Abstract
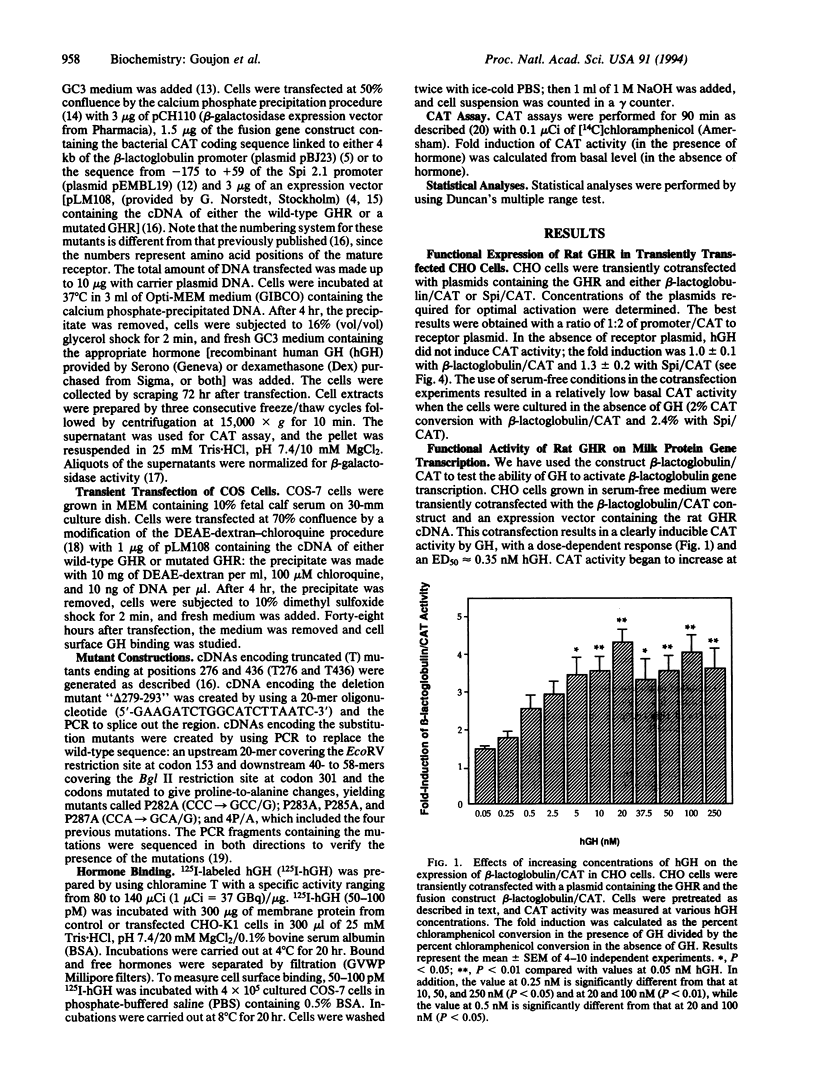
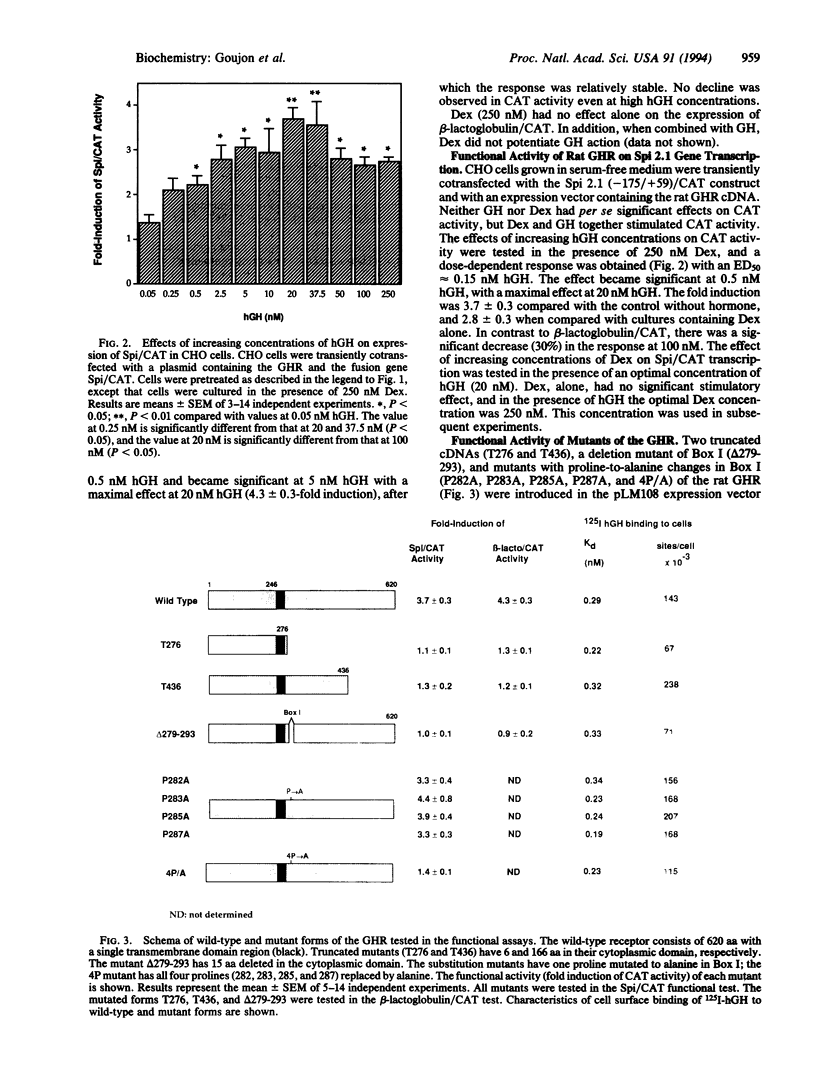
To study structure-function relationships of the growth hormone (GH) receptor (GHR), two functional systems have been developed. CHO cells were transiently cotransfected with the cDNA encoding the full-length rat GHR and with a construct consisting of the 5' flanking region of one of two GH-dependent genes encoding ovine beta-lactoglobulin or serine protease inhibitor 2.1 (Spi 2.1, formerly Spi.1; the corresponding rat gene has recently been redesignated Spin2a) coupled to the bacterial reporter gene encoding chloramphenicol acetyltransferase (CAT). Transfected cells were grown in the absence and presence of human GH and dexamethasone for the Spi 2.1 gene construct. GH was able to activate each promoter (with approximately 4-fold induction of CAT activity) in a dose-dependent manner. For both tests, the maximal effect was observed at 20 nM human GH. These tests have been used to identify functional domains of the GHR. Two truncated (T) GHRs, lacking most or part of the cytoplasmic domain [called T276 (ending at residue 276) and T436 (ending at residue 436)], were unable to stimulate CAT activity. The GHR contains a proline-rich region, called "Box I," conserved in the cytokine/GH/prolactin receptor family. Alanine substitutions for the four prolines of GHR Box I were introduced. Single proline-to-alanine mutations did not affect the functional activity of the GHR. However, modification of the four prolines together or deletion of the Box I (15 amino acids between positions 279 and 293) resulted in the complete absence of GH stimulation. Thus, the proline-rich region, shown to be important for other members of this receptor superfamily, is also critical for GH signal transduction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argetsinger L. S., Campbell G. S., Yang X., Witthuhn B. A., Silvennoinen O., Ihle J. N., Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993 Jul 30;74(2):237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- Bazan J. F. A novel family of growth factor receptors: a common binding domain in the growth hormone, prolactin, erythropoietin and IL-6 receptors, and the p75 IL-2 receptor beta-chain. Biochem Biophys Res Commun. 1989 Oct 31;164(2):788–795. doi: 10.1016/0006-291x(89)91528-3. [DOI] [PubMed] [Google Scholar]
- Billestrup N., Møldrup A., Serup P., Mathews L. S., Norstedt G., Nielsen J. H. Introduction of exogenous growth hormone receptors augments growth hormone-responsive insulin biosynthesis in rat insulinoma cells. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7210–7214. doi: 10.1073/pnas.87.18.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emtner M., Mathews L. S., Norstedt G. Growth hormone (GH) stimulates protein synthesis in cells transfected with GH receptor complementary DNA. Mol Endocrinol. 1990 Dec;4(12):2014–2020. doi: 10.1210/mend-4-12-2014. [DOI] [PubMed] [Google Scholar]
- Fukunaga R., Ishizaka-Ikeda E., Pan C. X., Seto Y., Nagata S. Functional domains of the granulocyte colony-stimulating factor receptor. EMBO J. 1991 Oct;10(10):2855–2865. doi: 10.1002/j.1460-2075.1991.tb07835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser F., Mulsant P., Gillois M. Long-term multiplication of the Chinese hamster ovary (CHO) cell line in a serum-free medium. In Vitro Cell Dev Biol. 1985 Oct;21(10):588–592. doi: 10.1007/BF02620890. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S. D., McGrath M. F., Collier R. J., Krivi G. G. Cloning and in vivo expression of bovine growth hormone receptor mRNA. Mol Cell Endocrinol. 1990 Sep 10;72(3):187–200. doi: 10.1016/0303-7207(90)90143-v. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Jammes H., Gaye P., Belair L., Djiane J. Identification and characterization of growth hormone receptor mRNA in the mammary gland. Mol Cell Endocrinol. 1991 Jan;75(1):27–35. doi: 10.1016/0303-7207(91)90242-k. [DOI] [PubMed] [Google Scholar]
- Lesueur L., Edery M., Ali S., Paly J., Kelly P. A., Djiane J. Comparison of long and short forms of the prolactin receptor on prolactin-induced milk protein gene transcription. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):824–828. doi: 10.1073/pnas.88.3.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D. W., Spencer S. A., Cachianes G., Hammonds R. G., Collins C., Henzel W. J., Barnard R., Waters M. J., Wood W. I. Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature. 1987 Dec 10;330(6148):537–543. doi: 10.1038/330537a0. [DOI] [PubMed] [Google Scholar]
- Lincoln D. T., Water M. J., Breipohl W., Sinowatz F., Lobie P. E. Growth hormone receptors expression in the proliferating rat mammary gland. Acta Histochem Suppl. 1990;40:47–49. [PubMed] [Google Scholar]
- Mathews L. S., Enberg B., Norstedt G. Regulation of rat growth hormone receptor gene expression. J Biol Chem. 1989 Jun 15;264(17):9905–9910. [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Murakami M., Narazaki M., Hibi M., Yawata H., Yasukawa K., Hamaguchi M., Taga T., Kishimoto T. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine receptor family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11349–11353. doi: 10.1073/pnas.88.24.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A., Gibson T., Lehto V. P., Saraste M. SH3--an abundant protein domain in search of a function. FEBS Lett. 1992 Jul 27;307(1):55–61. doi: 10.1016/0014-5793(92)80901-r. [DOI] [PubMed] [Google Scholar]
- Möller C., Hansson A., Enberg B., Lobie P. E., Norstedt G. Growth hormone (GH) induction of tyrosine phosphorylation and activation of mitogen-activated protein kinases in cells transfected with rat GH receptor cDNA. J Biol Chem. 1992 Nov 15;267(32):23403–23408. [PubMed] [Google Scholar]
- Møldrup A., Allevato G., Dyrberg T., Nielsen J. H., Billestrup N. Growth hormone action in rat insulinoma cells expressing truncated growth hormone receptors. J Biol Chem. 1991 Sep 15;266(26):17441–17445. [PubMed] [Google Scholar]
- Paquereau L., Vilarem M. J., Rossi V., Rouayrenc J. F., Le Cam A. Regulation of two rat serine-protease inhibitor gene promoters by somatotropin and glucocorticoids. Study with intact hepatocytes and cell-free systems. Eur J Biochem. 1992 Nov 1;209(3):1053–1061. doi: 10.1111/j.1432-1033.1992.tb17381.x. [DOI] [PubMed] [Google Scholar]
- Peel C. J., Bauman D. E. Somatotropin and lactation. J Dairy Sci. 1987 Feb;70(2):474–486. doi: 10.3168/jds.S0022-0302(87)80030-9. [DOI] [PubMed] [Google Scholar]
- Ren R., Mayer B. J., Cicchetti P., Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993 Feb 19;259(5098):1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- Sakamaki K., Miyajima I., Kitamura T., Miyajima A. Critical cytoplasmic domains of the common beta subunit of the human GM-CSF, IL-3 and IL-5 receptors for growth signal transduction and tyrosine phosphorylation. EMBO J. 1992 Oct;11(10):3541–3549. doi: 10.1002/j.1460-2075.1992.tb05437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Yoon J. B., Berry S. A., Seelig S., Towle H. C. An inducible nuclear factor binds to a growth hormone-regulated gene. J Biol Chem. 1990 Nov 15;265(32):19947–19954. [PubMed] [Google Scholar]