Abstract
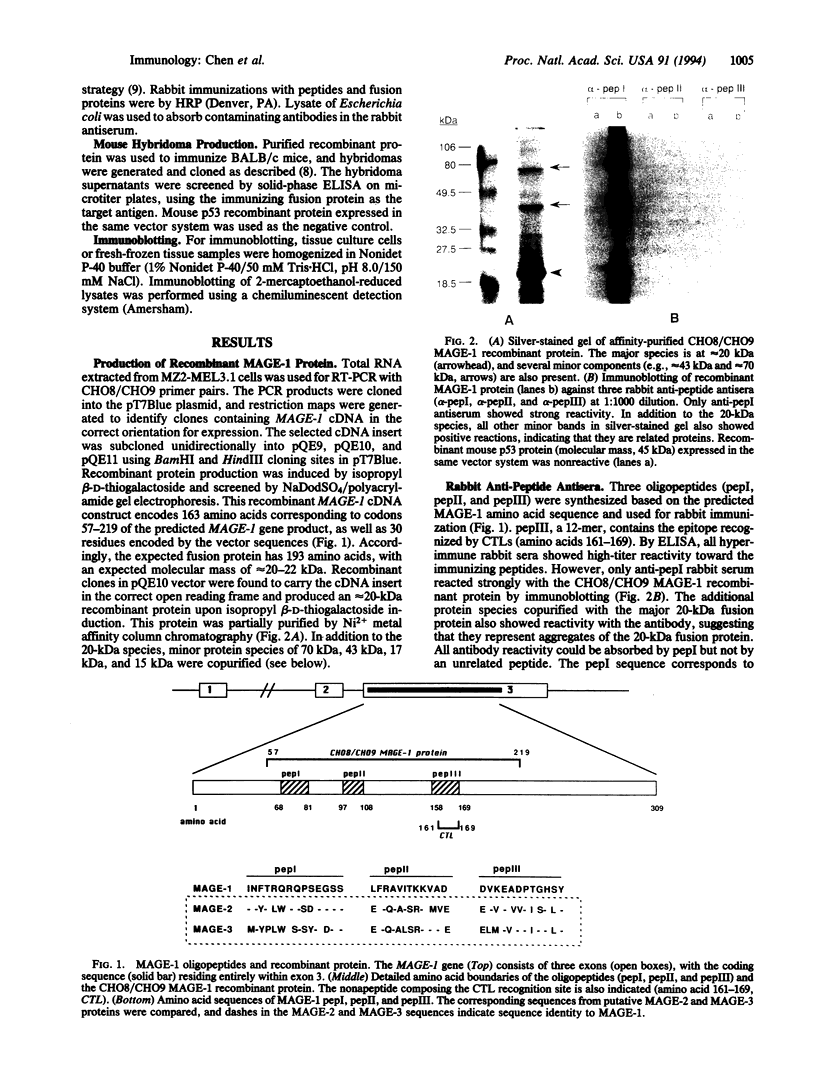
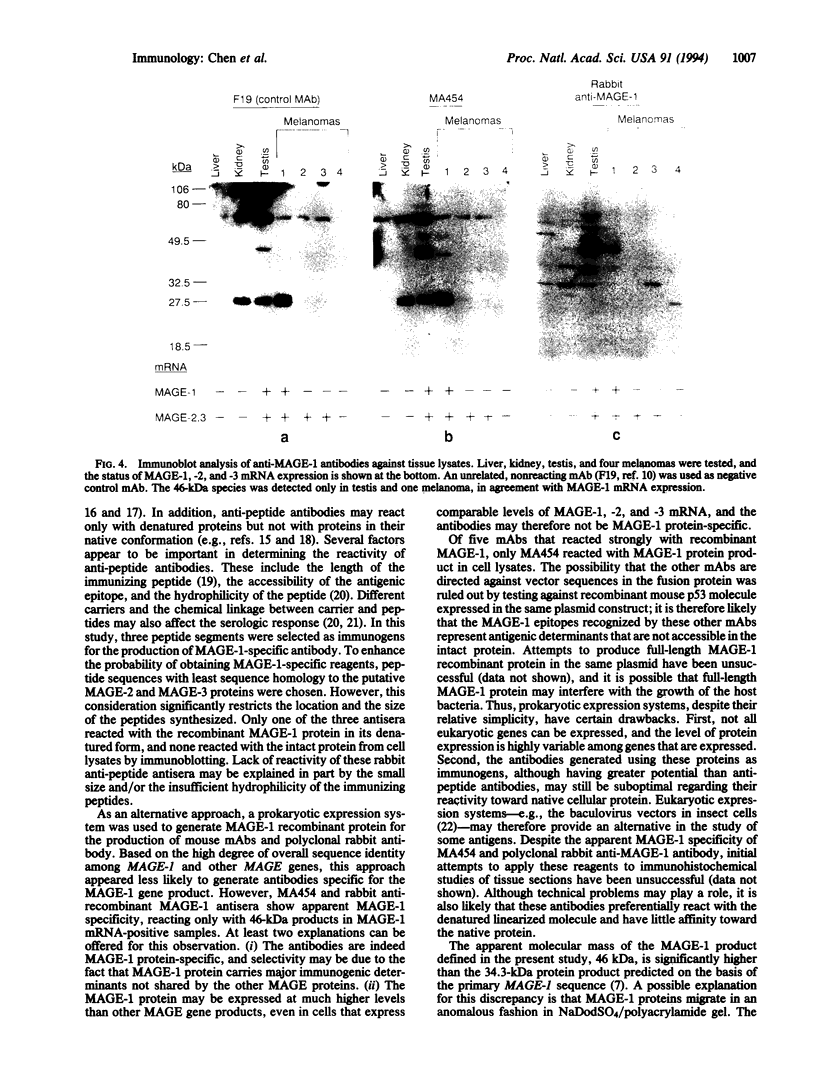
The human MAGE-1 gene encodes a melanoma peptide antigen recognized by autologous cytotoxic T lymphocytes. To produce antibodies against the MAGE-1 gene product, several approaches were taken. Three oligopeptides were synthesized based on predicted MAGE-1 amino acid sequences and were used to generate rabbit anti-peptide anti-sera. In addition, a truncated MAGE-1 cDNA was cloned into an Escherichia coli expression vector, and recombinant protein was produced and purified. All three rabbit anti-peptide antisera showed reactivity against the immunizing peptide, and one reacted with the recombinant MAGE-1 protein by immunoblotting, but none reacted with cell lysates from MAGE-1 mRNA-positive cells. The recombinant MAGE-1 protein was then used for the generation of mouse monoclonal and rabbit polyclonal antibodies. One IgG1 monoclonal antibody, MA454, as well as rabbit polyclonal antisera recognized a 46-kDa protein in extracts of MAGE-1 mRNA-positive melanoma cell lines. The antibodies showed no apparent cross-reactivity with products of the closely related MAGE-2 and MAGE-3 genes. Serological typing of normal and tumor cell lysates was in full agreement with mRNA analysis, showing expression of MAGE-1 protein in MAGE-1 mRNA-positive testis and a subset of melanomas but not in MAGE-1 mRNA-negative normal or tumor tissues. Transfection of the MAGE-1 gene into a MAGE-1 mRNA-negative melanoma cell line resulted in the expression of the 46-kDa protein, confirming the identity of this protein as the MAGE-1 gene product.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahraoui E. M., Granier C., Van Rietschoten J., Rochat H., el Ayeb M. Specificity and neutralizing capacity of antibodies elicited by a synthetic peptide of scorpion toxin. J Immunol. 1986 May 1;136(9):3371–3377. [PubMed] [Google Scholar]
- Brasseur F., Marchand M., Vanwijck R., Hérin M., Lethé B., Chomez P., Boon T. Human gene MAGE-1, which codes for a tumor-rejection antigen, is expressed by some breast tumors. Int J Cancer. 1992 Nov 11;52(5):839–841. doi: 10.1002/ijc.2910520528. [DOI] [PubMed] [Google Scholar]
- Dippold W. G., Lloyd K. O., Li L. T., Ikeda H., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: definition of six antigenic systems with mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6114–6118. doi: 10.1073/pnas.77.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson H. J., Lerner R. A., Wright P. E. The physical basis for induction of protein-reactive antipeptide antibodies. Annu Rev Biophys Biophys Chem. 1988;17:305–324. doi: 10.1146/annurev.bb.17.060188.001513. [DOI] [PubMed] [Google Scholar]
- Etlinger H. M., Renia L., Matile H., Manneberg M., Mazier D., Trzeciak A., Gillessen D. Antibody responses to a synthetic peptide-based malaria vaccine candidate: influence of sequence variants of the peptide. Eur J Immunol. 1991 Jun;21(6):1505–1511. doi: 10.1002/eji.1830210626. [DOI] [PubMed] [Google Scholar]
- Francis M. J., Hastings G. Z., Brown F., McDermed J., Lu Y. A., Tam J. P. Immunological evaluation of the multiple antigen peptide (MAP) system using the major immunogenic site of foot-and-mouth disease virus. Immunology. 1991 Jul;73(3):249–254. [PMC free article] [PubMed] [Google Scholar]
- Groome N., Lawrence M. Preparation of monoclonal antibodies to the beta A subunit of ovarian inhibin using a synthetic peptide immunogen. Hybridoma. 1991 Apr;10(2):309–316. doi: 10.1089/hyb.1991.10.309. [DOI] [PubMed] [Google Scholar]
- Hofmann S. L., Goldstein J. L., Orth K., Moomaw C. R., Slaughter C. A., Brown M. S. Molecular cloning of a histidine-rich Ca2+-binding protein of sarcoplasmic reticulum that contains highly conserved repeated elements. J Biol Chem. 1989 Oct 25;264(30):18083–18090. [PubMed] [Google Scholar]
- Kleinschmidt J. A., Dingwall C., Maier G., Franke W. W. Molecular characterization of a karyophilic, histone-binding protein: cDNA cloning, amino acid sequence and expression of nuclear protein N1/N2 of Xenopus laevis. EMBO J. 1986 Dec 20;5(13):3547–3552. doi: 10.1002/j.1460-2075.1986.tb04681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPan K. E., Klapper D. G., Frelinger J. A. Production and characterization of a peptide specific, anti-major histocompatibility complex class II, monoclonal antibody. Mol Immunol. 1991 Apr-May;28(4-5):499–504. doi: 10.1016/0161-5890(91)90164-f. [DOI] [PubMed] [Google Scholar]
- Lu Y. A., Clavijo P., Galantino M., Shen Z. Y., Liu W., Tam J. P. Chemically unambiguous peptide immunogen: preparation, orientation and antigenicity of purified peptide conjugated to the multiple antigen peptide system. Mol Immunol. 1991 Jun;28(6):623–630. doi: 10.1016/0161-5890(91)90131-3. [DOI] [PubMed] [Google Scholar]
- Ostrowski L. E., Pegram C. N., Von Wronski M. A., Humphrey P. A., He X. M., Shiota S., Mitra S., Brent T. P., Bigner D. D. Production and characterization of antipeptide antibodies against human O6-methylguanine-DNA methyltransferase. Cancer Res. 1991 Jul 1;51(13):3339–3344. [PubMed] [Google Scholar]
- Rettig W. J., Garin-Chesa P., Healey J. H., Su S. L., Ozer H. L., Schwab M., Albino A. P., Old L. J. Regulation and heteromeric structure of the fibroblast activation protein in normal and transformed cells of mesenchymal and neuroectodermal origin. Cancer Res. 1993 Jul 15;53(14):3327–3335. [PubMed] [Google Scholar]
- Rimoldi D., Romero P., Carrel S. The human melanoma antigen-encoding gene, MAGE-1, is expressed by other tumour cells of neuroectodermal origin such as glioblastomas and neuroblastomas. Int J Cancer. 1993 May 28;54(3):527–528. doi: 10.1002/ijc.2910540329. [DOI] [PubMed] [Google Scholar]
- Schmidt-Zachmann M. S., Franke W. W. DNA cloning and amino acid sequence determination of a major constituent protein of mammalian nucleoli. Correspondence of the nucleoplasmin-related protein NO38 to mammalian protein B23. Chromosoma. 1988;96(6):417–426. doi: 10.1007/BF00303035. [DOI] [PubMed] [Google Scholar]
- Schmidt-Zachmann M. S., Hügle-Dörr B., Franke W. W. A constitutive nucleolar protein identified as a member of the nucleoplasmin family. EMBO J. 1987 Jul;6(7):1881–1890. doi: 10.1002/j.1460-2075.1987.tb02447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth M. S., Trudgett A., Hoey E. M., Martin S. J., Brown F. Characterization of neutralizing antibodies to bovine enterovirus elicited by synthetic peptides. Arch Virol. 1992;126(1-4):21–33. doi: 10.1007/BF01309681. [DOI] [PubMed] [Google Scholar]
- Spangler B. D. Binding to native proteins by antipeptide monoclonal antibodies. J Immunol. 1991 Mar 1;146(5):1591–1595. [PubMed] [Google Scholar]
- Tanaka T., Slamon D. J., Cline M. J. Efficient generation of antibodies to oncoproteins by using synthetic peptide antigens. Proc Natl Acad Sci U S A. 1985 May;82(10):3400–3404. doi: 10.1073/pnas.82.10.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversari C., van der Bruggen P., Luescher I. F., Lurquin C., Chomez P., Van Pel A., De Plaen E., Amar-Costesec A., Boon T. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J Exp Med. 1992 Nov 1;176(5):1453–1457. doi: 10.1084/jem.176.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversari C., van der Bruggen P., Van den Eynde B., Hainaut P., Lemoine C., Ohta N., Old L., Boon T. Transfection and expression of a gene coding for a human melanoma antigen recognized by autologous cytolytic T lymphocytes. Immunogenetics. 1992;35(3):145–152. doi: 10.1007/BF00185107. [DOI] [PubMed] [Google Scholar]
- Van den Eynde B., Hainaut P., Hérin M., Knuth A., Lemoine C., Weynants P., van der Bruggen P., Fauchet R., Boon T. Presence on a human melanoma of multiple antigens recognized by autologous CTL. Int J Cancer. 1989 Oct 15;44(4):634–640. doi: 10.1002/ijc.2910440413. [DOI] [PubMed] [Google Scholar]
- Wilczynska M., Poniatowski J., Janecka A., Cierniewski C. S. Production and characteristics of human tissue plasminogen-activator antibodies with region-oriented synthetic peptides. Eur J Biochem. 1992 Jun 15;206(3):653–657. doi: 10.1111/j.1432-1033.1992.tb16970.x. [DOI] [PubMed] [Google Scholar]
- van der Bruggen P., Traversari C., Chomez P., Lurquin C., De Plaen E., Van den Eynde B., Knuth A., Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991 Dec 13;254(5038):1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]