Abstract
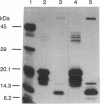
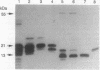
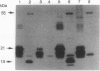
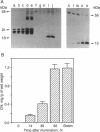
In addition to their application in organ transplantation, immunosuppressive drugs are valuable tools for studying signal transduction in eukaryotic cells. Using affinity chromatography, we have purified immunosuppressive drug receptors (immunophilins) from fava bean. Proteins belonging to both major classes of the immunophilin family identified from animal sources [FK506- and rapamycin-binding proteins (FKBPs) and cyclophilins] were present in this higher plant. FKBP13, the most abundant FKBP family member in leaf tissues, was not detected in root tissues, whereas other FKBPs were present in both tissues. While the abundance of cyclophilin A in leaves was similar to that in roots, cyclophilin B/C was expressed at a much higher level in leaf tissues than in root tissues. Subcellular localization of immunophilins in mesophyll cells showed that chloroplasts contained FKBP13 and cyclophilin B/C but not other members, which explains the preferential expression of these two proteins in leaves over roots. The abundance of chloroplast-localized immunophilins, FKBP13 and cyclophilin B/C, was regulated by light. Although etiolated leaves produced detectable levels of cyclophilin B/C, they did not express FKBP13. Illumination of etiolated plants dramatically increased the expression of both FKBP13 and cyclophilin B/C. The light-induced expression of FKBP13 is closely correlated with the accumulation of chlorophyll in the leaf tissue. Our findings suggest that FKBP13 and cyclophilin B/C may play a specific role in chloroplasts.
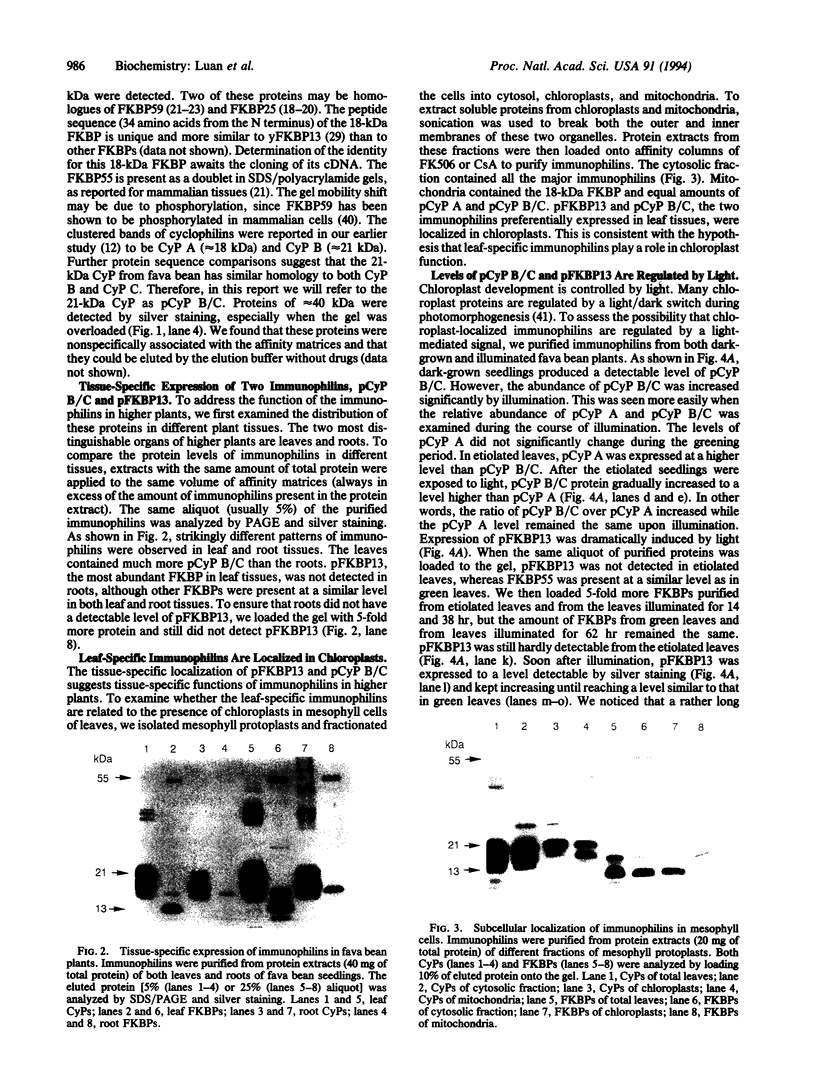
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer B. E., Mattila P. S., Standaert R. F., Herzenberg L. A., Burakoff S. J., Crabtree G., Schreiber S. L. Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9231–9235. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer B. E., Somers P. K., Wandless T. J., Burakoff S. J., Schreiber S. L. Probing immunosuppressant action with a nonnatural immunophilin ligand. Science. 1990 Oct 26;250(4980):556–559. doi: 10.1126/science.1700475. [DOI] [PubMed] [Google Scholar]
- Breiman A., Fawcett T. W., Ghirardi M. L., Mattoo A. K. Plant organelles contain distinct peptidylprolyl cis,trans-isomerases. J Biol Chem. 1992 Oct 25;267(30):21293–21296. [PubMed] [Google Scholar]
- Clipstone N. A., Crabtree G. R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992 Jun 25;357(6380):695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Dumont F. J., Melino M. R., Staruch M. J., Koprak S. L., Fischer P. A., Sigal N. H. The immunosuppressive macrolides FK-506 and rapamycin act as reciprocal antagonists in murine T cells. J Immunol. 1990 Feb 15;144(4):1418–1424. [PubMed] [Google Scholar]
- Ellis R. J., van der Vies S. M. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541. [DOI] [PubMed] [Google Scholar]
- Freskgård P. O., Bergenhem N., Jonsson B. H., Svensson M., Carlsson U. Isomerase and chaperone activity of prolyl isomerase in the folding of carbonic anhydrase. Science. 1992 Oct 16;258(5081):466–468. doi: 10.1126/science.1357751. [DOI] [PubMed] [Google Scholar]
- Friedman J., Weissman I. Two cytoplasmic candidates for immunophilin action are revealed by affinity for a new cyclophilin: one in the presence and one in the absence of CsA. Cell. 1991 Aug 23;66(4):799–806. doi: 10.1016/0092-8674(91)90123-g. [DOI] [PubMed] [Google Scholar]
- Galat A., Lane W. S., Standaert R. F., Schreiber S. L. A rapamycin-selective 25-kDa immunophilin. Biochemistry. 1992 Mar 3;31(8):2427–2434. doi: 10.1021/bi00123a031. [DOI] [PubMed] [Google Scholar]
- Gasser C. S., Gunning D. A., Budelier K. A., Brown S. M. Structure and expression of cytosolic cyclophilin/peptidyl-prolyl cis-trans isomerase of higher plants and production of active tomato cyclophilin in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9519–9523. doi: 10.1073/pnas.87.24.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby A. A., Ellis R. J. Chaperone function: the assembly of ribulose bisphosphate carboxylase-oxygenase. Annu Rev Cell Biol. 1990;6:125–149. doi: 10.1146/annurev.cb.06.110190.001013. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Haendler B., Hofer-Warbinek R., Hofer E. Complementary DNA for human T-cell cyclophilin. EMBO J. 1987 Apr;6(4):947–950. doi: 10.1002/j.1460-2075.1987.tb04843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haendler B., Keller R., Hiestand P. C., Kocher H. P., Wegmann G., Movva N. R. Yeast cyclophilin: isolation and characterization of the protein, cDNA and gene. Gene. 1989 Nov 15;83(1):39–46. doi: 10.1016/0378-1119(89)90401-0. [DOI] [PubMed] [Google Scholar]
- Hung D. T., Schreiber S. L. cDNA cloning of a human 25 kDa FK506 and rapamycin binding protein. Biochem Biophys Res Commun. 1992 Apr 30;184(2):733–738. doi: 10.1016/0006-291x(92)90651-z. [DOI] [PubMed] [Google Scholar]
- Jaenicke R. Folding and association of proteins. Prog Biophys Mol Biol. 1987;49(2-3):117–237. doi: 10.1016/0079-6107(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Jin Y. J., Albers M. W., Lane W. S., Bierer B. E., Schreiber S. L., Burakoff S. J. Molecular cloning of a membrane-associated human FK506- and rapamycin-binding protein, FKBP-13. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6677–6681. doi: 10.1073/pnas.88.15.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y. J., Burakoff S. J., Bierer B. E. Molecular cloning of a 25-kDa high affinity rapamycin binding protein, FKBP25. J Biol Chem. 1992 Jun 5;267(16):10942–10945. [PubMed] [Google Scholar]
- Keegstra K. Transport and routing of proteins into chloroplasts. Cell. 1989 Jan 27;56(2):247–253. doi: 10.1016/0092-8674(89)90898-2. [DOI] [PubMed] [Google Scholar]
- Lebeau M. C., Massol N., Herrick J., Faber L. E., Renoir J. M., Radanyi C., Baulieu E. E. P59, an hsp 90-binding protein. Cloning and sequencing of its cDNA and preparation of a peptide-directed polyclonal antibody. J Biol Chem. 1992 Mar 5;267(7):4281–4284. [PubMed] [Google Scholar]
- Lin C. S., Boltz R. C., Siekierka J. J., Sigal N. H. FK-506 and cyclosporin A inhibit highly similar signal transduction pathways in human T lymphocytes. Cell Immunol. 1991 Apr 1;133(2):269–284. doi: 10.1016/0008-8749(91)90103-i. [DOI] [PubMed] [Google Scholar]
- Liu J., Albers M. W., Wandless T. J., Luan S., Alberg D. G., Belshaw P. J., Cohen P., MacKintosh C., Klee C. B., Schreiber S. L. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry. 1992 Apr 28;31(16):3896–3901. doi: 10.1021/bi00131a002. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Liu J., Walsh C. T. Peptidyl-prolyl cis-trans-isomerase from Escherichia coli: a periplasmic homolog of cyclophilin that is not inhibited by cyclosporin A. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4028–4032. doi: 10.1073/pnas.87.11.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S., Li W., Rusnak F., Assmann S. M., Schreiber S. L. Immunosuppressants implicate protein phosphatase regulation of K+ channels in guard cells. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2202–2206. doi: 10.1073/pnas.90.6.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. B., Foor F., Siekierka J. J., Hsu M. J., Ramadan N., Morin N., Shafiee A., Dahl A. M., Brizuela L., Chrebet G. Yeast FKBP-13 is a membrane-associated FK506-binding protein encoded by the nonessential gene FKB2. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7471–7475. doi: 10.1073/pnas.89.16.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe S. J., Tamura J., Kincaid R. L., Tocci M. J., O'Neill E. A. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992 Jun 25;357(6380):692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- Peattie D. A., Harding M. W., Fleming M. A., DeCenzo M. T., Lippke J. A., Livingston D. J., Benasutti M. Expression and characterization of human FKBP52, an immunophilin that associates with the 90-kDa heat shock protein and is a component of steroid receptor complexes. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10974–10978. doi: 10.1073/pnas.89.22.10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price E. R., Zydowsky L. D., Jin M. J., Baker C. H., McKeon F. D., Walsh C. T. Human cyclophilin B: a second cyclophilin gene encodes a peptidyl-prolyl isomerase with a signal sequence. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1903–1907. doi: 10.1073/pnas.88.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneuwly S., Shortridge R. D., Larrivee D. C., Ono T., Ozaki M., Pak W. L. Drosophila ninaA gene encodes an eye-specific cyclophilin (cyclosporine A binding protein). Proc Natl Acad Sci U S A. 1989 Jul;86(14):5390–5394. doi: 10.1073/pnas.86.14.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991 Jan 18;251(4991):283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- Shieh B. H., Stamnes M. A., Seavello S., Harris G. L., Zuker C. S. The ninaA gene required for visual transduction in Drosophila encodes a homologue of cyclosporin A-binding protein. Nature. 1989 Mar 2;338(6210):67–70. doi: 10.1038/338067a0. [DOI] [PubMed] [Google Scholar]
- Stamnes M. A., Shieh B. H., Chuman L., Harris G. L., Zuker C. S. The cyclophilin homolog ninaA is a tissue-specific integral membrane protein required for the proper synthesis of a subset of Drosophila rhodopsins. Cell. 1991 Apr 19;65(2):219–227. doi: 10.1016/0092-8674(91)90156-s. [DOI] [PubMed] [Google Scholar]
- Standaert R. F., Galat A., Verdine G. L., Schreiber S. L. Molecular cloning and overexpression of the human FK506-binding protein FKBP. Nature. 1990 Aug 16;346(6285):671–674. doi: 10.1038/346671a0. [DOI] [PubMed] [Google Scholar]
- Starzl T. E., Demetris A. J., Van Thiel D. Liver transplantation (2). N Engl J Med. 1989 Oct 19;321(16):1092–1099. doi: 10.1056/NEJM198910193211606. [DOI] [PubMed] [Google Scholar]
- Swanson S. K., Born T., Zydowsky L. D., Cho H., Chang H. Y., Walsh C. T., Rusnak F. Cyclosporin-mediated inhibition of bovine calcineurin by cyclophilins A and B. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3741–3745. doi: 10.1073/pnas.89.9.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P. K., Albers M. W., Chang H., Faber L. E., Schreiber S. L. Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex. Science. 1992 May 29;256(5061):1315–1318. doi: 10.1126/science.1376003. [DOI] [PubMed] [Google Scholar]
- Tocci M. J., Matkovich D. A., Collier K. A., Kwok P., Dumont F., Lin S., Degudicibus S., Siekierka J. J., Chin J., Hutchinson N. I. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J Immunol. 1989 Jul 15;143(2):718–726. [PubMed] [Google Scholar]
- Tropschug M., Wachter E., Mayer S., Schönbrunner E. R., Schmid F. X. Isolation and sequence of an FK506-binding protein from N. crassa which catalyses protein folding. Nature. 1990 Aug 16;346(6285):674–677. doi: 10.1038/346674a0. [DOI] [PubMed] [Google Scholar]
- Wiederrecht G., Brizuela L., Elliston K., Sigal N. H., Siekierka J. J. FKB1 encodes a nonessential FK 506-binding protein in Saccharomyces cerevisiae and contains regions suggesting homology to the cyclophilins. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1029–1033. doi: 10.1073/pnas.88.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]