Abstract
Although the heart responds to estrogen, it is not clear whether estrogen acts directly on heart muscle or indirectly by means of the vascular, immune, or nervous system. No role for estrogen receptor (ER) β in the heart has been established, but ERβ–/– mice are hypertensive, and as they age, their hearts become enlarged. Histological and ultrastructural analysis of the heart revealed a disarray of myocytes, a disruption of intercalated discs, an increase in the number and size of gap junctions, and a profound alteration in nuclear structure, concomitantly with a loss of expression of lamin A/C from the nuclear envelope. In the lungs of ERβ–/– mice, lamin A/C was located in the nuclear membrane, indicating that lamin A/C is not an ERβ-regulated gene. Immunohistochemical studies with ERβ antibodies failed to detect ERβ in the myocardium. We conclude that abnormalities in heart morphology in ERβ–/– mice are likely due to stress on the nuclear envelope as a result of the chronic sustained systolic and diastolic hypertension observed in ERβ–/– mice. Because neither ERα nor ERβ could be detected in heart muscle, the effects of estrogen on the myocardium seem to be indirect.
Keywords: lamin A/C, N-cadherin, dilated cardiomyopathy, hypertension
Estrogen receptor (ER) β–/– mice become hypertensive by 5 months of age, and thereafter, systolic and diastolic pressure is sustained at about 30 mmHg above normal in males and 20 mmHg above normal in females (1 mmHg = 133 Pa) (1). The cause of the hypertension is not known. In response to hypertension, the heart adapts by increasing muscle mass. In the face of continued hypertension, the increased muscle mass can make the heart less effective as a pump, and dilated cardiomyopathy results (2).
Estrogen regulates heart rate by increasing vagal tone and decreasing sympathetic tone to the heart (3) and has cardiovascular protective effects through its modulation of endogenous vasoconstrictors such as angiotensin II and vasodilators such as nitric oxide in blood vessels (4).
Although ovariectomy and estrogen treatment influences expression of genes such as connexin 43 (Cx43) and α-myosin heavy chain (3–5), in the myocardium, there is little evidence for direct transcriptional regulation by estrogen of any genes in the heart. Analysis by Affymetrix of the effects of estrogen in the mouse heart showed very few estrogen-regulated RNAs (6), and in two separate lines of ERE-reporter mice (7, 8), estradiol did not induce reporter genes in the heart. The question is still whether there are functional estrogen receptors in the myocardium.
ERα and ERβ are expressed in the endothelium (9) and smooth muscle (10) of blood vessels and in neonatal cardiomyocytes in culture, but only when estradiol is added to the culture medium (11). Some studies report that ERβ cannot be detected in the adult heart (12), whereas in others ERβ is abundant in the nucleus (13–16), and in one study ERβ was exclusively mitochondrial (17). These diverse results obtained in different laboratories could be due to differences in antibody specificity. Part of it is due to the fact that ERβ is present at low levels in cells, and stringent conditions must be used to distinguish between background and specific signals.
In the present study, we have investigated whether ERα and ERβ are nuclear receptors in the myocardium of mouse hearts and whether any changes in gene expression or morphology in the hearts of ERβ–/– mice can be attributed to loss of ERβ from the heart.
Materials and Methods
Animals and Tissue Collection. ERβ–/– mice and WT littermates were generated from heterozygous breeding as described (18). All animals were housed in the animal-care facility with a 12-h light/12-h dark photoperiod with 50% humidity and given free access to tap water and rodent chow. Genotyping by PCR was performed as described in ref. 19. Mice were killed by CO2 asphyxiation. Hearts and lungs were excised, frozen immediately, and stored at –80°C until used for immunohistochemistry and Western blotting, or fixed in 4% paraformaldehyde overnight and routinely embedded in paraffin wax for immunohistochemistry.
Histology. After paraffin embedding, sections (4 μm) were mounted on organosilane-coated slides. Slides were stained with hematoxylin/eosin for histological evaluation under the light microscope. Transversal histological sections were prepared at equal distance from the heart apex (3 mm) and viewed under a Zeiss Axioplan 2 microscope.
Immunohistochemistry. ERβ IgY was prepared in this laboratory and characterized previously (20). It was raised against the whole human ERβ protein. For Western blotting, two ERβ antibodies were raised in rabbits, one against the ligand binding domain of human ERβ and one against the sequence of amino acids 1–50 of the human ERβ. N-cadherin (sc-1502, Santa Cruz Biotechnology, 1:100) and Cx43 (Zymed, 1:100) were detected in paraffin sections by immunofluorescence procedures. Briefly, sections were incubated with primary antibodies [in 3% (wt/vol) BSA in PBS] overnight at 4°C. PBS was used in place of primary antibodies in negative controls. Slides were washed with PBS and incubated with the respective FITC-conjugated donkey secondary antibodies (Jackson ImmunoResearch) for 45 min at 37°C. Nuclei were counterstained with propidium iodide in the case of Cx43 stains.
For visualization of f-actin, the sections were dewaxed and washed in PBS, and the cells were permeabilized by the addition of 0.1% Triton X-100 in PBS for 20 min. The sections were then stained with FITC-phalloidin (Molecular Probes) diluted 1:200 according to the manufacturer's instructions.
For lamin A/C (sc-7293, Santa Cruz Biotechnology, 1:100) and lamin B (sc-6217, Santa Cruz Biotechnology, 1:100) staining, frozen, 9-μm sections were air-dried for 30 min. After washing with ice-cold methanol and acetone, each for 3 min, sections were fixed for 10 min at room temperature in 4% paraformaldehyde, followed first by 0.1% Triton X-100 in PBS for 5 min, next by 10% normal donkey serum in PBS for 30 min at 25°C, and finally by incubation with primary antibodies and FITC-conjugated secondary antibodies. Nuclei were counterstained with propidium iodide. Sections were mounted in VECTA-SHIELD antifading medium (Vector Laboratories) and examined at comparable depths on a Leica TCSSP confocal microscope. At a ×63 magnification used to evaluate connexin expression, each fluorescent spot indicates a gap junction (GJ) plaque (21).
For ERβ staining, sections were incubated for 1 h at 4°C with normal goat serum diluted at 1:10 in PBS. ERβ IgY was diluted 1:200 in PBS containing 3% BSA. Sections were incubated with antibodies overnight at 4°C. For negative controls, the primary antibody was replaced with PBS alone or with primary antibody after absorption with ERβ protein. Before addition of the secondary antibody, sections were rinsed in PBS. Sections were then incubated in biotinylated goat anti-chicken Ig (1:200 dilution) for2hat room temperature, followed by washing with PBS and incubation in avidin-biotin-horseradish peroxidase for 1 h. After thorough washing in PBS, slides were developed with 3,3′-diaminobenzidine tetrahydrochloride, slightly counter-stained with Mayer's hematoxylin, and dehydrated through an ethanol series, followed by exposure to xylene and mounting.
Construction of Figures. Each single confocal image in a z-series captures the distribution of antibody-labeled protein in a 0.5-μm section of the tissue. The z-series was then projected to give a single image showing the distribution of the visualized protein in a tissue depth of up to 15 μm (see ref. 21 for methods). Quantitative analysis of GJ plaque size and numerical density was performed by using the Leica TCSSP image processing tool.
Transmission Electron Microscopy. Hearts were dissected and small pieces were cut and fixed in 2% glutaraldehyde plus 0.5% paraformaldehyde in 0.1 M sodium cacodylate buffer containing 0.1 M sucrose and 3 mM CaCl2 (pH 7.4) at room temperature for 30 min followed by 24 h at 4°C. Specimens were rinsed in 0.15 M sodium cacodylate buffer containing 3 mM CaCl2 (pH 7.4) and postfixed in 2% osmium tetroxide in 0.07 M sodium cacodylate buffer containing 1.5 mM CaCl2 (pH 7.4) at 4°C for 2 h, dehydrated in ethanol followed by acetone, and embedded in LX-112 (Ladd Research Laboratories, Burlington, VT). Semithin sections were cut and stained with toluidine blue and used for light-microscopic analysis. Ultrathin sections (≈40–50 nm) were cut and contrasted with uranyl acetate followed by lead citrate and examined in a Tecnai 10 (Fei, Eindhoven, The Netherlands) transmission electron microscope at 80 kV.
Calculation of Intercalated Disc (ID) Ratio. Ten randomly selected IDs in left ventricles from WT and ERβ–/– mice were analyzed. The total length of the ID was divided by the width of the disk, giving a ratio.
Western Blotting. Nuclear extracts, total cell extracts, and mitochondrial fractions were analyzed by Western blotting. Frozen tissues were homogenized with a Polytron PT3100 (Kinematica, Lucerne, Switzerland). Two tablets of mixture protease inhibitors (Boehringer Mannheim) were added per 50 ml of PBS before use. To prepare nuclear extracts, crude nuclear fractions were sedimented from tissue homogenates at 600 × g for 10 min. Nuclei were sedimented through a cushion of 25 ml of 30% sucrose at 100,000 × g for 1 h. Mitochondrial fractions were sedimented from the postnuclear supernatant by centrifugation at 10,000 × g for 20 min. Protein was extracted by incubating the pellets in 0.5% deoxycholate/0.1% Nonidet P-40 in PBS. Solubilized protein was separated from debris by centrifugation. Solubilized protein or total homogenates were dissolved in SDS sample buffer, and aliquots were used for Western blotting. Protein content was measured by the Bio-Rad protein assay with BSA as the standard. Aliquots of 30 μg of protein were loaded onto each lane of an 8% polyacrylamide gel. Western blotting was done according to the protocol described in ref. 22. Antibody dilutions were 1:1,000 for lamin A/C and lamin B, 1:3,000 for ERβ ligand binding domain antibody, and 1:5,000 for the peroxidase-conjugated goat anti-rabbit; anti-mouse and anti-chicken IgGs were used at 1:5,000. Signals were detected with ECL (Amersham Pharmacia).
RNA Extraction and cDNA Synthesis. Extraction of total RNA from frozen left ventricle tissue, DNase digestion, and cDNA synthesis were carried out as described in ref. 23.
Quantitative Real-Time RT-PCR. The primers and probes for the target genes were determined with the assistance of the computer program primer express (Applied Biosystems). The primers and probes used in this study are as follows: ANF forward 5′-ATTTCAAGAACCTGCTAGACCACCT-3′ (200 nM) and reverse 5′-CAGTCTGCTCACTCAGGGCC-3′ (200 nM), probe FAM-5′-TGACCTCATCTTCTACCGGCATCTTCTCC-3′-TAMRA (200 nM), beta-MHC forward 5′-GTGAAGGGCATGAGGAAGAGC-3′ (300 nM) and reverse 5′-AGGCCTTCACCTTCAGCTGC-3′ (300 nM), and probe FAM-5′-CACCTACCAGACAGAGGAAGACAGGAAGAACCTAC-3′-TAMRA (300 nM). The real-time PCR reactions were performed as described in ref. 24, and the amplified products were sequenced. Standard curves were generated by using serially diluted solutions of standard cDNA derived from the left ventricles of WT mice. Real-time PCR was done in triplicate for each sample. The 18S rRNA (PDAR, primer express, Applied Biosystems) was used as a reference gene. The target gene transcripts in each sample were normalized on the basis of their 18S rRNA transcript content. Each experimental group (WT and ERβ–/–) contained samples of three different animals (n = 3).
Statistical Analysis. The differences in mRNA expression levels of the target genes between the experimental groups were evaluated by using Student's t test.
Results
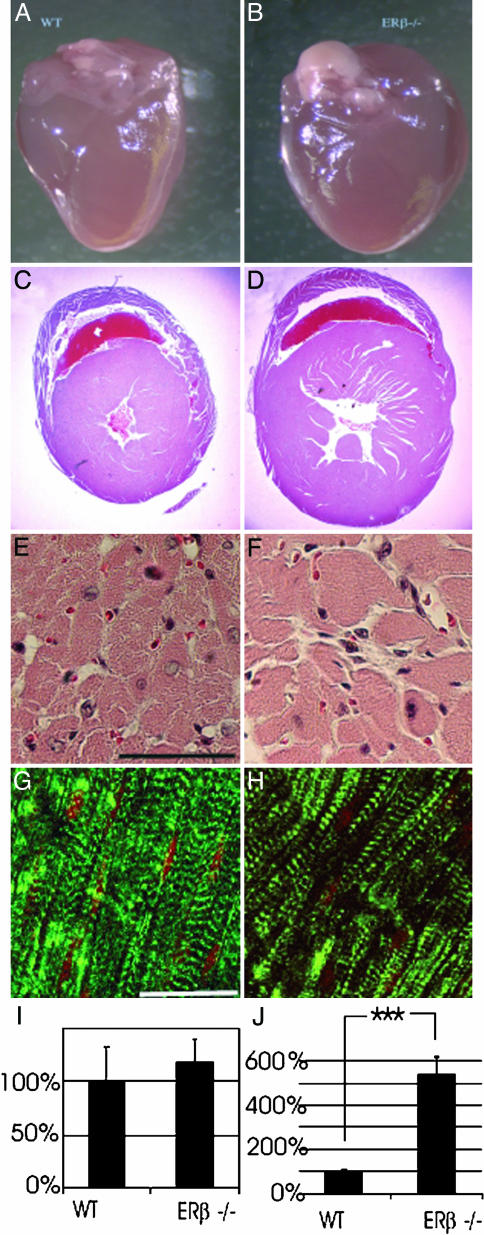
Altered Cardiac Morphology in ERβ–/– Mice. After 8 months of age, ERβ–/– mouse hearts (Fig. 1B) were larger than those of WT littermates (Fig. 1 A). Cross sections from hearts of ERβ–/– and WT mice documenting hypertrophy and dilation of ventricles are shown in Fig. 1 C and D. Histologically, there was an increase in myocyte diameter and an increase in the distance between myocytes (Fig. 1 E and F). In addition, there was an up-regulation of β-myosin heavy chain in hearts of ERβ–/– mice (Fig. 1 G and H), a clear indication of pressure and volume overload (25).
Fig. 1.
Morphology of ERβ–/– hearts. (A and B) The hearts of 6-month-old male mice showed that, compared with their WT littermates, ERβ–/– mouse hearts had enlarged ventricles with the rounded rather than the typical apical shape. (C and D) A cross section through the hearts illustrates the differences in diameter of the heart and the hypertrophy of the ERβ–/– mouse heart. Cardiomyocyte diameter and intercellular space were measured from seven individual hearts. (E and F) Cardiomyocytes were enlarged, and intercellular space was wider in mutant hearts. (Scale bar, 50 μm.) (G and H) When hearts were stained for f-actin with FITC-phalloidin, no alterations in the f-actin cross striations were observed in ERβ–/– hearts; however, the individual myofibrils do not run as strictly parallel in the ERβ–/– cardiomyocytes as they do in WT and are of heterogeneous size. (Scale bar, 20 μm.) (I and J) Levels of ANF (proatrial natriuretic factor) and β-MHC (β-myosin heavy chain) genes were measured by RT-PCR. The data are expressed as mean ± SEM. ***, P < 0.001. The expression of β-MHC mRNA was increased 5-fold in ERβ–/– mice.
Expression of ERs in WT Mice. There was no detectable expression of ERα or ERβ in the myocardium. Immunohistochemical studies with ERα-specific MC20 antibodies (data not shown) failed to detect ERα in the myocardium, but it was well expressed in the epithelial cells lining the large airways. ERβ-specific IgY also did not detect ERβ in the myocardium. ERβ was well expressed in the nucleus of cells in the alveoli and in the epithelial cells of the bronchioles (Fig. 2A). Western blots with mitochondrial fractions and nuclear extracts prepared from hearts of WT mice were probed with specific ERβ ligand binding domain and N-terminal antibodies. As shown previously (26), with the ligand binding domain antibody, ERβ was easily detected on Western blots with nuclear extracts from the lungs of WT mice. No specific signals of the correct size were found in mitochondrial fractions (Fig. 2B) or in nuclear extracts of hearts (Fig. 2C) when blots were probed with the N-terminal antibody. However, in mitochondrial fractions, a band that migrated at a molecular mass of 70 kDa was recognized by this antibody. This band was present in fractions prepared from hearts of ERβ–/– mice and is thus unlikely to be related to ERβ. To confirm equal loading in the lanes, the blot was probed for a specific mitochondrial marker, cytochrome oxidase-2. The antibody was a gift from Nils-Göran Larsson (Karolinska Institutet).
Fig. 2.
Expression of ERβ in the heart and lung of WT mice. (A) Immunohistochemical study with ERβ chicken 503 antibody revealed the presence of ERβ in the nucleus of alveolar and epithelial cells of bronchioles in the lung but no staining of the myocardium. Western blots of mitochondrial fractions (B Left) and nuclear extracts (C) were prepared from hearts of WT mice probed with a specific ERβ N-terminal antibody. Loading of the lanes was as follows: 1, ERβ (0.2 pmol); 2 and 4, heart mitochondrial fractions; 3 and 5, lung mitochondrial fractions. (B Right) Blot stripped and reprobed with a specific antibody raised against mitochondrial cytochrome oxidase-2.
Structure of the Heart of ERβ–/– Mice. By light microscopy, it was evident that the orientation of myofibrils was irregular and they were of heterogeneous length in ERβ–/– mice. However, as shown by phalloidin stain, which stains f-actin in the I-bands, there was no gross alteration of sarcomere striation (Fig. 1 G and H).
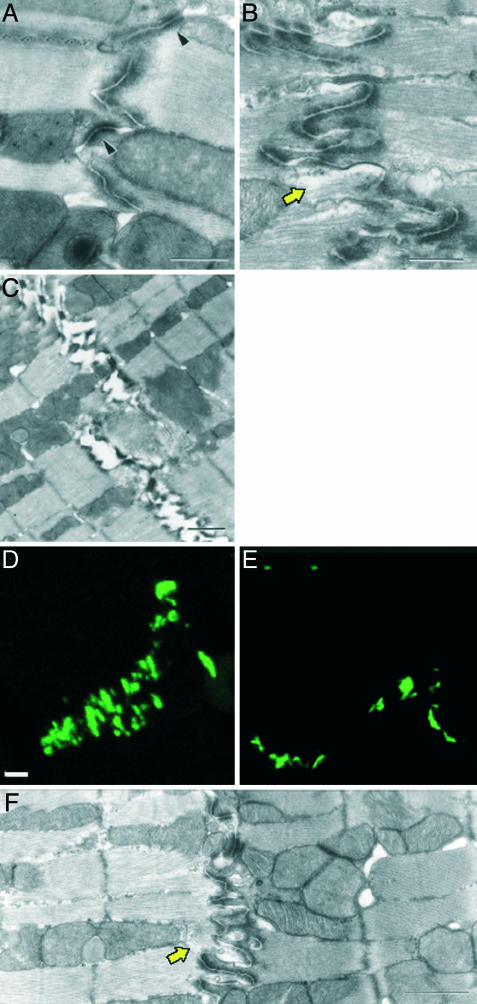
By transmission electron microscopy, the IDs were more convoluted in ERβ–/– mouse hearts (Fig. 3 B and C) than in hearts of WT littermates (Fig. 3A). Measurements of ID length showed an increase of 110 ± 15% (SD) in membrane length in ERβ–/– mice. The ratio of membrane length to width was 1.62 ± 0.26 for WT and 3.03 ± 0.62 for ERβ–/– hearts, confirming a higher degree of convolution of the IDs in ERβ–/– mice. N-cadherin was organized in a parallel pattern along the ID region of the plasma membrane in WT cardiomyocytes (Fig. 3D), but in ERβ–/– hearts, N-cadherin was dispersed (Fig. 3E). Often, regions of the ID were ruptured, and the distance between the myofibrils was increased (Fig. 3F).
Fig. 3.
Altered ID structure in ERβ–/– cardiomyocytes. (A) Electron micrograph of ID in WT mice displaying a normal morphology, i.e., containing desmosomes (arrowhead), fascia adherents, and cytoplasmic filament. (Scale bar, 0.5 μm.) (B) IDs in ERβ–/– mice are more convoluted but contain desmosomes and fascia adherents. (Scale bar, 0.5 μm.) (C) Frequently, the IDs in ERβ–/– mice were dissociated. An electron micrograph of adjacent cardiomyocytes with an ID with electron-dense plaques from 6-month-old ERβ–/– mice, to which filaments have attached, is shown. (Scale bar, 1 μm.) (F) In ERβ–/– hearts, apparently missing material (electronically less dense) between the filaments was a typical observation (arrow). (D and E) Changes of ID morphology as seen by immunofluorescence. Semithin cryosections of WT (D) and ERβ–/– (E) mice were stained with antibodies against N-cadherin. There was an increase of signal over a wider area in ERβ–/– hearts. (Scale bar, 4 μm.)
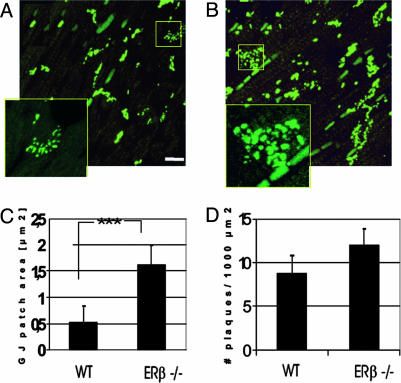
By confocal microscopy (Fig. 4A), Cx43 signals in WT mice were concentrated at points of intercellular apposition in a pattern consistent with the distribution of GJs and IDs. In sections of hearts from ERβ–/– mice, there was an increase in the amount of Cx43 signal at points of intercellular apposition, and the staining was more disperse (Fig. 4B). Such a distribution is consistent with the long and convoluted disks. Digital image processing confirmed that the proportion of total myocardial tissue area occupied by a strong Cx43 immunoreactive signal was higher in ERβ–/– than in WT mouse hearts. The mean plaque size was 0.65 ± 0.05 μm2 in WT mice and 1.62 ± 0.36 μm2 in ERβ–/– mice (Fig. 4C). The area densities in WT and ERβ–/– mice were 8.5 ± 1.3 and 12.1 ± 1.7 plaques per 1,000 μm2, respectively (Fig. 4D).
Fig. 4.
(A and B) Confocal fluorescence microscopy of anti-Cx43 immunostaining in left ventricle of WT (A) and ERβ–/– (B) mice. Projectional images were constructed from 20 optical sections. The patches of punctate staining represent GJs organized in the pattern of the ID between longitudinally oriented cardiomyocytes. Note the higher intensity of the immunolabeled GJs in ERβ–/– mice (B). (Scale bar, 20 μm.) (C and D) Quantification of Cx43 GJ area (C) and numerical densities (D), expressed from the values obtained from projection images of 20 levels (each interval of 0.5-μm optical section) in five randomly examined areas for each animal tissue. The data are from the left ventricle of five animals from each group and are expressed as mean ± SEM.
Nuclear Envelope Architecture. Ultrastructural and immunofluorescence analysis of nuclear lamina components in cardiomyocytes of 6-month-old WT mice (Fig. 5) showed a normal smooth nuclear envelope (Fig. 5A). The cardiomyocyte nuclei in ERβ–/– mice were of bizarre shapes with folded nuclear envelope (Fig. 5B) and convoluted membranous structures close to the nuclei (Fig. 5C). Immunohistochemical analysis revealed that lamin A/C was associated with the nuclear envelope in myocytes from WT mice (Fig. 5D), but in ERβ–/– mice, there was sparse patchy nuclear staining and intense cytoplasmic staining (Fig. 5E). In the lungs of WT (Fig. 5F) and ERβ–/– (Fig. 5G) mice, lamin A/C was localized as a clear ring surrounding the nucleus. On Western blots with nuclear fractions, lamin A/C was well expressed in WT mice and absent from ERβ–/– mouse hearts. Levels of lamin A/C in the total cell extract were similar in WT and ERβ–/– mice (Fig. 5H).
Fig. 5.
Altered nuclear structure in ERβ–/– cardiomyocytes. (A) WT nuclei display a normal elongated morphology with a smooth nuclear envelope. (B) Nuclei in ERβ–/– mice display an irregular, folded nuclear envelope. (C) Occasionally, close to the nuclear envelope, stacks of membrane are found (arrowheads). N, nucleus. [Scale bar: 2 μm(A and B), 0.2 μm(C), and 20 μm(D–G).] Immunohistochemical analysis of lamin A/C in cardiomyocytes and alveolar epithelial cells from WT (D and F) and ERβ–/– (E and G) mice. (E) In the ERβ–/– muscle, the signal intensity of lamin A/C around the nuclei appears either increased or weaker, but commonly delocalized and not confined to the nuclear envelope. These immunolabeled structures in the cytoplasm possibly constitute the counterparts of the excess membranous material observed by transmission electron microscopy. Nuclei are counterstained with propidium iodide. In the lungs of WT (F) and ERβ–/– (G) mice, lamin A/C is localized as a clear ring surrounding the nucleus. (H) Western blot analysis of lamin A/C protein expression levels in total cell extracts and nuclear extracts from WT (lanes 3–5) and ERβ–/– mice (lanes 1 and 2). Levels of lamin A/C in total cell extracts are similar in WT and ERβ–/– mice. Note the total lack of lamin A/C expression in nuclear extracts from ERβ–/– mice.
Discussion
ERβ–/– mice develop sustained systolic and diastolic hypertension as they age (1). In the present study, we have shown that there are abnormalities in the hearts of these mice and have investigated these abnormalities to determine whether they are simply normal responses to hypertension or whether the loss of ERβ from the heart has contributed to the morphological abnormalities.
We found that the hearts of ERβ–/– mice responded to the stress of chronic hypertension in a predictable fashion. One indicator of this stress was the increase in the expression of β-MHC, the fetal form of myosin in the mouse (27, 28). This is an energy conservation switch as β-MHC has a lower ATPase activity. In addition, there were changes in the IDs, a disarray in GJs, and an increase in the major ventricular GJ protein Cx43, all typical adjustments of the heart to hypertension (29–37). There was also rupture of the myocyte fibrillar apparatus, a condition that has been described in mice lacking muscle LIM protein (38) or plakoglobin (39).
The nuclei of ERβ–/– mouse hearts were of irregular shape with distended herniations of the nuclear membrane and presence of convoluted membranous structures close to the nucleus. These types of changes are observed in cardiomyocytes where lamin A/C gene is abnormal (40, 41). Nuclear lamins give the nucleus its shape and are involved in chromatin organization, DNA replication, gene expression, and transmission of mechanical signals from the cell surface to the nucleus (42–46). We found in the present study that although there was no reduction in the cellular content of lamin A/C, there was loss of lamin A/C from the nuclear envelope in ERβ–/– mice. Mislocation of lamins is the most likely cause of the observed abnormalities in nuclear shape.
Unlike what has been reported in previous studies from other laboratories (12–17), we could not detect ERβ in the nuclei of cardiac myocytes. The antibodies used in this study have been tested extensively for specificity for ERβ in immunochemical studies in the mammary gland, testes, salivary glands, prostate, and uterus. ERα was also undetectable. The levels of nuclear receptors in tissues are usually very low. For ERs, it is in the fmol/mg protein range. In breast cancer, the range is 10–1,000 fmol/mg protein. In some cancers, ERα levels can reach as high as 2–3 pmol/mg cytosolic protein (120–180 ng/mg protein), but this is very rare (47). The recent study (17) showing the content of ERα and ERβ in MCF-7 cell homogenates at 1 μg/mg protein (16 pmol/mg protein) seems to be unrealistic. It should be routine that standard amounts of ER proteins be used on Western blots, along with the samples being tested. An aliquot containing 30 μg of protein from tissue homogenates should not contain more than 60–90 fmol of ER.
As discussed above, there is no consensus on whether ERβ is present in the myocardium and little evidence for direct effects of estrogen in the myocardium. When estrogen is shown to increase transcription of genes in the heart, the fold increase is much smaller than it is in the uterus (48). In the present study, we examined normal male and female mouse hearts for the presence of ERs and could find no evidence for their presence. It remains possible that treatment with estradiol could induce ERs in the heart as it does in myocytes in culture, but this has not been examined because it was not relevant to the study of ERβ–/– mouse hearts.
We conclude that dilation of the heart, distortion of the nuclear shape, and displacement of lamins from the nuclear envelope to the cytoplasm in the heart of ERβ–/– mice are likely due to stress on the nuclear envelope as a result of the chronic sustained systolic and diastolic hypertension observed in ERβ–/– mice. Because we were unable to detect ERs in hearts of WT mice, the effects of estrogen on the myocardium seem to be indirect.
Acknowledgments
We thank Dr. Gil-Jin Shim (Karolinska Institutet, Stockholm), Dr. D. Becker (University College London), and Dr. Sari Makelä (University of Turku, Turku, Finland) for expert advice and stimulating discussions. Special thanks to Dr. Maria Eriksson for discussions of the implications of the cellular localization of lamins. This research was supported by grants from the Swedish Cancer Fund and KaroBio AB and by EC Marie Curie Individual Postdoctoral Fellowship MCFI-2000-01758 (to C.F.).
Abbreviations: ID, intercalated disc; GJ, gap junction; ER, estrogen receptor; Cx43, connexin 43.
References
- 1.Zhu, Y., Bian, Z., Lu, P., Karas, R. H., Bao, L., Cox, D., Hodgin, J., Shaul, P. W., Thoren, P., Smithies, O., et al. (2002) Science 295, 505–508. [DOI] [PubMed] [Google Scholar]
- 2.Pinto, Y. M., Paul, M. & Ganten, D. (1998) Cardiovasc. Res. 39, 77–88. [DOI] [PubMed] [Google Scholar]
- 3.Saleh, T. M. & Connell, B. J. (2003) Brain Res. 973, 161–170. [DOI] [PubMed] [Google Scholar]
- 4.Kaya, D., Cevrioglu, S., Onrat, E., Fenkci, I. V. & Yilmazer, M. (2003) J. Obstet. Gynaecol. Res. 29, 406–411. [DOI] [PubMed] [Google Scholar]
- 5.Yu, W., Dahl, G. & Werner, R. (1994) Proc. R. Soc. London Ser. B 255, 125–132. [DOI] [PubMed] [Google Scholar]
- 6.Otsuki, M., Gao, H., Dahlman-Wright, K., Ohlsson, C., Eguchi, N., Urade, Y. & Gustafsson, J.-Å. (2003) Mol. Endocrinol. 17, 1844–1855. [DOI] [PubMed] [Google Scholar]
- 7.Di Lorenzo, D., Villa, R., Biasiotto, G., Belloli, S., Ruggeri, G., Albertini, A., Apostoli, P., Raviscioni, M., Ciana, P. & Maggi, A. (2002) Endocrinology 143, 4544–4551. [DOI] [PubMed] [Google Scholar]
- 8.Lemmen, J. G., Arends, R. J., van Boxtel, A. L., van der Saag, P. T. & van der Burg, B. (2004) J. Mol. Endocrinol. 32, 689–701. [DOI] [PubMed] [Google Scholar]
- 9.Tschugguel, W., Dietrich, W., Zhegu, Z., Stonek, F., Kolbus, A. & Huber, J. C. (2003) J. Clin. Endocrinol. Metab. 88, 2281–2287. [DOI] [PubMed] [Google Scholar]
- 10.Maggi, A., Cignarella, A., Brusadelli, A., Bolego, C., Pinna, C. & Puglisi, L. (2003) Circulation 2, 211–217. [DOI] [PubMed] [Google Scholar]
- 11.Grohe, C., Kahlert, S., Lobbert, K. & Vetter, H. (1998) J. Endocrinol. 156, R1–R7. [DOI] [PubMed] [Google Scholar]
- 12.Jankowski, M., Rachelska, G., Donghao, W., McCann, S. M. & Gutkowska, J. (2001) Proc. Natl. Acad. Sci. USA 98, 11765–11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savolainen, H., Frosen, J., Petrov, L., Aavik, E. & Hayry, P. (2001) J. Heart Lung Transplant. 20, 1252–1264. [DOI] [PubMed] [Google Scholar]
- 14.Xu, Y., Arenas, I. A., Armstrong, S. J. & Davidge, S. T. (2003) Cardiovasc. Res. 57, 388–394. [DOI] [PubMed] [Google Scholar]
- 15.Saunders, P. T., Maguire, S. M., Gaughan, J. & Millar, M. R. (1997) J. Endocrinol. 154, R13–R16. [DOI] [PubMed] [Google Scholar]
- 16.Taylor, A. H. & Al-Azzawi, F. (2000) J. Mol. Endocrinol. 24, 145–155. [DOI] [PubMed] [Google Scholar]
- 17.Yang, S. H., Liu, R., Perez, E. J., Wen, Y., Stevens, S. M., Jr., Valencia, T., Brun-Zinkernagel, A. M., Prokai, L., Will, Y., Dykens, J. W., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 4130–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krege, J. H., Hodgin, J. B., Couse, J. F., Enmark, E., Warner, M., Mahler, J. F., Sar, M., Korach, K. S., Gustafsson, J.-Å. & Smithies, O. (1998) Proc. Natl. Acad. Sci. USA 95, 15677–15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Windahl, S. H., Vidal, O., Andersson, G., Gustafsson, J.-Å. & Ohlsson, C. (1999) J. Clin. Invest. 104, 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saji, S., Jensen, E. V., Nilsson, S., Rylander, T., Warner, M. & Gustafsson, J.-Å. (2000) Proc. Natl. Acad. Sci. USA 97, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker, D. L., Evans, W. H., Green, C. R. & Warner, A. (1995) J. Cell Sci. 108, 1455–1467. [DOI] [PubMed] [Google Scholar]
- 22.Weihua, Z., Saji, S., Makinen, S., Cheng, G., Jensen, E. V., Warner, M. & Gustafsson, J.-Å. (2000) Proc. Natl. Acad. Sci. USA 97, 5936–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller, P., Kietz, S., Gustafsson, J.-Å. & Ström, A. (2002) J. Biol. Chem. 277, 28376–28379. [DOI] [PubMed] [Google Scholar]
- 24.Förster, C., Makelä, S., Wärri, A., Becker, D., Hultenby, K., Warner, M. & Gustafsson, J.-Å. (2002) Proc. Natl. Acad. Sci. USA 99, 15578–15583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz, K., Boheler, K. R., de la Bastie, D., Lompre, A. M. & Mercadier, J. J. (1992) Am. J. Physiol. 262, R364–R369. [DOI] [PubMed] [Google Scholar]
- 26.Patrone, C., Cassel, T. N., Pettersson, K., Piao, Y. S., Cheng, G., Ciana, P., Maggi, A., Warner, M., Gustafsson, J.-Å. & Nord, M. (2003) Mol. Cell. Biol. 23, 8542–8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babiker, F. A., De Windt, L. J., van Eickels, M., Thijssen, V., Bronsaer, R. J., Grohe, C., van Bilsen, M. & Doevendans, P. A. (2004) Circulation 109, 269–276. [DOI] [PubMed] [Google Scholar]
- 28.Uzzaman, M., Honjo, H., Takagishi, Y., Emdad, L., Magee, A. I., Severs, N. J. & Kodama, I. (2000) Circ. Res. 86, 871–878. [DOI] [PubMed] [Google Scholar]
- 29.Peters, N. S. (1996) Clin. Sci. 90, 447–452. [DOI] [PubMed] [Google Scholar]
- 30.Forbes, M. S. & Sperelakis, N. (1985) Tissue Cell 17, 605–648. [DOI] [PubMed] [Google Scholar]
- 31.Peters, N. S. (1997) Eur. Heart J. 18, 1697–1702. [DOI] [PubMed] [Google Scholar]
- 32.Sepp, R., Severs, N. J. & Gourdie, R. G. (1996) Heart 76, 412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luque, E. A., Veenstra, R. D., Beyer, E. C. & Lemanski, L. F. (1994) J. Morphol. 222, 203–213. [DOI] [PubMed] [Google Scholar]
- 34.Ehler, E., Horowits, R., Zuppinger, C., Price, R. L., Perriard, E., Leu, M., Caroni, P., Sussman, M., Eppenberger, H. M. & Perriard, J.-C. (2001) J. Cell Biol. 153, 763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forbes, M. S. & Sperelakis, N. (1972) Am. J. Anat. 134, 271–289. [DOI] [PubMed] [Google Scholar]
- 36.Thornell, L., Carlsson, L., Li, Z., Mericskay, M. & Paulin, D. (1997) J. Mol. Cell. Cardiol. 29, 2107–2124. [DOI] [PubMed] [Google Scholar]
- 37.Angst, B. D., Khan, L. U., Severs, N. J., Whitely, K., Rothery, S., Thompson, R. P., Magee, A. I. & Gourdie, R. G. (1997) Circ. Res. 80, 88–94. [DOI] [PubMed] [Google Scholar]
- 38.Ecarnot-Laubriet, A., De Luca, K., Vandroux, D., Moisant, M., Bernard, C., Assem, M., Rochette, L. & Teyssier, J. R. (2000) J. Mol. Cell. Cardiol. 32, 2385–2395. [DOI] [PubMed] [Google Scholar]
- 39.Bierkamp, C., McLaughlin, K. J., Schwarz, H., Huber, O. & Kemler, R. (1996) Dev. Biol. 180, 780–785. [DOI] [PubMed] [Google Scholar]
- 40.Mounkes, L. C., Burke, B. & Stewart, C. L. (2001) Trends Cardiovasc. Med. 11, 280–285. [DOI] [PubMed] [Google Scholar]
- 41.Pendas, A. M., Zhou, Z., Cadinanos, J., Freije, J. M., Wang, J., Hultenby, K., Astudillo, A., Wernerson, A., Rodriguez, F., Tryggvason, K. & Lopez-Otin, C. (2002) Nat. Genet. 31, 94–99. [DOI] [PubMed] [Google Scholar]
- 42.Moir, R. D. & Spann, T. P. (2001) Cell. Mol. Life Sci. 58, 1748–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mounkes, L., Kozlov, S., Burke, B. & Stewart, C. L. (2003) Curr. Opin. Genet. Dev. 13, 223–230. [DOI] [PubMed] [Google Scholar]
- 44.Moir, R. D., Spann, T. P., Lopez-Soler, R. I., Yoon, M., Goldman, A. E., Khuon, S. & Goldman, R. D. (2000) J. Struct. Biol. 129, 324–334. [DOI] [PubMed] [Google Scholar]
- 45.Raharjo, W. H., Enarson, P., Sullivan, T., Stewart, C. L. & Burke, B. (2001) J. Cell Sci. 114, 4447–4457. [DOI] [PubMed] [Google Scholar]
- 46.Verga, L., Concardi, M., Pilotto, A., Bellini, O., Pasotti, M., Repetto, A., Tavazzi, L. & Arbustini, E. (2003) Virchows Arch. 443, 664–671. [DOI] [PubMed] [Google Scholar]
- 47.Palmieri, C., Cheng, G. J., Saji, S., Zelada-Hedman, M., Warri, A., Weihua, Z., Van Noorden, S., Wahlstrom, T., Coombes, R. C., Warner, M. & Gustafsson, J.-Å. (2002) Endocr. Relat. Cancer 9, 1–13. [DOI] [PubMed] [Google Scholar]
- 48.Liu, D., Zhang, Z., Gladwell, W. & Teng, C. T. (2003) Endocrinology 144, 4894–4904. [DOI] [PubMed] [Google Scholar]