Abstract
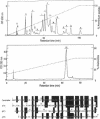
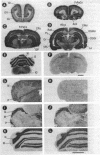
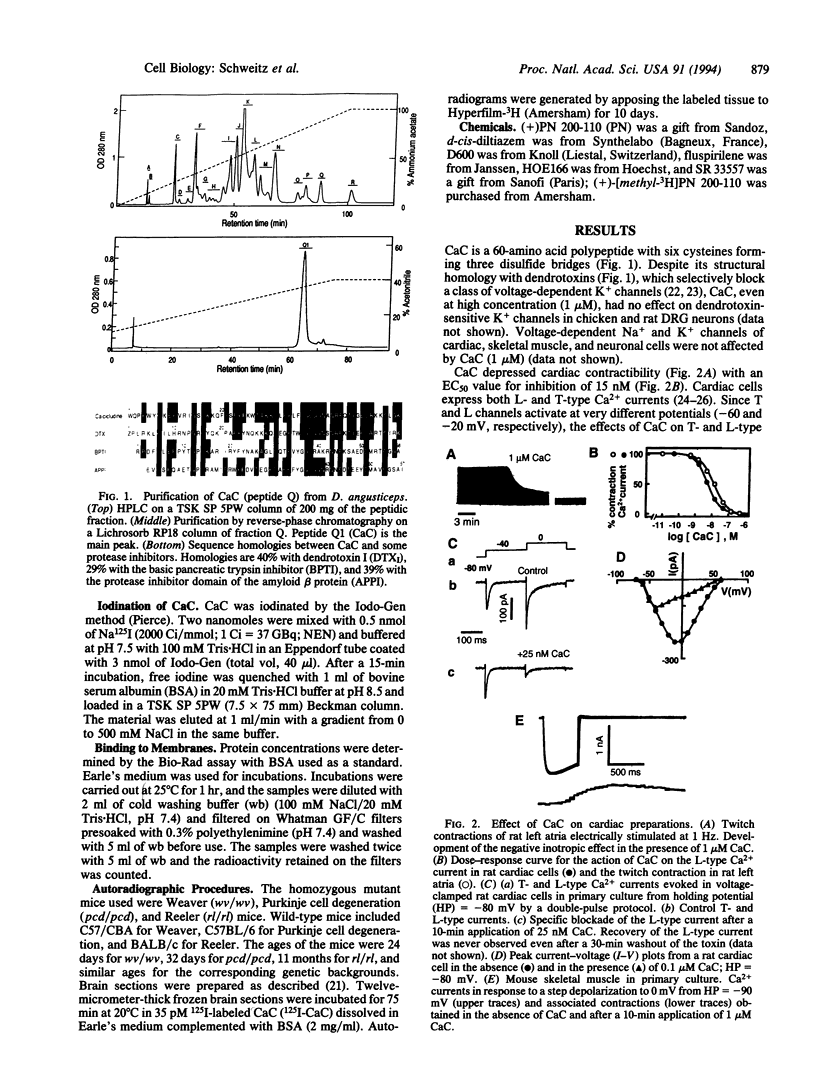
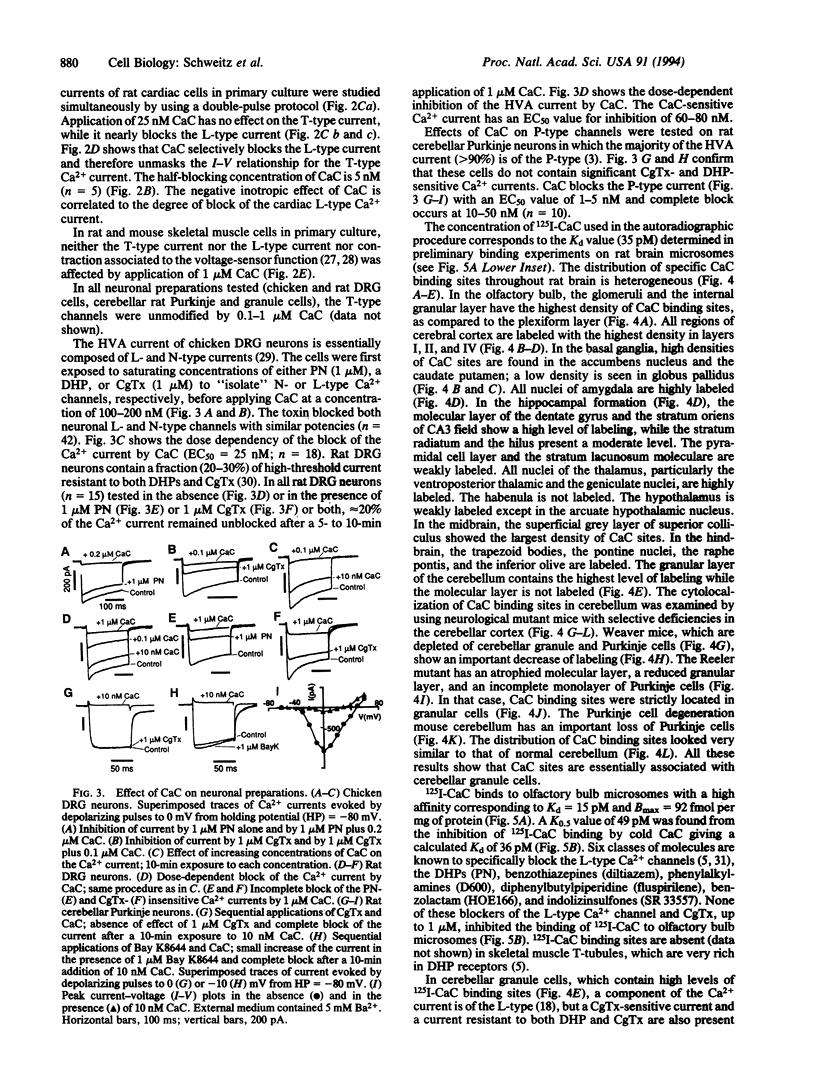
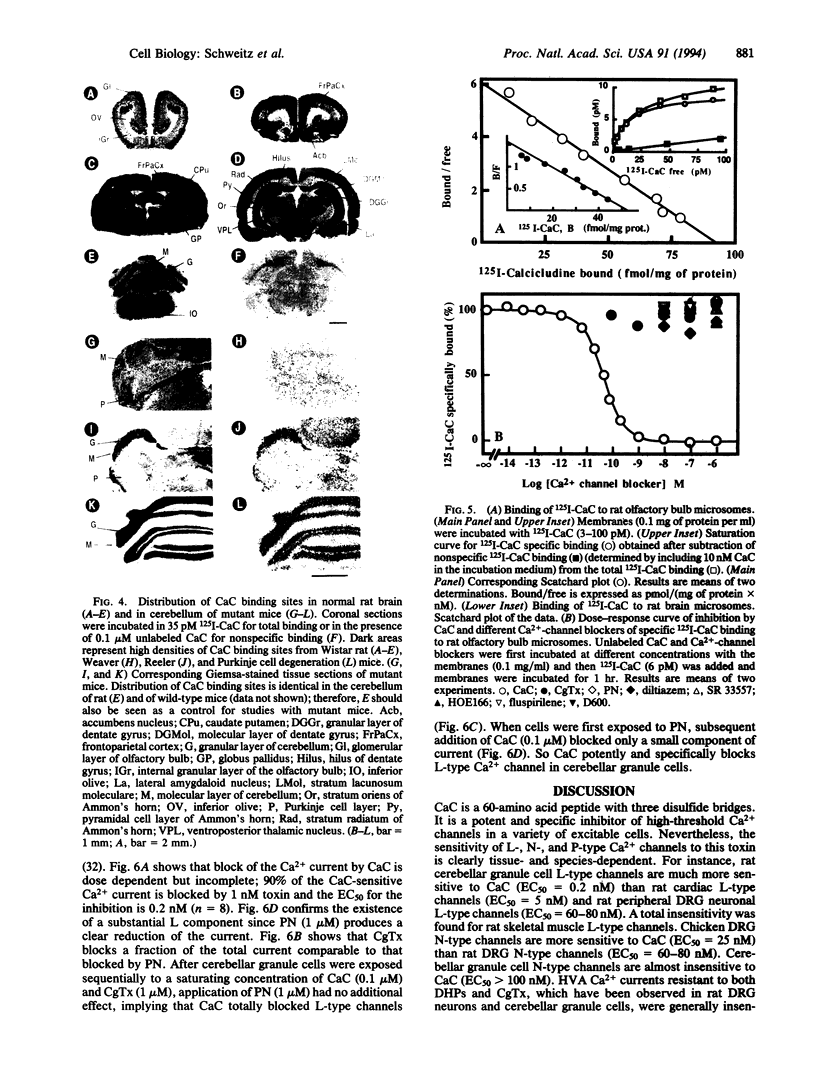
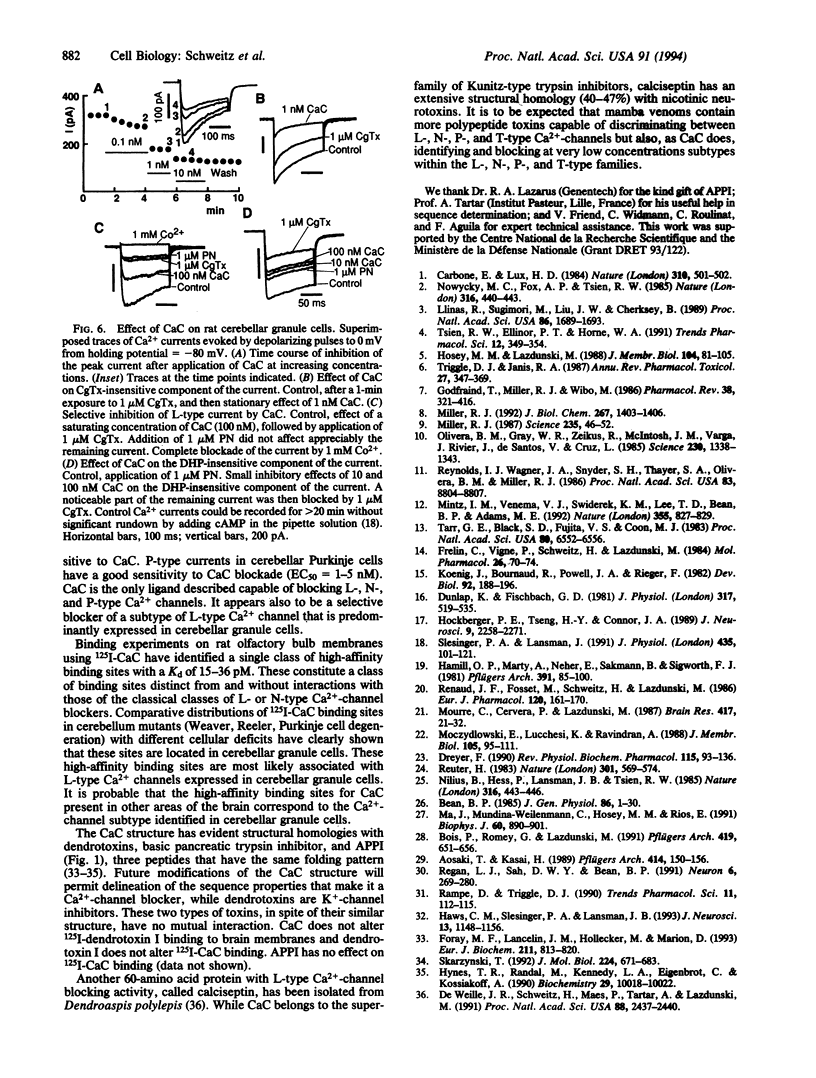
Calcicludine (CaC) is a 60-amino acid polypeptide from the venom of Dendroaspis angusticeps. It is structurally homologous to the Kunitz-type protease inhibitor, to dendrotoxins, which block K+ channels, and to the protease inhibitor domain of the amyloid beta protein that accumulates in Alzheimer disease. Voltage-clamp experiments on a variety of excitable cells have shown that CaC specifically blocks most of the high-threshold Ca2+ channels (L-, N-, or P-type) in the 10-100 nM range. Particularly high densities of specific 125I-labeled CaC binding sites were found in the olfactory bulb, in the molecular layer of the dentate gyrus and the stratum oriens of CA3 field in the hippocampal formation, and in the granular layer of the cerebellum. 125I-labeled CaC binds with a high affinity (Kd = 15 pM) to a single class of noninteracting sites in rat olfactory bulb microsomes. The distribution of CaC binding sites in cerebella of three mutant mice (Weaver, Reeler, and Purkinje cell degeneration) clearly shows that the specific high-affinity labeling is associated with granule cells. Electrophysiological experiments on rat cerebellar granule neurons in primary culture have shown that CaC potently blocks the L-type component of the Ca2+ current (K0.5 = 0.2 nM). Then CaC, in the nanomolar range, appears to be a highly potent blocker of an L-subtype of neuronal Ca2+ channels.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aosaki T., Kasai H. Characterization of two kinds of high-voltage-activated Ca-channel currents in chick sensory neurons. Differential sensitivity to dihydropyridines and omega-conotoxin GVIA. Pflugers Arch. 1989 Jun;414(2):150–156. doi: 10.1007/BF00580957. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bois P., Romey G., Lazdunski M. Indolizinsulphones. A class of blockers with dual but discriminative effects on L-type Ca2+ channel activity and excitation-contraction coupling in skeletal muscle. Pflugers Arch. 1991 Dec;419(6):651–656. doi: 10.1007/BF00370310. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Dreyer F. Peptide toxins and potassium channels. Rev Physiol Biochem Pharmacol. 1990;115:93–136. [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981 Aug;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foray M. F., Lancelin J. M., Hollecker M., Marion D. Sequence-specific 1H-NMR assignment and secondary structure of black mamba dendrotoxin I, a highly selective blocker of voltage-gated potassium channels. Eur J Biochem. 1993 Feb 1;211(3):813–820. doi: 10.1111/j.1432-1033.1993.tb17613.x. [DOI] [PubMed] [Google Scholar]
- Frelin C., Vigne P., Schweitz H., Lazdunski M. The interaction of sea anemone and scorpion neurotoxins with tetrodotoxin-resistant Na+ channels in rat myoblasts. A comparison with Na+ channels in other excitable and non-excitable cells. Mol Pharmacol. 1984 Jul;26(1):70–74. [PubMed] [Google Scholar]
- Godfraind T., Miller R., Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986 Dec;38(4):321–416. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Haws C. M., Slesinger P. A., Lansman J. B. Dihydropyridine- and omega-conotoxin-sensitive Ca2+ currents in cerebellar neurons: persistent block of L-type channels by a pertussis toxin-sensitive G-protein. J Neurosci. 1993 Mar;13(3):1148–1156. doi: 10.1523/JNEUROSCI.13-03-01148.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockberger P. E., Tseng H. Y., Connor J. A. Development of rat cerebellar Purkinje cells: electrophysiological properties following acute isolation and in long-term culture. J Neurosci. 1989 Jul;9(7):2258–2271. doi: 10.1523/JNEUROSCI.09-07-02258.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosey M. M., Lazdunski M. Calcium channels: molecular pharmacology, structure and regulation. J Membr Biol. 1988 Sep;104(2):81–105. doi: 10.1007/BF01870922. [DOI] [PubMed] [Google Scholar]
- Hynes T. R., Randal M., Kennedy L. A., Eigenbrot C., Kossiakoff A. A. X-ray crystal structure of the protease inhibitor domain of Alzheimer's amyloid beta-protein precursor. Biochemistry. 1990 Oct 30;29(43):10018–10022. doi: 10.1021/bi00495a002. [DOI] [PubMed] [Google Scholar]
- Koenig J., Bournaud R., Powell J. A., Rieger F. Appearance of contractile activity in muscular dysgenesis (mdg/mdg) mouse myotubes during coculture with normal spinal cord cells. Dev Biol. 1982 Jul;92(1):188–196. doi: 10.1016/0012-1606(82)90162-2. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Lin J. W., Cherksey B. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Mundiña-Weilenmann C., Hosey M. M., Ríos E. Dihydropyridine-sensitive skeletal muscle Ca channels in polarized planar bilayers. 1. Kinetics and voltage dependence of gating. Biophys J. 1991 Oct;60(4):890–901. doi: 10.1016/S0006-3495(91)82123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Voltage-sensitive Ca2+ channels. J Biol Chem. 1992 Jan 25;267(3):1403–1406. [PubMed] [Google Scholar]
- Mintz I. M., Venema V. J., Swiderek K. M., Lee T. D., Bean B. P., Adams M. E. P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature. 1992 Feb 27;355(6363):827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Lucchesi K., Ravindran A. An emerging pharmacology of peptide toxins targeted against potassium channels. J Membr Biol. 1988 Oct;105(2):95–111. doi: 10.1007/BF02009164. [DOI] [PubMed] [Google Scholar]
- Mourre C., Cervera P., Lazdunski M. Autoradiographic analysis in rat brain of the postnatal ontogeny of voltage-dependent Na+ channels, Ca2+-dependent K+ channels and slow Ca2+ channels identified as receptors for tetrodotoxin, apamin and (-)-desmethoxyverapamil. Brain Res. 1987 Aug 4;417(1):21–32. doi: 10.1016/0006-8993(87)90175-2. [DOI] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Rampe D., Triggle D. J. New ligands for L-type Ca2+ channels. Trends Pharmacol Sci. 1990 Mar;11(3):112–115. doi: 10.1016/0165-6147(90)90196-f. [DOI] [PubMed] [Google Scholar]
- Regan L. J., Sah D. W., Bean B. P. Ca2+ channels in rat central and peripheral neurons: high-threshold current resistant to dihydropyridine blockers and omega-conotoxin. Neuron. 1991 Feb;6(2):269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- Renaud J. F., Fosset M., Schweitz H., Lazdunski M. The interaction of polypeptide neurotoxins with tetrodotoxin-resistant Na+ channels in mammalian cardiac cells. Correlation with inotropic and arrhythmic effects. Eur J Pharmacol. 1986 Jan 21;120(2):161–170. doi: 10.1016/0014-2999(86)90536-4. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Wagner J. A., Snyder S. H., Thayer S. A., Olivera B. M., Miller R. J. Brain voltage-sensitive calcium channel subtypes differentiated by omega-conotoxin fraction GVIA. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8804–8807. doi: 10.1073/pnas.83.22.8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarzyński T. Crystal structure of alpha-dendrotoxin from the green mamba venom and its comparison with the structure of bovine pancreatic trypsin inhibitor. J Mol Biol. 1992 Apr 5;224(3):671–683. doi: 10.1016/0022-2836(92)90552-u. [DOI] [PubMed] [Google Scholar]
- Slesinger P. A., Lansman J. B. Inactivation of calcium currents in granule cells cultured from mouse cerebellum. J Physiol. 1991 Apr;435:101–121. doi: 10.1113/jphysiol.1991.sp018500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggle D. J., Janis R. A. Calcium channel ligands. Annu Rev Pharmacol Toxicol. 1987;27:347–369. doi: 10.1146/annurev.pa.27.040187.002023. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Ellinor P. T., Horne W. A. Molecular diversity of voltage-dependent Ca2+ channels. Trends Pharmacol Sci. 1991 Sep;12(9):349–354. doi: 10.1016/0165-6147(91)90595-j. [DOI] [PubMed] [Google Scholar]
- de Weille J. R., Schweitz H., Maes P., Tartar A., Lazdunski M. Calciseptine, a peptide isolated from black mamba venom, is a specific blocker of the L-type calcium channel. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2437–2440. doi: 10.1073/pnas.88.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]