Summary
Objective
During critical periods of brain development, both seizures and anticonvulsant medications can affect neurodevelopmental outcomes. In rodent models, many anticonvulsants trigger neuronal apoptosis. However, white matter apoptosis (WMA) has not been examined after anticonvulsant drug treatment. Here, we sought to determine if anticonvulsant drugs induced apoptosis in the developing white matter (WM) in rodent model.
Methods
Postnatal day (P)7 rats were treated with phenobarbital (PB-75), MK-801 (0.5), lamotrigine (LTG-20), carbamazepine (CBZ-100), phenytoin (PHT-50), levetiracetam (LEV-250) or saline; all doses are mg/kg. Brain tissue collected 24 h after treatment was stained using the TUNEL method. The number of degenerating cells within WM i.e. Anterior Commissure (AC), Corpus Callosum, Cingulum and Hippocampus-associated WM tracts was quantified.
Results
Saline-treated rats showed low baseline level of apoptosis in developing WM on P8 in all the areas examined. PB, PHT, and MK-801 significantly increased apoptosis in all 4 brain areas examined. Exposure to CBZ, LTG or LEV failed to increase apoptosis in all regions.
Significance
Commonly used anticonvulsants (PB, PHT) cause apoptosis in the developing WM in a rat model, the NMDA receptor antagonist MK-801 has a similar effect. These results are consistent with reports of anesthesia-induced WMA during brain development. Consistent with the lack of neuronal apoptosis caused by LTG, LEV and CBZ, these drugs did not not cause WMA. Many infants treated with anticonvulsant drugs have underlying neurological injury, including white matter damage (e.g., following intraventricular hemorrhage (IVH) or hypoxic ischemic encephalopathy (HIE)). The degree to which anticonvulsant drug treatment will alter outcomes in the presence of underlying injury remains to be examined, but avoiding drugs (when possible) that induce WMA may be beneficial.
Keywords: anticonvulsant, antiepileptic drug, apoptosis, cell death, neonatal
Introduction
The incidence of seizures during the neonatal period range from 1 to 3 per 1000 live births;1 these estimates are higher in premature and very low birth weight infants. Seizures in the newborn population arise from a variety of causes (e.g., intraventricular hemorrhage (IVH) or hypoxic ischemic encephalopathy (HIE)), and have been associated with poor neurodevelopmental outcome. Seizures in these populations are typically aggressively treated with anticonvulsant drugs.2
While data from animal models suggest that seizures may predispose the neonatal developing brain to damage,3 there is growing evidence that drugs used to treat these seizures may also damage the developing brain. For example, phenobarbital (PHB) and phenytoin (PHT), two of the most commonly utilized drugs for neonatal seizures2 trigger profound neuronal apoptosis in developing rodent models.4–8 A similar profile has been reported with other anticonvulsants, including valproate,4 which has been shown to produce long-term alterations after in utero exposure in humans.9 While the pruning of neurons by apoptosis is a normal component of brain development, several classes of common medications used in clinical neonatology significantly increase apoptosis. Sedatives (e.g., Midazolam, Diazepam),4,10 anesthetics (e.g. sevoflurane, isoflurane)11 and anticonvulsant drugs (e.g. phenobarbital, phenytoin)4–7 all trigger increased neuronal apoptosis in neonatal rats. It is worth noting that not all anticonvulsant drugs trigger this effect; for example, carbamazepine,6,7 topiramate,6,12 and lamotrigine5 avoid this effect at therapeutically relevant doses, only triggering apoptosis at supratherapeutic levels, or as part of polytherapy. Levetiracetam avoids induction of apoptosis even at supratherapeutic doses or as part of polytherapy.6
Neuronal apoptosis after neonatal exposure to PB and PHT has been observed in the multiple areas of the cortex, the thalamus, hypothalamus, and basal ganglia, amongst other areas.4,7 Apart from apoptosis, PB and PHT stunt synaptic maturation in the developing striatum.13 Moreover, these, and other anticonvulsant drugs trigger long-term alterations in behavioral function.13–16 These findings mirror clinical data showing that children with epilepsy (who are almost always aggressively treated with anticonvulsant drugs) have a variety of comorbid learning and neuropsychiatric conditions.17 Indeed, in animal models, phenobarbital exposure can actually worsen outcomes after repeated seizures in the prepubscent time period.18 However, the relative contribution of seizures, underlying etiology (e.g., hypoxic ischemic injury), and the per se effect of drugs can not be isolated in clinical populations.
Anesthetic agents such as isoflurane and sevoflurane injure not only the developing grey matter (e.g., trigger neuronal apoptosis) but also damage the developing white matter (e.g., oligodendrocyte apoptosis).19,20 In addition, exposure to these anesthetic agents in infancy and in the perinatal period has also been implicated in adverse neurological outcomes especially related to cognitive and behavioral domains. The effects on white matter remains wholly unexamined for anticonvulsant drugs, raising the possibility that white matter damage may contribute to the long-term behavioral and functional alterations reported after anticonvulsant treatment.
Here, we examined the white matter injury in otherwise normal neonatal rats with no underlying neurological condition. We selected postnatal day (P) 7 rats as a model of developing neonatal brain that corresponds to the period of brain growth in the late second trimester of pregnancy to the period after birth.21 This period also corresponds to the peak timing of anticonvulsant-induced neuronal apoptosis.4 We hypothesized that anticonvulsants that trigger neuronal apoptosis (e.g., phenobarbital, phenytoin) would also trigger apoptosis in the white matter, whereas treatments devoid of effect on neuronal apoptosis would likewise be benign with respect to the white matter changes.
Methods
Animals
Postnatal day (P)7, male and female Sprague-Dawley rat pups (Harlan, Indianapolis, IN, U.S.A.) were used. P7 was selected as this time point corresponds to the peak of the brain growth spurt in rodents and is equivalent to late third trimester/early life in humans.21 Treatments were counterbalanced within and across litters, as well as between sexes. Pups were born to timed-pregnant dams with P0 designated as the date of parturition. Animals were maintained in a temperature-controlled (21°C) room with a 12-h light cycle. Food (Lab Diet #5001) and water were available ad libitum. All experiments were approved by the Georgetown University Animal Care and Use Committee.
Drug treatments
Drug treatments were administered intraperitoneally at a volume of 0.01ml/g. Control groups received equivalent volumes of vehicle (0.01 ml/g body weight). Treatments occurred on P7, 24 h before sacrifice as in prior studies.5–7
Drug solutions
Phenobarbital sodium (75 mg/kg; Sigma-Aldrich) and MK-801 (0.5 mg/kg, Sigma-Aldrich) were dissolved in normal saline. Lamotrigine (20 mg/kg, GlaxoSmithKline) and carbamazepine (100 mg/kg, Sigma-Aldrich) were suspended in saline containing 1.0% Tween-80. Phenytoin (50 mg/kg, Sigma-Aldrich) was dissolved in alkalinized saline. Levetiracetam (250 mg/kg; Keppra Oral Solution, UCB Pharma) was diluted from stock in saline.
Selection of Drug Doses
The doses of phenobarbital, phenytoin, lamotrigine, carbamazepine and levetiracetam fell within the anticonvulsant dose range in neonatal rats.22–25 Moreover, the doses selected for phenobarbital, MK-801, and phenytoin trigger neuronal apoptosis in the developing rat brain. 4,5,7,26 By contrast, the doses selected of lamotrigine, carbamazepine and levetiracetam do not.5–7
Tissue preparation
Twenty-four hours after drug treatment, pups were decapitated, brains removed, and rapidly frozen in isopentane. Tissue was stored at −80°C until cryosectioning. 20 μm thick sections were stained using the TUNEL (Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling) method (ApopTag, in situ apoptosis detection kit) per the manufacturer’s instructions as we have previously described.5–7 This method of detecting apoptosis labels cells in the late stages of degeneration (i.e., those with DNA fragmentation). Several other studies have employed alternative methods to document that the degeneration caused by anticonvulsants/anesthetics is indeed apoptotic (i.e., caspase-mediated).13,19,27 Positive (e.g., phenytoin) and negative controls (i.e., vehicle) were included in each batch of staining.
Microscopy
Photomicrographs (10x and 4x) were collected on a Nikon 80i microscope with a Motic 5 mega-pixel camera. Images were acquired by two observers blinded to treatment status. Three photomicrographs, spaced at 200μm, were taken for each region.
Quantification of apoptosis within each region was performed by counting TUNEL-positive cells within anatomically-defined regions of interest. Fiber tracts were defined using a combination of the developmental atlases. Sections through the anterior commissure were selected anterior to its decussation. Sections through the corpus callosum, cingulum and hippocampal associated white matter tracts (stratum alveus/oriens and the ventral hippocampal commissure, fimbria, and dorsal fornix) were selected starting at the anterior hippocampus.
Image analysis was performed by investigators blinded to treatment, with a high inter-rated correlation (r = 0.80). Data used for statistical analyses were the counts of a single observer (SK). Counting was performed using IMAGEJ (National Institutes of Health, Bethesda, MD, U.S.A.), which was used to outline the regions of interest prior to counting.
Statistics
Statistical comparisons were performed using GraphPad Prism. Analysis of variance was used to determine differences amongst groups. Two-tailed P values less than 0.05 were considered statistically significant for Holm-Sidak-adjusted multiple comparison test results. Only the a priori hypotheses were tested (i.e., drug versus vehicle).
Results
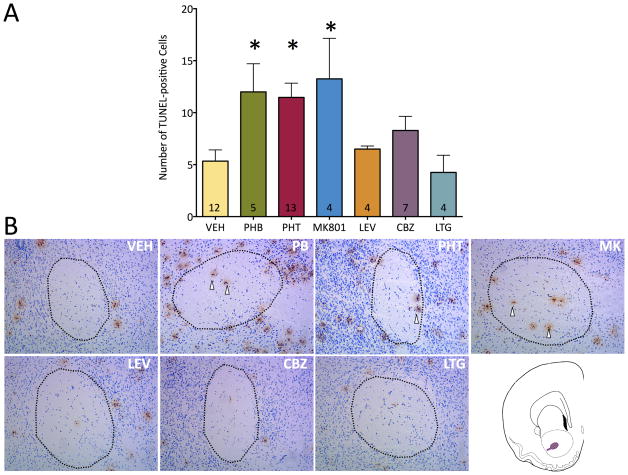
Vehicle-treated rats showed a low baseline level of apoptosis in the developing white matter on P8, i.e., 24 h after treatment. As shown in Figure 1, we first examined the profile of apoptosis within the anterior commissure, a major forebrain white matter bundle that serves as the principal connection between the amygdalae. When animals were treated with phenobarbital (75 mg/kg), the number of apoptotic cells within the anterior commissure was significantly elevated (2.3-fold). A similar elevation (2.2-fold, and 2.5-fold) was seen after treatment with phenytoin (50 mg/kg) or MK-801 (0.5 mg/kg). By contrast, exposure to carbamazepine (100 mg/kg), lamotrigine (20 mg/kg) or levetiracetam (250 mg/kg) failed to increase the number of apoptotic cells in the anterior commissure. These effects were revealed by analysis of variance, which showed a main effect of drug treatment (F6,42=3.71, P=0.0047). The elevation in cell death after phenobarbital, phenytoin, and MK-801 treatments, as compared to vehicle reached the level of statistical significance (Ps<0.05, Holm-Sidak test).
Figure 1. Neonatal exposure to phenobarbital, phenytoin or MK-801 induces apoptosis within the anterior commissure.
(A) Number of TUNEL-positive cells in the anterior commissure. Bars indicate mean + SEM. * = significantly increased white matter apoptosis above vehicle (ANOVA and Holm-Sidak test p<0.05). Numbers within the bars indicate the number of specimens analyzed. (B) Apoptosis noted in the anterior commissure as outlined by the black dotted line. Sections depicted are representative of the findings after treatment with anticonvulsants labeled. Anterior commissure is outlined in purple in the schematic in (B). Vehicle or control used is saline. White arrows denote apoptotic cells stained with TUNEL method. AC: Anterior commissure, PB: phenobarbital, PHT: phenytoin, MK: MK-801 NMDA receptor antagonist, LEV: levetiracetam, CBZ: carbamazepine, LTG: lamotrigine
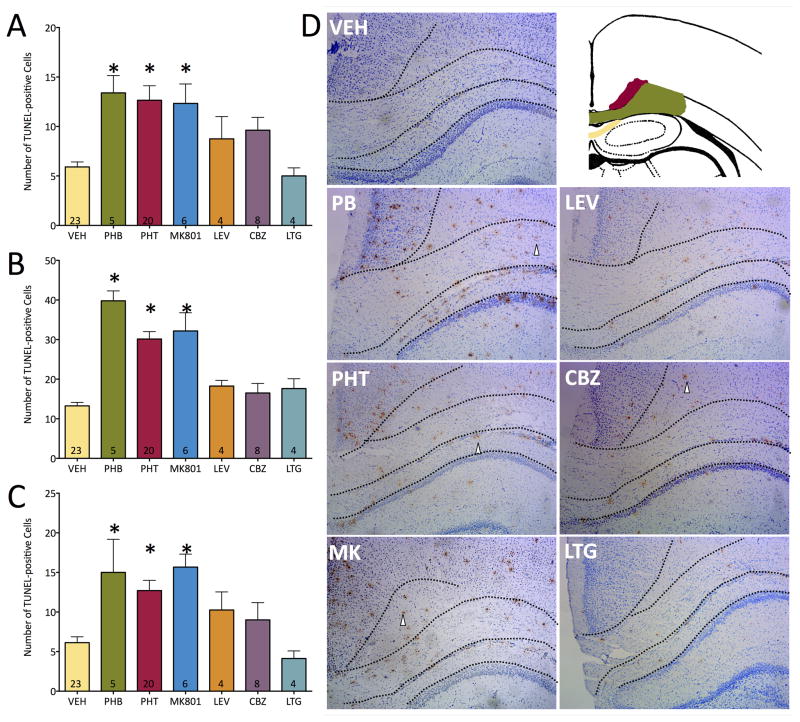
We next examined the profile of apoptosis in the cingulum (cingulate bundle). This fiber bundle contains a diversity of projections associated with the cingulate cortex, including connections with the thalamus, prefrontal cortex, insula, temporal cortex, amygdala. Moreover, this bundle contains projections from several modulatory neurotransmitter systems, including cholinergic fibers originating in the nucleus basalis, as well as ascending monoaminergic projections (dopamine, norepinephrine, and serotonin). This structure thus plays a major role in regulation of the limbic system. As in the anterior commissure, we found vehicle-treated pups to display a low baseline level of apoptosis within the developing cingulum. As shown in Figure 2A and D, phenobarbital induced a 2.3-fold increase in apoptosis; MK-801 and phenytoin both induced a 2.1-fold increase in apoptosis. Neither carbamazepine, lamotrigine nor levetiracetam increased apoptosis in the cingulum. These effects were revealed by analysis of variance; there was a main effect of drug treatment (F6,63=5.73, P<0.0001). Holm-Sidak corrected post-tests showed that the increases found for phenobarbital, phenytoin, and MK-801 reached the level of statistical significance (Ps = 0.007, <0.0001, and 0.012, respectively).
Figure 2. Neonatal exposure to phenobarbital, phenytoin, or MK-801 induces apoptosis within the cingulum, corpus callosum, and hippocampal-associated white matter tracts.
(A) Number of TUNEL-positive cells in the cingulum. (B) Number of TUNEL-positive cells in the corpus callosum. (C) Number of TUNEL-positive cells in the hippocampal-associated white matter. Bars indicate mean + SEM. * = significantly increased white matter apoptosis above vehicle (ANOVA and Holm-Sidak test p<0.0001). Numbers within the bars indicate the number of specimens analyzed. (D) Representative photomicrographs and schematic of regions of interest for each drug treatment. The black dotted line segregates the WM areas. In the schematic, red denotes cingulum, green color denotes corpus callosum and the yellow area depicts the hippocampus associated white matter tracts (stratum alveus/oriens, ventral hippocampal commissure, fimbria, and dorsal fornix). The sections are representative of the findings after treatment with the anticonvulsants labeled. Vehicle (control) is saline. White arrows denote apoptotic cells stained with TUNEL method. PB: phenobarbital, PHT: phenytoin, MK: MK-801 NMDA receptor antagonist, LEV: levetiracetam, CBZ: carbamazepine, LTG: lamotrigine
We next turned our attention to the corpus callosum (Figure 2B and D). This fiber bundle, located immediately subjacent to the cingulum is the major pathway for interhemispheric cortico-cortical projections. As in the other regions, vehicle treated animals displayed a low level of baseline apoptosis in the corpus callosum. Phenobarbital increased apoptosis 3-fold, and phenytoin and MK-801 each increased apoptosis 2.4-fold over vehicle. These effects were revealed by analysis of variance. We found a main effect of drug treatment (F6,63=20.58, P<0.0001). This effect was driven by significant elevations in apoptosis caused by phenobarbital, phenytoin and MK-801 (Ps <0.0001, Holm-Sidak test).
Finally, we examined the induction of apoptosis within the fiber tracts associated with the hippocampus (Figure 2C and D). Segregation of these fibers (located within the stratum alveus/oriens and the ventral hippocampal commissure, fimbria, and dorsal fornix) was not possible with our preparation, and we refer to them collectively as hippocampal associated white matter tracts. We found a low level of baseline apoptosis within this region (i.e., in vehicle treated animals) which was amplified 2.4-fold by phenobarbital, 2.1-fold by phenytoin, and 2.6-fold by MK-801. Neither lamotrigine, levetiracetam, nor carbamazepine increased the number of apoptotic cells in this region. Analysis of variance revealed a main effect of drug treatment (F6,63=5.96, P<0.0001), with Holm-Sidak post-tests showing that the elevation caused by MK-801, phenobarbital, and phenytoin reached the level of statistical significance (Ps = 0.009, 0.0036, and 0.0006, respectively).
Discussion
In this study we found that several anticonvulsant drugs commonly used to treat seizures in both pregnancy and in the neonatal period increased apoptosis within white matter tracts of the developing rat brain. In particular, phenobarbital and phenytoin triggered cell death in anterior commissure, corpus callosum, cingulum, and hippocampal-associated white matter tracts. A similar profile was found for the NMDA receptor antagonist, MK-801. By contrast, lamotrigine, levetiracetam and carbamazepine did not induce apoptosis in the developing white matter. Across drugs, the profile of white matter apoptosis mirrored that previously reported in the grey matter, i.e., those drugs that trigger grey matter apoptosis also triggered white matter apoptosis, although the fold increase in WM apoptosis was smaller than that previously reported in the GM.5–7,26 In the regions we examined, the pattern across WM regions was “all or none”; those drugs that induced significant WM apoptosis in one region did so in all regions examined. It remains to be seen if this pattern is true for other white matter tracts, such as those in the cerebellum. As has been reported for GM apoptosis, the extent of apoptosis differs based on region; in the present study, the absolute magnitude of increase was largest within the corpus callosum. It is worth noting that WM apoptosis does not appear to be more sensitive to proapoptic effects as compared to GM apoptosis. For example, the therapeutically-relevant dose of lamotrigine selected is below the threshold for induction of GM apoptosis, and it did not induce WM apoptosis. These data extend our knowledge of the mechanisms of injury caused by a subset of anticonvulsant drugs in the developing (neonatal) brain.
The P7 rat pups we used in the present study model a period of development extending from the late third trimester through early life in humans. Thus, our findings may be relevant both to gestational (in utero) exposure as well as postnatal exposure to anticonvulsants (as a treatment for seizures following neonatal neurological insult). Gestational exposure to some anticonvulsants has been associated with fetal malformation as well as a variety of neurocognitive deficits. 28
Premyelinating oligodendroglia are vulnerable to a number of factors, including hypoperfusion and local or systemic cytokine-related injury,29 anesthesia,19 and hyperoxia.30 In the present study, we did not assess the cell types impacted by our drug treatments. This was in large part due to the timing of our histological procedures; animals survived 24 h after drug treatment, allowing us to detect the late stages of apoptosis, a time by which many markers of cell lineage are likely cleared or destroyed. In support of this, we found relatively low degree of co-localization in a subset of tissue double stained for fraction and myelin basic protein (data not shown); this is consistent with the report of Brambrink and colleagues who found that late stage degeneration was associated with low abundance of co-localization between these markers, whereas early degeneration was associated with high co-localization.31 Indeed, after anesthesia exposure, within the white matter, oligodendrocytes are preferentially lost, as opposed to microglia or astrocytes. The degree to which various cell types within the WM are affected by anticonvulsant exposure therefore remains to be examined, ideally with shorter survival times than we employed in the present study.
Our present findings are consistent with a growing body of literature regarding white matter damage after early-life anesthesia exposure. For example, volatile anesthetics including sevoflurane and isoflurane trigger increased white matter apoptosis in the developing non-human primate brain.19 A similar pattern has been reported with propofol32 and NMDA receptor antagonist ketamine.20 It is worth noting that ketamine shares the same mechanism of action as MK-801. In addition to white matter injury, anesthetics also cause oxidative stress, apoptosis in the developing grey matter and long-term effects on behavior, learning, and memory.27,33,34
It is now well-established that many anticonvulsant drugs trigger apoptosis in the developing grey matter; excessive pruning of neurons has been reported after exposure to phenobarbital, phenytoin, valproate, vigabatrin, diazepam, clonazepam, and high doses of lamotrigine in rodent models of the perinatal period.4–7 Carbamazepine, levetiracetam, and clinically-relevant doses of lamotrigine avoid this effect.5–7 Above and beyond enhanced neuronal apoptosis, several of these drugs have been shown to cause long-term disruptions in striatal synaptic development,13 alterations in the cortical proteome35 as well as the induction of long-lasting behavioral changes and learning deficits.14–16,36–38
The present findings add another potential mechanism by which these drugs may exert long-lasting effects. The anterior commissure is the major route of information transfer between the amygdalae, which is key to fear learning and social behavior. Similarly, the corpus callosum is the major route of cortico-cortical information transfer between the cerebral hemispheres. Finally, the cingulum and hippocampal-associated white matter tracts are critical contributors to the circuit of Papez and the limbic system, which has been implicated in memory processing. Indeed, deficits in all of these behavioral domains have been reported after anticonvulsants in neonatal rodents.14–16,36–38
Only one study has examined the effects of developmental anticonvuslant exposure on structural brain development. Ikonomidou and colleagues39 examined brain volumes using a voxel-based morphometry approach in offspring of women with epilepsy. These individuals were exposed to a range of anticonvuslant drugs during gestation, including phenobarbital, phenytoin, carbamazepine, valproate, ethosuximide, and primidone. Some subjects were exposed to monotherapy, others to polytherapy. It is worth noting that gestational exposure to valproate,9 and neonatal exposure to phenobarbital have both been associated with cognitive deficits in humans.40 While the authors reported a significant decrease in volume of the putamen, pallidum, and hypothalamus in these subjects, they failed to detect significant changes in white matter. However, it is important to note that the authors stated, “Because there was not an a priori hypothesis for the white matter analysis, only voxels [in the white matter] surviving the correction for multiple comparisons at p<0.05 were considered significant.” By contrast, the authors examined grey matter regions (basal ganglia and hypothalamus) with a less restrictive threshold based on the a priori hypothesis of decreased volume in these areas. This a priori hypothesis was the result of studies in animal models demonstrating increased apoptosis in these regions after perinatal anticonvulsant exposure. Had preclinical data existed indicating white matter apoptosis triggered by anticonvulsant drugs, the outcome of the above referenced study may have differed. This is one of several explanations that may explain the apparent discrepancy between their findings and the present study. At least two other factors may have contributed: 1) the magnitude of anticonvulsant-induced apoptosis in most grey matter regions is equal to or larger than what we report in white matter (2–3 times baseline apoptosis; see results section). For example, in the study of Bittigau et al., P7 phenobarbital exposure triggered an average 6.4-fold increase in GM apoptosis across regions examined. This was as large as 33-fold in the ventromedial hypothalamus,4 one of the regions identified in imaging study. Similarly, 3 to 5 fold increases have been reported in other studies.5–8 2) The diversity of drug exposure in the Ikonomidou study, which may have reflected difficulty in recruiting participants, included carbamazepine, which does not cause white matter apoptosis. A final possibility, which merits further exploration, is that deficits in white matter induced by perinatal anticonvulsant exposure may be overcome by compensatory proliferation in the period after exposure. Arguing against this possibility are data from fetal alcohol spectrum disorders, which are associated with long-term alterations in the shape and volume of corpus callosum, as well as decreased fractional anisotropy in the corpus callosum (as reviewed in 41). Indeed, neonatal alcohol exposure, like neonatal anticonvulsant and anesthesia exposure, is associated with increased neuronal apoptosis.42 Thus, while our present study cannot directly comment on the long-term effects of white matter damage triggered by anticonvulsant drugs, it does provide a strong rationale for future focused examination of white matter volume, as well as white matter integrity using diffusion tensor imaging approaches in individuals exposed to anticonvulsants during brain development.
WMI is associated with behavioral deficits, learning impairment, cognitive abnormalities, and motor deficits in longitudinal follow up studies in clinical populations, including those with HIE 43. Moreover, overt white matter changes such as periventricular cystic leukomalacia is a strong predictor of future neurological impairment, although it is becoming rare.44 The more common diffuse (noncystic) WM injury is characterized by loss of early differentiating oligodendrocytes, astrogliosis, subsequent impairment of myelination, and thinning of corpus callosum. These changes are associated with long-term neurodevelopmental and cognitive impairments in middle to late childhood.43 Thus, the potential contribution of subtle changes in WM to long term neurodevelopmental impairments is gaining appreciation. The impact of subtle changes in WM structure on long-term behavior again underscores the importance of screening for potential anticonvulsant-induced WM damage in humans.
The associations noted between white matter injury and adverse outcomes in both human subjects and animal models provided a justification for us to look for white matter injury in rodents treated with anticonvulsant drugs. HIE and periventricular leukomalacia are frequent causes of both neonatal seizures and white matter damage.44 While the development of therapeutic hypothermia has provided neuroprotection and had a major impact on improving the neurodevelopmental outcome in HIE, seizures remain a significant problem in this population. A large population of infants with HIE continue to be exposed to phenobarbital and phenytoin along with other anticonvulsant drugs during the neonatal period. The adverse neurological outcomes associated with HIE/PVL are typically thought of as a consequence of frank neuronal injury. Others have suggested that the repeated seizures associated with these conditions are, in fact, the cause of adverse outcomes.17 A third possibility, that is gaining strong preclinical support, is that the drugs used to treat the seizures contributes to these adverse outcomes. Indeed, for infants with already compromised white matter, the degree to which drug exposure may exacerbate underlying injury remains important to explore. At the present time, we cannot conclusively associate long-term deficits with either grey matter or white matter apoptosis. This is in part due to the fact that a complete profile of behavioral effects of anticonvulsant exposure during brain development remains to be completed. WM damage caused by anticonvulsants cannot be considered in isolation, as these drugs cause a host of other changes, including loss of neurons and changes in synaptic function. Here, we add white matter injury to the potential list of contributing factors. There are many anticonvulsant drugs in current clinical use which have not been tested for induction of either neuronal or white matter apoptosis in the immature brain. This is a critical area for future research.45
Conclusions
Increased white matter apoptosis was seen in rat pups in the vulnerable period of rapid brain growth corresponding to the perinatal and neonatal period in humans when treated with phenobarbital, phenytoin and NMDA receptor antagonist MK-801. Carbamazepine, lamotrigine, and levetiracetam were devoid of this effect. These finding mirror the apoptosis that has been demonstrated in the grey matter in the same animal model. An important next step will be to elucidate the cell types that are affected in the white matter. White matter injury has been implicated in long term adverse neurodevelopmental outcomes both in the clinical world and animal research models. Here we have described another source of WMI, which may contribute to adverse outcomes. These findings may be relevant to the treatment of neonates diagnosed with seizures, as well as those exposed to anticonvulsants in utero (maternal use). Given the lack of clinical data regarding effects (or lack thereof) of anticonvulsant drugs on white matter development, our present findings indicate the importance and clear need for such studies in the future.
Key Point Box.
Phenobarbital and phenytoin exposure induces white matter apoptosis in P7 rats
NMDA receptor antagonist (MK-801) induces white matter apoptosis in P7 rats
Levetiracetam, carbamazepine and lamotrigine did not induce significant white matter apoptosis in P7 rats
White matter injury after anticonvulsant exposure may contribute to adverse neurological outcomes
Acknowledgments
PAF was supported in part by KL2TR001432 and T32HD046388.
Footnotes
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Conflicts of Interest
None of the authors has any conflict of interest to disclose.
References
- 1.Ronen GM, Penney S, Andrews W. The epidemiology of clinical neonatal seizures in Newfoundland: a population-based study. J Pediatr. 1999;134:71–5. doi: 10.1016/s0022-3476(99)70374-4. [DOI] [PubMed] [Google Scholar]
- 2.Bartha AI, Shen J, Katz KH, et al. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol. 2007;37:85–90. doi: 10.1016/j.pediatrneurol.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Holmes GL, Gairsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann Neurol. 1998;44:845–57. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- 4.Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A. 2002;99:15089–94. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz I, Kim J, Gale K, Kondratyev A. Effects of lamotrigine alone and in combination with MK-801, phenobarbital, or phenytoin on cell death in the neonatal rat brain. J Pharmacol Exp Ther. 2007;322:494–500. doi: 10.1124/jpet.107.123133. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Kondratyev A, Gale K. Antiepileptic drug-induced neuronal cell death in the immature brain: effects of carbamazepine, topiramate, and levetiracetam as monotherapy versus polytherapy. J Pharmacol Exp Ther. 2007;323:165–73. doi: 10.1124/jpet.107.126250. [DOI] [PubMed] [Google Scholar]
- 7.Forcelli PA, Kim J, Kondratyev A, Gale K. Pattern of antiepileptic drug-induced cell death in limbic regions of the neonatal rat brain. Epilepsia. 2011;52:e207–11. doi: 10.1111/j.1528-1167.2011.03297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown L, Gutherz S, Kulick C, Soper C, Kondratyev A, Forcelli P. Profile of retigabine-induced neuronal apoptosis in the developing rat brain. Epilepsia. doi: 10.1111/epi.13335. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meador KJ, Baker GA, Browning N, et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244–52. doi: 10.1016/S1474-4422(12)70323-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young C, Jevtovic-Todorovic V, Qin Y-Q, et al. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol. 2005;146:189–97. doi: 10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loepke AW, Istaphanous GK, McAuliffe JJ, 3rd, et al. The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg. 2009;108:90–104. doi: 10.1213/ane.0b013e31818cdb29. [DOI] [PubMed] [Google Scholar]
- 12.Glier C, Dzietko M, Bittigau P, Jarosz B, Korobowicz E, Ikonomidou C. Therapeutic doses of topiramate are not toxic to the developing rat brain. Exp Neurol. 2004;187:403–9. doi: 10.1016/j.expneurol.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Forcelli PA, Janssen MJ, Vicini S, Gale K. Neonatal exposure to antiepileptic drugs disrupts striatal synaptic development. Ann Neurol. 2012;72:363–72. doi: 10.1002/ana.23600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhardwaj SK, Forcelli PA, Palchik G, Gale K, Srivastava LK, Kondratyev A. Neonatal exposure to phenobarbital potentiates schizophrenia-like behavioral outcomes in the rat. Neuropharmacology. 2012;62:2337–45. doi: 10.1016/j.neuropharm.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutherz SB, Kulick CV, Soper C, Kondratyev A, Gale K, Forcelli PA. Brief postnatal exposure to phenobarbital impairs passive avoidance learning and sensorimotor gating in rats. Epilepsy Behav. 2014;37:265–9. doi: 10.1016/j.yebeh.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frankel S, Medvedeva N, Gutherz S, Kulick C, Kondratyev A, Forcelli P. Comparison of the long-term behavioral effects of neonatal exposure to retigabine or phenobarbital in rats. Epilepsy & Behavior. 2016 doi: 10.1016/j.yebeh.2016.01.018. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russ SA, Larson K, Halfon N. A national profile of childhood epilepsy and seizure disorder. Pediatrics. 2012;129:256–64. doi: 10.1542/peds.2010-1371. [DOI] [PubMed] [Google Scholar]
- 18.Mikati MA, Holmes GL, Chronopoulos A, et al. Phenobarbital modifies seizure-related brain injury in the developing brain. Ann Neurol. 1994;36:425–33. doi: 10.1002/ana.410360314. [DOI] [PubMed] [Google Scholar]
- 19.Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–41. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brambrink AM, Evers AS, Avidan MS, et al. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012;116:372–84. doi: 10.1097/ALN.0b013e318242b2cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 22.Bernásková K, Mares P. Similar effects of lamotrigine and phenytoin against cortical epileptic foci in immature rats. Physiol Res. 2010;59:113–9. doi: 10.33549/physiolres.931563. [DOI] [PubMed] [Google Scholar]
- 23.Kulick CV, Gutherz SB, Beck VC, Medvedeva N, Soper C, Forcelli PA. Profile of anticonvulsant action of levetiracetam, tiagabine and phenobarbital against seizures evoked by DMCM (methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate) in neonatal rats. Eur J Pharmacol. 2014;743:63–8. doi: 10.1016/j.ejphar.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forcelli PA, Soper C, Duckles A, Gale K, Kondratyev A. Melatonin potentiates the anticonvulsant action of phenobarbital in neonatal rats. Epilepsy Res. 2013;107:217–23. doi: 10.1016/j.eplepsyres.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubová H, Mares P. Anticonvulsant action of oxcarbazepine, hydroxycarbamazepine, and carbamazepine against metrazol-induced motor seizures in developing rats. Epilepsia. 1993;34:188–92. doi: 10.1111/j.1528-1157.1993.tb02397.x. [DOI] [PubMed] [Google Scholar]
- 26.Ikonomidou C, Bosch F, Miksa M, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–4. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 27.Yon J, Carter L, Reiter R, Jevtovic-Todorovic V. Melatonin reduces the severity of anesthesia-induced apoptotic neurodegeneration in the developing rat brain. Neurobiol Dis. 2006;21:522–30. doi: 10.1016/j.nbd.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Meador KJ, Baker GA, Finnell RH, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407–12. doi: 10.1212/01.wnl.0000227919.81208.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haynes RL, Folkerth RD, Keefe RJ, et al. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62:441–50. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz T, Ritter J, Mueller S, Felderhoff-Mueser U, Chew L-J, Gallo V. Cellular changes underlying hyperoxia-induced delay of white matter development. J Neurosci. 2011;31:4327–44. doi: 10.1523/JNEUROSCI.3942-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Creeley CE, Dikranian KT, Dissen GA, Back SA, Olney JW, Brambrink AM. Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology. 2014;120:626–38. doi: 10.1097/ALN.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creeley C, Dikranian K, Dissen G, Martin L, Olney J, Brambrink A. Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth. 2013;110(Suppl 1):i29–38. doi: 10.1093/bja/aet173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raper J, Alvarado MC, Murphy KL, Baxter MG. Multiple Anesthetic Exposure in Infant Monkeys Alters Emotional Reactivity to an Acute Stressor. Anesthesiology. 2015;123:1084–92. doi: 10.1097/ALN.0000000000000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaindl AM, Koppelstaetter A, Nebrich G, et al. Brief alteration of NMDA or GABAA receptor-mediated neurotransmission has long term effects on the developing cerebral cortex. Mol Cell Proteomics. 2008;7:2293–310. doi: 10.1074/mcp.M800030-MCP200. [DOI] [PubMed] [Google Scholar]
- 36.Forcelli PA, Gale K, Kondratyev A. Early postnatal exposure of rats to lamotrigine, but not phenytoin, reduces seizure threshold in adulthood. Epilepsia. 2011;52:e20–2. doi: 10.1111/j.1528-1167.2010.02971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forcelli PA, Kozlowski R, Snyder C, Kondratyev A, Gale K. Effects of neonatal antiepileptic drug exposure on cognitive, emotional, and motor function in adult rats. J Pharmacol Exp Ther. 2012;340:558–66. doi: 10.1124/jpet.111.188862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mikulecká A, Subrt M, Stuchlík A, Kubová H. Consequences of early postnatal benzodiazepines exposure in rats. I. Cognitive-like behavior. Front Behav Neurosci. 2014;8:101. doi: 10.3389/fnbeh.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikonomidou C, Scheer I, Wilhelm T, et al. Brain morphology alterations in the basal ganglia and the hypothalamus following prenatal exposure to antiepileptic drugs. Eur J Paediatr Neurol. 2007;11:297–301. doi: 10.1016/j.ejpn.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Farwell JR, Lee YJ, Hirtz DG, Sulzbacher SI, Ellenberg JH, Nelson KB. Phenobarbital for febrile seizures--effects on intelligence and on seizure recurrence. N Engl J Med. 1990;322:364–9. doi: 10.1056/NEJM199002083220604. [DOI] [PubMed] [Google Scholar]
- 41.Donald KA, Eastman E, Howells FM, et al. Neuroimaging effects of prenatal alcohol exposure on the developing human brain: a magnetic resonance imaging review. Acta Neuropsychiatr. 2015;27:251–69. doi: 10.1017/neu.2015.12. [DOI] [PubMed] [Google Scholar]
- 42.Ikonomidou C, Bittigau P, Ishimaru MJ, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–60. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- 43.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–94. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 44.Volpe JJ. Neurology of the newborn. 5. Philadelphia: Saunders/Elsevier; 2008. p. 1094. [Google Scholar]
- 45.Meador KJ, Loring DW. Developmental effects of antiepileptic drugs and the need for improved regulations. Neurology. 2015 doi: 10.1212/WNL.0000000000002119. [DOI] [PMC free article] [PubMed] [Google Scholar]