Abstract
Multiple sequence alignment of the 12 adenovirus endopeptidases known to date identified a number of conserved residues which might be important for enzyme activity. Eleven mutants were created in the cloned gene by site-directed mutagenesis to identify the active site of this thiol endopeptidase. Analysis of the proteolytic activity in a crude system using viral precursor proteins, as well as in a purified system with activated proteinases using a new chromophoric octapeptide substrate, yielded results consistent with Cys-104 and His-54 being two members of the active site. This result was confirmed by the carboxymethylation of the reactive Cys-104 and its prevention by the active-thiol-specific agent E64. Although Cys-122 and Cys-126 were also reactive cysteines, mutation of these residues did not affect enzyme activity. Replacement of the active-site Cys-104 by serine converted the enzyme into a serine-like proteinase, sensitive to serine proteinase inhibitors. The absence of homology to other proteinases, particularly at the active-site cysteine, coupled with the requirement for activation by a substrate cleavage fragment, indicates that the adenovirus endoproteinase may represent a new subclass of cysteine proteinases.
Full text
PDF

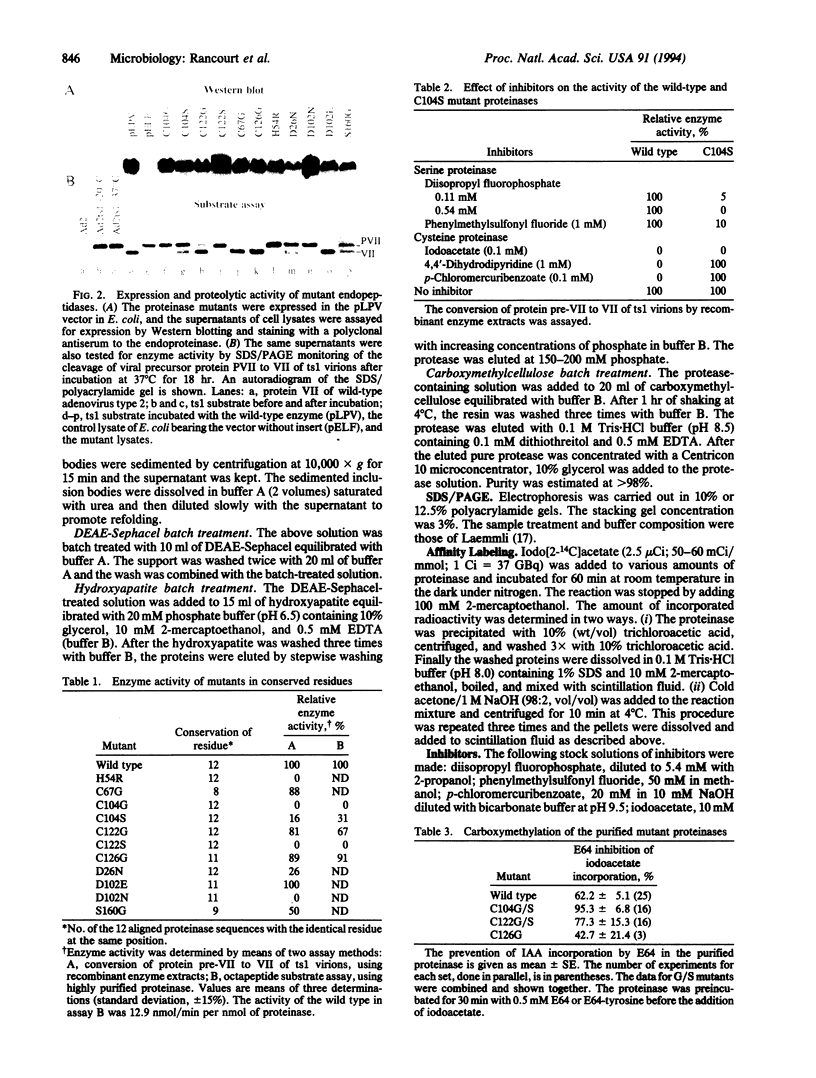

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W. The proteinase polypeptide of adenovirus serotype 2 virions. Virology. 1990 Jul;177(1):259–272. doi: 10.1016/0042-6822(90)90479-b. [DOI] [PubMed] [Google Scholar]
- Bazan J. F., Fletterick R. J. Detection of a trypsin-like serine protease domain in flaviviruses and pestiviruses. Virology. 1989 Aug;171(2):637–639. doi: 10.1016/0042-6822(89)90639-9. [DOI] [PubMed] [Google Scholar]
- Bazan J. F., Fletterick R. J. Viral cysteine proteases are homologous to the trypsin-like family of serine proteases: structural and functional implications. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7872–7876. doi: 10.1073/pnas.85.21.7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The molecular evolution of genes and proteins: a tale of two serines. Nature. 1988 Aug 11;334(6182):528–530. doi: 10.1038/334528a0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cai F., Weber J. M. Organization of the avian adenovirus genome and the structure of its endopeptidase. Virology. 1993 Sep;196(1):358–362. doi: 10.1006/viro.1993.1489. [DOI] [PubMed] [Google Scholar]
- Carter P., Wells J. A. Dissecting the catalytic triad of a serine protease. Nature. 1988 Apr 7;332(6164):564–568. doi: 10.1038/332564a0. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Donchenko A. P., Blinov V. M., Koonin E. V. Cysteine proteases of positive strand RNA viruses and chymotrypsin-like serine proteases. A distinct protein superfamily with a common structural fold. FEBS Lett. 1989 Jan 30;243(2):103–114. doi: 10.1016/0014-5793(89)80109-7. [DOI] [PubMed] [Google Scholar]
- Hahn C. S., Strauss J. H. Site-directed mutagenesis of the proposed catalytic amino acids of the Sindbis virus capsid protein autoprotease. J Virol. 1990 Jun;64(6):3069–3073. doi: 10.1128/jvi.64.6.3069-3073.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki J. N., Gibson B. W., Craik C. S. Evolution of catalysis in the serine proteases. Cold Spring Harb Symp Quant Biol. 1987;52:615–621. doi: 10.1101/sqb.1987.052.01.070. [DOI] [PubMed] [Google Scholar]
- Houde A., Weber J. M. Adenovirus proteinases: comparison of amino acid sequences and expression of the cloned cDNA in Escherichia coli. Gene. 1990 Apr 16;88(2):269–273. doi: 10.1016/0378-1119(90)90042-p. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawson M. A., Semler B. L. Poliovirus thiol proteinase 3C can utilize a serine nucleophile within the putative catalytic triad. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9919–9923. doi: 10.1073/pnas.88.22.9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel W. F., McGrath W. J., Toledo D. L., Anderson C. W. Viral DNA and a viral peptide can act as cofactors of adenovirus virion proteinase activity. Nature. 1993 Jan 21;361(6409):274–275. doi: 10.1038/361274a0. [DOI] [PubMed] [Google Scholar]
- Mirza A., Weber J. Infectivity and uncoating of adenovirus cores. Intervirology. 1980;13(5):307–311. doi: 10.1159/000149139. [DOI] [PubMed] [Google Scholar]
- Mott J. E., Grant R. A., Ho Y. S., Platt T. Maximizing gene expression from plasmid vectors containing the lambda PL promoter: strategies for overproducing transcription termination factor rho. Proc Natl Acad Sci U S A. 1985 Jan;82(1):88–92. doi: 10.1073/pnas.82.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A. D., Phylip L. H., Farmerie W. G., Scarborough P. E., Alvarez A., Dunn B. M., Hirel P. H., Konvalinka J., Strop P., Pavlickova L. Sensitive, soluble chromogenic substrates for HIV-1 proteinase. J Biol Chem. 1990 May 15;265(14):7733–7736. [PubMed] [Google Scholar]
- Tihanyi K., Bourbonnière M., Houde A., Rancourt C., Weber J. M. Isolation and properties of adenovirus type 2 proteinase. J Biol Chem. 1993 Jan 25;268(3):1780–1785. [PubMed] [Google Scholar]
- Tremblay M. L., Déry C. V., Talbot B. G., Weber J. In vitro cleavage specificity of the adenovirus type 2 proteinase. Biochim Biophys Acta. 1983 Mar 16;743(2):239–245. doi: 10.1016/0167-4838(83)90220-0. [DOI] [PubMed] [Google Scholar]
- Weber J. Genetic analysis of adenovirus type 2 III. Temperature sensitivity of processing viral proteins. J Virol. 1976 Feb;17(2):462–471. doi: 10.1128/jvi.17.2.462-471.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster A., Hay R. T., Kemp G. The adenovirus protease is activated by a virus-coded disulphide-linked peptide. Cell. 1993 Jan 15;72(1):97–104. doi: 10.1016/0092-8674(93)90053-s. [DOI] [PubMed] [Google Scholar]
- Webster A., Russell S., Talbot P., Russell W. C., Kemp G. D. Characterization of the adenovirus proteinase: substrate specificity. J Gen Virol. 1989 Dec;70(Pt 12):3225–3234. doi: 10.1099/0022-1317-70-12-3225. [DOI] [PubMed] [Google Scholar]
- Webster A., Russell W. C., Kemp G. D. Characterization of the adenovirus proteinase: development and use of a specific peptide assay. J Gen Virol. 1989 Dec;70(Pt 12):3215–3223. doi: 10.1099/0022-1317-70-12-3215. [DOI] [PubMed] [Google Scholar]
- Yeh-Kai L., Akusjärvi G., Aleström P., Pettersson U., Tremblay M., Weber J. Genetic identification of an endoproteinase encoded by the adenovirus genome. J Mol Biol. 1983 Jun 15;167(1):217–222. doi: 10.1016/s0022-2836(83)80044-8. [DOI] [PubMed] [Google Scholar]



