hamster cells and its possible role in tumor development. Proc Natl Acad Sci U S A. 1980 May;77(5):2886–2889. doi: 10.1073/pnas.77.5.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. W., Green M., Green M. R. An adenovirus E1a protein region required for transformation and transcriptional repression. Cell. 1986 Sep 26;46(7):1043–1051. doi: 10.1016/0092-8674(86)90704-x. [DOI] [PubMed] [Google Scholar]
- Moran E., Zerler B., Harrison T. M., Mathews M. B. Identification of separate domains in the adenovirus E1A gene for immortalization activity and the activation of virus early genes. Mol Cell Biol. 1986 Oct;6(10):3470–3480. doi: 10.1128/mcb.6.10.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. DETECTION OF SPECIFIC ANTIGEN IN SV40-TRANSFORMED CELLS BY IMMUNOFLUORESCENCE. J Exp Med. 1964 Aug 1;120:121–128. doi: 10.1084/jem.120.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston R., Bishop J. M. The protein products of the myc and myb oncogenes and adenovirus E1a are structurally related. Nature. 1983 Dec 22;306(5945):803–806. doi: 10.1038/306803a0. [DOI] [PubMed] [Google Scholar]
- Sarid J., Halazonetis T. D., Murphy W., Leder P. Evolutionarily conserved regions of the human c-myc protein can be uncoupled from transforming activity. Proc Natl Acad Sci U S A. 1987 Jan;84(1):170–173. doi: 10.1073/pnas.84.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyabhama S., Epstein A. L. Short-term efficient expression of transfected DNA in human hematopoietic cells by electroporation: definition of parameters and use of chemical stimulators. DNA. 1988 Apr;7(3):203–209. doi: 10.1089/dna.1988.7.203. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Föhring B., Shenk T. E., Raska K., Jr Tumorigenicity of adenovirus-transformed cells: region E1A of adenovirus 12 confers resistance to natural killer cells. Virology. 1985 Dec;147(2):413–421. doi: 10.1016/0042-6822(85)90143-6. [DOI] [PubMed] [Google Scholar]
- Schneider J. F., Fisher F., Goding C. R., Jones N. C. Mutational analysis of the adenovirus E1a gene: the role of transcriptional regulation in transformation. EMBO J. 1987 Jul;6(7):2053–2060. doi: 10.1002/j.1460-2075.1987.tb02470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabel S., Argos P., Philipson L. The release of growth arrest by microinjection of adenovirus E1A DNA. EMBO J. 1985 Sep;4(9):2329–2336. doi: 10.1002/j.1460-2075.1985.tb03934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg R., Peltenburg L. T., Plomp A. C., Schrier P. I. High expression of the c-myc oncogene renders melanoma cells prone to lysis by natural killer cells. J Immunol. 1989 Dec 15;143(12):4331–4337. [PubMed] [Google Scholar]
- Zerler B., Roberts R. J., Mathews M. B., Moran E. Different functional domains of the adenovirus E1A gene are involved in regulation of host cell cycle products. Mol Cell Biol. 1987 Feb;7(2):821–829. doi: 10.1128/mcb.7.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong W. H., Steerenberg P. A., Ursem P. S., Osterhaus A. D., Vos J. G., Ruitenberg E. J. The athymic nude rat. III. Natural cell-mediated cytotoxicity. Clin Immunol Immunopathol. 1980 Oct;17(2):163–172. doi: 10.1016/0090-1229(80)90084-7. [DOI] [PubMed] [Google Scholar]