Abstract
Loss of the tumor suppressor gene PTEN exerts diverse outcomes on cancer in different developmental contexts. To gain insight into the effect of its loss on outcomes in the brain, we conditionally inactivated the murine Pten gene in neonatal neural stem/progenitor cells. Pten inactivation created an abnormal perivascular proliferative niche in the cerebellum that persisted in adult animals but did not progress to malignancy. Proliferating cells showed undifferentiated morphology and expressed the progenitor marker Nestin but not Math1, a marker of committed granule neuron progenitors. Co-deletion of Pten and Trp53 resulted in fully penetrant medulloblastoma originating from the perivascular niche, which exhibited abnormal blood vessel networks and advanced neuronal differentiation of tumor cells. EdU pulse-chase experiments demonstrated a perivascular cancer stem cell population in Pten/Trp53 double mutant medulloblastomas. Genetic analyses revealed recurrent somatic inactivations of the tumor suppressor gene Ptch1 and a recapitulation of the sonic hedgehog subgroup of human medulloblastomas. Overall, our results showed that PTEN acts to prevent proliferation of a progenitor niche in postnatal cerebellum predisposed to oncogenic induction of medulloblastoma.
Introduction
PTEN, one of the most commonly mutated genes in human cancer, is the major negative regulator of the PI3-kinase signaling cascade, and engages multiple downstream mechanisms as a tumor suppressor (1). Importantly, PTEN also plays diverse roles in normal development. Within the brain, loss of PTEN can cause multiple complex phenotypes including megalencephaly, seizures, autism, or mental retardation (2). PTEN mutations also occur in brain tumors including glioblastoma and medulloblastoma (3–7). Here, we identified a novel function for Pten in regulating progenitor cells in the cerebellum.
Cells in the cerebellum arise from two germinal zones: the ventricular zone and the external granule layer (EGL). The ventricular zone is the embryonic germinal neuroepithelium that generates deep cerebellar nuclei, Purkinje cells and other GABAergic neurons in the cerebellum. At birth, the ventricular germinal neuroepithelium disappears, and proliferative precursors in the rhombic lip emigrate along the pial surface to form the EGL. The EGL proliferates most during early postnatal days and produces all the cells that differentiate to become internal granule neurons (8). Sonic hedgehog (Shh) is expressed by Purkinje cells and binds to its receptor, Ptch1, on granule cell precursors in the EGL, promoting proliferation and preventing differentiation (9, 10). Phosphoinositide 3-kinase (PI3K) and Shh signaling pathways can synergize to promote granule cell progenitor proliferation in vitro (11). Pten, the major negative regulator of PI3K signaling, is also required for normal cerebellar development. Deletion of Pten in EGL granule progenitors causes ectopic positioning and hypertrophy of cerebellar granule cells (12–14).
Aside from the two well characterized germinal zones in the developing cerebellum, multipotent neural stem cells have also been isolated from the cerebellum postnatally and are thought to be located in the white matter tracts (15), possibly arising from embryonic ventricular zone progenitors that emigrate into the white matter tracts (16). The identity and function of this pool of neural stem/progenitor cells are largely unknown.
Medulloblastoma is the most common malignant brain tumor of childhood. It is a collection of clinically and molecularly heterogeneous embryonal tumors, and can be divided into four molecular groups defined by gene expression or DNA methylation signatures: SHH, WNT, Group 3 and Group 4 (17). The SHH group of medulloblastomas contains tumors carrying mutations in PTCH1 or SUFU, tumor suppressor genes that negatively regulate SHH signaling, as well as tumors without detectable mutations in these components, but with similar gene expression signatures. The SHH subgroup of medulloblastoma is thought to originate from the EGL precursors, which is supported by Ptch1 mutant mouse models (18–21). The Wnt subgroup of medulloblastoma is likely to develop from cells of the dorsal brainstem (22). Expression of c-Myc and loss of p53 function in granule neuron progenitor cells or in mouse cerebellar stem cells, defined by expression of Prominin 1 and absence of neuronal and glial lineage markers, can drive medulloblastomas that resemble human MYC-driven Group 3 medulloblastomas (23, 24). It is unclear how many different cell types may serve as cells of origin for Group 3 tumors, and the cell of origin for Group 4 medulloblastoma is not known.
Cancer initiating cells, or cancer stem cells, are the subpopulation in the primary tumor that are capable of self-renewing, differentiating and regenerating the original tumor when transplanted in vivo. Cancer initiating cells have now been isolated from human cancers of the blood, breast, brain, skin, bone, and prostate (25). They are hypothesized to sustain tumor growth and to be responsible for relapse and metastasis. Cancer stem cells can also be isolated from human and mouse medulloblastomas (21, 26) and have been shown to reside in a perivascular niche (27). The perivascular cell population was found to account for the re-growth of Ptch1-mutant medulloblastoma after irradiation-induced apoptosis (28). However, whether there is a perivascular population of cells in the wild-type brain that is susceptible to oncogenic transformation, and whether this population could serve as cells of origin for medulloblastoma are unknown.
In the present study, we inactivated Pten in neural stem/progenitor cells in neonatal mice, and revealed a novel perivascular proliferative niche in the cerebellum. Combined deletion of Pten and Trp53 generated medulloblastomas with complete penetrance. Pten;Trp53-null medulloblastomas acquired somatic mutations in Ptch1, and had gene expression signatures that were similar to the human SHH subgroup of medulloblastoma. Paradoxically, Pten deletion induced abnormal proliferation of perivascular progenitor cells, but was associated with extensive neuronal differentiation of bulk tumor cells in the context of medulloblastomas. Thus Pten deletion may influence the expansion of progenitor cells that can serve as cells of origin for medulloblastoma, and may also contribute to the differentiation state of tumor cells.
Materials and Methods
Mice
For Cre activity mapping, Nestin-CreERT2 transgenic mice (29) were bred with R26R-EYFP (30) mice to generate Nestin-CreERT2;R26R-EYFP mice. Cre activity was detected by EYFP IF staining on frozen tissue sections. Nestin-CreERT2;PtenloxP/loxP (PtencKO) mice were generated by crossing Nestin-CreERT2 mice to PtenloxP mice (31), a gift from Tak Mak (University of Toronto, Toronto). Nestin-CreERT2;PtenloxP/loxP;Trp53loxP/loxP (PtencKO;Trp53cKO) mice were generated by breeding Nestin-CreERT2;PtenloxP/loxP with Trp53loxP mice (32). For all analyses including Nestin-CreERT2, the transgene was hemizygous to avoid possible variation from Cre transgene dosage. All procedures were reviewed and approved by the Animal Care and Use Committee at St. Jude Children’s Research Hospital.
Genotyping polymerase chain reaction assays
PCR was used to genotype mice. Primers used were:
Cre, forward primer: 5′AGCGATCGCTGCCAGGAT; reverse primer: 5′ACCAGCGTTTTCGTTCTGCC; expected product: 157 base pairs.
Pten, forward primer: 5′TTATCTGGATCAACTTTGGGCC; reverse primer: 5′TCCCACCAATGAACAAACAGT; expected products: 146 (wild-type) and 244 (floxed allele) base pairs.
Trp53, forward primer: 5′CACAAAAACAGGTTAAACCCAG; reverse primer: 5′AGCACATAGGAGGCAGAGAC; expected products: 288 (wild-type) and 370 (floxed allele) base pairs.
Induction of Cre activity
Tamoxifen (™) (Sigma) was dissolved at 5 mg/ml at 37°C in corn oil (Sigma), sterilized by filtering and stored at 4°C in the dark for up to 10 days. Pups were injected i.p. daily with 3 mg TM/40g body weight on P0 and P1. An equivalent volume of sterile filtered corn oil alone was used for vehicle injections. Two injections in the same mouse were separated by 24 hours.
5-ethynyl-2′-deoxyuridine injection and detection
5-ethynyl-2′-deoxyuridine (EdU) (Invitrogen) was dissolved at 2.5 mg/ml in sterile phosphate buffered saline (PBS) and stored at −20°C. EdU was injected i.p. at a dose of 5μg/g body weight every 2 hours for 5 injections. Fluorescent detection of EdU was performed using Click-iT EdU Cell Proliferation Assay kit (Invitrogen).
Immunohistochemistry and immunofluorescence
Mice were anesthetized and perfused transcardially with 1x PBS followed by 4% PFA in PBS. Following dissection, tissues were post-fixed overnight in 4% PFA in PBS at 4°C, and then equilibrated in 25% sucrose in PBS for an additional 24 hours at 4°C. Tissues were embedded in TBS embedding media (Triangle Biomedical Sciences) on dry ice and cut into 12 μm thick cryosections. Tissue slides were equilibrated at room temperature for 20 minutes then washed three times in PBS prior to staining. For paraffin sections, mice were processed the same way as above. Following perfusion, tissues were post-fixed for an additional 24 hours in 4% PFA, embedded in paraffin, and cut into 5 μm sections. Primary antibodies used for immunostaining were against GFP (1:1000, Invitrogen A6455; 1:2000, Abcam #13970), Pten (1:100, Cell Signaling #9559), p-Akt S473 (1:50, Cell Signaling #9271), Gfap (1:200, Sigma G3893), Ki67 (1:5000, Novocastra NCL-Ki67p), CD34 (1:100, BD Pharmingen #553731), CD45 (1:400, BD Pharmingen #553076), CD133 (1:100, eBioscience #12-1331) Nestin (1:100, Chemicon MAB353), PCNA (1:500, Santa Cruz #sc-56) and Math1 (1:200, Abcam, #ab105497). For IHC, microwave antigen retrieval was performed for all antibodies and biotinylated secondary antibodies were used in conjunction with horseradish peroxidase-conjugated streptavidin (Elite ABC, Vector Laboratories). Color development was conducted with substrates NovaRed, DAB or VIP (Vector
Laboratories) and counterstaining with hematoxylin or methyl green (Vector Laboratories), respectively. For IF, Alexa Fluor 488, 647 (Invitrogen) and cyanine 3, 5 (Jackson ImmunoResearch) conjugated secondary antibodies were employed along with Vectashield mounting media containing DAPI (Vector Laboratories).
Quantification of tumor vasculature
Representative H&E fields taken from medulloblastomas of both Pten;Trp53 dKO and Trp53cKO (4 tumors/group). Images are analyzed by ImageJ (version 1.50i) “Image -> Adjust -> Threshold Color” and “Analyze -> Measure” tools to quantify area of open blood vessels represented as percentage per field.
Comparative genomic hybridization and gene expression microarray
Genomic DNA was prepared by scraping tumor tissues from multiple deparaffinized sections, followed by Proteinase K-digestion and phenol-chloroform extraction. DNA quality was verified by gel electrophoresis. Control DNA was isolated from the spleens and livers of wild-type FVB mice. A total of 18 Pten;Trp53 dKO tumors were analyzed using the Roswell Park Cancer Institute (RPCI) 6.5K mouse BAC array comparative genomic hybridization (aCGH) as described (33).
Total RNA was extracted from frozen tumor tissue from Pten; Trp53 dKO using Trizol reagent (Invitrogen). RNA integrity was verified on all samples using the Agilent 2100 Bioanalyzer (Agilent Technologies). Gene expression profiles were generated using the Affymetrix Mouse Genome 430 v2.0 chip.
Array CGH and gene expression array data are deposited in Gene Expression Omnibus (GEO) under accession number GSE40106.
Gene expression analysis
Double knockout tumors resemble the human SHH-medulloblastomas. The top 25 genes most highly expressed in each of the four subgroups were derived as gene sets, as reported in Kawauchi et al (23) and used for Gene Set Enrichment Analysis (34). Comparing six Pten;Tp53 dKO tumors with three Pten cKO knockout cerebellum samples induced at P5, three P5 cerebellum samples and three Pten cKO samples induced at P30, only the gene set highly expressed in human SHH-subgroup is significantly enriched in the dKO tumors (p=0.082, FDR=0.0767). Gene sets for the other three medulloblastoma subgroups were not significantly enriched in dKO tumors (FDR>0.25).
Ptch1 sequence analysis
Total RNA was extracted from snap-frozen medulloblastoma samples using Trizol (Invitrogen). cDNA was synthesized from tumor RNA by reverse transcription using random hexamer primers and SuperScript II Reverse Transcriptase (Invitrogen). After cDNA synthesis, Ptch1 sequencing analysis was done as describe by Lee et al. (35).
Results
Induction of Nestin-CreERT2 in neonatal mice directed extensive cre activity in cerebellum
Tamoxifen injection of adult Nestin-CreERT2 mice induces cre activity in neural stem and progenitor cells (29). We bred these mice with R26R-LacZ or R26R-EYFP cre reporter mice to map the cre activity induced by tamoxifen administration in neonatal mice. Consistent with the fact that developing brain has stronger expression of Nestin and more numerous neural stem/progenitors, induction of Cre activity induced by Nestin-CreERT2 at postnatal days 0 and 1 (P0 and P1) was more robust than observed in adult brain, with strongest activities in the neural stem/progenitor cell niches including the subventricular zone of the lateral ventricle (SVZ) (29). The bulk of the cerebellum develops postnatally, and TM injection at P0 and P1 induced extensive Nestin-CreERT2 activity in the cerebellum including the EGL, IGL and white matter tracts very effectively (Fig. S1A–D). Cre activity was also detected by reporter expression in NG2-expressing glial progenitor cells adjacent to the blood vessel, but absent in CD34-expressing vascular endothelial cells.
Pten deletion causes cerebellar disorganization and hypertrophy
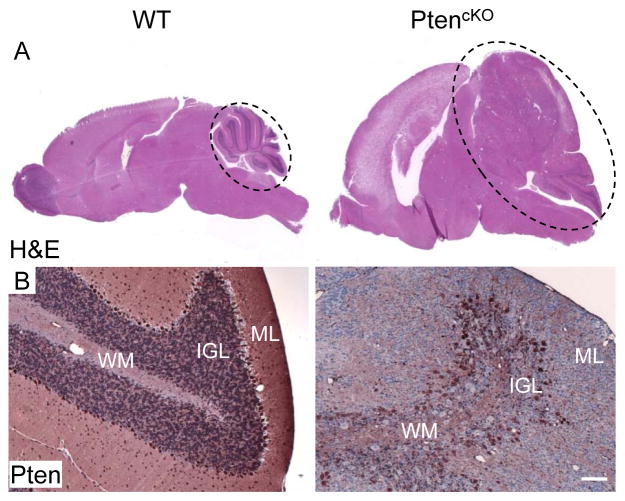
To determine the effect of broad Pten loss on developing brain, we induced deletion of Pten in Nestin-CreERT2;PtenloxP/loxP mice by TM injection at P0 and P1 (hereafter PtencKO mice). PtencKO mice appeared normal until 2–3 months of age, then began exhibiting lethargy, increasing macrocephaly and progressive neurological abnormalities including ataxia and seizure, requiring euthanasia with a median survival of 153 days. One hundred percent of the mice (24/24) induced at birth were affected, whereas none of the uninduced PtenloxP/loxP mice or TM-treated wild-type littermates showed these deficits. In addition to the previously described dramatic expansion of the SVZ and rostral migratory stream (29), brains from PtencKO mice showed profound hydrocephalus and dramatic increases in overall size especially in the cerebellum (Fig. 1A) where foliation was visible, but the organization of cell layers was disrupted. The molecular layer (ML) was dramatically thickened with many Pten-deficient ectopic granule cells. The majority of Purkinje cells expressed Pten, but were not well aligned at the interface of the IGL and ML. The IGL was thinner than wild-type control and composed of both Pten positive and negative granule cells (Fig. 1B). The size of the Pten-deficient granule cells was larger than the Pten positive granule cells in aging mice (not shown). All of these abnormalities mimic human Lhermitte-Duclos disease and are in agreement with previous findings but with a more severe manifestation compared to other Pten conditional knockout models in which Pten deletion was driven by different GFAP-cre mice (12, 13).
Figure 1. Pten deletion caused cerebellar disorganization and hypertrophy.
H&E (A) and Pten IHC (B) staining of the cerebella. (A) H&E staining of the wild-type (WT) and PtencKO brains showed dramatic hypertrophy of the PtencKO brain, especially cerebellum (dashed circle). (B) Pten IHC staining of the WT and PtencKO brains showed disrupted organization of the cerebellum with a much less well-defined IGL due to numerous ectopic Pten-deficient granule cells in the molecular layer (ML) in the PtencKO brain. Scale bar: B=100 μm. WM indicates white matter tracts.
Pten inactivation induced novel perivascular proliferative niches in the cerebellum
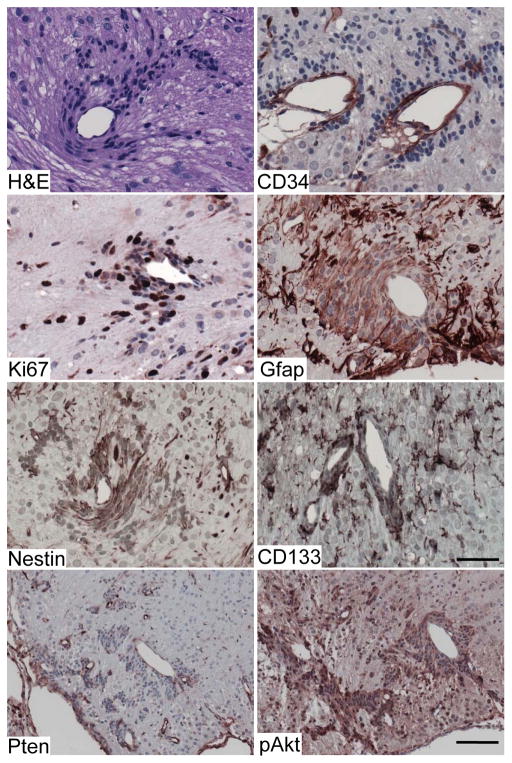
In addition to the cerebellar disorganization and hypertrophy, we identified multiple perivascular proliferative niches in PtencKO cerebellum, which were composed of hyperchromatic cells with progenitor-like morphology (20/24 mice) (Fig. 2). Previous models in which Pten conditional deletion was driven by GFAP-cre showed extensive deletion in cerebellar granule cells, but no evidence of hyperproliferative lesions. In these models, cre activity was limited to the majority of cerebellar granule neurons, as well as more variable activity in astrocytes, granule neurons of the dentate gyrus, and subsets of neurons in the cerebral cortex, and minimal activity in the subventricular zone despite the known expression of Gfap in the stem cells of the subventricular zone. The cre activity in GFAP-cre mice is not completely overlapping with endogenous Gfap expression, presumably due to integration site effects of the transgene (12, 13, 36). Nestin is endogenously expressed in a broader range of stem and progenitor cells than Gfap, including all cells within the subventricular zone, and the Nestin-creER driver used here showed strong induction of cre activity in these stem/progenitor compartments (32) as well as granule neurons and perivascular cells in the cerebellum (Fig. S1D). The perivascular hyperplastic lesions varied in size from as few as several cells to hundreds of cells, and were observed as early as 3 months of age but were not found in 30-day-old mice. These hyperplastic lesions grew surrounding CD34+ blood vessels and had a high percentage of Ki67+ cells (Figure 2). The cells did not express CD45, and therefore were not extravasated lymphocytes (Fig. S2). The lesions were distributed throughout cerebellum, but occurred more frequently in anterior and lateral cerebellar lobules and in areas around the pia between lobules. They expressed Nestin and CD133 (Fig. 2) which mark neural stem/progenitor cells or brain tumor initiating cells (26, 37), but did not express Math1, a marker for granule cell progenitors of the EGL (Fig. S2). They also expressed Gfap, which labels neural stem cells in the SVZ in addition to astrocytes (38)(Figure 2). The perivascular precursor-like cells are Pten-null and p-Akt positive, consistent with cells that underwent Cre-mediated recombination (Fig. 2). These perivascular hyperplastic lesions persisted, but did not develop into tumors in mice up to 10 months of age. Although Pten has been shown to influence stem cell self-renewal (39, 40), this is the first example of Pten loss inducing a novel progenitor niche.
Figure 2. Pten inactivation induced novel perivascular proliferative niches in the cerebellum.
H&E and IHC of perivascular lesions from the PtencKO cerebellum using antibodies against the indicated markers. The perivascular lesions surrounding CD34+ blood vessels were also often located adjacent to the pia and had a high percentage of Ki67+ cells. The perivascular cells had an embryonal morphology, expressed Gfap, Nestin and CD133, consistent with a neural stem/progenitor identity. The precursor-like cells were Pten-null and positive for pAkt consistent with cells that underwent Cre-mediated recombination. Scale bar: Pten, p-Akt and CD45: 100 μm; others: 50 μm.
Concurrent Pten and Trp53 deletion induced medulloblastoma with extensive vascularization and highly differentiated tumor cells
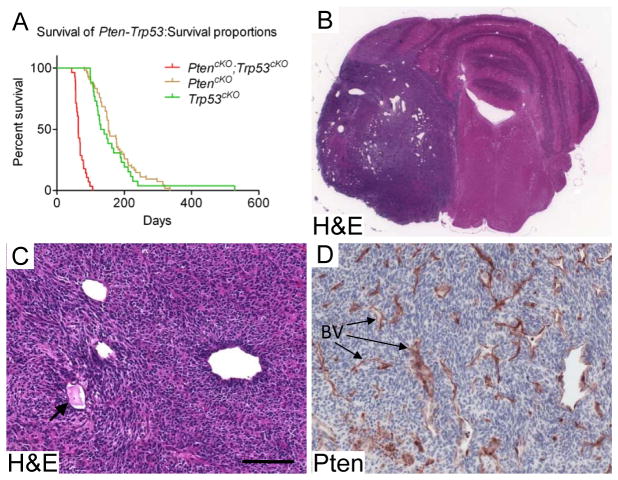
TP53 is one of the most frequently mutated genes in human cancer, and is also recurrently mutated in different types of brain tumors (7, 17). Therefore, we asked whether Trp53 deficiency would cooperate with Pten deletion to transform the perivascular proliferative niche into a full-blown cerebellar malignancy. Deletion of Trp53 alone by TM induction of Nestin-CreERT2;Trp53loxP/loxP (Trp53cKO) mice at P0 and P1, resulted in a median onset of morbidity of 135 days, with 47% of mice developing medulloblastoma, and the remainder developing non-CNS tumors or other types of brain tumors (Fig. 3A). Combined deletion of Pten and Trp53 accelerated tumor onset and increased the incidence of medulloblastoma. 100% (26/26) of induced Nestin-CreERT2;PtenloxP/loxP;Trp53loxP/loxP (hereafter dKO mice) developed medulloblastomas with a median onset of morbidity of 63 days of age (Fig. 3A). All dKO medulloblastomas had two striking features that distinguished them from Trp53cKO medulloblastomas; extensive and abnormal vascularization, and highly differentiated tumor cells surrounding a perivascular tumor stem cell niche (Fig. 3B–D and 4). Vascularization of dKO medulloblastomas was significantly more extensive (p<0.0009) and included abnormal blood vessels with dilated and angular shapes. Blood clots were often seen in the lumen of abnormal blood vessels (Fig. 3C and S3). Pten IHC staining showed that the vast majority of the tumor mass was devoid of Pten with only blood vessel endothelial cells retaining Pten (Fig. 3D). In addition to cerebellar tumors, dKO mice also developed gliomas in the forebrain (18/26) and brain stem (5/26).
Figure 3. Concurrent Pten and Trp53 deletion caused medulloblastoma with extensive abnormal vascularization.
(A) Kaplan-Meier survival curves show Pten;Trp53 dKO mice with a median tumor morbidity of 63 days of age and 100% penetrance of medulloblastoma (red curve), PtencKO mice with a median morbidity age of 153 days (brown curve) and Trp53cKO with a median morbidity age of 135 days (green curve). PtencKO mice were euthanized due to macrocephaly and hydrocephaly, but no detectable tumor masses. 47% of Trp53cKO developed medulloblastoma and the remainder developed non-CNS tumors or other types of brain tumors. n: dKO=26; PtencKO=24; Trp53cKO=26. p value for survival comparing dKO to p53 cKO is <0.0001. (B) and (C) H&E staining of medulloblastoma generated by co-deletion of Pten and Trp53 shows an extensive network of enlarged blood vessels and high vascularization. The arrow in (C) points to a blood vessel (BV) with clot in its lumen. (D) Pten IHC shows that medulloblastoma cells were devoid of Pten, while BV express Pten (arrows). Scale bar: C and D: 100 μm.
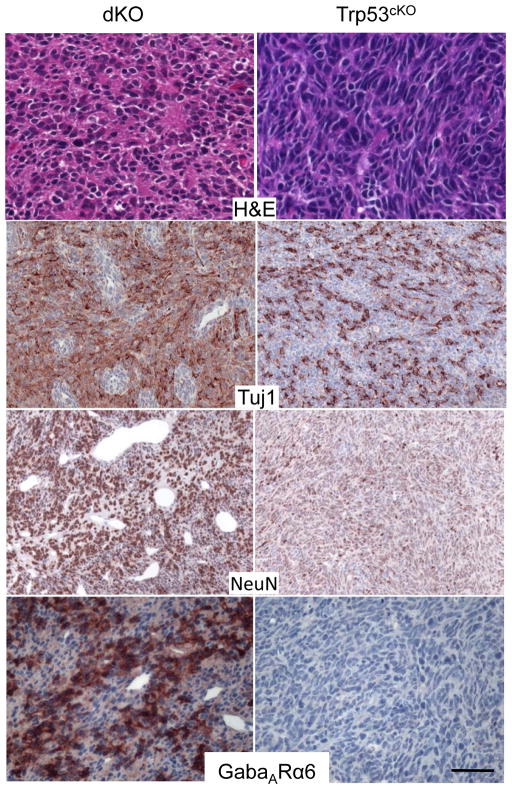
Figure 4. Pten loss drove abnormal angiogenesis and neuronal differentiation.
H&E and IHC of the medulloblastomas from Pten;Trp53 dKO and Trp53cKO mice. Pten;Trp53 dKO medulloblastomas show differentiated morphology in some areas (H&E), and a differentiated immunophenotype. Medulloblastomas from both genotypes express class III beta tubulin, detected by Tuj1, which is expressed in immature and mature neurons. However dKO medulloblastoma cells outside of the perivascular clusters show pronounced expression of NeuN, a marker of differentiated neurons and GabaARα6, which specifically labels mature cerebellar granule neurons. Trp53cKO medulloblastomas only exhibited weak NeuN and no GabaARα6 expression. Trp53cKO medulloblastomas did not show the extensive abnormal vascularization seen in dKO medulloblastomas. Scale bar: H&E; GabaARα6: 50 μm, Tuj1 and NeuN: 100 μm.
Both dKO and Trp53cKO medulloblastomas were positive for class III beta-tubulin, a marker for neurons. However, the dKO medulloblastomas exhibited an advanced neuronal immunophenotype, with the majority of tumor cells expressing NeuN and GabaARα6, markers that are normally expressed in mature cerebellar granule neurons that were not expressed in Trp53cKO medulloblastomas (Fig. 4). Thus, Pten loss drove abnormal angiogenesis and extensive neuronal differentiation in dKO medulloblastomas.
Early proliferative lesions showed that Pten;Trp53 dKO medulloblastoma initiates from a perivascular niche
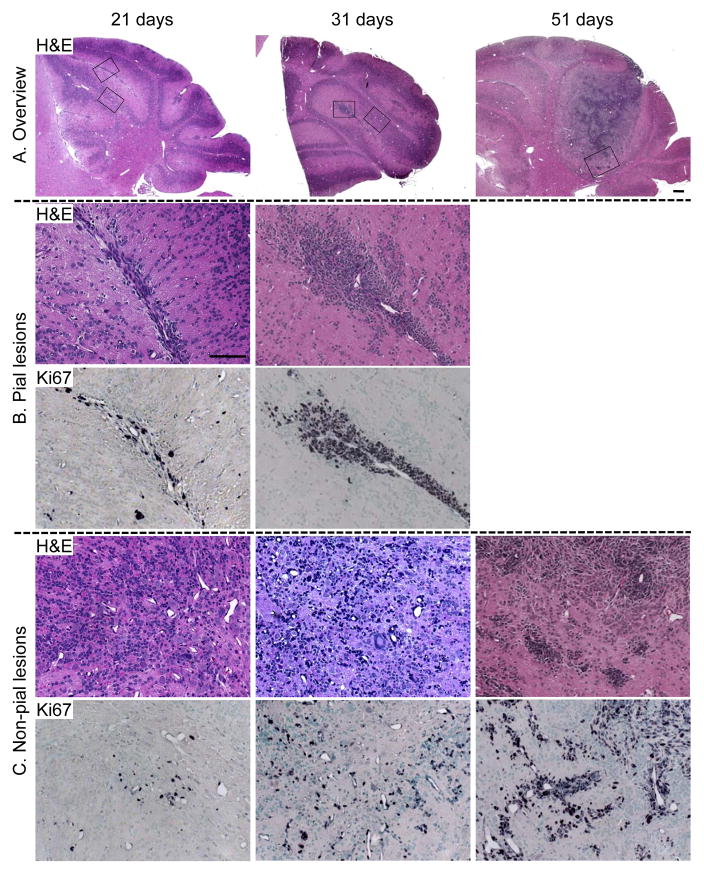
To investigate where the dKO medulloblastoma originates, and how it relates to the perivascular proliferative lesions in PtencKO cerebellum, we analyzed dKO cerebella at P21, P31 and P51, earlier than the median onset of morbidity at P63. Proliferation associated with development of wild-type cerebellum is complete by P21, with only rare Ki67+ proliferative cells detected under the pial surface or in the white matter tracts. In contrast, the dKO cerebellum at P21 showed disrupted lamination and abundant Ki67+ proliferative cells throughout the cerebellum which were concentrated around blood vessels either under the pial surface or in the parenchyma (Fig. 5). By P31, the number of Ki67+ cells dramatically increased. The hyperchromatic cell clusters around blood vessels were easily visible by H&E staining (Fig. 5). Although the dKO mice were not symptomatic by P51, there were sizable tumor masses present in the cerebellum. The perivascular growth pattern of tumor cells was maintained at the edges of medulloblastoma where tumor cells were invading brain parenchyma (Figure 5). All of the perivascular lesions were negative for Pten IHC, consistent with cells that have undergone Cre-mediated recombination (Fig. S4). Thus, the early perivascular proliferative lesions in the dKO cerebellum were reminiscent of those in PtencKO mice. dKO medulloblastomas emerged from these perivascular niches over time.
Figure 5. Early proliferative lesions showed that the Pten;Trp53 dKO medulloblastomas initiated from a perivascular niche.
H&E and Ki67 IHC of cerebella from 21, 31 and 51 day-old Pten;Trp53 dKO mice. Black boxes on the low-mag H&E image (A) indicate the area shown in high-mag H&E and Ki67 IHC images (B and C). The proliferative cells were hyperchromatic by H&E staining but not easily discernable from surrounding non-proliferative cells. At P21 and P31, dKO cerebella showed a progressive increase in proliferative lesions surrounding blood vessels either under the pial surface or in non-pia regions. By P51 days, tumor masses had formed. However, the perivascular growth pattern of tumor cells was maintained at the edge of the tumor where medulloblastoma cells were invading the brain parenchyma. Scale bar: 100 μm.
dKO medulloblastomas arose from a perivascular tumor stem cell niche
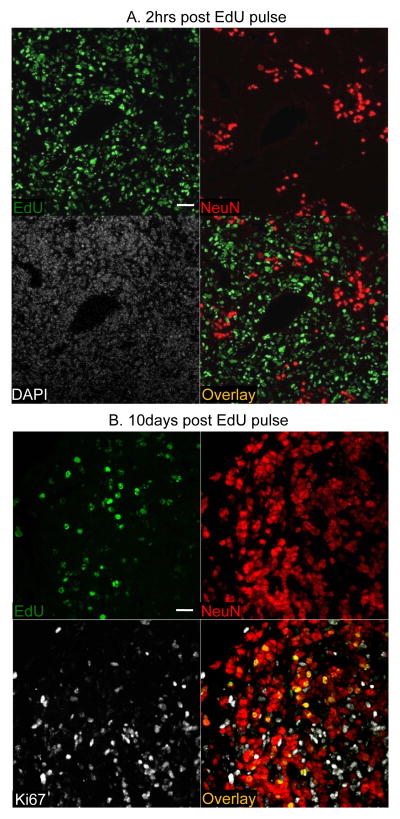
To investigate whether the proliferating perivascular cells gave rise to the non-proliferative NeuN+ medulloblastoma tumor bulk, we traced the fate of proliferative tumor cells 2 hours or 10 days after an EdU pulse in P50 dKO mice. Two hours after an EdU injection, EdU+ cells and NeuN+ cells were mutually exclusive, consistent with non-proliferating NeuN+ tumor cells (Fig. 6A). However, 10 days after an EdU injection, a significant proportion of EdU+ cells were also NeuN+, marking proliferating cells that had differentiated (Fig. 6B, S5A). There were also EdU+ cells that were positive for Ki67 but negative for NeuN. These EdU+ Ki67+ cells were usually less intensely stained for EdU, consistent with dilution of incorporated EdU with multiple rounds of cell division. These data demonstrate that the dKO medulloblastoma has its own lineage hierarchy in which a subpopulation of proliferative tumor cells exit the cell cycle and differentiate along the granule cell lineage, consistent with descriptions of cancer stem cells (25). Strikingly, many, but not all of the proliferating tumor cells expressed Math1, consistent with SHH subgroup medulloblastoma (Fig. S5B).
Figure 6. Lineage tracing demonstrates perivascular proliferating cells gave rise to non-proliferative NeuN+ medulloblastoma tumor cells.
(A) EdU+NeuN double IF labeling of medulloblastomas from the Pten;Trp53 dKO mice that were harvested 2 hrs after EdU pulse at P50. EdU+ and NeuN+ cells did not overlap at 2 hrs post EdU pulse showing that NeuN+ tumor cells were not proliferative. (B) EdU+NeuN+Ki67 triple IF labeling of medulloblastomas from the dKO mice that were harvested 10 days after EdU pulse at P50. There were large numbers of EdU+NeuN double positive cells but no Ki67+NeuN double positive cells 10 days after an EdU pulse. This indicates perivascular proliferating cells gave rise to the non-proliferative NeuN+ medulloblastoma tumor cells. Scale bar: A: 40 μm; B: 20μm.
Pten;Trp53 dKO medulloblastomas acquired somatic inactivation of Ptch1
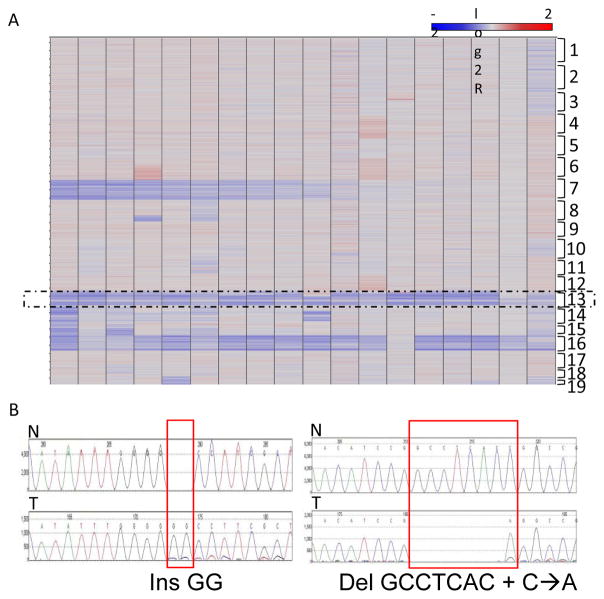
Tumorigenesis is considered a multi-step process involving initiation, promotion and progression. To investigate which other secondary genetic alterations in addition to the initiating Pten and Trp53 mutations were involved in the medulloblastoma formation, we used aCGH to compare genomic DNA from the tumors and reference genomic DNA from liver or spleen. All of the 18 medulloblastomas showed loss of chromosome 13, on which Ptch1 is located (Fig. 7A). In addition, 5 of the tumors had focal homozygous deletion of the BAC probe encompassing Ptch1 (Fig. S6). We also performed RT-PCR and direct sequencing to analyze Ptch1 mRNA and found mutations of Ptch1 in two medulloblastoma samples that had hemizygous deletion of Ptch1 (Fig. 7B). These data support the hypothesis that loss of heterozygosity on chromosome 13 was associated with Ptch1 tumor suppressor loss of function. Thirteen and 18 out of the 18 medulloblastomas also showed loss of the entire or part of chromosomes 7 and 16 respectively (Fig. 7A). No recurrent focal homozygous deletion on chromosome 7 or 16 could be identified.
Figure 7. Pten;Trp53 dKO medulloblastomas acquired somatic inactivation of Ptch1.
(A) Heatmap of array CGH shows Pten;Trp53 dKO medulloblastomas lost chromosomes 7, 13 and 16 at high frequencies. (B) Ptch1 cDNA sequencing of normal reference (N, above) or tumor (T, below), shows two dKO medulloblastomas with hemizygous Ptch1 deletion that also acquired somatic insertion (left) or deletion (right) mutations in the remaining Ptch1 allele.
Pten;Trp53 dKO medulloblastomas showed expresssion signatures of SHH subgroup medulloblastoma
Human medulloblastoma is classified into 4 molecular groups based on gene expression profiles; SHH, WNT, Group 3 and Group 4 (17).
We compared gene expression profiles of dKO medulloblastomas with the expression signatures of the four subgroups of human medulloblastoma using Gene Set Enrichment Analysis (34). Pten;Trp53 dKO medulloblastomas were significantly similar to the SHH subgroup (p=0.082, FDR=0.0766), and were not significantly similar to the other three subgroups of medulloblastoma (FDR>0.25).
Discussion
In this study, we used an inducible Cre driven by Nestin regulatory elements to delete Pten in a wide-spectrum of progenitor cells in the neonatal cerebellum, when the brain is still undergoing active development. We recapitulated the Pten-deficient phenotypes of neuronal hypertrophy and disrupted cerebellar lamination found in previous studies and confirmed that Pten regulates neuronal size and positioning (12, 13, 36). The more severe phenotypes of cerebellar disorganization and hypertrophy found in the current model compared to previous studies are likely caused by the more potent and ubiquitous Cre activity in neural stem/progenitor cells driven by the Nestin promoter than by the Gfap promoter used previously (12, 13, 36).
Unexpectedly, neonatal deletion of Pten revealed a novel perivascular proliferative progenitor niche in the cerebellum that was not found in previous Pten conditional knockout models. Deletion of Pten driven by the same Nestin-creERT2 induced at P30 did not generate this phenotype, suggesting the presence of an unknown perivascular germinal niche in the developing cerebellum that is normally regulated by Pten but regresses or diminishes upon completion of development. Combined deletion of Pten and Tp53 transformed this proliferative niche into medulloblastomas with expression of markers of terminal granule neuron differentiation. The proliferative niche in Pten-cKO cerebellum shared several features with Nestin-expressing progenitors (NEPs) of the EGL (41). The vast majority of granule neurons in the cerebellum are derived from Math1-expressing GNPs. However, 3–5% of EGL cells are NEPs, which express Nestin, but not Math1, and are committed to the granule lineage. These cells are quiescent postnatally and normally undetectable by P21, however, they were more efficiently transformed into sonic hedgehog subgroup medulloblastomas than GNPs in response to Ptch1-deletion (41). However, perivascular proliferative lesions in Pten cKO cerebella were frequently also found within the IGL or molecular layers, clearly isolated from the pial surface where EGL progenitors would reside, or from the white matter tracts, where another population of postnatal cerebellar stem cells has been identified (15). Neural stem cells of the SVZ also reside in a vascular niche (42–44). In Pten cKO cerebella, Pten deficiency may have expanded the location of the previously identified population of Nestin+ progenitors of the EGL, or may have revealed a novel population of perivascular progenitor cells not yet described.
Our study underlines the importance of Pten regulation in the neural stem/progenitor cells in the cerebellum indicating that Pten is required for silencing or withdrawing the neural stem cells upon completion of development under physiological conditions. In the nervous system, Pten negatively regulates proliferation and self-renewal of embryonic neural stem cells (39), and induces premature differentiation of neuroblasts in the subventricular zone of mice from neonatal ages through adulthood (29). Here we report the first example of Pten loss resulting in the presence of a normally undetectable ectopic niche with ongoing progenitor cell proliferation in adult mice.
The outcome of Pten deletion varies significantly in the stem cell niche of different tissues. Importantly, the cerebellar perivascular hyperplasia in Pten cKO cerebellum did not show progressive expansion up to 10 months of age, and was never found to undergo neoplastic transformation. This is consistent with our finding that Pten deletion is not sufficient to initiate tumorigenesis in neural stem/progenitor cells in the forebrain (29), and is in contrast to Pten function in other stem cells. For instance, deletion of Pten causes short-term expansion, but long-term decline of adult hematopoeitic stem cells, and is sufficient to induce eventual emergence of leukemia (40). Intringuingly, Pten deletion has no effect on hematopoietic stem cells in neonatal mice, demonstrating another example of the striking developmental dependence of Pten function (45).
PTEN loss of function or PI3K activating mutations contribute to multiple molecular groups of human medulloblastoma, and in some cases co-occur with TP53 or PTCH1 mutations (3–6). Proliferation of human medulloblastoma cell lines was decreased by a PI3K inhibitor, and medulloblastomas showed reduced PTEN expression (46). PI3K antagonists also inhibit tumor growth of a mouse model of Group 3 medulloblastoma (47). In the postnatal cerebellum, proliferating cells in Pten cKO mice constituted a pre-malignant niche that could be transformed to medulloblastoma with complete penetrance when combined with Tp53 deletion. The recurrent somatic loss of Ptch1 in the Pten;Tp53 dKO medulloblastomas is consistent with the cooperative effects of deregulated PI3K signaling and Ptch1 deletion inducing medulloblastoma in other mouse models. Mouse studies have shown cooperative effects of Shh pathway activation, driven by Ptch1 loss or SmoM2 expression, combined with PI3K activation, driven by PI3K or activated Akt expression, or Pten deletion, in generating medulloblastomas (28, 48–50). Combined inhibition of Shh and PI3K pathways synergize to inhibit tumor growth in a mouse model driven by combined deletion of Pten and Ptch1 (51).
Pten;Trp53 dKO medulloblastomas have a very well-defined organization of cancer stem/progenitor cells maintained in a perivascular niche that give rise to non-proliferative bulk tumor cells expressing markers of fully differentiated granule neurons. This spatial organization and extent of differentiation distinguishes them from other mouse medulloblastoma models associated with Ptch1 loss (18, 20, 49, 52–54). Cancer stem/progenitor cells are maintained in a perivascular niche and give rise to non-proliferative bulk tumor cells expressing markers of fully differentiated granule neurons. This is consistent with a previous study showing that perivascular Nestin-expressing tumor cells were responsible for the recurrence of Ptch1 and Pten dKO medulloblastoma after irradiation (28), and that combined Pten deletion and SHH pathway activation induced medulloblastoma with extensive nodularity in which bulk tumor cells showed differentiated morphology (28, 50). This association between Pten loss and differentiation is consistent with the premature differentiation of neuroblasts caused by Pten deletion in the subventricular zone (29).
The contribution of Pten loss to tumor phenotype also varies markedly between brain tumor types. PTEN and TRP53 are commonly co-mutated in human glioblastomas (7), and combined deletion of Pten and Trp53 driven by induction of GFAP-creER in adult brain resulted in high-grade astrocytomas (33). These tumors did not display the abnormal vasculature or the spatially organized hierarchy of perivascular progenitor cells and highly differentiated bulk tumor cells consistently observed in Pten; Trp53 dKO medulloblastomas. Thus, in keeping with the highly context-dependent outcomes of Pten loss (2), there is not a simple association between Pten loss and tumor phenotype, even among different tumor types within the brain.
Mouse models of spontaneous medulloblastoma seem to over-represent the SHH subgroup of human disease in that the majority of mouse medulloblastomas harbor Ptch1 mutation (20, 49, 53, 55) especially when involving Trp53 mutation. Our Pten and Trp53 dKO medulloblastoma also spontaneously developed Ptch1 mutations as well as other genomic abnormalities. One hundred percent penetrance of medulloblastoma and Ptch1 mutation demonstrates strong cooperation between PI3K, P53 and SHH pathways. Although the subgroup of SHH medulloblastomas are shown to be able to arise from the EGL (18–20, 41), our study indicates an potential alternative origin from nestin positive cells within a perivascular niche that may contribute to potential heterogeneity among human SHH medulloblastoma. This niche may give rise to human medulloblastomas of the SHH molecular subgroup that can arise in the midline of the cerebellum, and not on the cerebellar cortical surface where the EGL is located.
Supplementary Material
Acknowledgments
We thank Dr. Michael Wang for assistance with array figures, Kristen Cox for genotyping and the Hartwell Center for Biotechnology for array experiments. This work was supported by NIH grant P01 CA096832 to SJB, JZ and DWE, the St Jude Cancer Center support grant P30 CA021765, and ALSAC of St Jude Children’s Research Hospital.
Footnotes
The authors declare no potential conflicts of interest
References
- 1.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nature reviews Molecular cell biology. 2012;13:283–96. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 2.Endersby R, Baker SJ. PTEN signaling in brain: neuropathology and tumorigenesis. Oncogene. 2008;27:5416–30. doi: 10.1038/onc.2008.239. [DOI] [PubMed] [Google Scholar]
- 3.Parsons DW, Li M, Zhang X, Jones S, Leary RJ, Lin JC, et al. The genetic landscape of the childhood cancer medulloblastoma. Science. 2011;331:435–9. doi: 10.1126/science.1198056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson G, Parker M, Kranenburg TA, Lu C, Chen X, Ding L, et al. Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488:43–8. doi: 10.1038/nature11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones DT, Jager N, Kool M, Zichner T, Hutter B, Sultan M, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488:100–5. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pugh TJ, Weeraratne SD, Archer TC, Pomeranz Krummel DA, Auclair D, Bochicchio J, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488:106–10. doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturm D, Bender S, Jones DT, Lichter P, Grill J, Becher O, et al. Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nature reviews Cancer. 2014;14:92–107. doi: 10.1038/nrc3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatten ME, Roussel MF. Development and cancer of the cerebellum. Trends Neurosci. 2011;34:134–42. doi: 10.1016/j.tins.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–8. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- 10.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–14. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 11.Kenney AM, Widlund HR, Rowitch DH. Hedgehog and PI-3 kinase signaling converge on Nmyc1 to promote cell cycle progression in cerebellar neuronal precursors. Development. 2004;131:217–28. doi: 10.1242/dev.00891. [DOI] [PubMed] [Google Scholar]
- 12.Backman SA, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J, et al. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet. 2001;29:396–403. doi: 10.1038/ng782. [DOI] [PubMed] [Google Scholar]
- 13.Kwon CH, Zhu X, Zhang J, Knoop LL, Tharp R, Smeyne RJ, et al. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat Genet. 2001;29:404–11. doi: 10.1038/ng781. [DOI] [PubMed] [Google Scholar]
- 14.Marino S, Krimpenfort P, Leung C, van der Korput HA, Trapman J, Camenisch I, et al. PTEN is essential for cell migration but not for fate determination and tumourigenesis in the cerebellum. Development. 2002;129:3513–22. doi: 10.1242/dev.129.14.3513. [DOI] [PubMed] [Google Scholar]
- 15.Lee A, Kessler JD, Read TA, Kaiser C, Corbeil D, Huttner WB, et al. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci. 2005;8:723–9. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Goldman JE. Generation of cerebellar interneurons from dividing progenitors in white matter. Neuron. 1996;16:47–54. doi: 10.1016/s0896-6273(00)80022-7. [DOI] [PubMed] [Google Scholar]
- 17.Northcott PA, Jones DT, Kool M, Robinson GW, Gilbertson RJ, Cho YJ, et al. Medulloblastomics: the end of the beginning. Nature reviews Cancer. 2012;12:818–34. doi: 10.1038/nrc3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang ZJ, Ellis T, Markant SL, Read TA, Kessler JD, Bourboulas M, et al. Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer Cell. 2008;14:135–45. doi: 10.1016/j.ccr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, et al. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14:123–34. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y, Miller HL, Jensen P, Hernan R, Connelly M, Wetmore C, et al. A molecular fingerprint for medulloblastoma. Cancer Res. 2003;63:5428–37. [PubMed] [Google Scholar]
- 21.Read TA, Fogarty MP, Markant SL, McLendon RE, Wei Z, Ellison DW, et al. Identification of CD15 as a marker for tumor-propagating cells in a mouse model of medulloblastoma. Cancer Cell. 2009;15:135–47. doi: 10.1016/j.ccr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468:1095–9. doi: 10.1038/nature09587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawauchi D, Robinson G, Uziel T, Gibson P, Rehg J, Gao C, et al. A mouse model of the most aggressive subgroup of human medulloblastoma. Cancer Cell. 2012;21:168–80. doi: 10.1016/j.ccr.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pei Y, Moore CE, Wang J, Tewari AK, Eroshkin A, Cho YJ, et al. An animal model of MYC-driven medulloblastoma. Cancer Cell. 2012;21:155–67. doi: 10.1016/j.ccr.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward RJ, Dirks PB. Cancer stem cells: at the headwaters of tumor development. Annu Rev Pathol. 2007;2:175–89. doi: 10.1146/annurev.pathol.2.010506.091847. [DOI] [PubMed] [Google Scholar]
- 26.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 27.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22:436–48. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu G, Chow LM, Bayazitov IT, Tong Y, Gilbertson RJ, Zakharenko SS, et al. Pten deletion causes mTorc1-dependent ectopic neuroblast differentiation without causing uniform migration defects. Development. 2012;139:3422–31. doi: 10.1242/dev.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–34. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 32.Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–25. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 33.Chow LM, Endersby R, Zhu X, Rankin S, Qu C, Zhang J, et al. Cooperativity within and among Pten, p53, and Rb pathways induces high-grade astrocytoma in adult brain. Cancer Cell. 2011;19:305–16. doi: 10.1016/j.ccr.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Y, Miller HL, Russell HR, Boyd K, Curran T, McKinnon PJ. Patched2 modulates tumorigenesis in patched1 heterozygous mice. Cancer Res. 2006;66:6964–71. doi: 10.1158/0008-5472.CAN-06-0505. [DOI] [PubMed] [Google Scholar]
- 36.Fraser MM, Zhu X, Kwon CH, Uhlmann EJ, Gutmann DH, Baker SJ. Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res. 2004;64:7773–9. doi: 10.1158/0008-5472.CAN-04-2487. [DOI] [PubMed] [Google Scholar]
- 37.Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, et al. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci U S A. 2008;105:1026–31. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–16. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 39.Groszer M, Erickson R, Scripture-Adams DD, Dougherty JD, Le Belle J, Zack JA, et al. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc Natl Acad Sci U S A. 2006;103:111–6. doi: 10.1073/pnas.0509939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–22. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 41.Li P, Du F, Yuelling LW, Lin T, Muradimova RE, Tricarico R, et al. A population of Nestin-expressing progenitors in the cerebellum exhibits increased tumorigenicity. Nat Neurosci. 2013;16:1737–44. doi: 10.1038/nn.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–78. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magee JA, Ikenoue T, Nakada D, Lee JY, Guan KL, Morrison SJ. Temporal changes in PTEN and mTORC2 regulation of hematopoietic stem cell self-renewal and leukemia suppression. Cell Stem Cell. 2012;11:415–28. doi: 10.1016/j.stem.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hartmann W, Digon-Sontgerath B, Koch A, Waha A, Endl E, Dani I, et al. Phosphatidylinositol 3′-kinase/AKT signaling is activated in medulloblastoma cell proliferation and is associated with reduced expression of PTEN. Clin Cancer Res. 2006;12:3019–27. doi: 10.1158/1078-0432.CCR-05-2187. [DOI] [PubMed] [Google Scholar]
- 47.Pei Y, Liu KW, Wang J, Garancher A, Tao R, Esparza LA, et al. HDAC and PI3K Antagonists Cooperate to Inhibit Growth of MYC-Driven Medulloblastoma. Cancer Cell. 2016;29:311–23. doi: 10.1016/j.ccell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao G, Pedone CA, Del Valle L, Reiss K, Holland EC, Fults DW. Sonic hedgehog and insulin-like growth factor signaling synergize to induce medulloblastoma formation from nestin-expressing neural progenitors in mice. Oncogene. 2004;23:6156–62. doi: 10.1038/sj.onc.1207818. [DOI] [PubMed] [Google Scholar]
- 49.Frappart PO, Lee Y, Russell HR, Chalhoub N, Wang YD, Orii KE, et al. Recurrent genomic alterations characterize medulloblastoma arising from DNA double-strand break repair deficiency. Proc Natl Acad Sci U S A. 2009;106:1880–5. doi: 10.1073/pnas.0806882106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castellino RC, Barwick BG, Schniederjan M, Buss MC, Becher O, Hambardzumyan D, et al. Heterozygosity for Pten promotes tumorigenesis in a mouse model of medulloblastoma. PLoS One. 2010;5:e10849. doi: 10.1371/journal.pone.0010849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Metcalfe C, Alicke B, Crow A, Lamoureux M, Dijkgraaf GJ, Peale F, et al. PTEN loss mitigates the response of medulloblastoma to Hedgehog pathway inhibition. Cancer Res. 2013;73:7034–42. doi: 10.1158/0008-5472.CAN-13-1222. [DOI] [PubMed] [Google Scholar]
- 52.Zindy F, Nilsson LM, Nguyen L, Meunier C, Smeyne RJ, Rehg JE, et al. Hemangiosarcomas, medulloblastomas, and other tumors in Ink4c/p53-null mice. Cancer Res. 2003;63:5420–7. [PubMed] [Google Scholar]
- 53.Wetmore C, Eberhart DE, Curran T. The normal patched allele is expressed in medulloblastomas from mice with heterozygous germ-line mutation of patched. Cancer Res. 2000;60:2239–46. [PubMed] [Google Scholar]
- 54.Uziel T, Zindy F, Xie S, Lee Y, Forget A, Magdaleno S, et al. The tumor suppressors Ink4c and p53 collaborate independently with Patched to suppress medulloblastoma formation. Genes Dev. 2005;19:2656–67. doi: 10.1101/gad.1368605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zindy F, Uziel T, Ayrault O, Calabrese C, Valentine M, Rehg JE, et al. Genetic alterations in mouse medulloblastomas and generation of tumors de novo from primary cerebellar granule neuron precursors. Cancer Res. 2007;67:2676–84. doi: 10.1158/0008-5472.CAN-06-3418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.