Abstract
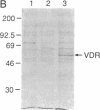
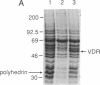
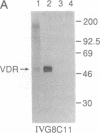
The rat 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3] receptor has been expressed at elevated levels in Spodoptera frugiperda cells using the baculovirus expression vector system. The recombinant 1,25-(OH)2D3 receptor is full-length, binds 1,25-(OH)2D3, and is recognized by a monoclonal antibody specific for 1,25-(OH)2D3 receptor. Densitometric scanning of Coomassie brilliant blue-stained SDS/polyacrylamide gels indicated a recombinant receptor protein level comprising 5% of the total soluble protein from the insect cells. The hydroxylapatite binding assay revealed average levels of 2 nmol of unoccupied 1,25-(OH)2D3 receptor per mg of protein in insect cells at 72 hr after infection with recombinant baculovirus. A measure of total 1,25-(OH)2D3 receptor using a ligand-independent, immunoradiometric assay disclosed average levels of 2.3 nmol of receptor per mg of protein produced by these same cells. A monoclonal antibody directed against the 1,25-(OH)2D3 receptor, and reported to cross-react with this receptor derived from several species, recognized the recombinant rat 1,25-(OH)2D3 receptor upon Western analysis. A monoclonal antibody directed specifically against the porcine receptor failed to recognize the recombinant rat 1,25-(OH)2D3 receptor protein. The cytosolic preparation of insect cells infected with recombinant baculovirus exhibited an equilibrium dissociation constant of 1 x 10(-11) M as determined by a 1,25-(OH)2D3 saturation analysis plotted by the method of Scatchard. This expression system provides an adequate source from which abundant quantities of 1,25-(OH)2D3 receptor can be purified for subsequent x-ray crystallographic analyses.
Full text
PDF
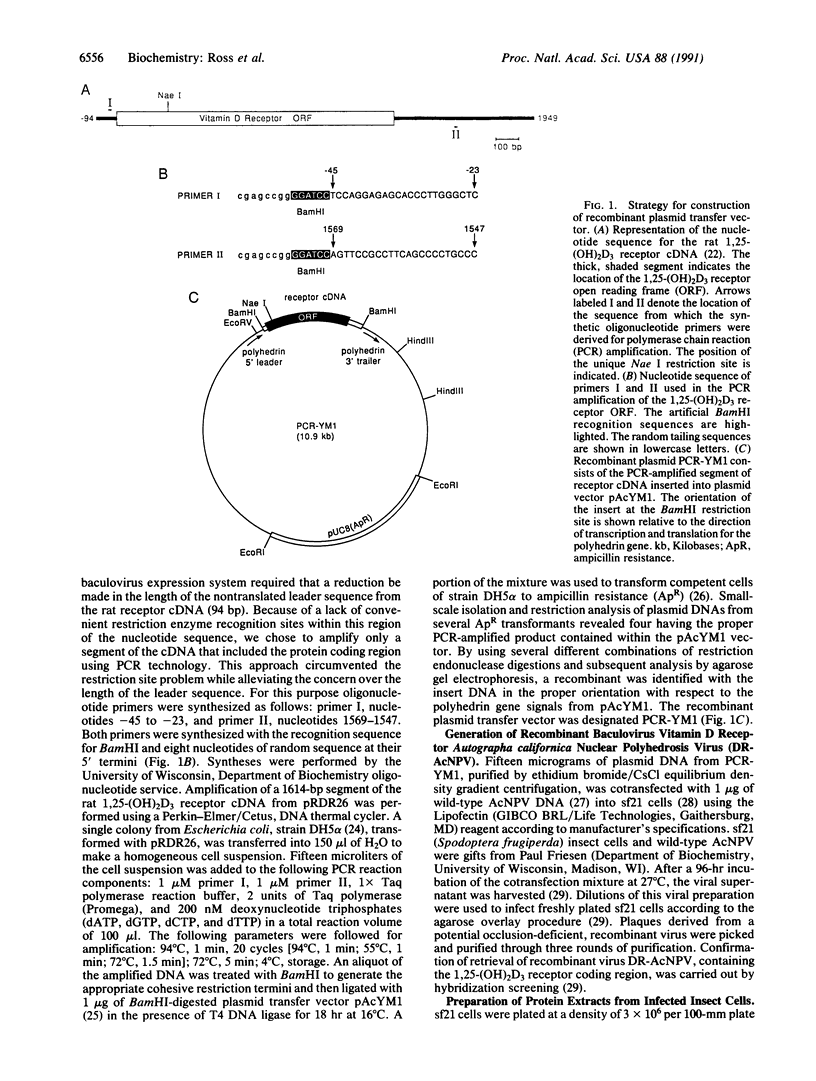

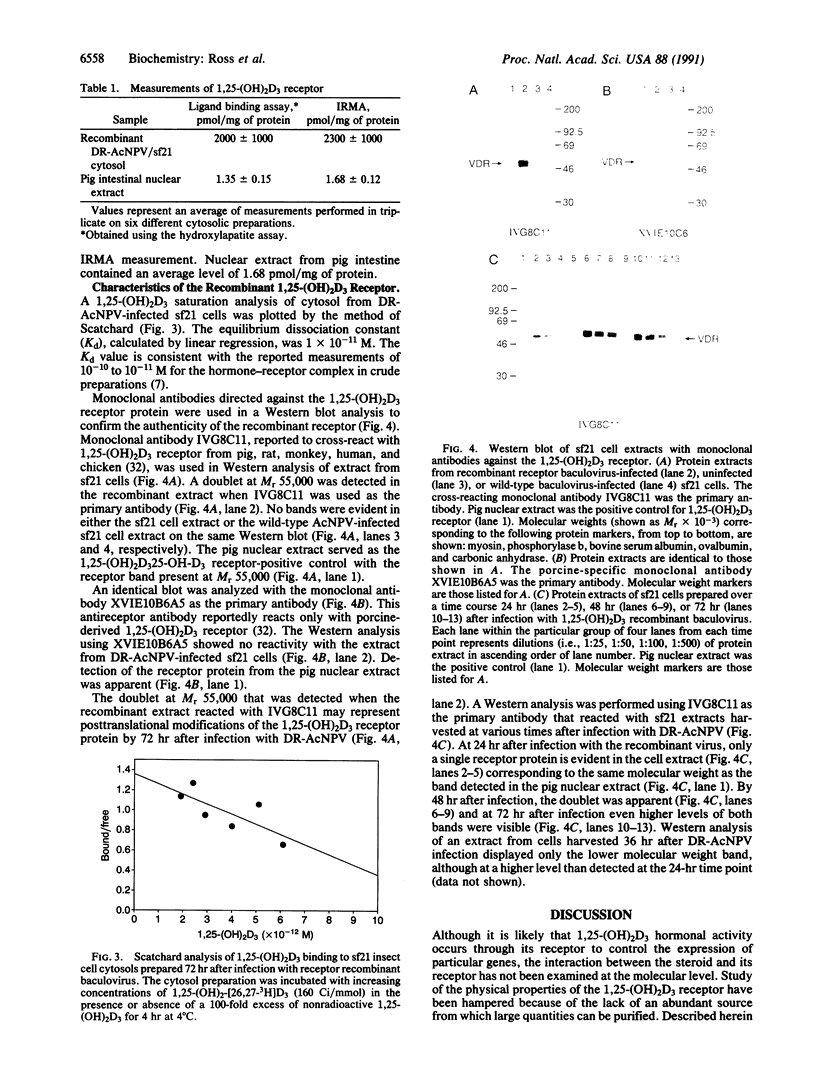

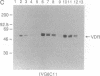
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker A. R., McDonnell D. P., Hughes M., Crisp T. M., Mangelsdorf D. J., Haussler M. R., Pike J. W., Shine J., O'Malley B. W. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci U S A. 1988 May;85(10):3294–3298. doi: 10.1073/pnas.85.10.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brake A. J., Merryweather J. P., Coit D. G., Heberlein U. A., Masiarz F. R., Mullenbach G. T., Urdea M. S., Valenzuela P., Barr P. J. Alpha-factor-directed synthesis and secretion of mature foreign proteins in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4642–4646. doi: 10.1073/pnas.81.15.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks M. H., Bell N. H., Love L., Stern P. H., Orfei E., Queener S. F., Hamstra A. J., DeLuca H. F. Vitamin-D-dependent rickets type II. Resistance of target organs to 1,25-dihydroxyvitamin D. N Engl J Med. 1978 May 4;298(18):996–999. doi: 10.1056/NEJM197805042981804. [DOI] [PubMed] [Google Scholar]
- Brown M., Sharp P. A. Human estrogen receptor forms multiple protein-DNA complexes. J Biol Chem. 1990 Jul 5;265(19):11238–11243. [PubMed] [Google Scholar]
- Brown T. A., DeLuca H. F. Phosphorylation of the 1,25-dihydroxyvitamin D3 receptor. A primary event in 1,25-dihydroxyvitamin D3 action. J Biol Chem. 1990 Jun 15;265(17):10025–10029. [PubMed] [Google Scholar]
- Burmester J. K., Maeda N., DeLuca H. F. Isolation and expression of rat 1,25-dihydroxyvitamin D3 receptor cDNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1005–1009. doi: 10.1073/pnas.85.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester J. K., Wiese R. J., Maeda N., DeLuca H. F. Structure and regulation of the rat 1,25-dihydroxyvitamin D3 receptor. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9499–9502. doi: 10.1073/pnas.85.24.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame M. C., Pierce E. A., DeLuca H. F. Identification of the porcine intestinal 1,25-dihydroxyvitamin D3 receptor on sodium dodecyl sulfate/polyacrylamide gels by renaturation and immunoblotting. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7825–7829. doi: 10.1073/pnas.82.23.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame M. C., Pierce E. A., Prahl J. M., Hayes C. E., DeLuca H. F. Monoclonal antibodies to the porcine intestinal receptor for 1,25-dihydroxyvitamin D3: interaction with distinct receptor domains. Biochemistry. 1986 Aug 12;25(16):4523–4534. doi: 10.1021/bi00364a011. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F., Schnoes H. K. Vitamin D: recent advances. Annu Rev Biochem. 1983;52:411–439. doi: 10.1146/annurev.bi.52.070183.002211. [DOI] [PubMed] [Google Scholar]
- Demay M. B., Gerardi J. M., DeLuca H. F., Kronenberg H. M. DNA sequences in the rat osteocalcin gene that bind the 1,25-dihydroxyvitamin D3 receptor and confer responsiveness to 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1990 Jan;87(1):369–373. doi: 10.1073/pnas.87.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eil C., Liberman U. A., Marx S. J. The molecular basis for resistance to 1,25-dihydroxyvitamin D: studies in cells cultured from patients with hereditary hypocalcemic 1,25(OH)2D3-resistant rickets. Adv Exp Med Biol. 1986;196:407–422. doi: 10.1007/978-1-4684-5101-6_27. [DOI] [PubMed] [Google Scholar]
- Garabedian M., Tanaka Y., Holick M. F., Deluca H. F. Response of intestinal calcium transport and bone calcium mobilization to 1,25-dihydroxyvitamin D3 in thyroparathyroidectomized rats. Endocrinology. 1974 Apr;94(4):1022–1027. doi: 10.1210/endo-94-4-1022. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Kerner S. A., Scott R. A., Pike J. W. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4455–4459. doi: 10.1073/pnas.86.12.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liao J., Ozono K., Sone T., McDonnell D. P., Pike J. W. Vitamin D receptor interaction with specific DNA requires a nuclear protein and 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9751–9755. doi: 10.1073/pnas.87.24.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markose E. R., Stein J. L., Stein G. S., Lian J. B. Vitamin D-mediated modifications in protein-DNA interactions at two promoter elements of the osteocalcin gene. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1701–1705. doi: 10.1073/pnas.87.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura Y., Possee R. D., Overton H. A., Bishop D. H. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J Gen Virol. 1987 May;68(Pt 5):1233–1250. doi: 10.1099/0022-1317-68-5-1233. [DOI] [PubMed] [Google Scholar]
- McDonnell D. P., Mangelsdorf D. J., Pike J. W., Haussler M. R., O'Malley B. W. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science. 1987 Mar 6;235(4793):1214–1217. doi: 10.1126/science.3029866. [DOI] [PubMed] [Google Scholar]
- McDonnell D. P., Pike J. W., Drutz D. J., Butt T. R., O'Malley B. W. Reconstitution of the vitamin D-responsive osteocalcin transcription unit in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Aug;9(8):3517–3523. doi: 10.1128/mcb.9.8.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell D. P., Scott R. A., Kerner S. A., O'Malley B. W., Pike J. W. Functional domains of the human vitamin D3 receptor regulate osteocalcin gene expression. Mol Endocrinol. 1989 Apr;3(4):635–644. doi: 10.1210/mend-3-4-635. [DOI] [PubMed] [Google Scholar]
- Napoli J. L., Mellon W. S., Fivizzani M. A., Schnoes H. K., DeLuca H. F. Direct chemical synthesis of 1 alpha,25-dihydroxy[26,27-3H]vitamin D3 with high specific activity: its use in receptor studies. Biochemistry. 1980 May 27;19(11):2515–2521. doi: 10.1021/bi00552a033. [DOI] [PubMed] [Google Scholar]
- Nilsson B., Abrahmsén L., Uhlén M. Immobilization and purification of enzymes with staphylococcal protein A gene fusion vectors. EMBO J. 1985 Apr;4(4):1075–1080. doi: 10.1002/j.1460-2075.1985.tb03741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Vogel R. L., Craig A. M., Prahl J., DeLuca H. F., Denhardt D. T. Identification of a DNA sequence responsible for binding of the 1,25-dihydroxyvitamin D3 receptor and 1,25-dihydroxyvitamin D3 enhancement of mouse secreted phosphoprotein 1 (SPP-1 or osteopontin) gene expression. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9995–9999. doi: 10.1073/pnas.87.24.9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman A. W., Roth J., Orci L. The vitamin D endocrine system: steroid metabolism, hormone receptors, and biological response (calcium binding proteins). Endocr Rev. 1982 Fall;3(4):331–366. doi: 10.1210/edrv-3-4-331. [DOI] [PubMed] [Google Scholar]
- Omdahl J. L., DeLuca H. F. Regulation of vitamin D metabolism and function. Physiol Rev. 1973 Apr;53(2):327–372. doi: 10.1152/physrev.1973.53.2.327. [DOI] [PubMed] [Google Scholar]
- Perlman K., Kutner A., Prahl J., Smith C., Inaba M., Schnoes H. K., DeLuca H. F. 24-homologated 1,25-dihydroxyvitamin D3 compounds: separation of calcium and cell differentiation activities. Biochemistry. 1990 Jan 9;29(1):190–196. doi: 10.1021/bi00453a026. [DOI] [PubMed] [Google Scholar]
- Pike J. W., Sleator N. M. Hormone-dependent phosphorylation of the 1,25-dihydroxyvitamin D3 receptor in mouse fibroblasts. Biochem Biophys Res Commun. 1985 Aug 30;131(1):378–385. doi: 10.1016/0006-291x(85)91813-3. [DOI] [PubMed] [Google Scholar]
- Sandgren M. E., Deluca H. F. An immunoradiometric assay for 1,25-dihydroxyvitamin D3 receptor. Anal Biochem. 1989 Nov 15;183(1):57–63. doi: 10.1016/0003-2697(89)90171-1. [DOI] [PubMed] [Google Scholar]
- Sone T., McDonnell D. P., O'Malley B. W., Pike J. W. Expression of human vitamin D receptor in Saccharomyces cerevisiae. Purification, properties, and generation of polyclonal antibodies. J Biol Chem. 1990 Dec 15;265(35):21997–22003. [PubMed] [Google Scholar]
- Srinivasan G., Thompson E. B. Overexpression of full-length human glucocorticoid receptor in Spodoptera frugiperda cells using the baculovirus expression vector system. Mol Endocrinol. 1990 Feb;4(2):209–216. doi: 10.1210/mend-4-2-209. [DOI] [PubMed] [Google Scholar]
- Vaughn J. L., Goodwin R. H., Tompkins G. J., McCawley P. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro. 1977 Apr;13(4):213–217. doi: 10.1007/BF02615077. [DOI] [PubMed] [Google Scholar]
- Vialard J., Lalumière M., Vernet T., Briedis D., Alkhatib G., Henning D., Levin D., Richardson C. Synthesis of the membrane fusion and hemagglutinin proteins of measles virus, using a novel baculovirus vector containing the beta-galactosidase gene. J Virol. 1990 Jan;64(1):37–50. doi: 10.1128/jvi.64.1.37-50.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]