Abstract
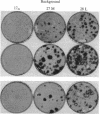
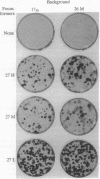
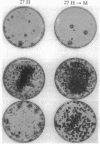
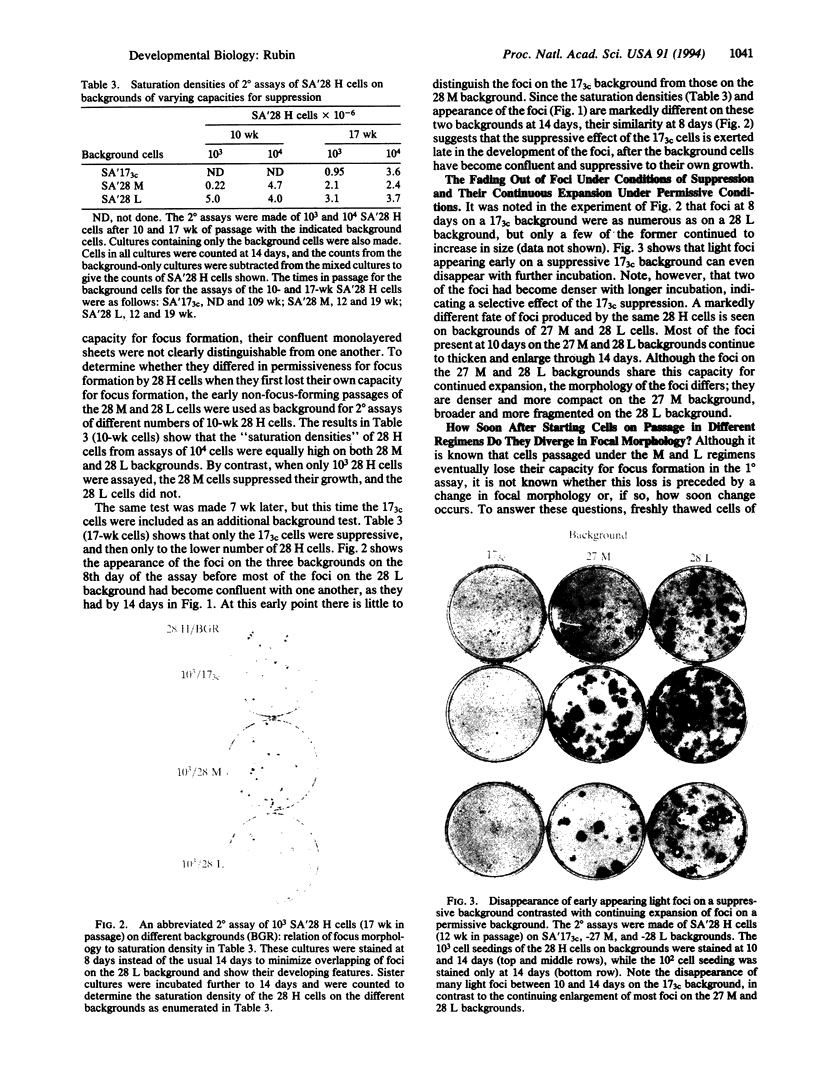
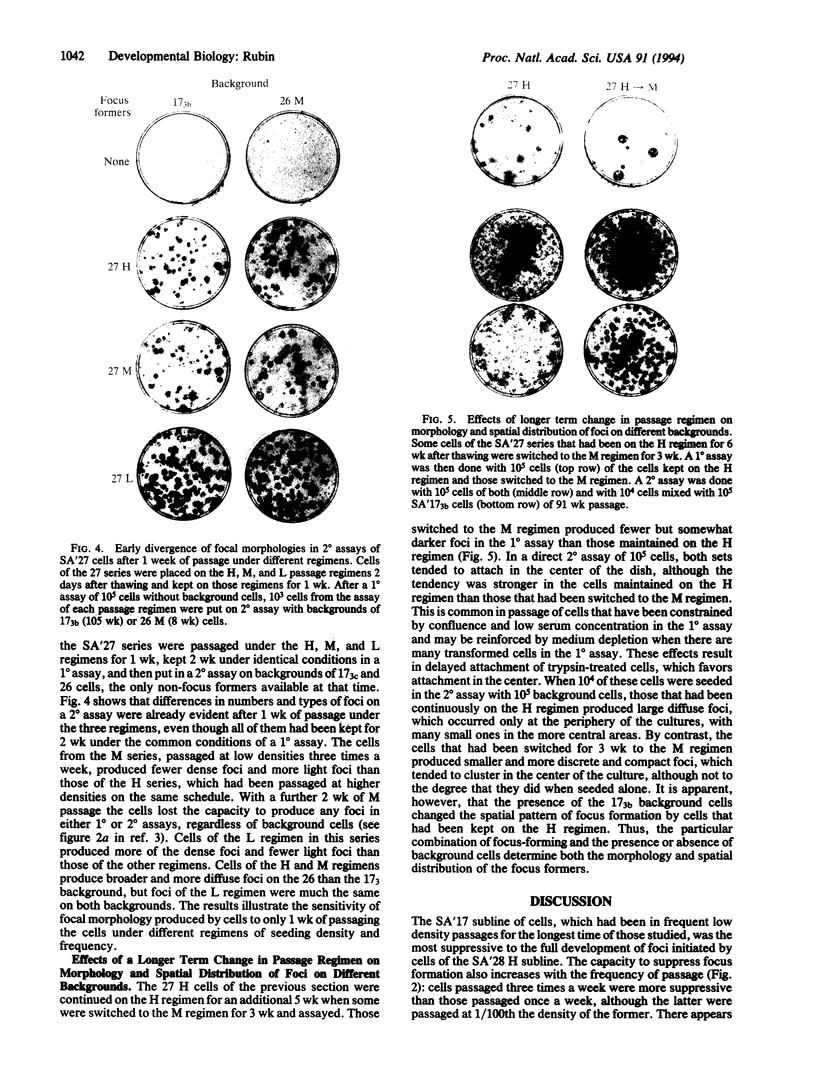
NIH 3T3 cells that are passaged frequently at low density in high (10%) calf serum lose their original capacity to produce transformed foci on a monolayer of nontransformed cells. They can then be used to form a monolayered background for the assay of the number of focus-forming cells from a transformed population. Continuation of the low-density passages for many weeks gives rise to a population that can suppress the full development of foci by a transformed line. The suppression appears to occur only after the background cells have become confluent and contact inhibited. It can also cause the disappearance of light foci that had developed before suppression began. Another subline of cells that were passaged at cloning density only once a week lose their focus-forming capacity more slowly than those passaged thrice weekly. When used as a background for the assay of a transformed line, they permit continuous expansion of the foci, with no sign of suppression. Not only the number and size of foci but also their detailed morphology is influenced by the background on which they are formed. A suppressive background can also determine the spatial distribution of foci, presumably as a result of gradients in local cell density of the background. The permissiveness of a nontransformed cell population for focus formation by transformed cells appears to be related to the capacity of the nontransformed population itself to undergo transformation when exposed to the constraints used to induce transformation. These findings indicate there are many degrees of capacity to suppress focus formation and to overcome suppression. They have significance for tumor development and for the epigenetic interactions of normal development.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chow M., Yao A., Rubin H. Cellular epigenetics: topochronology of progressive "spontaneous" transformation of cells under growth constraint. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):599–603. doi: 10.1073/pnas.91.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEAN A. C., HINSHELWOOD C. INTEGRATION OF CELL REACTIONS. Nature. 1963 Jul 6;199:7–11. doi: 10.1038/199007a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Embryonic induction--molecular prospects. Development. 1987 Mar;99(3):285–306. doi: 10.1242/dev.99.3.285. [DOI] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan M. L., Sachs T. Development of immature stomata: evidence for epigenetic selection of a spacing pattern. Dev Biol. 1991 Jul;146(1):100–105. doi: 10.1016/0012-1606(91)90450-h. [DOI] [PubMed] [Google Scholar]
- Mehta P. P., Bertram J. S., Loewenstein W. R. Growth inhibition of transformed cells correlates with their junctional communication with normal cells. Cell. 1986 Jan 17;44(1):187–196. doi: 10.1016/0092-8674(86)90497-6. [DOI] [PubMed] [Google Scholar]
- Rubin A. L., Yao A., Rubin H. Relation of spontaneous transformation in cell culture to adaptive growth and clonal heterogeneity. Proc Natl Acad Sci U S A. 1990 Jan;87(1):482–486. doi: 10.1073/pnas.87.1.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. 'Spontaneous' transformation as aberrant epigenesis. Differentiation. 1993 Jun;53(2):123–137. doi: 10.1111/j.1432-0436.1993.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Rubin H. Cellular epigenetics: effects of passage history on competence of cells for "spontaneous" transformation. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10715–10719. doi: 10.1073/pnas.90.22.10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. On the nature of enduring modifications induced in cells and organisms. Am J Physiol. 1990 Feb;258(2 Pt 1):L19–L24. doi: 10.1152/ajplung.1990.258.2.L19. [DOI] [PubMed] [Google Scholar]
- Rubin H., Xu K. Evidence for the progressive and adaptive nature of spontaneous transformation in the NIH 3T3 cell line. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1860–1864. doi: 10.1073/pnas.86.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOKER M. REGULATION OF GROWTH AND ORIENTATION IN HAMSTER CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Oct;24:165–174. doi: 10.1016/0042-6822(64)90099-6. [DOI] [PubMed] [Google Scholar]
- Sachs T. Epigenetic selection: an alternative mechanism of pattern formation. J Theor Biol. 1988 Oct 21;134(4):547–559. doi: 10.1016/s0022-5193(88)80056-0. [DOI] [PubMed] [Google Scholar]
- Shipley G. D., Ham R. G. Improved medium and culture conditions for clonal growth with minimal serum protein and for enhanced serum-free survival of Swiss 3T3 cells. In Vitro. 1981 Aug;17(8):656–670. doi: 10.1007/BF02628401. [DOI] [PubMed] [Google Scholar]
- Weiss R. A. The influence of normal cells on the proliferation of tumour cells in culture. Exp Cell Res. 1970 Nov;63(1):1–18. doi: 10.1016/0014-4827(70)90326-5. [DOI] [PubMed] [Google Scholar]