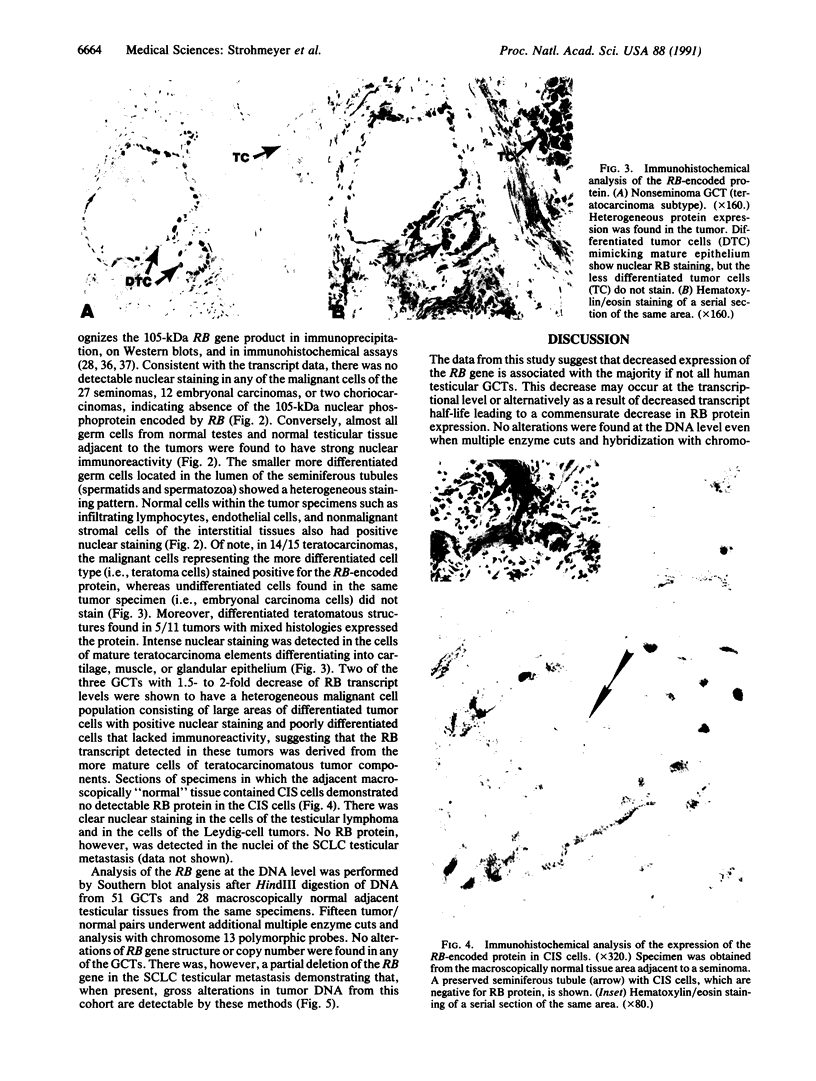
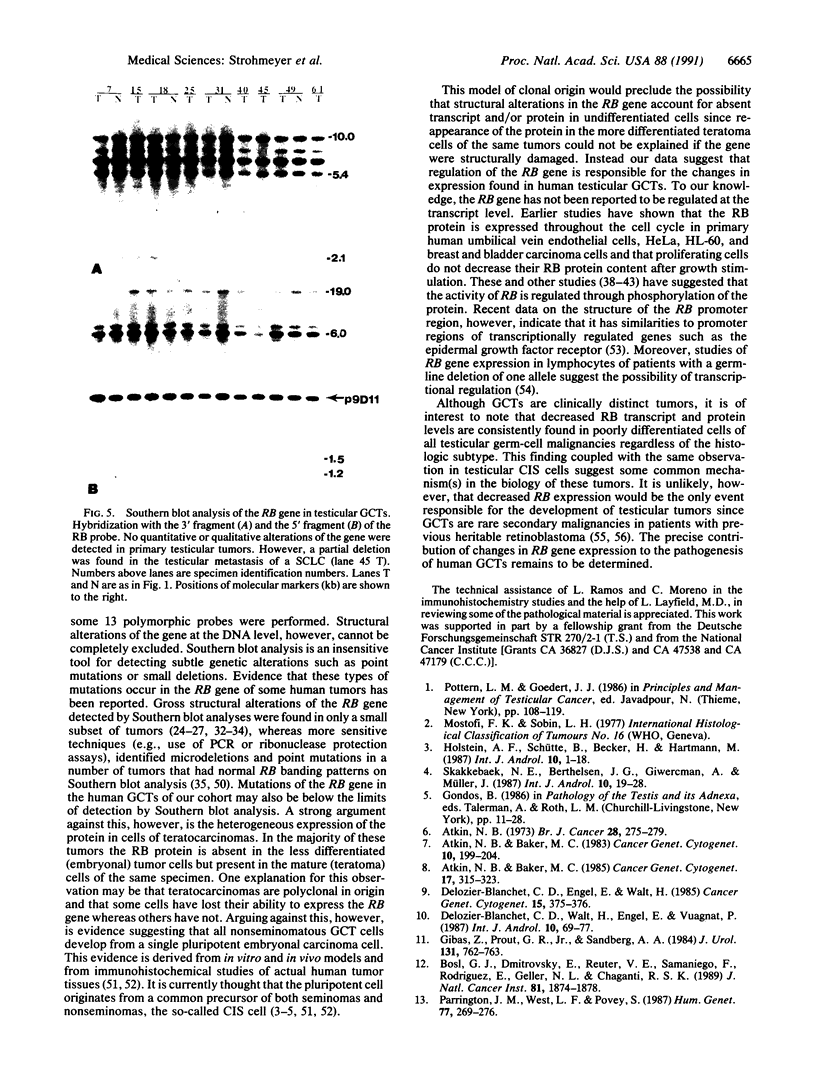
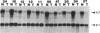Abstract
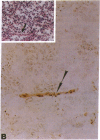
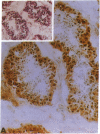
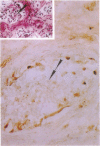
Inactivation of the retinoblastoma gene (RB gene) is associated with the development of several human malignancies including retinoblastomas, some osteo- and soft tissue sarcomas, small cell lung cancer, and possibly breast and bladder cancers. To our knowledge, this gene has not been evaluated in human germ-cell malignancies. In this study 67 primary testicular germ-cell tumors and 4 testicular non-germ-cell malignancies were examined to determine the prevalence and nature of RB gene alterations. Decreased expression of RB gene mRNA was found in all testicular germ-cell tumors (both seminomas and nonseminomas) examined. The RB protein could not be detected by immunohistochemical analysis in the undifferentiated cells of any germ-cell tumors whereas the differentiated malignant cells present in 14/15 teratocarcinomas expressed the protein. No gross alterations of the RB gene were found at DNA level in any of the examined specimens. This and the presence of the RB protein in the more differentiated tumor cells of teratocarcinomas suggest that changes in transcript levels rather than mutation(s) of the gene may be responsible for the absent or decreased RB expression in human germ-cell tumors. To date studies on the mechanism of RB regulation have demonstrated that it occurs at the protein level by phosphorylation of the p105 gene product. The findings presented here indicate that additional regulation might occur at the transcript level.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson D. H., Ellsworth R. M., Kitchin F. D., Tung G. Second nonocular tumors in retinoblastoma survivors. Are they radiation-induced? Ophthalmology. 1984 Nov;91(11):1351–1355. doi: 10.1016/s0161-6420(84)34127-6. [DOI] [PubMed] [Google Scholar]
- Atkin N. B., Baker M. C. Chromosome analysis of three seminomas. Cancer Genet Cytogenet. 1985 Aug;17(4):315–323. doi: 10.1016/0165-4608(85)90115-3. [DOI] [PubMed] [Google Scholar]
- Atkin N. B., Baker M. C. i(12p): specific chromosomal marker in seminoma and malignant teratoma of the testis? Cancer Genet Cytogenet. 1983 Oct;10(2):199–204. doi: 10.1016/0165-4608(83)90125-5. [DOI] [PubMed] [Google Scholar]
- Atkin N. B. High chromosome numbers of seminomata and malignant teratomata of the testis: a review of data on 103 tumours. Br J Cancer. 1973 Sep;28(3):275–279. doi: 10.1038/bjc.1973.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban G., Gilbert F., Nichols W., Meadows A. T., Shields J. Abnormalities of chromosome #13 in retinoblastomas from individuals with normal constitutional karyotypes. Cancer Genet Cytogenet. 1982 Jul;6(3):213–221. doi: 10.1016/0165-4608(82)90058-9. [DOI] [PubMed] [Google Scholar]
- Bookstein R., Shew J. Y., Chen P. L., Scully P., Lee W. H. Suppression of tumorigenicity of human prostate carcinoma cells by replacing a mutated RB gene. Science. 1990 Feb 9;247(4943):712–715. doi: 10.1126/science.2300823. [DOI] [PubMed] [Google Scholar]
- Bosl G. J., Dmitrovsky E., Reuter V. E., Samaniego F., Rodriguez E., Geller N. L., Chaganti R. S. Isochromosome of the short arm of chromosome 12: clinically useful markers for male germ cell tumors. J Natl Cancer Inst. 1989 Dec 20;81(24):1874–1878. doi: 10.1093/jnci/81.24.1874. [DOI] [PubMed] [Google Scholar]
- Buchkovich K., Duffy L. A., Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989 Sep 22;58(6):1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Cance W. G., Brennan M. F., Dudas M. E., Huang C. M., Cordon-Cardo C. Altered expression of the retinoblastoma gene product in human sarcomas. N Engl J Med. 1990 Nov 22;323(21):1457–1462. doi: 10.1056/NEJM199011223232105. [DOI] [PubMed] [Google Scholar]
- Cavenee W., Leach R., Mohandas T., Pearson P., White R. Isolation and regional localization of DNA segments revealing polymorphic loci from human chromosome 13. Am J Hum Genet. 1984 Jan;36(1):10–24. [PMC free article] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Lynch D., Furukawa Y., Griffin J., Piwnica-Worms H., Huang C. M., Livingston D. M. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989 Sep 22;58(6):1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- Delozier-Blanchet C. D., Engel E., Walt H. Isochromosome 12p in malignant testicular tumors. Cancer Genet Cytogenet. 1985 Feb 15;15(3-4):375–376. doi: 10.1016/0165-4608(85)90182-7. [DOI] [PubMed] [Google Scholar]
- Delozier-Blanchet C. D., Walt H., Engel E., Vuagnat P. Cytogenetic studies of human testicular germ cell tumours. Int J Androl. 1987 Feb;10(1):69–77. doi: 10.1111/j.1365-2605.1987.tb00167.x. [DOI] [PubMed] [Google Scholar]
- Dunn J. M., Phillips R. A., Becker A. J., Gallie B. L. Identification of germline and somatic mutations affecting the retinoblastoma gene. Science. 1988 Sep 30;241(4874):1797–1800. doi: 10.1126/science.3175621. [DOI] [PubMed] [Google Scholar]
- Dunn J. M., Phillips R. A., Zhu X., Becker A., Gallie B. L. Mutations in the RB1 gene and their effects on transcription. Mol Cell Biol. 1989 Nov;9(11):4596–4604. doi: 10.1128/mcb.9.11.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Horowitz J. M., Gerber M. R., Wang X. F., Bogenmann E., Li F. P., Weinberg R. A. Deletions of a DNA sequence in retinoblastomas and mesenchymal tumors: organization of the sequence and its encoded protein. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9059–9063. doi: 10.1073/pnas.84.24.9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Murphree A. L., T'Ang A., Qian J., Hinrichs S. H., Benedict W. F. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987 Jun 26;236(4809):1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Gibas Z., Prout G. R., Jr, Sandberg A. A. Malignant teratoma of the testis with an isochromosome no. 12, i(12p), as the sole structural cytogenetic abnormality. J Urol. 1984 Apr;131(4):762–763. doi: 10.1016/s0022-5347(17)50613-8. [DOI] [PubMed] [Google Scholar]
- Hansen M. F., Koufos A., Gallie B. L., Phillips R. A., Fodstad O., Brøgger A., Gedde-Dahl T., Cavenee W. K. Osteosarcoma and retinoblastoma: a shared chromosomal mechanism revealing recessive predisposition. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6216–6220. doi: 10.1073/pnas.82.18.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour J. W., Lai S. L., Whang-Peng J., Gazdar A. F., Minna J. D., Kaye F. J. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988 Jul 15;241(4863):353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein A. F., Schütte B., Becker H., Hartmann M. Morphology of normal and malignant germ cells. Int J Androl. 1987 Feb;10(1):1–18. doi: 10.1111/j.1365-2605.1987.tb00160.x. [DOI] [PubMed] [Google Scholar]
- Horowitz J. M., Yandell D. W., Park S. H., Canning S., Whyte P., Buchkovich K., Harlow E., Weinberg R. A., Dryja T. P. Point mutational inactivation of the retinoblastoma antioncogene. Science. 1989 Feb 17;243(4893):937–940. doi: 10.1126/science.2521957. [DOI] [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr, Meadows A. T., Nichols W. W., Hill R. Chromosomal deletion and retinoblastoma. N Engl J Med. 1976 Nov 11;295(20):1120–1123. doi: 10.1056/NEJM197611112952007. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., To H., Shew J. Y., Bookstein R., Scully P., Lee W. H. Inactivation of the retinoblastoma susceptibility gene in human breast cancers. Science. 1988 Jul 8;241(4862):218–221. doi: 10.1126/science.3388033. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Shew J. Y., Hong F. D., Sery T. W., Donoso L. A., Young L. J., Bookstein R., Lee E. Y. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987 Oct 15;329(6140):642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- Ludlow J. W., DeCaprio J. A., Huang C. M., Lee W. H., Paucha E., Livingston D. M. SV40 large T antigen binds preferentially to an underphosphorylated member of the retinoblastoma susceptibility gene product family. Cell. 1989 Jan 13;56(1):57–65. doi: 10.1016/0092-8674(89)90983-5. [DOI] [PubMed] [Google Scholar]
- Meadows A. T., Baum E., Fossati-Bellani F., Green D., Jenkin R. D., Marsden B., Nesbit M., Newton W., Oberlin O., Sallan S. G. Second malignant neoplasms in children: an update from the Late Effects Study Group. J Clin Oncol. 1985 Apr;3(4):532–538. doi: 10.1200/JCO.1985.3.4.532. [DOI] [PubMed] [Google Scholar]
- Mihara K., Cao X. R., Yen A., Chandler S., Driscoll B., Murphree A. L., T'Ang A., Fung Y. K. Cell cycle-dependent regulation of phosphorylation of the human retinoblastoma gene product. Science. 1989 Dec 8;246(4935):1300–1303. doi: 10.1126/science.2588006. [DOI] [PubMed] [Google Scholar]
- Parrington J. M., West L. F., Povey S. Loss of heterozygosity in hypotriploid cell cultures from testicular tumours. Hum Genet. 1987 Nov;77(3):269–276. doi: 10.1007/BF00284484. [DOI] [PubMed] [Google Scholar]
- Reissmann P. T., Simon M. A., Lee W. H., Slamon D. J. Studies of the retinoblastoma gene in human sarcomas. Oncogene. 1989 Jul;4(7):839–843. [PubMed] [Google Scholar]
- Robbins P. D., Horowitz J. M., Mulligan R. C. Negative regulation of human c-fos expression by the retinoblastoma gene product. Nature. 1990 Aug 16;346(6285):668–671. doi: 10.1038/346668a0. [DOI] [PubMed] [Google Scholar]
- Sikora K., Evan G., Stewart J., Watson J. V. Detection of the c-myc oncogene product in testicular cancer. Br J Cancer. 1985 Aug;52(2):171–176. doi: 10.1038/bjc.1985.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebaek N. E., Berthelsen J. G., Giwercman A., Müller J. Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl. 1987 Feb;10(1):19–28. doi: 10.1111/j.1365-2605.1987.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Strohmeyer T., Peter S., Hartmann M., Munemitsu S., Ackermann R., Ullrich A., Slamon D. J. Expression of the hst-1 and c-kit protooncogenes in human testicular germ cell tumors. Cancer Res. 1991 Apr 1;51(7):1811–1816. [PubMed] [Google Scholar]
- T'Ang A., Wu K. J., Hashimoto T., Liu W. Y., Takahashi R., Shi X. H., Mihara K., Zhang F. H., Chen Y. Y., Du C. Genomic organization of the human retinoblastoma gene. Oncogene. 1989 Apr;4(4):401–407. [PubMed] [Google Scholar]
- Tanaka T., Slamon D. J., Battifora H., Cline M. J. Expression of p21 ras oncoproteins in human cancers. Cancer Res. 1986 Mar;46(3):1465–1470. [PubMed] [Google Scholar]
- Varley J. M., Armour J., Swallow J. E., Jeffreys A. J., Ponder B. A., T'Ang A., Fung Y. K., Brammar W. J., Walker R. A. The retinoblastoma gene is frequently altered leading to loss of expression in primary breast tumours. Oncogene. 1989 Jun;4(6):725–729. [PubMed] [Google Scholar]
- Wang L. C., Vass W., Gao C. L., Chang K. S. Amplification and enhanced expression of the c-Ki-ras2 protooncogene in human embryonal carcinomas. Cancer Res. 1987 Aug 1;47(15):4192–4198. [PubMed] [Google Scholar]
- Wang N., Trend B., Bronson D. L., Fraley E. E. Nonrandom abnormalities in chromosome 1 in human testicular cancers. Cancer Res. 1980 Mar;40(3):796–802. [PubMed] [Google Scholar]
- Weichselbaum R. R., Beckett M., Diamond A. Some retinoblastomas, osteosarcomas, and soft tissue sarcomas may share a common etiology. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2106–2109. doi: 10.1073/pnas.85.7.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Tsutsumi M., Sakamoto H., Miyagawa K., Teshima S., Sugimura T., Terada M. Expression of the HST1 oncogene in human germ cell tumors. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1324–1329. doi: 10.1016/s0006-291x(88)81286-5. [DOI] [PubMed] [Google Scholar]