Abstract
Background
Medication non-adherence is a well-studied and known cause of late allograft loss, but is difficult to measure and prospectively monitor. The aim of this study was to assess if appointment non-adherence was correlated with medication non-adherence and a predictor of graft outcomes.
Methods
This was a longitudinal cohort study using national USRDS and VA health records data with time to event analyses conducted to assess impact on graft and patient survival.
Results
4,646 transplants were included in the analysis (3,656 with complete records); 14.6% of patients had an appointment no show rate of ≥12% (non-adherence). Appointment and medication non-adherence were highly correlated and both were significant independent predictors of outcomes. Those with appointment non-adherence had 1.5 times the risk of acute rejection (22.0% vs. 14.7%, p<0.0001) and a 65% higher risk of graft loss (aHR 1.65, 95% CI 1.38–1.97, p<0.0001). There was a significant interaction between appointment and medication non-adherence; those with appointment and medication non-adherence were at very high risk of graft loss (aHR 4.18, 95% CI 3.39–5.15; p<0.0001), compared to those with only appointment non-adherence (aHR 1.39, 95% CI 0.97–2.01; p=0.0766) or only medication non-adherence (aHR 2.44, 95% CI 2.11–2.81; p<0.0001).
Conclusion
These results demonstrate that non-adherence to health care appointments is a significant and independent risk factor for graft loss.
Keywords: Kidney Transplant, Nonadherence, Acute rejection, Graft Survival
INTRODUCTION
As the prevailing etiologies of late kidney allograft loss continue to be elucidated, the role of late antibody mediated rejection and immunologically-mediated graft dysfunction due to medication non-adherence has gained increasing recognition as a predominant, and potentially modifiable, risk-factor.1–3 Non-adherence to immunosuppression medications is common after kidney transplant, with rates estimated at 36 cases per 100 patients per year.4 Medication non-adherence has been established as a predominant risk factor for graft loss; a meta-analysis demonstrated that more than a third of graft losses were associated with medication non-adherence. The odds of graft loss in those deemed non-adherent to medications is five to seven times higher, as compared to those that are adherent.5,6
Although non-adherence is clearly established as a major risk factor for late graft loss, prospective tracking and early identification of those that are non-adherent to medications in standard clinical practice is a difficult and time-intensive endeavor. Anecdotally, outside of trials, non-adherence in the clinic is usually identified after a patient develops negative sequelae from this behavior, represented by rejection or, if not identified early, substantial and irreversible graft inflammation and damage.7 Most studies assessing medication non-adherence use indirect measures, such as self-report, medication refill assessment (through medication possession ratios [MPRs]), or electronic home-based surveillance systems.8 Tracking of timely refills through MPRs using pharmacy claims data appears to be a valid indirect measure of medication non-adherence and one of the more promising and logistically feasible mechanisms for population-based surveillance.9–11 However, it is still difficult to do within the U.S. health care environment, as health care is fragmented and patients may refill medications at multiple pharmacies and across different systems of care.12
In comparison, relatively few studies have assessed non-adherence to follow-up appointments as a risk factor for graft loss. The estimated rate of appointment non-adherence is 5.8 cases per 100 patients per year; however, this estimate relies on a small number of dated studies.4 Theoretically, tracking appointment non-adherence is a much easier endeavor, as this type of non-adherence can be confined to one health care system and electronic health records have the ability to report this data in real-time to clinicians or as aggregated reports. Thus, the aim of this study was to determine if appointment non-adherence during routine follow-up is correlated with medication non-adherence and if it is associated with deleterious clinical outcomes in a large veteran kidney transplant population.
METHODS
Study Design and Patients
This was a retrospective longitudinal cohort study which utilized a previously described dataset of a national sample of veteran kidney transplant recipients. We linked the VA corporate data warehouse (CDW), which contains detailed information aggregated from electronic health records, including outpatient, inpatient, laboratory, radiology and pharmacy data, to the United States Renal Data System (USRDS), which contains all of the data available from the Organ Procurement and Transplantation Network (OPTN) registry system. Thus, a national dataset of kidney transplants recipients containing detailed baseline data from OPTN and comprehensive follow-up clinical data from the VA CDW was created. The study population included recipients of kidney transplants between Jan 2001 and Dec 2007 that received care within the VA system, with follow-up through Dec 2010. Non-renal transplants (pancreas, liver, heart, lung), those that were not African-American or Caucasian (due to previous study definitions), events occurring outside of the designated time period and those with graft loss or follow-up <1 year were excluded. Further details on how the dataset and study cohort was created can be found elsewhere.13
Non-Adherence Definitions
Medication non-adherence was assessed through the use of medication possession ratios (MPRs). The VA is a closed health care system; thus, pharmacy administrative data, including all prescribing and refill information, is available at a national and comprehensive level. MPRs were determined using previously published and validated methodology, by calculating patient and medication-specific sum for days’ supplies and dividing this by the total days in that given year.14,15 We assessed both immunosuppression and cardiovascular medication classes for this analysis. For appointment non-adherence, all patient with “no shows” to both clinic and laboratory visits were counted and divided by the total number of these types of visits that occurred on an annual basis per patient. Within the VA system, missed or cancelled appointments can be assessed at a granular level. If a clinic or provider cancels an appointment, it is listed as “cancelled by clinic.” If a patient calls ahead of time and cancels or reschedules an appointment, it is listed as “cancelled by patient.” If a patient fails to show up or misses an appointment without calling ahead or after the appointment, it is listed as “no show.” For this assessment and subsequent analyses, only appointments listed in the VA system as “no shows” were counted as a missed appointment. All cancelled or rescheduled appointments were not counted in this metric. Non-adherence was aggregated by post-transplant year for each patient. Once a patient developed graft loss, non-adherence to either medications or appointments was censored at that time point. To ensure accurate and unbiased estimates of non-adherence, we utilized categorical (with cut points) and continuous assessments of these measures, in both cross-sectional and longitudinal analyses.
Outcomes
The primary outcome of interest was graft loss, defined as either retransplantation, a return to chronic dialysis or death. Mortality was also assessed as an outcome. Graft loss and death were assessed as time to event outcomes. Acute rejection (defined as biopsy proven or treated) and delayed graft function (defined as the need for dialysis within 7 days of transplant) was also assessed.
Statistical Analysis
First, we aggregated non-adherence data across the entire follow-up period and assessed the correlation between appointment and medication non-adherence. Due to the skewed non-normal distributions of both these measures, median values were utilized and the correlation (PROC CORR) was assessed by comparing the median of each after stratifying patients into ventiles (PROC RANK). Formal spline and knot analysis revealed a non-linear correlation, and one knot was utilized to create two linear correlations (PROC TRANSREG, PBSSPLINE).
Next, we assessed for the dose-response of both appointment and medication non-adherence using Cox regression analysis (PROC PHREG) and categorizing patients based on median non-adherence rates during post-transplant follow-up. MPR values were aggregated for immunosuppression and cardiovascular medications and grouped by 5% increments, from ≥95% to ≥40%; appointment non-adherence was aggregated for both clinic and lab visits and grouped in 4% increments from ≥4% to ≥44%. These were selected based on review of frequency histograms. From this, an MPR threshold of 80% and appointment threshold of 12% were chosen as valid cut points to dichotomize the cohorts. Baseline characteristics were compared using standard univariate analyses (PROC FREQ (chisq), PROC NPAR1WAY (wilcoxon)). Sequential modeling with Cox regression (PROC PHREG) was utilized to assess for the independent effect of appointment and medication non-adherence on graft loss and death, adjusted for baseline characteristics. The final complete model contains both the medication non-adherence and appointment non-adherence variables.
To ensure estimates were robust and minimally influenced by missing data, two sensitivity analyses were conducted. First, we utilized multiple imputation (PROC MI, PROC MIANALYZE) and conducted the same iterative modeling as above to compare estimates obtained using the complete case dataset. Second, we conducted longitudinal analysis through joint modeling to estimate the random intercepts and slopes for appointment non-adherence in each patient and then entered these into Cox regression models. Covariance from repeat transplants in the same individual was accounted for during modeling (CONS(aggregate)). A two-sided p-value of <0.05 was considered statistically significant and all analyses were carried out using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Between January 2001 and December 2007, 5,757 veteran kidney transplant patients receiving care through the VA system were capable of being linked to the USRDS registry data. Of these, 494 were excluded for race/ethnicity, 345 were excluded because of a transplant outside the defined study time period and 272 were excluded for graft loss or follow-up <1 year post-transplant, leaving 4,646 included in the analysis (Supplemental Figure 1, Consort diagram). For the primary multivariable analyses, 3,656 patients with complete data were included. The sensitivity analysis using multiple imputation included all 4,646 patients. Table 1 displays the baseline characteristics of the study population compared between appointment non-adherence cohorts. Patients with a missed appointment rate of ≥12% were, in general, 7 years younger and significantly more likely to be AA, unmarried, the recipient of a deceased donor transplant, spent a longer time on dialysis, and less likely to have received a preemptive transplant.
Table 1.
Baseline characteristics compared between the appointment adherent and appointment non-adherent cohorts for the population with complete data
| Characteristics | No Show Rate <12% (N=3,124) | No Show Rate ≥12% (N=532) | p-Value |
|---|---|---|---|
| Age (yrs, median [IQR]) | 59 (53,66) | 52 (45,59) | <0.0001 |
| Male Gender | 98.1% | 97.2% | 0.1763 |
| African American | 27.8% | 61.3% | <0.0001 |
| Married | 68.2% | 51.9% | <0.0001 |
| History of Hypertension | 83.9% | 87.2% | 0.0534 |
| History of Diabetes | 37.2% | 35.5% | 0.4608 |
| History of Angina or Coronary Artery Disease | 13.8% | 10.7% | 0.0556 |
| Living Donor | 35.0% | 29.0% | 0.0066 |
| Expanded Criteria Donor | 14.1% | 12.2% | 0.2566 |
| Donor after Cardiac Death | 4.3% | 4.7% | 0.6431 |
| Cold Ischemic Time (hrs, median [IQR]) | 14 (3,21) | 14 (5,22.5) | 0.1767 |
| Warm Ischemic Time (min, median [IQR]) | 35 (27,50) | 37 (30,50) | 0.0693 |
| HLA mismatches (median [IQR]) | 4 (2,5) | 4 (3,5) | 0.0007 |
| Panel Reactive Antibody (%, median [IQR]) | 0 (0,0) | 0 (0,0) | 0.0746 |
| PRA >20% | 5.1% | 9.2% | 0.0002 |
| Years on Dialysis (yrs, median [IQR]) | 1.6 (0.2,3.3) | 2.3 (0.9,4.3) | <0.0001 |
| Pre-emptive Transplant | 22.4% | 14.9% | <0.0001 |
| IL-2 Receptor Antagonist Antibody Induction | 31.8% | 25.6% | 0.0039 |
| Cytolytic Induction | 35.1% | 38.2% | 0.1663 |
| Tacrolimus | 71.8% | 71.4% | 0.8488 |
| Cyclosporine | 22.6% | 22.7% | 0.9450 |
| Mycophenolate | 87.2% | 82.9% | 0.0067 |
| Azathioprine | 1.6% | 2.8% | 0.0573 |
| mTOR | 10.4% | 12.2% | 0.2187 |
| Prednisone | 94.3% | 96.4% | 0.0447 |
The estimated frequency of appointment non-adherence is displayed in Supplemental Figure 2; 14.6% of patients (532 of 3,656) had an appointment no show rate of ≥12% during the follow-up period (prior to graft loss). The median appointment non-adherence rate for the entire cohort was 0% (IQR 0%, 6.7%), with the top 10% of non-adherent patients having a median appointment no show rate of 16.7%. There was a strong dose-response between appointment non-adherence and risk of graft loss (R2=0.856, Supplemental Figure 3), such that for every 1% increase in appointment non-adherence, there was an estimated 5% increase in the adjusted hazard ratio (aHR) for graft loss (95% CI 3.4–6.5%; p<0.001). This was particularly the case once the appointment non-adherence rate was greater than 12%. Appointment non-adherence also tightly correlated with medication non-adherence. Supplemental Figure 4 displays the median appointment non-adherence rate vs. the ranked median MPR rate in ventiles. In the top 50% of adherent patients, the median MPR rate was ≥80%, with the median missed appointment rate being 0%. Once the MPR fell below 80%, missed appointment rates increased above 0% in a linear fashion, such that a 1% increase in missed appointment rate translated into an estimated 1.6% decrease in MPR (95% CI 1.4 to 1.8%; p<0.0001).
A missed appointment rate of ≥12% was a significant risk factor for deleterious clinical outcomes, including delayed graft function, acute rejection, graft loss, and death (Table 2). Those with a missed appointment rate of ≥12% had 1.5 times the risk of acute rejection (22.0% vs. 14.7%, p<0.0001) and a 27% higher risk of delayed graft function (19.9% vs. 15.7%, p=0.0152). In the unadjusted univariate analysis, appointment non-adherence was associated with a 71% higher risk of graft loss (HR 1.714, 95% CI 1.47–2.00, p<0.0001) and a 31% higher risk of death (HR 1.314, 95% CI 1.09–1.59, p=0.0050).
Table 2.
Post-transplant outcomes compared between the appointment adherent and appointment non-adherent cohorts for the population with complete data
| Outcomes | No Show Rate <12% (N=3,124) | No Show Rate ≥12% (N=532) | p-Value | |
|---|---|---|---|---|
| Delayed Graft Function | 15.7% | 19.9% | 0.0152 | |
|
| ||||
| Acute Rejection | 14.7% | 22.0% | <0.0001 | |
|
| ||||
| Estimated Graft Survival | ||||
| 3-year | 92.2% | 87.0% | <0.0001 | |
| 5-year | 81.8% | 70.9.% | ||
| 7-year | 71.1% | 55.8% | ||
|
| ||||
| Estimated Patient Survival | ||||
| 3-year | 94.5% | 92.8% | 0.0050 | |
| 5-year | 86.7% | 82.9% | ||
| 7-year | 77.0% | 71.6% | ||
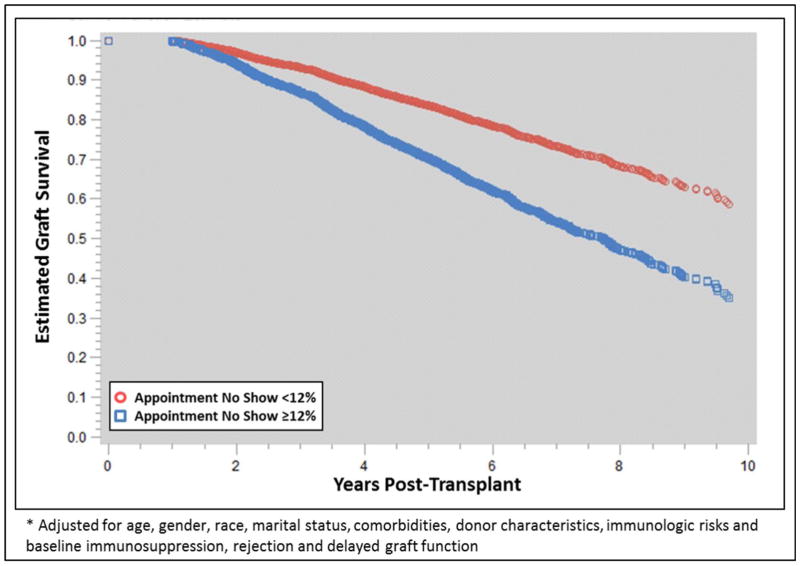
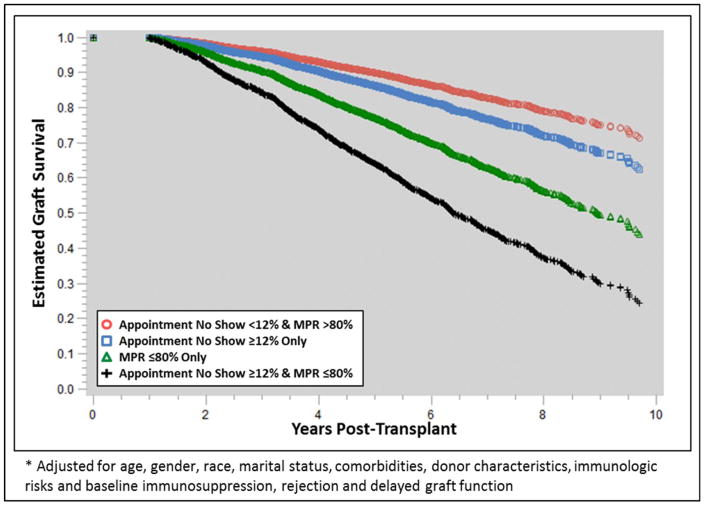
The sequential multivariable modeling results are displayed in Supplemental Table 1. Both appointment and medication non-adherence were significant and independent risk factors for graft loss even after adjusting for baseline covariates and post-transplant events, including DGF and acute rejection. In the fully adjusted model, which included medication non-adherence, appointment non-adherence was independently associated with a 65% higher risk of graft loss (aHR 1.650, 95% CI 1.38–1.97, p<0.0001, Figure 1). In general, medication non-adherence tended to be a stronger risk factor for graft loss (aHRs 2.63 to 2.51), as compared to appointment non-adherence (aHRs 1.65 to 2.04, Supplemental Table 1). There was also significant effect modification between appointment and medication non-adherence, as visualized in Figure 2. Those with high appointment non-adherence and low medication adherence were at a significantly higher risk of graft loss (aHR 4.18, 95% CI 3.39–5.15; p<0.0001), as compared to those with only appointment non-adherence (aHR 1.39, 95% CI 0.97–2.01; p=0.0766) or only medication non-adherence (aHR 2.44, 95% CI 2.11–2.81; p<0.0001).
Figure 1.
Adjusted* graft survival estimates compared between appointment adherence cohorts
Figure 2.
Adjusted* graft survival estimates compared between appointment and medication adherence cohorts
The two sensitivity analyses, one to assess the impact of missing data and one to assess the impact of using longitudinal data analysis, demonstrated that the estimates were robust and not likely to be significantly biased by missingness or type of analysis. Supplemental Table 2 displays the iterative modeling outcomes using the imputed data set (N=4,646, mimics Supplemental Table 1). The impact of non-adherence on outcomes are similar to those estimated using the complete case analysis, demonstrating low likelihood of bias due to missingness. The longitudinal data analysis demonstrated that a 1% increase in appointment non-adherence trajectory per year of follow-up was associated with a 30% increased risk of graft loss (slope aHR 1.303, 95% CI 1.17–1.46, p<0.001), with an estimate of 2.6% increased risk of graft loss for every 1% increase in appointment non-adherence at baseline (intercept aHR 1.026, 95% CI 1.01–1.04, p<0.001). These longitudinal estimates were similar, in both direction and magnitude, to those obtained using the aggregated follow-up data.
DISCUSSION
These results demonstrate that there is a strong correlation between appointment and medication non-adherence in kidney transplant recipients and that appointment non-adherence is a robust predictor for graft loss and death, even after accounting for medication non-adherence and acute rejection. Being non-adherent to both medications and appointment visits was associated with four times the risk of graft loss. Because appointment non-adherence is an easier measure to prospectively track in the real-life clinical care and follow-up of a transplant patient, these results suggest that interventions focused on identifying and resolving barriers causing appointment non-adherence may lead to improved long-term outcomes.
Although medication adherence is an important predictor of graft loss, it is difficult to define and measure. When reviewing previous literature assessing non-adherence after transplant, the estimated incidence is widely variable based on the method of measurement. For example, in one cohort, physician-suspected non-adherence was low (9%) compared to non-adherence based off of self-report questionnaires (31–37%), both of which are subjective measures.16,17 The MPR is an objective method of non-adherence detection that has been demonstrated to be associated with adverse health outcomes and can be used optimally within a closed healthcare system, such as the VA.11,12,18,19 Based on frequency histograms, we used a cut point MPR of 80% as our indicator value for non-adherence. Because an MPR of 80% has been recognized consistently as a definition of non-adherence in prescription refill record analyses and is generally recognized as acceptable across all adherence measures in solid organ transplant, this appears to validate our results.20–23 With the accuracy of MPR being questionable outside of closed healthcare systems, more optimal real-time objective measures of non-adherence are necessary. To further this endeavor, we chose to investigate appointment non-adherence.
Appointment non-adherence is a method to quantify medical adherence that has been measured infrequently in adults in the modern immunosuppression era. Additionally, the definitions of clinic non-adherence have varied in existing reports. Liu, et al. identified only 9 out of 246 kidney transplant patients that self-reported missing any clinic visits, none of whom also had self-reported non-adherence to immunosuppressants.24 The results from Liu et al differ significantly from our own, which may be explained by the method of data capture (self-report vs. hospital-records), as well as culture and healthcare system (Malaysian vs United States). More recently, in a cohort from Poland, 110 kidney transplant recipients were interviewed regarding adherence to multiple lifestyle recommendations. The researchers found that self-reported non-adherence to clinic visits increased over time from the transplant (5% at 2 months, increasing to 10% at 2 years).25 Although the incidence of clinic non-adherence was more similar to our own, the methods, population, and purpose for the analysis differed as there was no attempt to associate appointment non-adherence to graft outcomes. Other studies have defined non-adherence to clinic visits as missing >20% of visits, which differs from the cut point identified in our study. Yavuz, et al. found that 7.7% of 226 kidney transplant patients at their center in Turkey missed more than 20% of clinic visits, which is lower than the incidence of 14.6% that was discovered in our study.26 This may be due to the difference in the cut point chosen to identify non-adherence (20 vs. 12%). Additionally, it has been identified that studies addressing non-adherence in Europe report significantly less non-adherence to medical therapies than studies performed in the United States.4 However, within the US, Kiley et al. found that none of the patients in their cohort missed >20% of clinic visits, despite almost 25% of the 110 patients demonstrating non-adherence to medications.27 Unfortunately, this cohort was from over 20 years ago, when immunosuppression, transplant follow-up, and documentation were quite different than they are today.
There were a number of significant risk factors for appointment non-adherence. These included African American race, younger age, and not being married. We were not able to measure socioeconomic status (SES) in these patients. However, missed appointments may be a reflection of other SES measures, because at times, Veterans need to travel long distances for these appointments. Thus, transportation or costs of travel may be a barrier. Beyond this, there may be health literacy issues. Missed appointments may be a reflection of not understanding how to navigate the complex VA system, with multiple clinics, locations and providers. Education and health literacy may also impact behavioral components due to a lack of understanding of importance of good follow up and/or motivation to keep appointments.27–29 Unfortunately, these are issues we could not measure in retrospective review. They do represent areas of future study, as these are clearly modifiable, if they truly are mediators of non-adherence.
Overall, it is interesting to note that there are no recent comparable studies that measure non-adherence to clinic and laboratory visits and its relationship to allograft outcomes in the adult population. This is particularly thought-provoking, given the recent focus on medication non-adherence as a major risk factor for late allograft loss and the fact that medication non-adherence is quite difficult to track.1,11 Although there are fewer studies addressing non-adherence in pediatric transplant recipients, they more commonly report on clinic and laboratory visit non-adherence. A recent meta-analysis on the subject identified that non-adherence to clinic and laboratory visits in pediatrics are higher than reported medication non-adherence (13–19 cases/100 persons per year (PPY) vs 6 cases/100 PPY), although the impact on outcomes was not assessed.28 We believe that, through this analysis, we have identified a simple and objective indicator of non-adherent behavior, which can be tracked real-time in any electronic health record. By using an unbiased, easily replicable indicator of non-adherence, it should be possible to accumulate more homogenous data on the prevalence of non-adherence, as well as the effects of potential interventions.
There are a number of limitations to our analysis. As a retrospective longitudinal study, some of the baseline data utilized was dependent on documentation in the medical records and reporting to the OPTN. It is important to note, however, that the sensitivity analyses to evaluate the effect of the missing data demonstrated a lack of evidence suggesting missingness bias.30 Additionally, the population studied included solely veterans who are seen in the VA healthcare system. The demographics of the veteran population may limit the external validity to the national kidney transplant population, as the cohort was almost exclusively male, elderly, and had access to health care. Finally, this analysis solely focused on medication and appointment non-adherence within the VA system and did not assess other non-adherent behaviors (diet, lifestyle, smoking) or non-adherence to visits at other health care settings.
In conclusion, these results demonstrate that non-adherence to health care appointments is a significant and independent risk factor for graft loss and death in kidney transplant recipients. As appointment non-adherence is an easy and objective measure to monitor, as compared to medication, diet and lifestyle non-adherence, these data suggest this may be a promising method to identify high-risk patient cohorts for future interventional studies.
Supplementary Material
Footnotes
Conflict of Interest Statement: Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number K23DK099440.
References
- 1.Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 2.Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009;9:2520–2531. doi: 10.1111/j.1600-6143.2009.02799.x. [DOI] [PubMed] [Google Scholar]
- 3.Gaynor J, Ciancio G, Guerra G, Sageshima J, Hanson L, et al. Graft failure due to noncompliance among 628 kidney transplant recipients with long-term follow-up: a single-center observational study. Transplantation. 2014;97:925–933. doi: 10.1097/01.TP.0000438199.76531.4a. [DOI] [PubMed] [Google Scholar]
- 4.Dew MA, DiMartini A, De Vito Dabbs A, Myaskobsky L, Steel J, et al. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83:858–873. doi: 10.1097/01.tp.0000258599.65257.a6. [DOI] [PubMed] [Google Scholar]
- 5.Butler JA, Roderick P, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation. 2004;77:769–776. doi: 10.1097/01.tp.0000110408.83054.88. [DOI] [PubMed] [Google Scholar]
- 6.Chisholm MA, Kwong WJ, Spivey CA. Associations of characteristics of renal transplant recipients with clinicians’ perceptions of adherence to immunosuppressant therapy. Transplantation. 2007;84:1145–1150. doi: 10.1097/01.tp.0000287189.33074.c8. [DOI] [PubMed] [Google Scholar]
- 7.Pullalarevu R, Taber D, Chokkalingam A, Browning R, Kamel M, et al. Non-adherence is associated with increased risk of rejection and graft loss. Am J Transplant. 2016;16:321. [Google Scholar]
- 8.Doyle IC, Maldonado AQ, Heldenbrand S, Tichy EM, Trofe-Clark J. Nonadherence to therapy after adult solid organ transplantation: a focus on risks and mitigation strategies. Am J Health Syst Pharm. 2016;73:909–920. doi: 10.2146/ajhp150650. [DOI] [PubMed] [Google Scholar]
- 9.Cutler DM, Everett W. Thinking outside the pillbox – medication adherence as a priority for health care reform. N Engl J Med. 2010;362:1553–1555. doi: 10.1056/NEJMp1002305. [DOI] [PubMed] [Google Scholar]
- 10.Lehmann A, Aslani P, Ahmed R, Celio J, Gauchet A, et al. Assessing medication adherence: options to consider. Int J Clin Pharm. 2014;36:55–69. doi: 10.1007/s11096-013-9865-x. [DOI] [PubMed] [Google Scholar]
- 11.Stewart K, McNamara KP, George J. Challenges in measuring adherence: experiences from a controlled trial. Int J Clin Pharm. 2014;36:15–19. doi: 10.1007/s11096-013-9877-6. [DOI] [PubMed] [Google Scholar]
- 12.Sattler ELP, Lee JS, Perri M., III Medication (re)fill adherence measures derived from pharmacy claims data in older Americans: a review of the literature. Drugs Aging. 2013;30:383–399. doi: 10.1007/s40266-013-0074-z. [DOI] [PubMed] [Google Scholar]
- 13.Taber DJ, Gebregziabher M, Payne EH, Srinivas T, Baliga PK, Egede LE. Overall graft loss versus death-censored graft loss: unmasking the magnitude of racial disparities in outcomes among US kidney transplant recipients. Transplantation. 2016 doi: 10.1097/TP.0000000000001119. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. An empirical basis for standardizing adherence measures derived from administrative claims data among diabetic patients. Med Care. 2008;46:1125–1133. doi: 10.1097/MLR.0b013e31817924d2. [DOI] [PubMed] [Google Scholar]
- 15.Kim N, Agostini JV, Justice AC. Refill adherence to oral hypoglycemic agents and glycemic control in veterans. Ann Pharmacother. 2010;44:800–808. doi: 10.1345/aph.1M570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pabst S, Bertram A, Zimmermann T, Schiffer M, de Zwaan M. Physician reported adherence to immunosuppressants in renal transplant patients: prevalence, agreement, and correlates. J Psychosom Res. 2015;79:364–371. doi: 10.1016/j.jpsychores.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Massey EK, Tielen M, Laging M, et al. Discrepancies between beliefs and behavior: a prospective study into immunosuppressive medication adherence after kidney transplantation. Transplantation. 2015;99:375–380. doi: 10.1097/TP.0000000000000608. [DOI] [PubMed] [Google Scholar]
- 18.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:106–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 19.Pinsky BW, Takemoto SK, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant. 2009;9:2597–2606. doi: 10.1111/j.1600-6143.2009.02798.x. [DOI] [PubMed] [Google Scholar]
- 20.Van Wijk BLG, Klungel OH, Heerdink ER, de Boer A. The association between compliance with antihypertensive drugs and modification of antihypertensive drug regimen. J Hypertens. 2004;22:1831–1837. doi: 10.1097/00004872-200409000-00029. [DOI] [PubMed] [Google Scholar]
- 21.Steiner JF. J Clin Epidem [Google Scholar]; Su GC, Greanya ED, Partovi N, Yoshida EM, Shapiro RJ, Levy RD. Assessing medication adherence in solid-organ transplant recipients. Exp Clin Transplant. 2016;11:475–481. doi: 10.6002/ect.2013.0060. [DOI] [PubMed] [Google Scholar]
- 22.Chisholm MA, Vollenweider LJ, Mulloy LL. Renal transplant patient compliance with free immunosuppressive medications. Transplantation. 2000;70:1240–1244. doi: 10.1097/00007890-200010270-00020. [DOI] [PubMed] [Google Scholar]
- 23.Hillbrands LB, Hoitsma AJ, Koene RA. Medication compliance after renal transplantation. Transplantation. 1995;60:914. [PubMed] [Google Scholar]
- 24.Liu WJ, Zaki M. Medication compliance among renal transplant patients: a Hospital Kuala Lumpur experience. Med J Malaysia. 2004;59:649–658. [PubMed] [Google Scholar]
- 25.Kobus G, Malyszko J, Malyszko JS, Puza E, Bachorzewska-Gajewska H, Mysliwiec M. Compliance with lifestyle recommendations in kidney allograft recipients. Transplant Proc. 2011;43:2930–2934. doi: 10.1016/j.transproceed.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 26.Yavuz A, Tuncer M, Erdogan O, et al. Is there any effect of compliance on clinical parameters of renal transplant recipients? Transplant Proc. 2004;36:120–121. doi: 10.1016/j.transproceed.2003.11.052. [DOI] [PubMed] [Google Scholar]
- 27.Kiley DJ, Lam CS, Pollak R. A study of treatment compliance following kidney transplantation. Transplantation. 1993;55:51–56. doi: 10.1097/00007890-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Dew MA, Dabbs AD, Myaskovsky L, et al. Meta-analysis of medical regimen adherence outcomes in pediatric transplant recipients. Transplantation. 2009;88:736–746. doi: 10.1097/TP.0b013e3181b2a0e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kizer KW. The “new VA”: a national laboratory for health care quality management. Am J Med Qual. 14:3–20. doi: 10.1177/106286069901400103. 199. [DOI] [PubMed] [Google Scholar]
- 30.Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. Brit Med J. 2009;338:b2393–b2404. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.