Abstract
Small felids influence ecosystem dynamics through prey and plant population changes. Although most of these species are threatened, they are accorded one of the lowest research efforts of all felids, and we lack basic information about them. Many felids occur in sympatry, where intraguild competition is frequent. Therefore, assessing the role of interspecific interactions along with the relative importance of landscape characteristics is necessary to understand how these species co-occur in space. Here, we selected three morphologically similar and closely related species of small Neotropical cats to evaluate the roles of interspecific interactions, geomorphometry, environmental, and anthropogenic landscape characteristics on their habitat use. We collected data with camera trapping and scat sampling in a large protected Atlantic forest remnant (35,000 ha). Throughout occupancy modeling we investigated whether these species occur together more or less frequently than would be expected by chance, while dealing with imperfect detection and incorporating possible habitat preferences into the models. We used occupancy as a measure of their habitat use. Although intraguild competition can be an important determinant of carnivore assemblages, in our system, we did not find evidence that one species affects the habitat use of the other. Evidence suggested that proximity to the nature reserve (a more protected area) was a more important driver of Neotropical spotted cats’ occurrence than interspecific interactions or geomorphometry and environmental landscape characteristics—even though our entire study area is under some type of protection. This suggests that small felids can be sensitive to the area protection status, emphasizing the importance of maintaining and creating reserves and other areas with elevated protection for the proper management and conservation of the group.
Introduction
Predators play an important role in regulating ecosystem functioning and dynamics [1, 2]. They affect prey populations and, as a result, alter plant abundance, composition, succession, dispersion, and diversity [2, 3]. Consequently, the loss of this key group can lead to regime shifts, alternative states of ecosystems, and possible losses of ecosystem services [2]. Given the role of carnivores on ecosystem functioning and their sensitivity to the environment, landscapes with these animals—implying a relatively intact food web—have high potential for ecological integrity [4], which encompasses ecosystem health, biodiversity, stability, and sustainability [5]. Therefore, maintenance of carnivores can serve as a useful tool for protected area design and conservation planning [6].
Among the carnivores, felids are extreme as obligatory flesh eaters [7]. Historically, felids have suffered several anthropogenic impacts, which represent a major cause of felid mortality in several regions of the globe and account for up to 70% of deaths in some populations [8, 9]. For example, larger felids are frequently involved in conflicts with humans due to preying on domestic animals or livestock [9–13], and small felids are constantly suffering from fur trade [8, 14]. Although several countries banned the export of wildcat skins and signed the Convention on International Trade in Endangered Species of Wild Flora and Fauna (CITES) [9], illegal hunting of felids still occurs (e.g., [11]). Felids can also be exposed to diseases carried by domestic carnivores and to poaching, which even at moderate levels over a relatively short period of time can lead to massive population decline [9, 15, 16]. Hunting and these other human-related pressures are most likely to occur in areas with high accessibility or low (or inefficient) protection status, and in unrestricted areas surrounding protected areas [9, 17–19]. In highly protected populations, anthropogenic mortality is rare [9]. More recently, mammalian carnivores face local extinction due to habitat loss and fragmentation, exacerbated by their relatively large home ranges, low densities, and direct persecution by humans [4, 20–22].
Given the current scenario, understanding how species relate spatially to the environment and to human disturbances is critical to the assignment of areas for conservation and the development of conservation strategies [23–26]. Besides landscape characteristics, interspecific interactions may also regulate the occurrence, distribution or persistence of species [27]. In carnivore assemblages, intraguild interference competition and killing are potentially important determinants of species abundance and distribution and can lead to adaptive responses in use of space and activity patterns [7, 28, 29], which enable the coexistence of morphologically similar species [30].
Because of relatively recent divergence and constraints imposed by foraging and diet, felids present similar morphologies [31, 32]. Thus, they are a good model to understand how closely related and morphologically similar species can coexist. However, though felids are frequently sympatric, with the largest assemblages occurring in the tropical regions of the Americas, little attention has been given to the coexistence of these species and its implications [8]. The Neotropical spotted cats are the main sympatric small felids in Neotropical rainforests: Leopardus pardalis—ocelot, L. wiedii—margay, and L. guttulus—oncilla (formerly known as L. tigrinus [33]). These species have discrete differences in body sizes (8–15 kg, 3-9kg, and 1.5-3kg, respectively [34]) and share an important portion of their food sources [35, 36], therefore differential food exploitation is unlikely and intraguild competition is probably high [37]. They have been accorded one of the lowest research efforts of all felids, and more basic information on their biology and ecology is urgently needed [7, 8].
Frequently, studies on species occurrence and distribution assume that all species present at a location are detected with certainty. However, accounting for imperfect detection is fundamental to avoid omission errors (false absences) [38, 39] and resultant bias in parameter estimation [40]. Such omission errors may lead to incorrect inferences about species-habitat relationships or patterns of species co-occurrence [41]. A recent adaptation to occupancy models [42] allows such problems to be dealt with by incorporating detection probability, as well as possible habitat preferences, directly into the model set and evaluating co-occurrence patterns among pairs of species [41].
We investigated the role of geomorphometry, environmental and anthropogenic landscape attributes at multiple scales and interspecific interactions in the habitat use of Neotropical spotted cats in a large Atlantic forest remnant. Using a likelihood-based framework, we estimated the probability of occurrence and co-occurrence while accounting explicitly for imperfect detectability and habitat preferences. We used occupancy as a measure of habitat use, and developed specific models based on different hypotheses about effects of landscape characteristics and competition on the habitat use of Neotropical spotted cats. We have two alternative hypotheses: 1) The habitat use of Neotropical spotted cats is mainly driven by landscape characteristics—If landscape characteristics are important factors determining how spotted cats use the habitat, we would expect anthropogenic-related variables to be the main predictors of occurrence, given the sensitivity of carnivores to anthropogenic impacts [8, 9]. More specifically, because human-related pressures are most likely to occur in areas with low protection status or with high accessibility [9, 17–19], we would predict a negative association between habitat use and road density (a measure of human accessibility) and between habitat use and distance to a more protected area (a reserve). We would also expect that prey, hydrographic density, and forest cover would have a positive effect on habitat use, since those variables represent important resources (food and water availability) and high-quality habitats, but they would have a smaller influence than the human-related variables. We would also expect elevation to have a weak influence on how Neotropical spotted cats use the habitat, unless species are segregating in altitude due to the montaneous terrain in our study area; this phenomenon has been observed in other taxa (e.g., [41]). 2) The habitat use of Neotropical spotted cats is mainly driven by interspecific interactions—If competition is a major determinant of the habitat use of Neotropical spotted cats, we would expect their occupancy and/or detection probability to be lower when another spotted cat is present or detected; co-occurrence should be less than expected by chance, predicting avoidance, considering the commonness of interference intraguild competition and killing among carnivores [7, 28, 29]. We believe that knowledge of these potential relationships will be useful for planning management actions towards the conservation of this key group in Neotropical forests and helpful in clarifying the role of interspecific interactions on the occurrence of small felids, which could benefit our understanding of how small felids occur in other ecosystems worldwide.
Methods
Study area
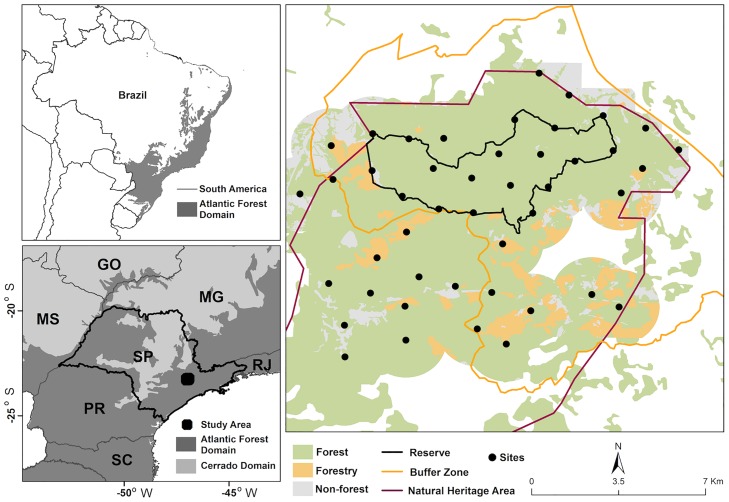
Serra do Japi (southeast Brazil, coordinates 47°03'40"W to 46°52'20"W and 23°22'30"S to 23°11'35"S; Fig 1) is one of the few large remnants of Atlantic Forest. The Atlantic Forest represents a global hotspot for biodiversity conservation [43], and currently it is highly fragmented (more than 80% of the remnants are < 50 ha in size), highly isolated (average distance between fragments is 1,440 m), and under negative edge influences (73% of remnants are 250 m from any forest edge) [44]. The study area is a Natural Heritage Area (35,000 ha) considered part of the UNESCO’s Atlantic Forest Biosphere Reserve [45]. Located within this area is the Biological Municipal Reserve (REBIO—2,071 ha) surrounded by a Buffer Zone (11,946 ha) (Fig 1). The REBIO presents the highest protection status in the area, where the only permitted activities are research and education. In the Natural Heritage Area and in the Buffer Zone, however, agriculture, horticulture and other forms of economic activities are present. The vegetation of the area is characterized as semideciduous mesophilic forest with mountainous terrain and a seasonal climate [46]. The mean temperature is 19.7°C and the mean annual rainfall is 1,422 mm, with a dry and cold season occurring from April to September and a wet and warm season from October to March [46].
Fig 1. Study area.
Study area and the sampling sites’ locations at Serra do Japi (Brazil) where Neotropical spotted cats were sampled using camera trap and scat sampling.
Data collection
From April 2013 to September 2014, we collected data on three Neotropical spotted cats (Leopardus pardalis, L. wiedii, and L. guttulus) at 45 sampling sites (spaced approx. 1.5 km apart) distributed in a regular grid across the forest remnant. This distance between the sampling sites provided a good coverage of our study area, and it is in agreement with the TEAM “terrestrial vertebrate (camera trap) monitoring protocol implementation manual” [47]. Furthermore, we had no evidence for spatial autocorrelation in data while using this spacing (S1 Table).
We conducted three campaigns at each sampling site to collect data: 1- April 2013 to September 2013; 2- October 2013 to March 2014; 3- April 2014 to September 2014. A different group of 15 sampling sites was surveyed every two months within each campaign (see S1 Fig for details). Hence, 45 sites were sampled during each campaign. We used camera trapping (passive infrared camera traps; Bushnell Trophy Cam; N = 5,198 trap days) and scat sampling during the first and the second campaigns, and only scat sampling during the third campaign. All cameras were fixed about 20 cm above ground and no bait was used. Traps were installed with a minimum distance of approx. 50 m from roads or trails, and none of the sampling sites was located close to roads that were highly used or open to the public. We visited each sampling site six times and collected all scats found along a 1-km segment in the dirt road closest to each site. There was no overlap between neighboring sampled areas because each sampling site was usually located in the center of its 1-km segment.
In addition to the felids’ data, we collected information on their main prey using the records from the camera traps. This was possible due to the height at which the cameras were positioned and the number of records (i.e., detections: Nsmall mammals = 77; Nmedium-sized mammals = 897; Nground-dwelling birds = 1,936). Although cameras are more commonly used to survey medium- and large-sized mammals, they can also be used to collect data on small mammals [48–50] and ground-dwelling birds [51–53]. They may provide a new and cost-effective technique for surveying terrestrial small mammals, particularly when presence data are the main requirement of the survey [54–55]. The use of camera traps to collect data on small mammals to assess prey availability for carnivores was already performed [56], but here we went a step further and included imperfect detection on prey estimates (through occupancy modeling, see “Single-species occupancy models” section) instead of using the number of captures. We considered small-sized mammals (< 1 kg; mainly small rodents and small marsupials) and small birds (< 0.2 g; mainly passerines and doves) as the main prey for margay and oncilla, and small and medium-sized mammals (< 13 kg; mainly small rodents, small marsupials, opossums, Brazilian rabbit, paca, armadillo, and porcupine) as well as small and medium-sized birds (< 0.5 g; passerines, doves, and tinamous) for ocelot [35, 57–61].
Identification of felids using tricology and genetics
Because felids defecate conspicuously to signal their presence [62, 63] and groom frequently [64], samples of scats with hairs are particularly easy to obtain. After washing the scats with running water and drying them, we collected the guard hairs found in each sample to identify the species to which the hair belonged. We cleaned the guard hairs with ethyl alcohol, and the cuticular impressions were obtained by pressing the hairs against a thin layer of nail varnish and leaving them to dry for three to five minutes on glass slides with the help of a bench vise (adapted from [65]). We photographed the cuticular impressions at 400x magnification (S2 Fig) and compared the pattern of the cuticles with our reference collection (obtained from hairs collected from museum specimens) and published guides [66, 67].
Hair sampling can lead to reliable detections of rare and cryptic animals [68], and the use of mammalian hair for identification of taxa, known as tricology, is an established low-cost method (e.g., [69–74]). This technique has also been proven to be as consistent as molecular methods for identification of some Neotropical felids [75]. To test the accuracy of our identification through tricology, we conducted molecular analysis for 74% of the samples (N = 49). We used mini-barcoding for molecular identification, comparing two markers from mitochondrial DNA [ATP6 (126 bp) and cytochrome oxidase I gene (COI) (187 bp)], applying the primers developed by [76]. The obtained sequences were compared with reference sequences from tissue samples of each species. We achieved confirmation for 100% of the samples, giving us confidence in our identification and confirming the reliability of the method.
Landscape covariates
We mapped with Quantum Gis software [77] the vegetation cover, hydrography and roads of the study area using high resolution satellite image interpretation at a 1:5,000 scale and cartographic maps (Secretariat of Economy and Planning—São Paulo State Government, at 1:10,000). We validated the cover map by extensive field verification with a botanist (see acknowledgements).
Because observed responses of the organisms to the environment may depend on the extent at which the environment is being perceived (and thus measured; [21, 78, 79]), we adopted a multi-spatial extent—also found in the literature as multiple scales [78]–approach while testing the influence of the covariates on felid occupancy. The extents were defined as concentric circles (buffers) of 500 and 1,000 m radius around each sampling site, and we calculated each site covariate for each extent. The smaller buffer (which covers 78 ha) is equivalent to the minimum known size for an ocelot home range (see [80]). This extent is likely smaller than the average home range of the other two cats as well [80]. The area of the larger buffer (314 ha) is closer to the home range of some ocelots, but might be also regarded as spatially conservative, considering the huge variation in home range size known from this and the other two cats [80]. Further, the larger buffer is the maximum we can use without overlapping with buffers of adjacent sampling points.
We determined five landscape site covariates: mean elevation, percentage of high-quality forest cover, hydrographic density, road density, and ‘weighted distance to reserve border’. The percentage of high-quality forest cover was measured as the percentage of intermediate and advanced forest succession cover within each buffer, which was calculated using Geographical Resources Analysis Support System (GRASS) [81]. Road and hydrographic densities within each buffer were calculated with the Kernel density function in ArcGIS software [82]. We obtained the mean elevation at each buffer from digital elevation models (DEM) available from Topodata Geomorphic database of Brazil [83]. We measured the distance from each sampling site to the nearest boundary of the Biological Municipal Reserve (REBIO), giving negative distances (in meters) to sites within the REBIO and positive distances otherwise (i.e., the center of the reserve received the smallest value). Then the covariate ‘weighted distance to reserve border’ was obtained by multiplying these distances by the protection status weight of the subarea in which each sampling site was located (REBIO = 1; REBIO’s Buffer Zone = 2; within the Natural Heritage Area but outside these two areas = 3; outside these three areas = 4). For ocelot, we used the distance to reserve border instead of ‘weighted distance to reserve border’ (due to the lack of convergence in the models with this latter covariate). We normalized all covariates and used only covariates with low correlation (S2 Table) in the final model sets (i.e., group of models being tested together).
Single-species occupancy models
We used occupancy modeling [42]—with a likelihood-based approach—to estimate the site occupancy (ψ) of each spotted cat species and their main potential prey, and to evaluate the influential factors on the felids’ occupancy while accounting for detection probability (p). Because the size of the home ranges of ocelots, margays, and oncillas likely exceed that of our sampling unit [84–87], we used occupancy as a measure of their habitat use instead of “true occupancy” [38, 88]. A basic assumption of the occupancy models is that the sites are closed to changes in occupancy during the repeated surveys [89]. This assumption can be relaxed when occupancy is interpreted as “use” and movement in and out of the sampled area is random [41, 89], similarly to our approach for the felids. We assessed the usage made of sites with various habitat characteristics within their home range, therefore we should interpret our result in a third-order selection level [90]. However, because individual animals were not identified, measures were made at the population level (i.e., it is a Design I study type; [91]).
We adopted a single-season approach [42], wherein data from all three campaigns were combined. Data from both methods (camera trapping and scat sampling) were included, but in different sampling occasions. The decision to adopt a single-season modeling instead of a multi-season approach was based on our previous analyses, which provided no evidence of changes in occupancy across the three campaigns. Specifically, we fit both full multi-season models and models where colonization and extinction were fixed to zero (no change), and the latter models were ranked as the best (S3 Table). We also developed models that included an effect of campaign/time on detection probabilities, but model selection results provided no evidence that this effect was needed (S4 Table).
We used a two-step approach while modeling the occupancy of each species of felid and prey: 1) assess spatial scale that best represents species’ response to each covariate; and 2) investigate sources of variation in ψ, determining the most influential covariates for occupancy, the parameter with biological meaning and in which we were most interested; as detailed next. To determine the scale that best represents each species’ response to the habitat, we used a general model for p (that consisted of as many potential covariates as possible) and allowed occupancy (ψ) to vary (following [92]) by only the focal habitat covariate measured at the extents of the two buffer sizes (S5 Table). After selecting the appropriate extent for each covariate, we developed another model set to investigate the variation in occupancy (S6 Table). In this second step, we allowed ψ to be constant (ψ(.)) or to vary as a function of either a single covariate or a combination of two (only additive effects). We built only models that translated plausible biological hypotheses regarding the effects of variables on each species’ occupancy (ψ). We used each covariate at its extent of stronger response for each species (from the previous step) and a general model for p. The potential covariates used in the general model for p were: method used to survey each sampling occasion (camera trapping or scat sampling), degree of soil coverage by plants or leaf litter on the roads where scats were sampled (0- no coverage; 1- low to medium coverage; and 2- high coverage), and percentage of high-quality forest cover at the 500 m buffer around each sampling site. By using a general model for the parameters that were not investigated within a specific model set, we reduced the possibility that imposed constraints (on p, for example) would result in residual sampling variation being attributed to a variation in occupancy.
We evaluated candidate models and estimated parameters using PRESENCE software [93] to determine the covariates that best explain occupancy. We ranked candidate models using the Akaike's Information Criterion adjusted for small sample size (AICc; N = number of sites) [94] and excluded all models that did not converge. We considered the covariate(s) from the top-ranked model(s) (i.e., models with ΔAICc<2) as the most likely determinant(s) of the species’ occupancy. When different spatial extents were equally plausible (ΔAICc<2), we chose to use the extent closer to the home range size of the spotted cats (1,000 m) in the final models. Additionally, we assessed the relative importance of each covariate by summing the Akaike weights (wi) of all the models (i) in which that covariate was present [94]. We applied model averaging [94] to estimate the overall occupancy of each felid at our study area and to estimate the site occupancy of each prey. The prey index was obtained by summing the site occupancy of all potential prey for each felid species.
Co-occurrence models
We investigated whether the presence of one felid influences the occupancy and detection probability of another felid by pair-wise comparisons for all felid species using two-species single-season occupancy models [41]. We used the ψBa/rBa parameterization in PRESENCE software [93], assuming that the dominant species was always the larger (i.e., greater mean body mass) in the analyzed pair (ocelot in ocelot-margay and ocelot-oncilla pairs, and margay in the margay-oncilla pair; [34]). The parameters estimated for occupancy were: ψA (occupancy of dominant species), ψBA (occupancy of subordinate species when the dominant species is present), and ψBa (occupancy of subordinate species when the dominant species is absent). We modeled ψA, ψBA, and ψBa, incorporating the best covariate revealed in the single-species models for each species to account for possible differences in habitat preferences. We also incorporated the method used to survey each sampling occasion (camera trapping and scat sampling) as a covariate for p in all co-occurrence analyses. We built models that assumed that the occupancy of the subordinate species was influenced by the dominant species (ψBA≠ψBa) or was independent of the dominant species (ψBA = ψBa).
For detection probability, the parameters estimated were: rA (probability of dominant species being detected when the subordinate species is present), pA (probability of dominant species being detected when the subordinate species is absent), pB (probability of subordinate species being detected when the dominant species is not present), rBA (probability of subordinate species being detected when the dominant species is present and detected), rBa (probability of subordinate species being detected when the dominant species is present but not detected). We built models where the detection probability of the subordinate species was influenced by the presence (pB≠rBa; pB≠rBA) or detection (rBa≠rBA) of the dominant species or was independent of the dominant species (pB = rBa = rBA), as well as models that assumed that the detection of the dominant species was influenced by the detection of the subordinate species (rA≠pA) or independent (rA = pA).
We calculated the species interaction factor (SIF) for occupancy (phi; [95]) and detection probability (; adapted from the formula for phi from [95]). The SIF is a ratio of how likely the two species are to co-occur compared to what would be expected under a hypothesis of independence [94]. We obtained the parameter estimates by model averaging [95] the estimates of each species-pair model set (i.e., group of all models tested for each species-pair). If two species occur or are detected independently, SIF = 1. If SIF<1, species co-occur or are detected less frequently than would be expected if they were independent (i.e., avoidance). If SIF>1, species co-occur or are detected more frequently than expected (i.e., aggregation) [95].
To rank candidate models, we adopted the same procedure as the single-species analysis. To infer about the co-occurence patterns, we considered the estimated parameters (ψA, ψBA, ψBa, rA, pA, pB, rBa, rBA), the relationships among them, the top-ranked model(s) (ΔAICc<2), and the SIF calculated for each species pair.
Ethics statement
Jundiaí City Hall and private owners provided permission to conduct this project at Serra do Japi. During this research, the animals were observed in their natural environment and none of them were captured or handled. Therefore, there are no protocols to be reported to institutional or governmental agencies that regulate animal research.
Results
Spatial scale
Models with the same covariate measured at different extents (500 m vs. 1,000 m) were usually equally supported, with the exception of high-quality forest cover for ocelot, which was better explained by the 500 m extent than the 1,000 m extent (S5 Table).
Single-species occupancy
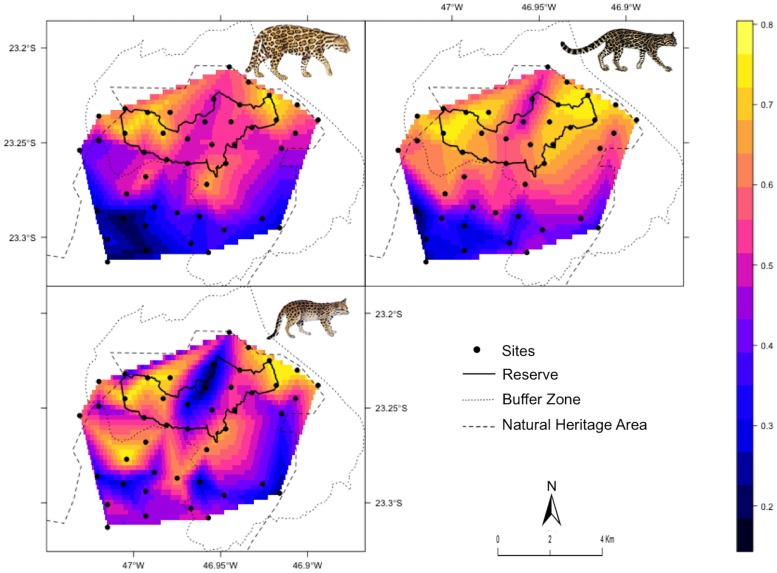
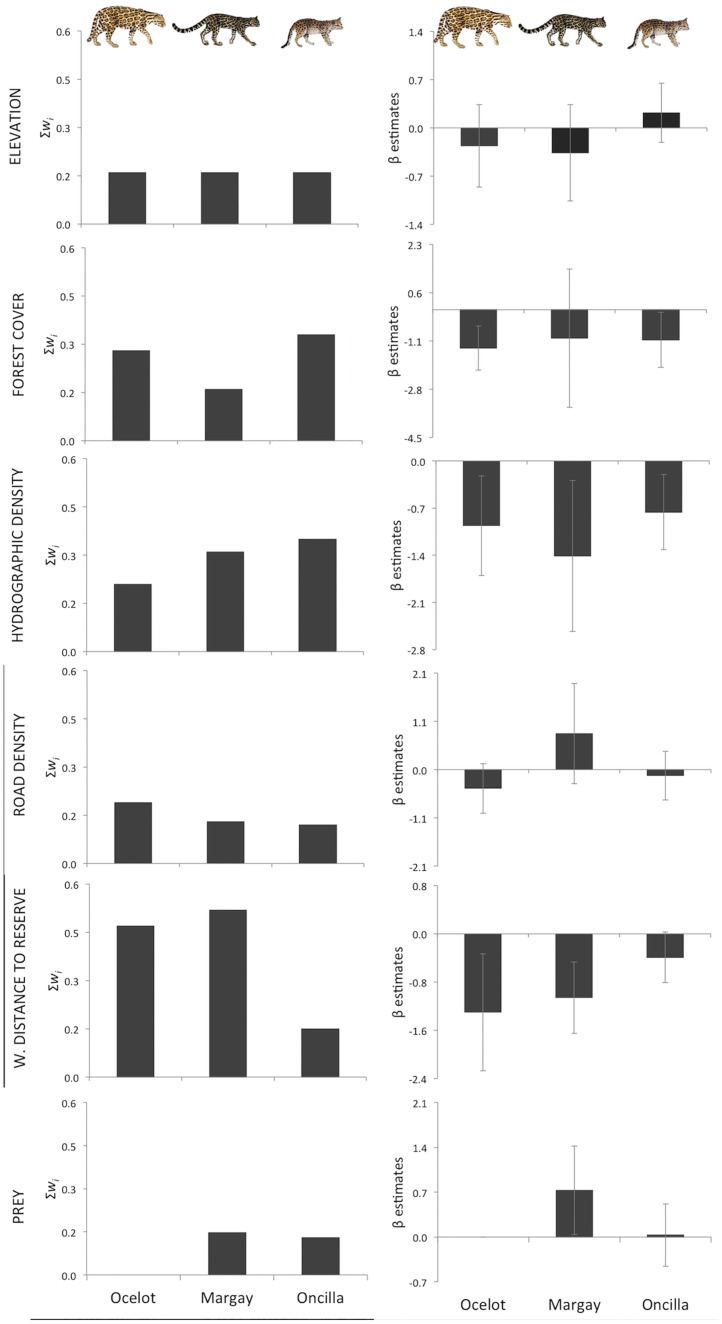
We had 123 detections (N = 5,198 trap days) of the three Neotropical spotted cats (Table 1; S7 Table). We had some evidence that the habitat use of the cats varied according to landscape characteristics (Fig 2). Margay and ocelot had higher occupancies closer to a more protected area (i.e., reserve), and weighted distance to reserve border was the main factor influencing their habitat use, emerging as the top-ranked model with high relative importance (Fig 3; S6 Table). All other analyzed covariates had overall low relevance (Fig 3; S6 Table).
Table 1. Number of records (detections) by each method (scat sampling and camera trap), number of sampling sites with detections, naïve occupancy, estimated occupancy probability () from single-season single-species models, and relative increase above naïve occupancy when using estimates of the three Neotropical spotted cats in a large Atlantic Forest remnant.
| N detections | N sites w. detections | Naïve occup. | Detection probability (p) | Rel. increase above naïve occup. (%)2 | ||||
|---|---|---|---|---|---|---|---|---|
| Scats | Camera traps | Scats | Camera traps | Occup. prob. 1 | ||||
| Ocelot | 8 | 16 | 12 | 0.27 | 0.07 | 0.08 | 0.48 (±0.15) | 80 |
| Margay | 27 | 12 | 17 | 0.38 | 0.11 | 0.05 | 0.64 (±0.17) | 69 |
| Oncilla | 33 | 29 | 21 | 0.47 | 0.14 | 0.07 | 0.64 (±0.13) | 37 |
1Occupancy probability and standard deviation estimated by model averaging.
2Percentage increase in estimated proportion of occupied sites when incorporating detection probability (p) [(estimated occupancy probability/naïve occupancy)-1*100].
Fig 2. Neotropical spotted cats’ site occupancy.
Interpolated site occupancy of the three spotted cats at an Atlantic Forest site in Brazil: ocelot—Leopardus pardalis (top left), margay—L. wiedii (top right), oncilla—L. guttulus (bottom left).
Fig 3. Covariates effect on the occupancy of Neotropical spotted cats.
Influence of geomorphometry, environmental, and anthropogenic covariates on the occupancy of spotted cats in a large Atlantic Forest remnant, showing the sum of wi (which indicates the relative importance of covariates) and the associated beta estimates with standard error estimated from the single-season single-species models.
Margay and oncilla, the two smaller and more cryptic cats, had more detections from scat sampling, a method that had a detection probability of twice the detection probability of camera traps (Table 1).
Co-occurrence patterns
There was no evidence that one species affects the occupancy of the other (Tables 2 and 3). For all three pairs of species (ocelot-margay, ocelot-oncilla, and margay-oncilla), the models where the pair had similar occupancies and the subordinate species had similar occupancy regardless of the dominant being at the sampling site or not (i.e., ψA = ψBA = ψBa) were ranked as the top models (Table 2). Furthermore, models where the covariate ‘weighted distance to reserve border’ was incorporated with ocelot and margay occupancy were always ranked better than the models without this covariate, reinforcing the importance of the reserve for both species (Table 2).
Table 2. Co-occurrence occupancy models used to evaluate the role of interspecific interactions on the habitat use of three sympatric Neotropical spotted cats in a large Atlantic Forest remnant.
| Model | AIC | ΔAIC | wi | K | -2LL |
|---|---|---|---|---|---|
| Ocelot-Margay | |||||
| ψA(reserve dist) = ψBA(reserve dist) = ψBa(reserve dist) p(global1) | 349.56 | 0 | 0.76 | 9 | 326.42 |
| ψA(reserve dist)≠ψBA(reserve dist) = ψBa(reserve dist) p(global) | 352.25 | 2.69 | 0.20 | 10 | 325.78 |
| ψA = ψBA = ψBa p(global) | 355.87 | 6.31 | 0.03 | 8 | 335.87 |
| ψA≠ψBA = ψBa p(global) | 358.03 | 8.47 | 0.01 | 9 | 334.89 |
| ψA≠ψBA≠ψBa p(global) | 361.32 | 11.76 | 0.00 | 10 | 334.85 |
| Ocelot-Oncilla | |||||
| ψA(reserve dist) = ψBA = ψBa p(global2) | 386.13 | 0 | 0.34 | 8 | 366.13 |
| ψA(reserve dist)≠ψBA = ψBa p(global) | 389.20 | 3.07 | 0.07 | 9 | 366.06 |
| ψA(reserve dist)≠ψBA≠ψBa p(global) | 392.53 | 6.40 | 0.01 | 10 | 366.06 |
| Margay-Oncilla | |||||
| ψA(reserve dist) = ψBA = ψBa p(global3) | 443.56 | 0 | 0.60 | 5 | 432.02 |
| ψA(reserve dist)≠ψBA = ψBa p(global) | 445.84 | 2.28 | 0.19 | 6 | 431.63 |
| ψA(reserve dist)≠ψBA≠ψBa p(global) | 447.44 | 3.88 | 0.09 | 7 | 430.41 |
| ψA,ψBA = ψBa p(global) | 447.61 | 4.05 | 0.08 | 5 | 436.07 |
| ψA≠ψBA≠ψBa p(global) | 448.84 | 5.28 | 0.04 | 6 | 434.63 |
| ψA = ψBA = ψBa p(global) | 452.62 | 9.06 | 0.01 | 4 | 443.62 |
p(general1) = pA≠rA≠pB≠rBA≠rBa; method;
p(general2) = pA = rA≠pB≠rBA≠rBa; method;
p(general3) = pA = rA = pB = rBA = rBa; method.
ψA = occupancy of dominant species; ψBA = occupancy of subordinate species when the dominant species is present; ψBa = occupancy of subordinate species when the dominant species is absent. Reserve dist = Covariate ‘weighted distance to reserve border’. We used “=” to designate that two or more parameters were set as equal (e.g., ψBA = ψBa means that the occupancy of the subordinate species is independent of that of the dominant species). We used “≠” to designate that two or more parameters were set as different (e.g., ψBA≠ψBa models assumed that the occupancy of the subordinate species was influenced by the dominant species).
Table 3. Occupancy (ψ), detection probability (p and r), and species interaction factor (SIF—phi and delta) estimated from co-occurrence occupancy models of three sympatric Neotropical spotted cats in a large Atlantic Forest remnant.
| ψA | ψBA | ψBa | rA | pA | pB | rBA | rBa | Phi | Delta | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ocelot—Margay | 0.60 | 0.63 | 0.63 | 0.06 | 0.06 | 0.08 | 0.08 | 0.08 | 1.00 | 1.00 |
| Ocelot—Oncilla | 0.67 | 0.80 | 0.80 | 0.05 | 0.05 | 0.04 | 0.41 | 0.10 | 1.00 | 3.53 |
| Margay—Oncilla | 0.61 | 0.68 | 0.63 | 0.09 | 0.08 | 0.08 | 0.13 | 0.12 | 1.03 | 1.11 |
ψA = occupancy of dominant species; ψBA = occupancy of subordinate species when the dominant species is present; ψBa = occupancy of subordinate species when the dominant species is absent; rA = probability of dominant species being detected when the subordinate species is present; pA = probability of dominant species being detected when the subordinate species is absent; pB = probability of subordinate species being detected when the dominant species is not present; rBA = probability of subordinate species being detected when the dominant species is present and detected; rBa = probability of subordinate species being detected when the dominant species is present but not detected; Phi = ratio of how much more (>1) or less (<1) likely the species are to co-occur at a site compared to what would be expected if the species occurred independently of each other; Delta = ratio of how much more (>1) or less (<1) likely the species are to be detected together in a survey compared to what would be expected if they were detected independently.
We also found no evidence that the presence of margay or oncilla have an effect on the detection probability of the more dominant species, the ocelot (i.e., that pA≠rA), or that the presence or detection of ocelot has an effect on the detection of margay (i.e., that pB≠rBA = rBa or pB≠rBA≠rBa; Tables 3 and 4). However, we found evidence that the presence of margay and the presence and detection of ocelot increases the detection probability of oncilla (i.e., margay-oncilla: pB≠rBA = rBa; ocelot-oncilla: pB≠rBA≠rBa; Tables 3 and 4).
Table 4. Co-occurrence occupancy models used to evaluate the role of interspecific interactions on the detection probability of three sympatric Neotropical spotted cats in a large Atlantic Forest remnant.
| Model | AIC | ΔAIC | wi | K | -2LL |
|---|---|---|---|---|---|
| Ocelot-Margay | |||||
| ψ(top) pA = rA = pB = rBA = rBa | 339.14 | 0 | 0.77 | 5 | 327.60 |
| ψ(top) pA = rA,pB = rBA = rBa | 341.81 | 2.67 | 0.20 | 6 | 327.60 |
| ψ(top) pA≠rA≠pB≠rBA = rBa | 346.45 | 7.31 | 0.02 | 8 | 326.45 |
| ψ(top) pA≠rA≠pB≠rBA≠rBa | 349.56 | 10.42 | 0.00 | 9 | 326.42 |
| Ocelot-Oncilla | |||||
| ψ(top) pA = rA≠pB≠rBA≠rBa | 386.13 | 0 | 0.21 | 8 | 366.13 |
| ψ(top) pA = rA = pB = rBA = rBa | 391.69 | 5.56 | 0.01 | 5 | 380.15 |
| ψ(top) pA≠rA≠pB = rBA = rBa | 393.20 | 7.07 | 0.01 | 7 | 376.17 |
| ψ(top) pA = rA≠pB = rBA = rBa | 394.36 | 8.23 | 0.00 | 6 | 380.15 |
| Margay-Oncilla | |||||
| ψ(top) pA = rA = pB = rBA = rBa | 443.56 | 0 | 0.28 | 5 | 432.02 |
| ψ(top) pA = rA≠pB≠rBA = rBa | 444.05 | 0.49 | 0.22 | 7 | 427.02 |
| ψ(top) pA = rA≠pB = rBA = rBa | 444.96 | 1.40 | 0.14 | 6 | 430.75 |
| ψ(top) pA≠rA≠pB≠rBA = rBa | 445.41 | 1.85 | 0.11 | 8 | 425.41 |
| ψ(top) pA≠rA≠pB = rBA = rBa | 445.46 | 1.90 | 0.11 | 7 | 428.43 |
| ψ(top) pA = rA≠pB≠rBA≠rBa | 445.69 | 2.13 | 0.10 | 8 | 425.69 |
| ψ(top) pA≠rA≠pB≠rBA≠rBa | 447.42 | 3.86 | 0.04 | 9 | 424.28 |
While modeling detection probability (p) for each pair of species, we incorporated the covariate “method” in p and kept occupancy (ψ) as it was in the top-ranked model from the co-occurrence occupancy models used to evaluate ψ (Table 2): Ocelot vs. Margay ψ(top) = [ψA(reserve dist) = ψBA(reserve dist) = ψBa(reserve dist)]; Ocelot vs. Oncilla ψ(top) = [ψA(reserve dist)≠ψBA = ψBa]; Margay vs. Oncilla ψ(top) = ψA = ψBA = ψBa.
Discussion
When investigating species’ distribution and use of habitat, assessing the relative importance of landscape attributes and the role of interspecific interactions is often difficult. Here, we used occupancy modeling [41, 42] to explicitly incorporate detection probability and habitat variables while examining co-occurrence patterns and landscape use by three sympatric and morphologically similar species of Neotropical cats. Predators can be considered a key group because they affect prey and plant populations, influencing ecosystem dynamics [1–3]. However, most species of felids are threatened, or we lack basic information about them. All three Neotropical spotted cats analyzed here are believed to be suffering from population decrease, and two (L. wiedii and L. guttulus) are considered ‘Near Threatened’ or ‘Vulnerable’ [96]. We demonstrated that the proximity to a more protected area (nature reserve) is the main factor influencing the habitat use of Neotropical spotted cats within their home ranges in a large Atlantic forest remnant, and we had low support for the hypothesis that interspecific interactions modulate how they use the landscape.
Our first prediction that among the landscape characteristics, the human-related variables would be more important predictors of landscape use by Neotropical spotted cats was in part corroborated. Roads and human accessibility, for instance, are important determinants of the occupancy of sensitive species (e.g., game species—[97]); and road kills can be common among felids and other carnivores [98, 99]. Here, we had some evidence that road density, which is also a measure of human accessibility, may have a negative effect on occupancy of some spotted cats (given its negative beta estimates for two out of the three species analyzed), particularly on ocelots; however, it did not have a strong effect and was not a major factor for them. Besides area accessibility, higher protection status is also usually associated with lower human-related pressures [9, 17, 18]. Our results showed that more strict protection status is important for the Neotropical spotted cats, especially for ocelots and margays, which used more areas closer to the reserve even though our entire study area is under some type of protection. Although ‘weighted distance to reserve border’ was not a high-ranked model for oncilla, the influence of this covariate on its occupancy was also in the predicted direction (negative). In previous studies, only ocelots or larger felids (pumas and jaguars) were more commom in areas with higher legal protection than in less protected areas [100, 101]. Whether these distinct outcomes are derived from differences in the felid community structure (e.g., more larger felids occuring in these previous studies than in our study area) or arise from some intrinsic local characteristic of the sites (e.g., level of anthropogenic pressures) can only be answered with further studies.
Prey availability can usually influence the abundance, density, occupancy and habitat use of carnivores [56, 102–105]. Although the relationship between the prey index and occupancy was in the predicted direction (positive), we did not have enough evidence that this variable, hydrographic density, high-quality forest cover, and elevation influence the spotted cats’ habitat use. The possible opportunistic feeding behavior of Neotropical small cats [36] could explain the lack of effect of prey on their habitat use. However, we also note that prey availability, measured as the sum of prey occupancy at each sampling site, varied only slightly across sites (mean 1.31±0.12) and did not account for differences in prey density. Therefore, we cannot yet discard a possible effect of prey on spotted cats’ habitat use.
Intraguild competition can be an important determinant of carnivore abundance and distribution, as it can lead to spatial or temporal segregation among species [7, 28, 106]. However, when examining species co-occurrence patterns, it is often difficult to distinguish the difference between habitat preferences and competitive exclusions. Furthermore, since species present at a location are not always detected with certainty, incorporating detection probability along with habitat preferences directly into the model set may avoid incorrect inferences about co-occurrence patterns [41]. By adopting this approach, we found no evidence that the presence of either ocelot, margay, or oncilla have a negative influence on how the other species use the habitat. Thus competitive exclusion among them is unlikely, at least within the analyzed scale, and at a relatively conserved and protected area such as ours. The lack of spatial partitioning based on interspecific interaction was also found for North American carnivores, and similarly to our study, habitat preferences were more important in structuring the community [107, 108]. Nonetheless, because occupancy does not account for variations in density, it is still possible that the presence of one species affects the density of the other, as previously suggested [109]. We also encourage further research to analyze the effects of larger felids (e.g., pumas and jaguars) on the habitat use of smaller cats, which unfortunately, we did not have data to investigate (only six detections of pumas and none of jaguar). In addition, since land use and human activity can alter occupancy patterns [110] and behavior [111] of carnivores, it would be of interest to explore whether interspecific relations and co-occurrence patterns among small felids are affected by different degrees of human disturbances, which unfortunately we did not have enough data to investigate. Finally, although the models used here can yield strong inferences about species co-occurrence patterns, this does not imply strong inference about the processes that generated the observed patterns, and it would be useful to observe the system dynamics over time [41].
We found evidence that one species affects the behavior of the other in at least one respect, as oncilla is more likely to be detected if margay is present or if ocelot is either present or detected. Felids are highly territorial and use scent marks such as urine and feces to mark their territories [62]. Two things should be noted from the fact that most of the felids’ detections came from scat sampling, and therefore, their territorial scent mark: first, since we collected the feces as they were detected, sites with scat detections became unmarked after each sampling occasion. Second, scent marks are frequently over-marked either by the same animal or other individuals [62]. Therefore, we suggest that the presence of a spotted cat (and consequently, its feces) increased the detection of another spotted cat as it was attracted to either over-mark the feces (before it was removed) or use an area rendered unmarked (through collection of the scats). The small felids may be able to avoid direct confrontations by marking recently used areas within their home range and using different parts of their home range according to where the other is currently marking (i.e., using the area). However, to clarify the mechanisms underlying our findings, more detailed studies on the behavior of the Neotropical spotted cats are necessary. What we can suggest so far is that territorial demarcation may potentially help to regulate how these small felids share their habitats, consequently alleviating or reducing the competition among them.
In conclusion, our results suggest that human-related factors, such as distance to a reserve, are more important drivers of Neotropical spotted cats’ habitat use than are interspecific interactions, environmental landscape attributes, geomorphometry or, potentially, prey availability. We suggest that the dietary overlap of the three species might be small enough to allow co-existence [57–61], or that another behavioral mechanism in addition to differences in habitat preference, such as time partitioning, may allow them to co-exist [100, 112, 113].
Management recommendations
Given the importance and vulnerability of spotted cats in Neotropical forests, several actions are required for their conservation, including enhancing forest connectivity and gathering basic information on their ecology and behavior [4, 114]. Here we underline the main management recommendations resulting from our study:
We demonstrated that the most important factor for managing small felids can be the maintenance and establishment of nature reserves and other areas that are managed to ensure minimal disturbance and human presence (i.e., IUCN Protected Area Category Ia—Strict Nature Reserve [115]). Protected areas can decrease habitat loss [116] and anthropogenic pressures [17, 18] as well as improve the occupancy of key groups (Nagy-Reis et al. present study). However, because poaching may still occur illegally, effective law enforcement and other management actions such as environmental education are also important to ensure the conservation of small felids.
The use of methods that incorporate detection probability is critical to the understanding of species’ responses to habitat and interspecific interactions. We showed how dealing with imperfect detection results in a substantial relative increase above naïve occupancy for rare, cryptic, and elusive animals such as small felids, reinforcing the importance of accounting for detection probability in ecological and behavioral studies on such species. Our research also emphasizes the feasibility of alternative methods for surveying felids, such as scat sampling combined with tricology, which has a higher detection probability than camera traps and supplies non-invasive material for studying diet and performing molecular analysis, providing essential information on population parameters and ecology [63, 117]. We also confirmed the reliability of tricology as a low cost alternative to molecular methods for identification of Neotropical felids, as long as meticulous procedure is adopted by a trained researcher.
Supporting Information
Sampling sites represented with a same color were sampled simultaneously within each campaign. Campaign 1 (April 2013 to September 2013)–Group A (black): Apr-Mai; Group B (blue): Jun-Jul; Group C (green): Ago-Sep. Campaign 2 (October 2013 to March 2014)–Group A: Oct-Nov; Group B: Dec-Jan; Group C: Feb-Mar. Campaign 3 (April 2014 to September 2014)–Group A: Apr-Mai; Group B: Jun-Jul; Group C: Ago-Sep.
(PDF)
From the left to the right: ocelot (Leopardus pardalis), margay (L. wiedii), and oncilla (L. guttulus).
(PDF)
Results of Moran’s I autocorrelation tests [1]—based on the number of detection records of each species and the geographic position of each site. We used R 2.13.0 software [2] and the ape package [3]. 1. Legendre P & Legendre L. Numerical Ecology. New York: Elsevier; 1998. 2. R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2014. Available: <www.R-project.org>. Accessed 2 Oct 2014. 3. Paradis E, Blomberg S, Boljer B, Claude J, Cuong HS. et al. Analyses of phylogenetics and evolution: package “ape”. Available: <ape-package.ird.fr>. Accessed 10 Jul 2015.
(PDF)
The four landscape site covariates (elevation, hydrographic density, road density and percentage of high-quality forest cover) were measured at 500 and 1,000 m spatial extents (buffer sizes). Highly correlated (rs<0.50) outcomes are in bold. S prey = prey index for margay and oncilla; O prey = prey index for ocelot; * = excluded covariates.
(PDF)
p(general) = campaign + method + soil coverage + percentage of high-quality forest cover at 500 m buffer size; “gamma” = colonization; “eps” = extinction; “.” = no covariate included; “0” = parameter was fixed to 0.
(PDF)
“Campaign” = campaign in which data was collected (1- April 2013 to September 2013; 2- October 2013 to March 2014; 3- April 2014 to September 2014); “Groups” = order within the campaigns that sampling sites were surveyed; “Method” = method used in each sampling occasion (camera trapping or scat sampling); “.” = no covariate included (i.e., null model).
(PDF)
p(general) = method + soil coverage + percentage of high-quality forest cover at 500 m buffer size.
(PDF)
p(general) = method + soil coverage + percentage of high-quality forest cover at 500 m buffer size.
(PDF)
(PDF)
Acknowledgments
We are thankful to Jundiaí City Hall and all private owners for permission to conduct this project at Serra do Japi, and to Coordination for the Improvement of Higher Education Personnel (CAPES), São Paulo Research Foundation (FAPESP; grant 2013/07162-6), and Idea Wild for their financial and equipment support. We are also grateful to J. E. Hines for assisting in our data analysis; M. F. Rossi for land cover mapping; J. W. Ribeiro for all the landscape metrics and relief information computing; V. H. Iwakami, M. F. Rossi and M. C. S. Canhoto for helping with scat sampling and tricology; C. A. Estevo for aiding with prey data; B. H. Saranholi and Molecular Biodiversity and Conservation Lab (BMC/Ufscar) for molecular analysis; J. Godinho for identifying vegetation cover in the field; M. J. F. Penteado for confirming species photo identification; and the Traldi family and Benê for assisting during data collection. The Brazilian Science Council (CNPq) provided a scholarship to AGC (process 305902/2014-8). MCR has been continuously supported by grants and scholarships from CNPq (process 312045/2013-1) and FAPESP (grant 2013/50421-2). Megan King, a native English speaker from British Columbia, CA did the proof reading of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
MBNR received funding from the Coordination for the Improvement of Higher Education Personnel (CAPES; www.capes.gov.br) and Idea Wild (www.ideawild.org). EZFS received funding from the São Paulo Research Foundation (FAPESP; grant 2013/07162-6; www.fapesp.br). AGC received funding from The Brazilian Science Council (CNPq; process 305902/2014-8; www.cnpq.br). MCR has been continuously supported by grants and scholarships from The Brazilian Science Council (CNPq; process 312045/2013-1) and São Paulo Research Foundation (FAPESP; grant 2013/50421-2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Crooks KR & Soulé ME. Mesopredator release and avifaunal extinctions in a fragmented system. Nature. 1999; 400(6744): 563–566. [Google Scholar]
- 2.Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, et al. Trophic downgrading of planet Earth. Science. 2011; 333(6040): 301–306. 10.1126/science.1205106 [DOI] [PubMed] [Google Scholar]
- 3.Terborgh J, Lopez L, Nunez P, Rao M, Shahabuddin G, Orihuela G, et al. Ecological meltdown in predator-free forest fragments. Science. 2001; 294(5548): 1923–1926. 10.1126/science.1064397 [DOI] [PubMed] [Google Scholar]
- 4.Noss RF, Quigley HB, Hornocker MG, Merril T, Paquet PC. Conservation biology and carnivore conservation in the Rocky Mountains. Conserv Biol. 1996; 10(4): 949–963. [Google Scholar]
- 5.Andreasen JK, O’Neill RV, Noss R, Slosser NC. Considerations for the development of a terrestrial index of ecological integrity. Ecol Indic. 2001; 1(1): 21–35. [Google Scholar]
- 6.Soulé ME & Terborgh J. Continental conservation: Scientific foundations of regional reserve networks. Washington: Island Press; 1999. [Google Scholar]
- 7.Macdonald DW, Loveridge AJ, Nowell K. Dramatis personae: an introduction to the wild felids In: Macdonald DW & Loveridge AJ, editors. Biology and conservation of wild felids. New York: Oxford University Press; 2011. pp. 3–58. [Google Scholar]
- 8.Nowell K & Jackson P. Wild cats: Status survey and conservation action plan. IUCN/SSC Cat Specialist Group; Switzerland: IUCN; 1996. [Google Scholar]
- 9.Loveridge AJ, Wang SW, Frank LG, Seidensticker J. People and felids: conservation of cats and management of conflicts In: Macdonald DW & Loveridge AJ, editors. Biology and conservation of wild felids. New York: Oxford University Press; 2011. pp. 161–196. [Google Scholar]
- 10.Murphy T & Macdonald DW. Pumas and people: lessons in the landscape of tolerance from a widely distributed felid In: Macdonald DW & Loveridge AJ, editors. Biology and conservation of wild felids. New York: Oxford University Press; 2011. pp. 431–452. [Google Scholar]
- 11.Michalski F, Boulhosa RLP, Faria A, Peres CA. Human–wildlife conflicts in a fragmented Amazonian forest landscape: determinants of large felid depredation on livestock. Anim Conserv. 2006; 9(2): 179–188. [Google Scholar]
- 12.Cavalcanti SMC, Marchini S, Zimmermann A, Gese EM, Macdonald DW. Jaguars, livestock, and people in Brazil: realities and perceptions behind the conflict In: Macdonald DW & Loveridge AJ, editors. Biology and conservation of wild felids. New York: Oxford University Press; 2011. pp. 383–402. [Google Scholar]
- 13.Jackson RM, Mishra C, McCarthy TM, Ale SB. Snow leopards: conflict and conservation In: Macdonald DW & Loveridge AJ, editors. Biology and conservation of wild felids. New York: Oxford University Press; 2011. pp. 417–430. [Google Scholar]
- 14.Sanderson JG & Watson P. Small wild cats: The animal answer guide. Baltimore: The Johns Hopkins University Press; 2011. [Google Scholar]
- 15.Craft ME. Ecology of infectious diseases in Serengeti lions In: Macdonald DW & Loveridge AJ, editors. Biology and conservation of wild felids. New York: Oxford University Press; 2011. pp. 263–282. [Google Scholar]
- 16.Munson L, Terio KA, Ryser-Degiorgis M-P, Lane EP, Courchamp F. Wild felid diseases: conservation implications and management strategies In: Macdonald DW & Loveridge AJ, editors. Biology and conservation of wild felids. New York: Oxford University Press; 2011. pp. 237–262. [Google Scholar]
- 17.Bruner AG, Gullinson RE, Rice RE, Fonseca GAB. Effectiveness of parks in protecting tropical biodiversity. Science. 2001; 291: 125–128. 10.1126/science.291.5501.125 [DOI] [PubMed] [Google Scholar]
- 18.Peres CA & Palacios E. Basin-wide effects of game harvest on vertebrate population densities in Amazonian forest: implications for animal-mediated seed dispersal. Biotropica. 2007; 39(3): 304–315. [Google Scholar]
- 19.Ewers RM & Rodrigues ASL. Estimates of reserve effectiveness are confounded by leakage. Trends Ecol Evol. 2008; 23(3): 113–116. 10.1016/j.tree.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 20.Woodroffe R & Ginsberg JR. Edge effects and the extinction of populations inside protected areas. Science. 1998; 280(5372): 2126–2128. [DOI] [PubMed] [Google Scholar]
- 21.Lyra-Jorge MC, Ribeiro MC, Giocheti G, Tambosi LR, Pivello VR. Influence of multi-scale landscape structure on the occurrence of carnivorous mammals in a human-modified savanna, Brazil. Eur J Wildl Res. 2010; 56: 359–368. [Google Scholar]
- 22.Jorge MLSP, Galetti M, Ribeiro MC, Ferraz KMPMB. Mammal defaunation as surrogate of trophic cascades in a biodiversity hotspot. Biol Conserv. 2013; 163: 1–9. [Google Scholar]
- 23.Cabeza M, Araújo MB, Wilson RJ, Thomas CD, Cowley MJR, Moilanen A. Combining probabilities of occurrence with spatial reserve design. J Appl Ecol. 2004; 41: 252–262. [Google Scholar]
- 24.Catullo G, Masia M, Falcucci A, Maiorano L, Rondinini C, Boitani L. A gap analysis of Southeast Asian mammals based on habitat suitability models. Biol Conserv. 2008; 141: 2730–2744. [Google Scholar]
- 25.Kanagaraj R, Wiegand T, Kramer-Schadt S, Anwar M, Goyal SP. Assessing habitat suitability for tiger in the fragmented Terai Arc Landscape of India and Nepal. Ecography. 2011; 34: 970–981. [Google Scholar]
- 26.Spencer W, Rustigian-Romsos H, Strittholt J, Scheller R, Zielinski W, Truex R. Using occupancy and population models to assess habitat conservation opportunities for an isolated carnivore population. Biol Conserv. 2011; 144: 788–803. [Google Scholar]
- 27.Schoener TW. The controversy over interspecific competition. Am Sci. 1982; 70: 586–590. [Google Scholar]
- 28.Palomares F & Caro TM. Interspecific killing among mammalian carnivores. Am Nat. 1999; 153: 492–508. [DOI] [PubMed] [Google Scholar]
- 29.Donadio E & Buskirk SW. Diet, morphology, and interspecific killing in Carnivora. Am Nat. 2006; 167: 524–536. 10.1086/501033 [DOI] [PubMed] [Google Scholar]
- 30.Schoener TW. Resource partitioning in ecological communities. Science. 1974; 185(4145): 27–39. 10.1126/science.185.4145.27 [DOI] [PubMed] [Google Scholar]
- 31.Johnson WE, Eizirik E, Pecon-Slattery J, Murphy WJ, Antunes A, Teeling E, et al. The late Miocene radiation of modern Felidae: a genetic assessment. Science. 2006; 311(5757): 73–77. 10.1126/science.1122277 [DOI] [PubMed] [Google Scholar]
- 32.Kitchener AC, Driscoli C, Eizirik E, Spong G. 2011. Felid form and function In: Macdonald DW & Loveridge AJ, editors. Biology and conservation of wild felids. New York: Oxford University Press; 2011. pp. 83–106. [Google Scholar]
- 33.Trigo TC, Schneider A, Oliveira TG, Lehugeur LM, Silveira L, Freitas TRO, et al. Molecular data reveal complex hybridization and cryptic species of Neotropical wild cat. Curr Biol. 2013; 23: 2528–2533. 10.1016/j.cub.2013.10.046 [DOI] [PubMed] [Google Scholar]
- 34.Emmons LH & Feer F. Neotropical Rainforest Mammals: A Field Guide. Chicago: University of Chicago Press; 1997. [Google Scholar]
- 35.Wang E. Diets of ocelots (Leopardus pardalis), margays (L. wiedii), and oncillas (L. tigrinus) in the Atlantic rainforest in Southeast Brazil. Stud Neotrop Fauna E. 2002; 37(3): 207–212. [Google Scholar]
- 36.Silva-Pereira JE, Moro-Rios RF, Bilski DR, Passos FC. Diets of three sympatric Neotropical small cats: Food niche overlap and interspecies differences in prey consumption. Mamm Biol. 2011; 76(3): 308–312. [Google Scholar]
- 37.Rosenzweig ML. Community structure in sympatric Carnivora. J Mammal. 1966; 47:602–612. [Google Scholar]
- 38.MacKenzie DI. What are the issues with presence-absence data for wildlife managers? J Wildl Manage. 2005; 69(3): 849–860. [Google Scholar]
- 39.Rondinini C, Wilson KA, Boitani L, Grantham H, Possingham HP. Tradeoffs of different types of species occurrence data for use in systematic conservation planning. Ecol Lett. 2006; 9: 1136–1145. 10.1111/j.1461-0248.2006.00970.x [DOI] [PubMed] [Google Scholar]
- 40.Gu W & Swihart RK. Absent or undetected? Effects of non-detection of species occurrence on wildlife–habitat models. Biol Conserv. 2004; 116: 195–203. [Google Scholar]
- 41.MacKenzie DI, Bailey LL, Nichols J. Investigating species co-occurrence patterns when species are detected imperfectly. J Anim Ecol. 2004; 73(3): 546–555. [Google Scholar]
- 42.Mackenzie DI, Nichols JD, Lachman GB, Droege S, Royle JA, Langtimm CA. Estimating site occupancy rates when detection probabilities are less than one. Ecology. 2002; 83: 2248–2255. [Google Scholar]
- 43.Myers N, Mittermeier RA, Mittermeier CG, Mittermeier CG, Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000; 403: 853–858. 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- 44.Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM. The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol Conserv. 2009; 142: 1141–1153. [Google Scholar]
- 45.SMPMA—Secretaria Municipal de Planejamento e Meio Ambiente. Plano de manejo—Reserva Biológica Municipal da Serra do Japi-Jundiaí-SP. Jundiaí: Prefeitura de Jundiaí; 2008. [Google Scholar]
- 46.Morellato LPC. História natural da Serra do Japi: Ecologia e preservação de uma área florestal no Sudeste do Brasil. Campinas: Editora da Unicamp; 1992. [Google Scholar]
- 47.TEAM Network. Terrestrial Vertebrate Protocol Implementation Manual, v. 3.1. Arlington: Tropical Ecology, Assessment and Monitoring Network, Conservation International; 2011. <www.teamnetwork.org/protocol/terrestrial-vertebrate-camera-trapping-monitoring-protocol> [Google Scholar]
- 48.Carley CJ, Fleharty ED, Mares MA. Occurrence and activity of Reithrodontomys megalotis, Microtus ochrogaster, and Peromyscus maniculatus as recorded by a photographic device. The Southwest Nat. 1970; 15(2): 209–216. [Google Scholar]
- 49.Hayes RA, Nahrung HF, Wilson JC. The response of native Australian rodents to predator odours varies seasonally: a by-product of life history variation? Anim Behav. 2006; 71(6): 1307–1314. [Google Scholar]
- 50.Oliveira-Santos LGR, Tortato MA, Graipel ME. Activity pattern of Atlantic Forest small arboreal mammals as revealed by camera traps. J Trop Ecol. 2008; 24(5): 563–567. [Google Scholar]
- 51.Dinata Y, Nugroho A, Haidir IA, Linkie M. Camera trapping rare and threatened avifauna in west-central Sumatra. Bird Conserv Int. 2008; 18(01): 30–37. [Google Scholar]
- 52.O’Brien TG & Kinnaird MF. A picture is worth a thousand words: the application of camera trapping to the study of birds. Bird Conserv Int. 2008; 18:144–162. [Google Scholar]
- 53.Samejima H, Ong R, Lagan P, Kitayama K. Camera-trapping rates of mammals and birds in a Bornean tropical rainforest under sustainable forest management. Forest Ecol Manage. 2012; 270: 248–256. [Google Scholar]
- 54.Torre I, Peris A, Tena L. Estimating the relative abundance and temporal activity patterns of wood mice (Apodemus sylvaticus) by remote photography in Mediterranean post-fire habitats. Galemys. 2005; 17: 41–52. [Google Scholar]
- 55.De Bondi N, White JG, Stevens M, Cooke R. A comparison of the effectiveness of camera trapping and live trapping for sampling terrestrial small-mammal communities. Wildl Res. 2010; 37(6): 456–465. [Google Scholar]
- 56.Sarmento PB, Cruz J, Eira C, Fonseca C. Modeling the occupancy of sympatric carnivorans in a Mediterranean ecosystem. Eur J Wildl Res. 2011; 57(1): 119–131. [Google Scholar]
- 57.Meza AV, Meyer EM, González CAL. Ocelot (Leopardus pardalis) food habits in a tropical deciduous forest of Jalisco, Mexico. Am Midl Nat. 2002; 148(1): 146–154. [Google Scholar]
- 58.Moreno RS, Kays RW, Samudio R Jr. Competitive release in diets of ocelot (Leopardus pardalis) and puma (Puma concolor) after jaguar (Panthera onca) decline. J Mammal. 2006; 87(4): 808–816. [Google Scholar]
- 59.Bianchi RD & Mendes SL. Ocelot (Leopardus pardalis) predation on primates in Caratinga Biological Station, Southeast Brazil. Am J Primatol. 2007; 69(10): 1178. [DOI] [PubMed] [Google Scholar]
- 60.Bianchi RDC, Rosa AF, Gatti A, Mendes SL. Diet of margay, Leopardus wiedii, and jaguarundi, Puma yagouaroundi, (Carnivora: Felidae) in Atlantic Rainforest, Brazil. Zoologia (Curitiba). 2011; 28(1): 127–132. [Google Scholar]
- 61.Trigo TC, Tirelli FP, Machado LF, Peters FB, Indrusiak CB, Mazim FD, et al. Geographic distribution and food habits of Leopardus tigrinus and L. geoffroyi (Carnivora, Felidae) at their geographic contact zone in southern Brazil. Stud Neotrop Fauna Environ. 2013; 48(1): 56–67. [Google Scholar]
- 62.Kitchener A. The natural history of the wild cats. New York: Cornell University Press; 1991. [Google Scholar]
- 63.Karanth KU, Funston P, Sanderson E. Many ways of skinning a cat: tools and techniques for studying wild felids In: Macdonald DW & Loveridge AJ, editors. Biology and conservation of wild felids. New York: Oxford University Press; 2011. pp. 197–216. [Google Scholar]
- 64.Eckstein RA & Hart BL. The organization and control of grooming in cats. Appl Anim Behav Sci. 2000; 68(2): 131–140. [DOI] [PubMed] [Google Scholar]
- 65.Quadros T & Monteiro-Filho ELA. Coleta e preparação de pêlos de mamíferos para identificação em microscopia óptica. Rev Bras Zool. 2006; 23(1): 274–278. [Google Scholar]
- 66.Quadros J & Monteiro-Filho ELA. Identificação dos mamíferos de uma área de floresta atlântica utilizando a microestrutura de pelos-guarda de predadores e presas. Arq Museu Nacional. 2010; 68(1–2): 47–66. [Google Scholar]
- 67.Vanstreels RET, Ramalho FDP, Adania CH. Microestrutura de pelos-guarda de felídeos brasileiros: considerações para a identificação de espécies. Biota Neotrop. 2010; 10(1): 333–337. [Google Scholar]
- 68.Kendall KC & McKelvey KS. Hair collection In: Long RA, MacKay P, Ray J, Zielinski W, editors. Noninvasive survey methods for carnivores. Washington: Island Press; 2008. pp. 135–176. [Google Scholar]
- 69.Hilton H & Kutscha NP. Distinguishing characteristics of the hairs of eastern coyote, domestic dog, red fox and bobcat in Maine. Am Midl Nat. 1978;100(1): 223–227. [Google Scholar]
- 70.Kennedy AJ. Distinguishing characteristics of the hairs of wild and domestic canids from Alberta. Can J Zoolog. 1982; 60(4): 536–541. [Google Scholar]
- 71.Mukherjee S, Goyal SP, Chellam R. Standardisation of scat analysis techniques for leopard (Panthera pardus) in Gir National Park, Western India. Mammalia. 1994; 58(1): 139–143. [Google Scholar]
- 72.Meng J & Wyss AR. Multituberculate and other mammal hair recovered from Palaeogene excreta. Nature. 1997; 385: 712–714. 10.1038/385712a0 [DOI] [PubMed] [Google Scholar]
- 73.Chernova OF. Architectonics of the medulla of guard hair and its importance for identification of taxa. Dokl Biol Sci. 2001; 376(1): 81–85. [Google Scholar]
- 74.De Marinis AM & Asprea A. Hair identification key of wild and domestic ungulates from Southern Europe. Wildl Biol. 2006; 12(3): 305–320. [Google Scholar]
- 75.Miotto RA, Ciocheti G, Rodrigues FP, Galetti PM Jr. Identification of pumas (Puma concolor (Linnaeus, 1771)) through faeces: a comparison between morphological and molecular methods. Braz J Biol. 2007; 67(4): 963–965. [DOI] [PubMed] [Google Scholar]
- 76.Chaves PB, Graeff VG, Lion MB, Oliveira LR, Eizirik E. DNA barcoding meets molecular scatology: short mtDNA sequences for standardized species assignment of carnivore noninvasive samples. Mol Ecol Resour. 2012; 12(1): 18–35. 10.1111/j.1755-0998.2011.03056.x [DOI] [PubMed] [Google Scholar]
- 77.Álvarez B. Plataforma QGIS; 2013. <www.qgis.org>
- 78.Boscolo D & Metzger JP. Is bird incidence in Atlantic forest fragments influenced by landscape patterns at multiple scales? Landsc Ecol. 2009; 24: 907–918. [Google Scholar]
- 79.Lesmeister DB, Nielsen CK, Schauber EM, Hellgren EC. Spatial and temporal structure of a mesocarnivore guild in Midwestern Nord America. Wildl Monogr. 2015; 191:1–61. [Google Scholar]
- 80.Oliveira TG. Neotropical cats: ecology and conservation. São Luís: Editora da Universidade Federal do Maranhão; 1994. [Google Scholar]
- 81.Neteler M, Bowman MH, Landa M, Metz M. GRASS GIS: A multi-purpose open. Environ Modell Softw. 2012; 31: 124–130. [Google Scholar]
- 82.ESRI. ArcGIS. Redlands, CA: Environmental Systems Research Institute; 2009. [Google Scholar]
- 83.INPE (BR). Topodata—Banco de dados geomorfométricos do Brasil [Internet]. Brasil: Instituto Nacional de Pesquisas Espaciais (BR) 2014. <www.dsr.inpe.br/topodata/index.php>. Accessed 20 November 2014. [Google Scholar]
- 84.Emmons LH. A field study of ocelots (Felis pardalis) in Peru. Rev Ecol-Terre Vie. 1988: 43:133–157. [Google Scholar]
- 85.Konecny MJ. Movement patterns and food habits of four sympatric carnivore species in Belize, Central America. Advances in Neotropical Mammology. 1989; 243: 264. [Google Scholar]
- 86.de Oliveira T, Eizirik E, Schipper J, Valderrama C, Leite-Pitman R, Payan E. Leopardus tigrinus The IUCN Red List of Threatened Species 2014–3 [Internet]. Cambridge: IUCN; 2008. <www.iucnredlist.org>. Accessed 10 December 2014. [Google Scholar]
- 87.Carvajal-Villarreal S, Caso A, Downey P, Moreno A, Tewes ME, Grassman LI. Spatial patterns of the margay (Leopardus wiedii; Felidae, Carnivora) at “El Cielo” Biosphere Reserve, Tamaulipas, Mexico. Mammalia. 2012; 76: 237–244. [Google Scholar]
- 88.MacKenzie DI, Royle JA, Brown JA, Nichols JD. Occupancy estimation and modeling for rare and elusive populations In: Thompson WL, editor. Sampling rare or elusive species: Concepts, designs, and techniques for estimating population parameters. Washington: Island Press; 2004. pp. 149–171. [Google Scholar]
- 89.Mackenzie DI & Royle JA. Designing occupancy studies: general advice and allocating survey effort. J Appl Ecol 2005; 42: 1105–1114. [Google Scholar]
- 90.Johnson DH. The Comparison of usage and availability measurements for evaluating resource preference. Ecology 1980; 61(1):65–71. [Google Scholar]
- 91.Manly BFJ, McDonald LL, Thomas DL, McDonald TL, Erickson WP. Resource selection by animals: statistical design and analysis for field studies. Dordrecht: Kluwer Academic Publishers; 2002. [Google Scholar]
- 92.MacKenzie DI. Modeling the probability of use: The effect of, and dealing with, detecting a species imperfectly. J Wildl Manage. 2006; 70(2): 367–374. [Google Scholar]
- 93.Hines JE. PRESENCE—Software to estimate patch occupancy and related parameters [Internet]. USGS-PWRC. 2006. <www.mbr-pwrc.usgs.gov/software/presence.html>. Accessed 2 December 2014.
- 94.Burnham KP & Anderson D. Model selection and multi-model inference: A practical information–theoretic approach. New York: Springer Science; 2002. [Google Scholar]
- 95.Richmond OMW, Hines JE, Beissinger SR. Two-species occupancy models: a new parameterization applied to co-occurrence of secretive rails. Ecol Appl. 2010; 20(7): 2036–2046. [DOI] [PubMed] [Google Scholar]
- 96.IUCN. IUCN Red List of Threatened Species 2014–3 [Internet]. Cambridge: IUCN; 2015. <www.iucnredlist.org>. Accessed 20 September 2015. [Google Scholar]
- 97.Licona M, McCleery R, Collier B, Brightsmith DJ, Lopez R. Using ungulate occurrence to evaluate community-based conservation within a biosphere reserve model. Anim Conserv. 2011; 14: 206–214. [Google Scholar]
- 98.Haines AM, Tewes ME, Laack LL. Survival and sources of mortality in ocelots. J Wildl Manage. 2005; 69(1): 255–263. [Google Scholar]
- 99.Ciocheti G. Spatial and temporal influences of road duplication on wildlife road kill using habitat suitability models. PhD Thesis, Universidade Federal de São Carlos. 2014. <www.bdtd.ufscar.br>
- 100.Di Bitetti MS, De Angelo CD, Di Blanco YE, Paviolo A. Niche partitioning and species coexistence in a Neotropical felid assemblage. Acta Oecol. 2010; 36(4): 403–412. [Google Scholar]
- 101.Carrillo E, Wong G, Cuarón AD. 2000. Monitoring mammal populations in Costa Rican protected areas under different hunting restrictions. Conserv Biol. 14(6): 1580–1591. [DOI] [PubMed] [Google Scholar]
- 102.Litvaitis JA, Sherburne JA, Bissonette JA. Bobcat habitat use and home range size in relation to prey density. J Wildl Manage. 1986; 50(1): 110–117. [Google Scholar]
- 103.Karanth KU & Nichols JD. Estimation of tiger densities in India using photographic captures and recaptures. Ecology. 1998; 79(8): 2852–2862. [Google Scholar]
- 104.Carbone C & Gittleman JL. A common rule for the scaling of carnivore density. Science. 2002; 295(5563): 2273–2276. 10.1126/science.1067994 [DOI] [PubMed] [Google Scholar]
- 105.Hetherington DA & Gorman ML. Using prey densities to estimate the potential size of reintroduced populations of Eurasian lynx. Biol Conserv. 2007; 137(1): 37–44. [Google Scholar]
- 106.Tannerfeldt M, Elmhagen B, Angerbjörn A. Exclusion by interference competition? The relationship between red and arctic foxes. Oecologia. 2002; 132(2): 213–220. [DOI] [PubMed] [Google Scholar]
- 107.Lesmeister DB, Nielsen CK, Schauber EM, Hellgren EC. Spatial and temporal structure of a mesocarnivore guild in Midwestern North America. Wildl Monogr 2015; 191:1–61. [Google Scholar]
- 108.Gompper ME, Lesmeister DB, Ray JC, Malcolm JR, Kays R. Differential habitat use or intraguild interactions: What structures a carnivore community? PLoS ONE. 2016; 11(1): e0146055 10.1371/journal.pone.0146055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oliveira TG, Tortato MA, Silveira L, Kasper CB, Mazim FD, Lucherini M, et al. Ocelot ecology and its effect on the small-felid guild in the lowland neotropics In: Macdonald DW & Loveridge AJ, editors. Biology and conservation of wild felids. New York: Oxford University Press; 2011. pp. 559–580. [Google Scholar]
- 110.Schuette P, Wagner AP, Wagner ME, Creel S. Occupancy patterns and niche partitioning within a diverse carnivore community exposed to anthropogenic pressures. Biol Conserv. 2013; 158: 301–312. [Google Scholar]
- 111.Boydston EE, Kapheim KM, Watts HE, Szykman M, Holekamp KE. Altered behaviour in spotted hyenas associated with increased human activity. Anim Conserv. 2003; 6: 207–219. [Google Scholar]
- 112.Oliveira-Santos LGR, Graipel ME, Tortato MA, Zucco CA, Cáceres NC, Goulart FV. Abundance changes and activity flexibility of the oncilla, Leopardus tigrinus (Carnivora: Felidae), appear to reflect avoidance of conflict. Zoologia (Curitiba). 2012: 29(2): 115–120. [Google Scholar]
- 113.Nagy-Reis MB, Estevo CA, Setz EZF. Activity of four sympatric Neotropical felids and its relation to their main preys. Anchorage, AK: 52ndAnimal Behavior Meeting; 2015.
- 114.Macdonald DW, Loveridge AJ, Rabinowitz A. Felid futures: crossing disciplines, borders, and generations In: Macdonald DW & Loveridge AJ, editors. Biology and conservation of wild felids. New York: Oxford University Press; 2011. pp. 599–650. [Google Scholar]
- 115.Phillips A. The history of the international system of protected area management categories. Parks. 2004; 14(3):4–14. [Google Scholar]
- 116.Andam KS, Ferraro PJ, Pfaff A, Sanchez-Azofeifa GA, Robalino JA. Measuring the effectiveness of protected area networks in reducing deforestation. P Natl Acad Sci USA. 2008; 105(42): 16089–16094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Taberlet P, Luikart G, Geffen E. New methods for obtaining and analyzing genetic data from free-ranging carnivores In: Gittleman JL, Funk SM, Macdonald D, Wayne RK, editors. Carnivore conservation. Cambridge: Cambridge University Press; 2001. pp. 313–334. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sampling sites represented with a same color were sampled simultaneously within each campaign. Campaign 1 (April 2013 to September 2013)–Group A (black): Apr-Mai; Group B (blue): Jun-Jul; Group C (green): Ago-Sep. Campaign 2 (October 2013 to March 2014)–Group A: Oct-Nov; Group B: Dec-Jan; Group C: Feb-Mar. Campaign 3 (April 2014 to September 2014)–Group A: Apr-Mai; Group B: Jun-Jul; Group C: Ago-Sep.
(PDF)
From the left to the right: ocelot (Leopardus pardalis), margay (L. wiedii), and oncilla (L. guttulus).
(PDF)
Results of Moran’s I autocorrelation tests [1]—based on the number of detection records of each species and the geographic position of each site. We used R 2.13.0 software [2] and the ape package [3]. 1. Legendre P & Legendre L. Numerical Ecology. New York: Elsevier; 1998. 2. R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2014. Available: <www.R-project.org>. Accessed 2 Oct 2014. 3. Paradis E, Blomberg S, Boljer B, Claude J, Cuong HS. et al. Analyses of phylogenetics and evolution: package “ape”. Available: <ape-package.ird.fr>. Accessed 10 Jul 2015.
(PDF)
The four landscape site covariates (elevation, hydrographic density, road density and percentage of high-quality forest cover) were measured at 500 and 1,000 m spatial extents (buffer sizes). Highly correlated (rs<0.50) outcomes are in bold. S prey = prey index for margay and oncilla; O prey = prey index for ocelot; * = excluded covariates.
(PDF)
p(general) = campaign + method + soil coverage + percentage of high-quality forest cover at 500 m buffer size; “gamma” = colonization; “eps” = extinction; “.” = no covariate included; “0” = parameter was fixed to 0.
(PDF)
“Campaign” = campaign in which data was collected (1- April 2013 to September 2013; 2- October 2013 to March 2014; 3- April 2014 to September 2014); “Groups” = order within the campaigns that sampling sites were surveyed; “Method” = method used in each sampling occasion (camera trapping or scat sampling); “.” = no covariate included (i.e., null model).
(PDF)
p(general) = method + soil coverage + percentage of high-quality forest cover at 500 m buffer size.
(PDF)
p(general) = method + soil coverage + percentage of high-quality forest cover at 500 m buffer size.
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.