Abstract
Common variable immunodeficiency (CVI) is characterized by hypogammaglobulinemia and recurrent bacterial infections due to failure of CVI B cells to differentiate in vivo into immunoglobulin-secreting plasma cells. We hypothesized that T-cell dysfunction resulting in abnormal contact-mediated B-cell activation may play a prominent role in the failure of CVI B cells to produce specific antibody. We have previously shown that B-cell proliferation and IgE production after stimulation with anti-CD40 and interleukin (IL) 4 were normal in 22 CVI patients evaluated, indicating that CVI B cells respond to signals delivered via CD40. Here we report that CD40 ligand (gp39) mRNA expression by activated lymphocytes from CVI patients (n = 31) as a group was significantly depressed (P < 0.0001) compared with normal controls (n = 32). gp39 mRNA expression by activated lymphocytes from 13 CVI patients fell below the normal control range. T-cell surface expression of functional gp39 protein was correspondingly low in those patients with gp39 mRNA levels below normal control range and normal in patients with gp39 mRNA levels within normal control range. In CVI patients as a group, gp39 mRNA levels correlated with IL-2 mRNA levels (P < 0.002, r = 0.6) and production (P < 0.001, r = 0.7) but not with gene expression or production of other lymphokines evaluated, suggesting an as-yet-undetermined association between gp39 and IL-2 gene regulation. Of the 13 patients whose activated T cells exhibited gp39 mRNA expression below the normal control range, 2 had normal T-cell-derived lymphokine production, whereas the remaining 11 exhibited broader T-cell dysfunction, resulting in IL-2 deficiency, and in some patients deficient production of other lymphokines as well, reflecting a heterogeneity in the underlying mechanisms leading to depressed gp39 expression in these patients. The observation that both gene and surface expression of gp39 by activated T cells is depressed in a subgroup of CVI patients suggests that inefficient signaling via CD40 may be responsible, in part, for failure of B-cell differentiation in these patients.
Full text
PDF
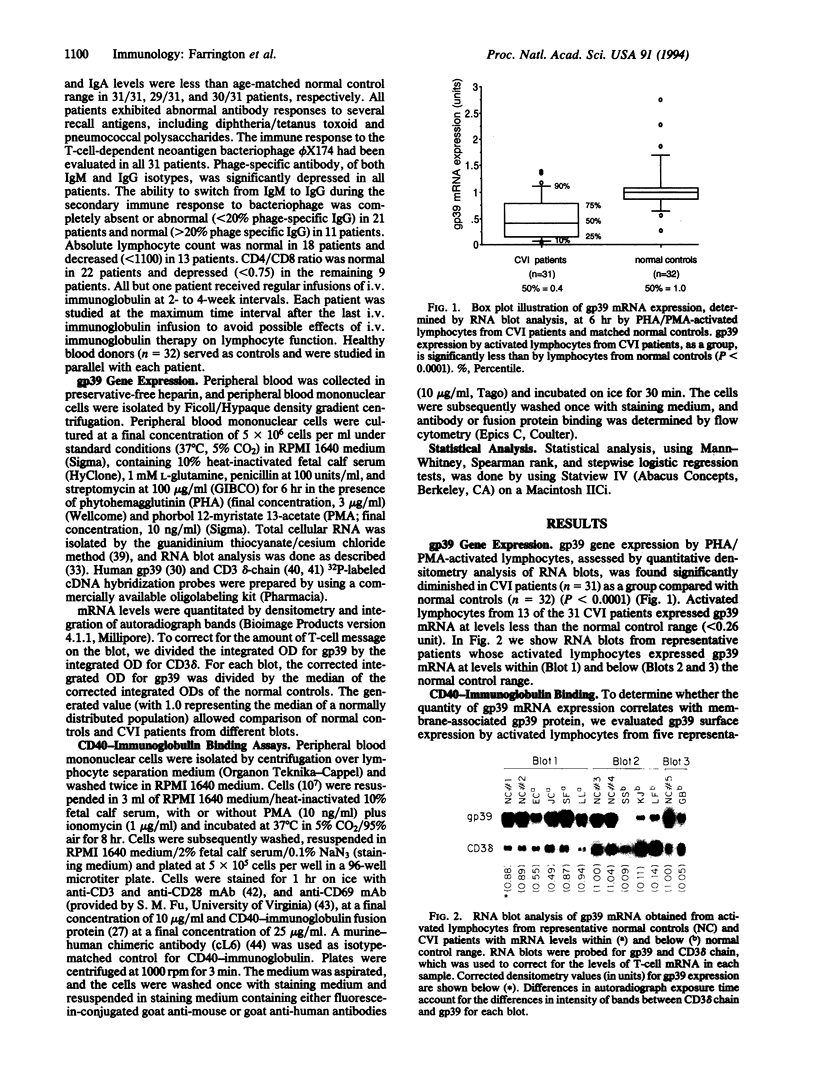
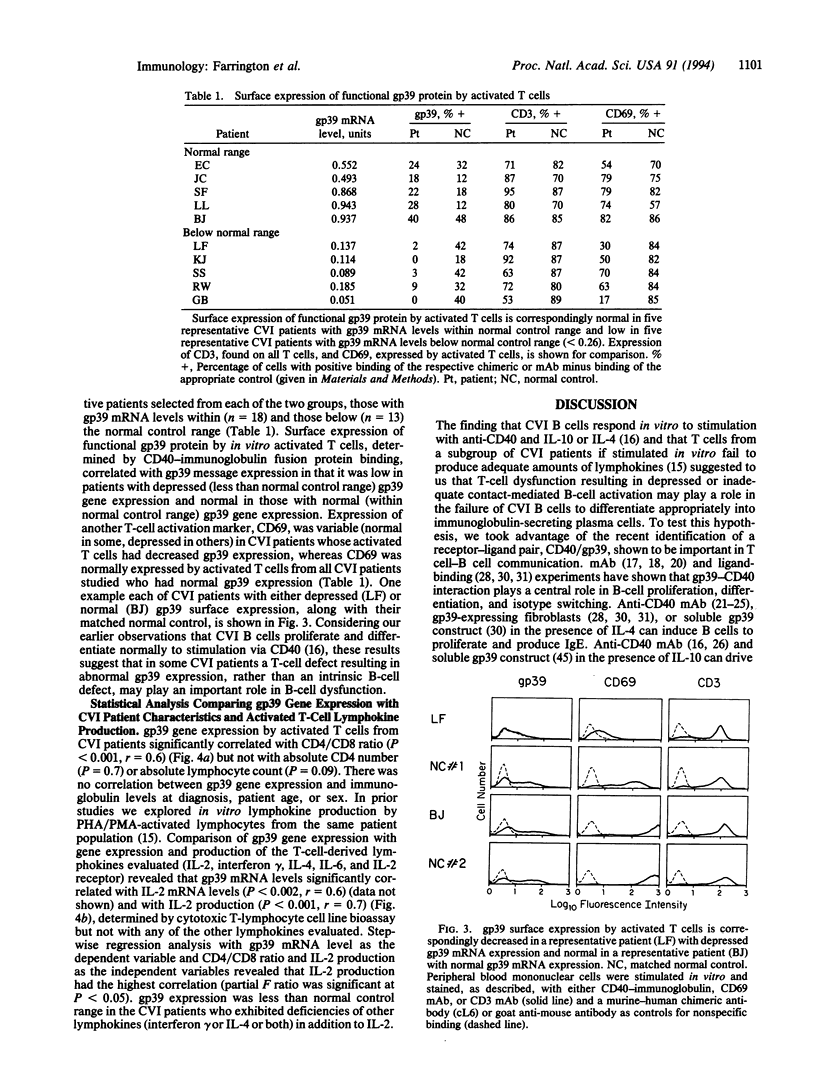
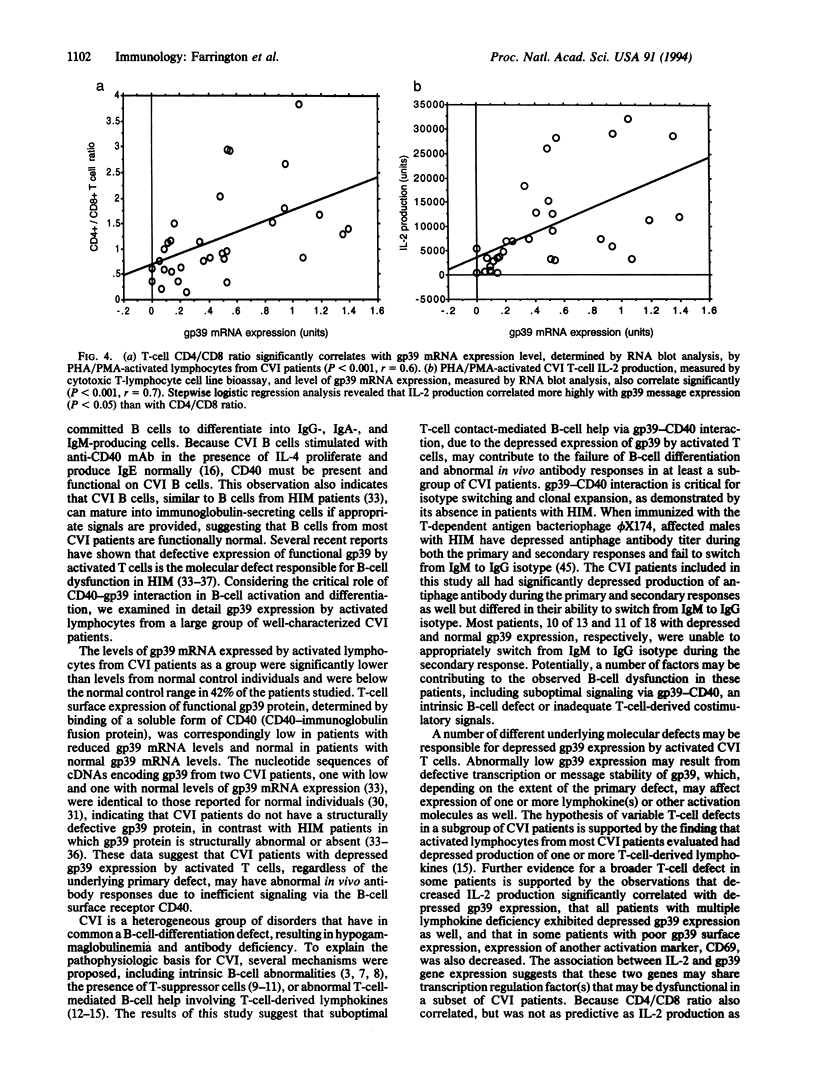

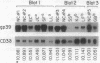
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Armitage R. J., Conley M. E., Rosenblatt H., Jenkins N. A., Copeland N. G., Bedell M. A., Edelhoff S., Disteche C. M., Simoneaux D. K. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993 Feb 12;259(5097):990–993. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- Armitage R. J., Fanslow W. C., Strockbine L., Sato T. A., Clifford K. N., Macduff B. M., Anderson D. M., Gimpel S. D., Davis-Smith T., Maliszewski C. R. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992 May 7;357(6373):80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Farrington M., Hollenbaugh D., Li X., Milatovich A., Nonoyama S., Bajorath J., Grosmaire L. S., Stenkamp R., Neubauer M. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993 Jan 29;72(2):291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Lane P. J. Regulation of human B-cell activation and adhesion. Annu Rev Immunol. 1991;9:97–127. doi: 10.1146/annurev.iy.09.040191.000525. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Ledbetter J. A. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4494–4498. doi: 10.1073/pnas.83.12.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Lawton A. R. Circulating B-cells in patients with immunodeficiency. Am J Pathol. 1972 Dec;69(3):513–528. [PMC free article] [PubMed] [Google Scholar]
- Cunningham-Rundles C. Clinical and immunologic analyses of 103 patients with common variable immunodeficiency. J Clin Immunol. 1989 Jan;9(1):22–33. doi: 10.1007/BF00917124. [DOI] [PubMed] [Google Scholar]
- DiSanto J. P., Bonnefoy J. Y., Gauchat J. F., Fischer A., de Saint Basile G. CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM. Nature. 1993 Feb 11;361(6412):541–543. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- Fell H. P., Gayle M. A., Yelton D., Lipsich L., Schieven G. L., Marken J. S., Aruffo A., Hellström K. E., Hellström I., Bajorath J. Chimeric L6 anti-tumor antibody. Genomic construction, expression, and characterization of the antigen binding site. J Biol Chem. 1992 Aug 5;267(22):15552–15558. [PubMed] [Google Scholar]
- Fuleihan R., Ramesh N., Loh R., Jabara H., Rosen R. S., Chatila T., Fu S. M., Stamenkovic I., Geha R. S. Defective expression of the CD40 ligand in X chromosome-linked immunoglobulin deficiency with normal or elevated IgM. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2170–2173. doi: 10.1073/pnas.90.6.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascan H., Gauchat J. F., Aversa G., Van Vlasselaer P., de Vries J. E. Anti-CD40 monoclonal antibodies or CD4+ T cell clones and IL-4 induce IgG4 and IgE switching in purified human B cells via different signaling pathways. J Immunol. 1991 Jul 1;147(1):8–13. [PubMed] [Google Scholar]
- Gordon J., Millsum M. J., Guy G. R., Ledbetter J. A. Synergistic interaction between interleukin 4 and anti-Bp50 (CDw40) revealed in a novel B cell restimulation assay. Eur J Immunol. 1987 Oct;17(10):1535–1538. doi: 10.1002/eji.1830171026. [DOI] [PubMed] [Google Scholar]
- Graf D., Korthäuer U., Mages H. W., Senger G., Kroczek R. A. Cloning of TRAP, a ligand for CD40 on human T cells. Eur J Immunol. 1992 Dec;22(12):3191–3194. doi: 10.1002/eji.1830221226. [DOI] [PubMed] [Google Scholar]
- Hollenbaugh D., Grosmaire L. S., Kullas C. D., Chalupny N. J., Braesch-Andersen S., Noelle R. J., Stamenkovic I., Ledbetter J. A., Aruffo A. The human T cell antigen gp39, a member of the TNF gene family, is a ligand for the CD40 receptor: expression of a soluble form of gp39 with B cell co-stimulatory activity. EMBO J. 1992 Dec;11(12):4313–4321. doi: 10.1002/j.1460-2075.1992.tb05530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabara H. H., Fu S. M., Geha R. S., Vercelli D. CD40 and IgE: synergism between anti-CD40 monoclonal antibody and interleukin 4 in the induction of IgE synthesis by highly purified human B cells. J Exp Med. 1990 Dec 1;172(6):1861–1864. doi: 10.1084/jem.172.6.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthäuer U., Graf D., Mages H. W., Brière F., Padayachee M., Malcolm S., Ugazio A. G., Notarangelo L. D., Levinsky R. J., Kroczek R. A. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature. 1993 Feb 11;361(6412):539–541. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- Kruger G., Welte K., Ciobanu N., Cunningham-Rundles C., Ralph P., Venuta S., Feldman S., Koziner B., Wang C. Y., Moore M. A. Interleukin-2 correction of defective in vitro T-cell mitogenesis in patients with common varied immunodeficiency. J Clin Immunol. 1984 Jul;4(4):295–303. doi: 10.1007/BF00915297. [DOI] [PubMed] [Google Scholar]
- Lane P., Traunecker A., Hubele S., Inui S., Lanzavecchia A., Gray D. Activated human T cells express a ligand for the human B cell-associated antigen CD40 which participates in T cell-dependent activation of B lymphocytes. Eur J Immunol. 1992 Oct;22(10):2573–2578. doi: 10.1002/eji.1830221016. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., June C. H., Grosmaire L. S., Rabinovitch P. S. Crosslinking of surface antigens causes mobilization of intracellular ionized calcium in T lymphocytes. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1384–1388. doi: 10.1073/pnas.84.5.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L., Fu S. M., Cunningham-Rundles C., Kunkel H. G. Polyclonal immunoglobulin secretion in patients with common variable immunodeficiency using monoclonal B cell differentiation factors. J Clin Invest. 1984 Dec;74(6):2115–2120. doi: 10.1172/JCI111636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Sung S. S., Bjorndahl J. M., Fu S. M. Human T cell activation. IV. T cell activation and proliferation via the early activation antigen EA 1. J Exp Med. 1989 Mar 1;169(3):677–689. doi: 10.1084/jem.169.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noelle R. J., Ledbetter J. A., Aruffo A. CD40 and its ligand, an essential ligand-receptor pair for thymus-dependent B-cell activation. Immunol Today. 1992 Nov;13(11):431–433. doi: 10.1016/0167-5699(92)90068-I. [DOI] [PubMed] [Google Scholar]
- Noelle R. J., Roy M., Shepherd D. M., Stamenkovic I., Ledbetter J. A., Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama S., Farrington M., Ishida H., Howard M., Ochs H. D. Activated B cells from patients with common variable immunodeficiency proliferate and synthesize immunoglobulin. J Clin Invest. 1993 Sep;92(3):1282–1287. doi: 10.1172/JCI116701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama S., Hollenbaugh D., Aruffo A., Ledbetter J. A., Ochs H. D. B cell activation via CD40 is required for specific antibody production by antigen-stimulated human B cells. J Exp Med. 1993 Sep 1;178(3):1097–1102. doi: 10.1084/jem.178.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs H. D., Davis S. D., Wedgwood R. J. Immunologic responses to bacteriophage phi-X 174 in immunodeficiency diseases. J Clin Invest. 1971 Dec;50(12):2559–2568. doi: 10.1172/JCI106756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padayachee M., Feighery C., Finn A., McKeown C., Levinsky R. J., Kinnon C., Malcolm S. Mapping of the X-linked form of hyper-IgM syndrome (HIGM1) to Xq26 by close linkage to HPRT. Genomics. 1992 Oct;14(2):551–553. doi: 10.1016/s0888-7543(05)80270-8. [DOI] [PubMed] [Google Scholar]
- Preud'Homme J. L., Griscelli C., Seligmann M. Immunoglobulins on the surface of lymphocytes in fifty patients with primary immunodeficiency diseases. Clin Immunol Immunopathol. 1973 Jan;1(2):241–256. doi: 10.1016/0090-1229(73)90025-1. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H., Lefranc M. P., Stinson M. A., Sims J. E., Schroder J., Steinmetz M., Spurr N. L., Solomon E., Goodfellow P. N. The chromosomal location of T-cell receptor genes and a T cell rearranging gene: possible correlation with specific translocations in human T cell leukaemia. EMBO J. 1985 Jun;4(6):1461–1465. doi: 10.1002/j.1460-2075.1985.tb03803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Cooper M. D., Schlossman S. F., Rosen F. S. Abnormalities of T cell maturation and regulation in human beings with immunodeficiency disorders. J Clin Invest. 1981 Sep;68(3):699–705. doi: 10.1172/JCI110305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset F., Garcia E., Banchereau J. Cytokine-induced proliferation and immunoglobulin production of human B lymphocytes triggered through their CD40 antigen. J Exp Med. 1991 Mar 1;173(3):705–710. doi: 10.1084/jem.173.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset F., Garcia E., Defrance T., Péronne C., Vezzio N., Hsu D. H., Kastelein R., Moore K. W., Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki O., Ralph P., Cunningham-Rundles C., Good R. A. Three distinct stages of B-cell defects in common varied immunodeficiency. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6008–6012. doi: 10.1073/pnas.79.19.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira S. K., Vercelli D., Jabara H. H., Fu S. M., Geha R. S. Molecular analysis of the induction of immunoglobulin E synthesis in human B cells by interleukin 4 and engagement of CD40 antigen. J Exp Med. 1992 Jan 1;175(1):289–292. doi: 10.1084/jem.175.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal F. P., Siegal M., Good R. A. Suppression of B-cell differentiation by leukocytes from hypogammaglobulinemic patients. J Clin Invest. 1976 Jul;58(1):109–122. doi: 10.1172/JCI108439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneller M. C., Strober W. Abnormalities of lymphokine gene expression in patients with common variable immunodeficiency. J Immunol. 1990 May 15;144(10):3762–3769. [PubMed] [Google Scholar]
- Spickett G. P., Webster A. D., Farrant J. Cellular abnormalities in common variable immunodeficiency. Immunodefic Rev. 1990;2(3):199–219. [PubMed] [Google Scholar]
- Spriggs M. K., Armitage R. J., Strockbine L., Clifford K. N., Macduff B. M., Sato T. A., Maliszewski C. R., Fanslow W. C. Recombinant human CD40 ligand stimulates B cell proliferation and immunoglobulin E secretion. J Exp Med. 1992 Dec 1;176(6):1543–1550. doi: 10.1084/jem.176.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]
- Zhang K., Clark E. A., Saxon A. CD40 stimulation provides an IFN-gamma-independent and IL-4-dependent differentiation signal directly to human B cells for IgE production. J Immunol. 1991 Mar 15;146(6):1836–1842. [PubMed] [Google Scholar]
- de la Concha E. G., Oldham G., Webster A. D., Asherson G. L., Platts-Mills T. A. Quantitative measurements of T- and B-cell function in "variable" primary hypogammaglobulinaemia: evidence for a consistent B-cell defect. Clin Exp Immunol. 1977 Feb;27(2):208–215. [PMC free article] [PubMed] [Google Scholar]
- van den Elsen P., Shepley B. A., Borst J., Coligan J. E., Markham A. F., Orkin S., Terhorst C. Isolation of cDNA clones encoding the 20K T3 glycoprotein of human T-cell receptor complex. 1984 Nov 29-Dec 5Nature. 312(5993):413–418. doi: 10.1038/312413a0. [DOI] [PubMed] [Google Scholar]