Abstract
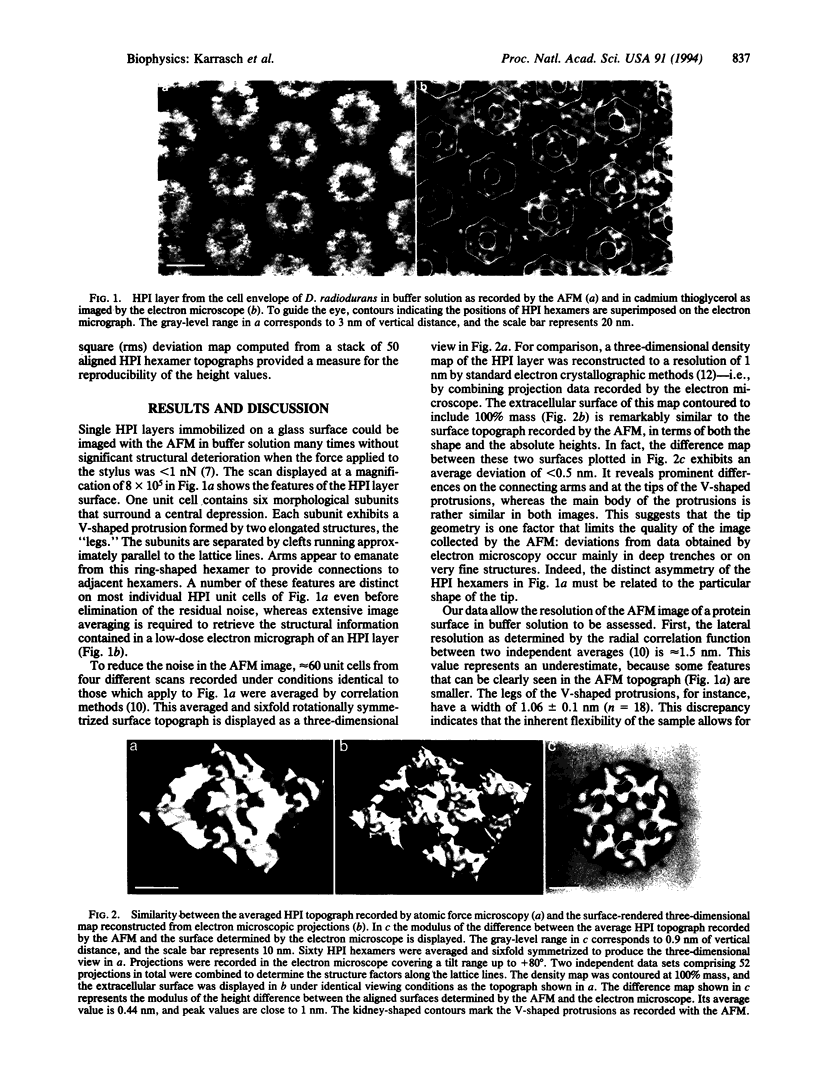
The atomic force microscope has the potential to monitor structural changes of a biological system in its native environment. To correlate them with the biological function at a molecular level, high lateral and vertical resolution are required. Here we demonstrate that the atomic force microscope is capable of imaging the surface of the hexagonally packed intermediate layer of Deinococcus radiodurans in buffer solution with a lateral resolution of 1 nm and a vertical resolution of 0.1 nm. On average, these topographs differ from those determined by electron microscopy by <0.5 nm.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumeister W., Barth M., Hegerl R., Guckenberger R., Hahn M., Saxton W. O. Three-dimensional structure of the regular surface layer (HPI layer) of Deinococcus radiodurans. J Mol Biol. 1986 Jan 20;187(2):241–250. doi: 10.1016/0022-2836(86)90231-7. [DOI] [PubMed] [Google Scholar]
- Baumeister W., Karrenberg F., Rachel R., Engel A., ten Heggeler B., Saxton W. O. The major cell envelope protein of Micrococcus radiodurans (R1). Structural and chemical characterization. Eur J Biochem. 1982 Jul;125(3):535–544. doi: 10.1111/j.1432-1033.1982.tb06715.x. [DOI] [PubMed] [Google Scholar]
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Butt H. J., Downing K. H., Hansma P. K. Imaging the membrane protein bacteriorhodopsin with the atomic force microscope. Biophys J. 1990 Dec;58(6):1473–1480. doi: 10.1016/S0006-3495(90)82492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A., Baumeister W., Saxton W. O. Mass mapping of a protein complex with the scanning transmission electron microscope. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4050–4054. doi: 10.1073/pnas.79.13.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Hoh J. H., Hansma P. K. Atomic force microscopy for high-resolution imaging in cell biology. Trends Cell Biol. 1992 Jul;2(7):208–213. doi: 10.1016/0962-8924(92)90248-l. [DOI] [PubMed] [Google Scholar]
- Hoh J. H., Sosinsky G. E., Revel J. P., Hansma P. K. Structure of the extracellular surface of the gap junction by atomic force microscopy. Biophys J. 1993 Jul;65(1):149–163. doi: 10.1016/S0006-3495(93)81074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Peters M., Lottspeich F., Schäfer W., Baumeister W. Nucleotide sequence analysis of the gene encoding the Deinococcus radiodurans surface protein, derived amino acid sequence, and complementary protein chemical studies. J Bacteriol. 1987 Nov;169(11):5216–5223. doi: 10.1128/jb.169.11.5216-5223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W., Hahn M. Three-dimensional reconstruction of imperfect two-dimensional crystals. Ultramicroscopy. 1984;13(1-2):57–70. doi: 10.1016/0304-3991(84)90057-3. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Yang J., Tamm L. K., Tillack T. W., Shao Z. New approach for atomic force microscopy of membrane proteins. The imaging of cholera toxin. J Mol Biol. 1993 Jan 20;229(2):286–290. doi: 10.1006/jmbi.1993.1033. [DOI] [PubMed] [Google Scholar]