Abstract
Depression rates surge in adolescence, particularly among females. Recent findings suggest that depressed adolescents are characterized by hypersensitivity to negative outcomes and blunted responsiveness to rewards. However, our understanding of the pathophysiology and time course of these abnormalities remains limited. Due to their high temporal resolution, event-related potentials (ERPs) provide an ideal probe to investigate these processes. In the present study, healthy (n = 25) and depressed (n = 26) female adolescents (13-18 years) completed a gambling task during 128-channel ERP recording. Time-domain analyses focused on ERPs linked to initial processing of negative versus rewarding outcomes (feedback-related negativity; FRN), and later, elaborative processing (late positive potential; LPP). Additionally, time-frequency analyses were used to decompose the FRN into its two constituent neural signals: loss-related theta and reward-related delta activity, thereby allowing us to separately probe these two putative mechanisms underlying FRN abnormalities in depression. Relative to healthy adolescents, depressed youth showed potentiated FRN (loss versus reward) responses. Time-frequency analyses revealed that this group difference in the FRN was driven by increased loss-related theta activity in depressed youth, and not by reward-related delta activity. For the LPP, healthy adolescents exhibited sustained positivity to rewards versus losses, whereas depressed adolescents showed the opposite pattern. Moreover, an enhanced LPP to losses was associated with rumination. In summary, the LPP may be a sensitive probe of depressive rumination, whereas FRN-linked theta activity may represent a neural marker of hypersensitivity to negative outcomes in depressed youth. Implications for treatment and future ERP research are discussed.
Keywords: depression, feedback-related negativity, time-frequency decomposition, late positive potential, rumination
Introduction
Depression rates surge during adolescence, particularly among females (e.g., Avenevoli et al., 2015; Costello, Copeland, & Angold, 2011). Data from the National Comorbidity Survey – Adolescent Supplement indicate that the 12-month prevalence of major depressive disorder (MDD) is 11% among female adolescents as compared to 5% for adolescent males, and moreover, female adolescents experience a nearly 4-fold increased risk of severe MDD relative to males (Avenevoli et al., 2015). Despite these alarming statistics, surprisingly little is known about the psychological and pathophysiological processes characterizing depression in youth. A greater understanding of neural markers associated with abnormalities in cognitive-affective processes among depressed adolescents may ultimately inform efforts to identify youth at risk of depression and help guide the development of more targeted and efficacious interventions.
A growing body of research implicates hypersensitivity to negative stimuli (Silk et al., 2013) as well as blunted responsiveness to reward-related stimuli (Forbes & Dahl, 2012) in adolescent depression. Given their excellent temporal resolution, event-related potentials (ERPs) may serve as useful probes of the time-course and neural substrates of these abnormalities. Importantly, ERPs do not rely on an individual’s ability to access and report on cognitive or affective processes underlying depression that may be at least partially outside conscious awareness. In the present study, we focused on two well-established ERP components: the first linked to initial processing of negative versus rewarding outcomes (the feedback-related negativity; FRN) and the second associated with relatively later, elaborative processing of salient or emotional content (the late positive potential; LPP).
The Feedback-Related Negativity (FRN)
The FRN is an early ERP component elicited by unfavorable or unexpected outcomes. It peaks approximately 250-300 milliseconds (ms) following the onset of a feedback stimulus and is maximal at fronto-central electrodes. Gambling tasks involving the receipt of monetary rewards versus losses have frequently been used to elicit the FRN. Within these tasks, the FRN is observed as a larger negative deflection to monetary losses relative to wins. Previous studies have found that the FRN to negative outcomes is enhanced in both current (e.g., Cavanagh et al., 2011; Tucker et al., 2003) and remitted (e.g., Santesso et al., 2008) depression. Accordingly, the FRN has traditionally been described as a neural marker sensitive to unfavorable outcomes (Heldmann et al., 2008; Holroyd & Coles, 2002; Holroyd et al., 2003). An alternative conceptualization of the FRN is that it is a positive deflection in the ERP waveform that is larger for rewards than losses (i.e., a reward positivity; see Proudfit, 2015). In other words, the FRN may reflect a reward-related positivity rather than a loss-related negativity. Indeed, studies using temporo-spatial principal component analyses (PCA) indicate that the FRN may be a positive polarity ERP component that is increased for rewarding outcomes and blunted for unfavorable outcomes (Carlson et al., 2011; Foti and Hajcak, 2009; Foti et al., 2011a). In line with this reward-related conceptualization of the FRN, studies reporting a blunted difference in FRN amplitude to rewarding versus negative feedback in depression (i.e., a smaller FRN difference wave/score) have interpreted such findings as evidence of reduced reward sensitivity in the disorder (Bress et al., 2015; Foti & Hajcak, 2009; Liu et al., 2014; but see Mueller et al., 2015).
As a means of reconciling these two competing conceptualizations of the FRN, recent studies employing time-frequency decomposition methods indicate that the FRN is a composite of two signals: one more sensitive to negative outcomes and the other more sensitive to rewards (Bernat et al., 2015; Foti et al., 2015). Specifically, in unselected samples of undergraduates, Foti et al. and Bernat et al. found that theta activity (4-7 Hz) in the time range of the FRN was increased for monetary losses relative to wins, whereas delta activity (< 3 Hz) was larger for wins than losses. EEG source localization analyses further suggested that loss-related theta activity was generated from the anterior cingulate cortex (ACC), whereas reward-related delta activity was localized to a possible source in the striatum (Foti et al., 2015; but see Cohen et al., 2011). Taken together, these findings suggest that delta activity to rewarding feedback may be a neural marker of reward sensitivity, whereas theta activity to negative feedback may be a neural index of sensitivity to negative outcomes. Critically, these two neural signals share extensive temporal and spatial overlap and consequently cannot be isolated using standard ERP analyses but can be parsed via time-frequency decomposition (Foti et al., 2015).
In comparison to other imaging modalities (e.g., functional magnetic resonance imaging [fMRI] and Positron Emission Tomography [PET]), these two EEG-derived time-frequency measures may serve as relatively easy-to-measure and noninvasive probes of abnormal incentive processing in depression. Although previous studies employing gambling paradigms involving the receipt of monetary rewards and losses have reported that depressed participants are characterized by abnormal FRNs (e.g., Foti et al., 2014; Liu et al., 2013), such findings could be attributable to either aberrant neural processing of rewards and/or loss feedback. Time-frequency decomposition can be used to isolate distinct loss- and reward-related neural signals, although this has not yet been examined in relation to adolescent depression. In an effort to replicate and extend the abovementioned Foti et al. and Bernat et al. findings, we conducted a time-frequency decomposition of the FRN in both healthy and depressed adolescents to examine whether group differences are observed in neural sensitivity to losses (i.e., as reflected by a larger theta-FRN response in depression) and/or to rewards (i.e., a blunted delta-FRN in depression). Such findings may help inform our understanding of the extent to which neural systems subserving the processing of reward and/or aversive stimuli exhibit abnormalities in depressed adolescents. To our knowledge, this is the first study to examine theta- versus delta-FRN abnormalities in clinical depression.
The Late Positive Potential (LPP)
In contrast to the early time course of the FRN, the LPP is a sustained positive-going ERP waveform (beginning ~ 300 ms post-stimulus and lasting several hundred ms or seconds) hypothesized to index elaborative processing of motivationally salient stimuli. The scalp distribution of the LPP is initially maximal over parietal regions but propagates to frontal electrodes several hundred ms following stimulus presentation (Foti et al., 2009). The LPP is enhanced to both emotional images (Foti, Hajcak, & Dien, 2009) and words (Fischler & Bradley, 2006), and shows test-retest stability over time (Auerbach et al., 2016). Within the context of a self-referential encoding task, Shestyuk and Deldin (2010) found that healthy adults displayed enhanced LPPs to self-relevant positive adjectives relative to negative adjectives; depressed adults exhibited the opposite pattern (i.e., enhanced LPPs to negative adjectives). When using the same self-referential task in a sample of female adolescents, Auerbach et al. (2015) found a similar pattern of potentiated LPPs to positive relative to negative adjectives in healthy youth, and the opposite pattern among depressed adolescents.
Interestingly, previous studies have found that the LPP can be modulated by emotion regulation strategies. For example, the LPP elicited by unpleasant images is reduced when participants are instructed to use cognitive reappraisal to reframe the images (Hajcak & Nieuwenhuis, 2006) or to redirect their attention to less unpleasant aspects of the images (Hajcak, Dunning & Foti, 2009). Conversely, the LPP can be enhanced through the use of maladaptive emotion regulation strategies, such as rumination. For example, in a sample of unselected undergraduates, Lewis et al. (2015) found that the LPP to unpleasant images was enhanced when participants were experimentally induced to ruminate. The authors failed to find an association between a measure of trait rumination and the LPP to unpleasant images. However, this study relied on a sample of unselected undergraduates, yielding limited variability and severity in trait rumination, which may have limited their ability to detect an underlying association. In their discussion, the authors highlighted the need to include depressed samples in future studies testing abnormalities in the LPP and its link to rumination. In light of the relatively late timecourse of the LPP, coupled with the above findings in healthy and depressed individuals, the LPP to negative stimuli may serve as a neural index of the propensity to ruminate.
Relatively little research has examined the LPP within gambling paradigms involving the receipt of monetary rewards and losses, which typically focus on the FRN instead. However, monetary rewards may elicit an enhanced LPP in healthy individuals insofar as such feedback is perceived as emotionally/motivationally salient and, in particular, if participants are cognitively elaborating on their successes during reward trials. In line with this conceptualization, the LPP has been shown to be enhanced to monetary rewards among healthy adolescents and young adults (Broyd et al., 2012). The LPP is also enhanced in healthy participants instructed to up-regulate their emotional responses to cues indicating monetary rewards (via cognitive elaboration strategies; Langeslag, & van Strien, 2013). Thus, healthy individuals may exhibit enhanced LPPs to rewards relative to losses, whereas depressed participants may display either an “anhedonic effect” (i.e., no differences in LPP to wins versus losses) or a potentiated LPP to losses insofar as they are cognitively elaborating (e.g., ruminating) on these negative outcomes. Given (1) the relatively late timeframe of the LPP, (2) evidence that this component is moderated by cognitive elaboration (potentiated LPP) and reappraisal/distraction (decreased LPP) and (3) recent evidence linking the LPP to unpleasant stimuli to state rumination in an unselected sample (Lewis et al., 2015), we expected that a larger LPP to losses (relative to wins) would be associated with higher rumination.
The Present Study
The goal of the present study was to use a combination of time-domain and time-frequency analyses to investigate electrophysiological markers characterizing initial and sustained responses to rewards and losses within a gambling paradigm (Balodis, Lockwood, Magrys, & Olmstead, 2010; Cox, Andrade & Johnsrude, 2005; Johnsrude et al., 2000). In addition to eliciting ERPs (FRN, LPP) linked to incentive processing, the gambling task employed in the current study incorporates an implicit conditioning component designed to probe the integrity of appetitive conditioning (stimulus-reward learning). Appetitive conditioning is hypothesized to underlie approach motivation and impairments in reward-related conditioning are proposed to play an important role in etiology of depression and in particular anhedonia (Martin-Soelch et al., 2007; Pizzagalli, 2014). Although there is a relatively large literature on the role of appetitive, and in particular aversive, conditioning in adult depression, the role of impairments in appetitive conditioning in depression has been neglected in the adolescent literature (Ernst et al., 2011). Appetitive conditioning has been linked to orbitofrontal cortex (OFC) and ventral striatal functioning, as well as other nodes in the mesocorticolimbic dopamine pathway (Berridge, & Kringelbach, 2015; Martin-Soelch et al., 2007), regions strongly implicated in the pathophysiology of depression and anhedonia (Pizzagalli, 2014). The appetitive conditioning task used in the present study has previously been shown to recruit both the OFC and ventral striatum (Cox et al., 2005). Within the task, neutral stimuli (abstract black and white patterns) are repeatedly paired with appetitive (monetary reward) and/or aversive (monetary loss) feedback in pre-specified ways at different pattern-reward/pattern-loss contingencies. At the end of the 180-trial gambling task, behavioral data are collected assessing participants’ preferences for the conditioned patterns. The task is described as an “implicit” conditioning paradigm as previous studies indicate that the majority of participants are unaware of the pattern-outcome contingencies embedded in the gambling task (Balodis et al., 2010; Cox et al., 2005; Johnsrude et al., 1999; 2000). The implicit nature of the conditioning procedure also reduces the likelihood that confounds such as task demands influence responses regarding pattern preferences. The behavioral pattern preference data allowed us to test for impairments in implicit appetitive conditioning in depressed relative healthy adolescents. To our knowledge this is the first study to do so.
Owing to prior findings summarized above, the following hypotheses were proposed:
Behavioral Hypotheses
-
(1)
Relative to healthy control adolescents, depressed youth were expected to report a reduced preference for the most frequently rewarded patterns, highlighting impaired appetitive conditioning.
FRN Hypotheses
-
(2)
Consistent with prior studies (Bress et al., 2015; Foti & Hajcak, 2009; Liu et al., 2014; but see Mueller et al., 2015), we hypothesized that FRN difference scores (losses minus wins) would be smaller in depressed relative to healthy participants.
-
(3a)
Following a time-frequency decomposition of the FRN, and paralleling the findings of Foti et al. (2015) and Bernat et al. (2015), we predicted that theta power would be greater for losses than wins across the full sample, whereas delta power would be greater for wins than losses.
-
(3b)
We expected greater theta power to losses but blunted delta power to wins, in depressed relative to the healthy participants.
LPP Hypotheses
-
(4)
We expected a significant group by condition interaction for the LPP, such that healthy youth would exhibit a larger LPP to wins than losses and depressed adolescents would show the opposite pattern (i.e., a larger LPP to losses than wins).
-
(5)
Finally, we hypothesized that rumination would be associated with later (LPP) but not earlier (FRN) ERP components.
Method
Participants
Female adolescents (healthy controls [HC] = 25, depressed adolescents [MDD] = 26) were recruited from the Greater Boston area. Participants were right-handed, female adolescents aged 13-18 years with English fluency. For HC participants, exclusion criteria included a history of depression, mania/hypomania, anxiety, eating disorders, substance use disorders, ADHD, psychosis, mental retardation, organic brain syndrome, and head injury resulting in loss of consciousness for 5 minutes or seizures. Depressed participants had the same exclusion criteria, with the exception of having to meet for a current major depressive episode at the time of the diagnostic assessment (a secondary diagnosis of generalized anxiety disorder (GAD) was allowed). The present sample partially overlaps with the HC and MDD samples from a previously published study investigating self-referential processing in youth using a different task (Auerbach et al., 2015). Specifically, 15/22 of the MDD participants and 10/30 of the HC participants from the latter study were included in this study.
As expected, there were significant differences in Beck Depression Inventory-II (BDI-II) scores (Beck, Steer, & Brown, 1996) between the HC (1.48 ± 1.92) and MDD (30.58 ± 10.47) participants, t(26.74) = -13.67, p < .001; Cohen’s d = 3.87. Nine (34.6%) participants in the MDD group met criteria for current GAD. The MDD and HC sample did not significantly differ in terms of age (15.88 ± 1.73 vs. 15.00 ± 1.56 years; t(49) = -1.92, p = .06), race (χ2(4) = 2.54, p = .64), or family income (χ2(5) = 8.73, p = .12). Participants endorsed the following races: 80.4% White, 5.9 % Asian, 2.0% Black or African-American, 9.8% multiple races, and 2.0% not reported. The income distribution in the sample included: 49.0% $100,000 or more, 15.7% $75,000-100,000, 13.7% $50,000-75,000, 2.0% $25,000-50,000, 0% $10,000-$25,000, and 3.9% less than $10,000. SSRI antidepressants were allowed provided that participants were on a stable dose for a minimum of 4 weeks at the time of enrollment. Seven participants in the MDD group were on SSRI antidepressants when enrolled in the study. Because there were no significant differences in ERP (FRN, LPP) or time-frequency (Theta, Delta) measures between those MDD patients on versus off medications, data were pooled.
Procedure
The Partners Healthcare Institutional Review Board provided approval for this study. Assent was obtained from participants aged 13-17, and written consent was obtained from 18-year-old participants and parents. The study assessment was completed over 2 days. During the first day of the assessment, participating adolescents were administered The Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997) to assess current and past Axis I psychopathology according to the Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; DSM–IV–TR; American Psychiatric Association, 2000) and completed self-report measures assessing depressive symptoms and rumination. On the second assessment day, participants completed an experimental task while 128-channel EEG was recorded. The median length between the first (diagnostic) and second (EEG) assessment day was 8 (SD = 6.18) for the HC group and 5 (SD = 4.03) for the MDD participants. Given that this difference was significant (t(49) = 2.75, p = .008), the number of days between assessments was added a covariate in the statistical models below. Participants were remunerated $40 for the two assessments, in addition to their earnings on the experimental task ($12.45 - $14.70, depending on the task version).
Measures
The Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present (K-SADS-PL; Kaufman et al., 1997). The K-SADS-PL was administered to assess current and past DSM-IV disorders. Participants were administered the semi-structured clinical interview during the initial session, and 26/51 adolescents met diagnostic criteria for MDD while 25/51 reported no current or past psychopathology (HC). Clinical psychology doctoral students and BA-level research assistants administered clinical interviews after receiving 40 hours of training (i.e., didactics, mock interviews, direct supervision). All interviews were digitally recorded. Twenty percent of the audiotaped interviews were selected at random to assess inter-rater reliability, and the Cohen’s kappa coefficients for depressive disorders were excellent (κ = 1.00). Depressed participants reported the following: (a) estimated number of major depressive episodes (Mean = 3.68, SD = 3.54) and (b) duration of current major depressive episode (Median = 11.5 weeks, SD = 39.27).
Beck Depression Inventory (BDI-II; Beck et al., 1996)
The BDI-II is a widely used 21-item self-report measure assessing depressive symptoms over the last 2 weeks. Scores on each item range from 0 to 3, with higher scores indicating higher levels of depressive symptoms. In this study, the Cronbach’s alpha for the BDI-II was .97, suggesting excellent internal consistency.
Children’s Response Style Questionnaire (CRSQ; Abela et al., 2007)
The CRSQ is a 25-item self-report measure that includes a Rumination subscale (13 items). The rumination subscale is designed to assess an adolescent’s tendency to respond to sad feelings with self-focused thoughts and to perseverate on their depressive state (e.g., “When I am sad, I think about how alone I feel”). Scores on each item range from 1 (almost never) to 4 (almost always), with higher scores reflecting a greater likelihood of engaging in a particular response style. Prior research has found that the CRSQ exhibits adequate reliability and validity (Abela et al., 2007; Hankin, 2008). In the current study, the Cronbach’s alpha for rumination was .96, indicating excellent internal consistency.
Experimental Task
Participants completed an implicit conditioning task while EEG data were recorded (Cox, Andrade, & Johnsrude, 2005; Johnsrude et al., 1999; 2000; see Figure 1). Over the course of 180 trials, participants were shown three black boxes on a computer screen and were informed that, “one of the boxes is hiding a green ball, and the other two are hiding red balls.” Participants were asked to guess the location of the green ball by selecting one of the boxes via button press. If the box with the green ball was selected, it “opened” revealing the green ball (presented for 2,500 ms), and participants heard a rising tone (500 ms) indicating a monetary gain of 30 cents (the tone was constructed from a sine wave of linearly increasing frequency: 440 to 1320 Hz; Audacity software, http://audacity.sourceforge.net). If participants chose incorrectly (i.e., revealing a red ball) they lost 15 cents, and the same 500 ms sound was played but in reverse (i.e., the tone frequency rose for monetary reward feedback but fell for loss feedback). Each trial was separated by a fixation cross (1000 ms). The original version of the task (Johnsrude et al., 1999; 2000) involved a food reward (candy or raisin) following each win trial, but this was replaced with monetary outcomes to minimize muscle artifacts (due to head movement and chewing) in the EEG. Similar to other monetary reward gambling tasks (Bress et al., 2013; Foti et al., 2015), and in light of research on human loss aversion (Tversky & Kahneman, 1992), the magnitude of monetary rewards was double that of losses in order to approximately equate subjective value of wins and losses. The task consisted of 90 monetary reward trials and 90 loss trials, separated into six blocks of 24-36 trials per block. Consistent with previous studies using this task (Johnsrude et al., 1999; 2000), in between each block participants were asked to estimate how many times they saw the green balls (i.e., reward feedback) in each of the three boxes in the preceding block. At the start of the task participants were instructed to keep track of how many green balls they found.1 This working memory component was intended to minimize participants noticing a preference conditioning procedure embedded in the gambling task (see Behavioral Data from Judgment Phase).
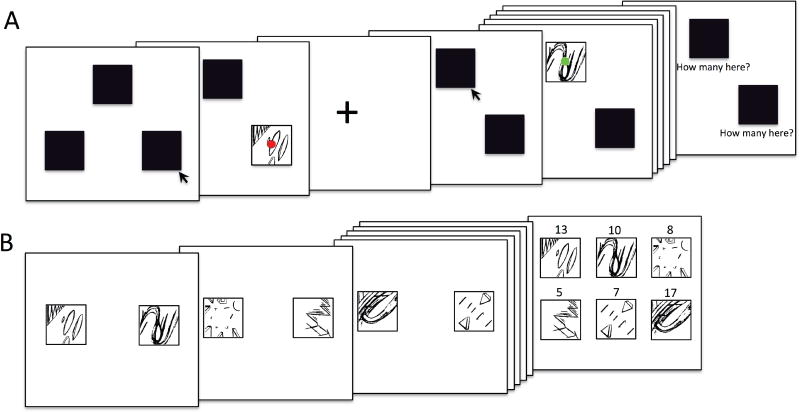
Figure 1.
(A). A schematic displaying one block of the 180-trial gambling paradigm. Participants were presented with three black boxes on a computer screen and were informed that, “one of the boxes is hiding a green ball [win 30 cents], and the other two are hiding red balls [lose 15 cents].” Participants were asked to guess the location of the green ball by selecting one of the boxes. In the first trial of the example displayed, the participant selects the bottom right box, which reveals a red ball (loss trial). In the second trial, the participant selects the top box, revealing a green ball (win trial). At the end of each block participants were asked to estimate how many times they saw the green balls (i.e., win feedback) in each of the three boxes in the preceding block. (B) The lower panel displays the “Judgment Phase” of the task, in which participants were presented with two patterns at a time and asked to choose their preferred pattern. A total of 6 patterns were presented (patterns A-C from the above gambling phase, as well as three novel patterns). Preference scores were computed for each pattern by totaling the number of times it was selected (maximum score = 20; see Methods for additional details).
Behavioral Data from Judgment Phase
The red and green balls revealed after each guess were superimposed on one of three abstract monochrome pattern backgrounds (Johnsrude et al., 1999; 2000). Unbeknownst to participants, the guessing game was embedded within an implicit preference conditioning procedure in which the abstract pattern backgrounds were paired with reward and loss feedback in pre-specified ways at different pattern-reward/pattern-loss contingencies. Specifically, Pattern A was paired with monetary reward feedback on 90% of trials and with loss feedback on 10% of trials; Pattern B was accompanied by rewards on 50% of trials and by losses on 50% of trials: and Pattern C was paired with rewards on 10% of trials and with losses on 90% of trials. To avoid pattern-specific effects on preferences, participants were randomly assigned to three different versions of the task (i.e., in version 2 the ratios were Pattern A, 10:90; Pattern B, 90:10; Pattern C, 50:50, while in version 3, the corresponding ratios were: 50:50; 10:90, and 90:10). Task version (A-C) was included as a covariate in the analyses presented in the results section. Following the completion of the 180-trial guessing game task and EEG recording, participants completed a behavioral task (“Judgment Phase”) assessing whether they developed preferences for these patterns. On each trial, a pair of patterns was presented on the screen, side-by-side. Participants were given the following instructions: “You will see two patterns on the screen. I would like you to choose the one that you prefer [using the button box]. Don’t think too hard; just go with your first impression.” In this judgment phase, a total of 6 patterns was presented (patterns A-C, as well as three novel patterns; see Johnsrude et al., 1999). There were a total of 60 trials, and each pattern was presented 20 times; 10 times on the left and 10 times on the right, in equal combination with each of the other five patterns. Preference scores were computed for each pattern by totaling the number of times it was selected (maximum score = 20). Consistent with previous studies, at the end of this task, participants were asked questions querying why they selected their most preferred and least preferred patterns (“You picked this pattern the most/least, why?”) in order to probe knowledge of pattern-outcome contingencies.
EEG Recording and Data Reduction
The continuous EEG was recorded in an electrically and acoustically shielded room using a 128-channel net from HydroCel GSN Electrical Geodesics, Inc. (EGI). EEG data were sampled at 250 Hz (referenced to Cz) and electrode impedances were kept below 75 k Ω. EEG data were re-referenced to the average of the two mastoid electrodes and offline filters (0.1 to 30 Hz) were applied. An independent component analysis (ICA) was performed to identify and correct for vertical and horizontal eye movement artifacts. In addition, EEG channels with a high number of channel-specific artifacts were removed and interpolated (spline interpolation; Perrin et al., 1987). The median number of interpolated channels was 5 (3.9% of 128 channels). To be included in the present study no more than 15% of channels could be interpolated. All EEG data processing (time-domain analyses and time-frequency decompositions) were conducted in BrainVision Analyzer 2.1.1 (Brain Products, Germany).
Time-Domain Analyses
For time-domain analyses, EEG data were segmented in epochs beginning 200 ms before stimulus (win or loss feedback) onset and up to 1000 ms after stimulus onset. The average amplitude 200 ms prior to stimulus onset was used for baseline correction. Intervals for individual channels were rejected using a semi-automated procedure, with artifacts identified using the following criteria: (1) a voltage step greater than 50 μV between sample points, (2) a voltage difference greater than 300 μV within a trial, and (3) a maximum voltage difference of less than 0.50 μV within a 100 ms interval. In addition, all trials were visually inspected for manual channel-specific artifact rejection.
In line with prior research (Bress et al., 2013; Kujawa, Proudfit, & Klein, 2014; Liu et al., 2014), the FRN was scored using a 100-ms time window surrounding the peak of the loss minus win grand average difference wave (250-350 ms post-stimulus at FCz). FRN analyses tested for group differences in this difference wave (loss minus wins). The LPP was examined across the average of fronto-central midline electrode sites Fz, FCz, and Cz from 600-1000 ms post-stimulus (Auerbach et al., 2015; Auerbach et al., 2016; Dennis & Hajcak, 2009). Similar to Auerbach et al. (2015), a Group (MDD, HC) x Condition (Wins, Losses) interaction was conducted to test LPP differences.
Time-Frequency Decomposition
To isolate theta and delta power, a continuous wavelet transformation was implemented. The processing stream was similar to the Time-Domain Analyses, however, a wider time window was utilized (-1500 ms to 1500 ms) to allow for the discarding of edge effects (Bernat et al., 2015; Foti et al. 2015). After applying the automatic artifact rejection parameters described earlier, a complex Morlet wavelet transformation was implemented using a Morlet parameter c of 3.5 applied to the data from 0.5 to 20 Hz in 30 frequency steps distributed on a logarithmic scale and with a baseline correction of -500 to -300 ms pre-stimulus (Cohen, 2014). The results of the wavelet transformations were averaged within each subject and condition (Wins, Losses), yielding a measure of total power. To test for group and condition differences, we extracted wavelet layers corresponding to delta (central frequency: 2.3 Hz; spectral bandwidth: 1.32 Hz) and theta (central frequency: 5.6 Hz; spectral bandwidth: 3.2 Hz) activity. Similar to previous studies, theta power was maximal at fronto-central electrodes and was scored as the mean activity from 250-350 ms at electrodes FCz (Bernat et al., 2015; Cavanagh et al., 2011). The relatively slower delta activity was more centro-parietally distributed and was scored as the mean activity from 200-400 ms at CPz.
Scores from the time-windowed FRN variable, time-frequency (theta and delta) factors and self-report measures were evaluated statistically using SPSS (version 20.0). Paired t-tests were employed to examine within-group effects of condition (Wins, Losses). Group differences in the FRN difference wave (and to wins and losses separately) were tested by means of one-way ANCOVAs. Two-way mixed ANCOVAs with Group (MDD, HC) and Condition (Wins, Losses) as factors were run to test for differences in theta power, delta power and the LPP. Task version (A-C) and number of days between the first and second assessment were included as covariates in the above ANCOVAs.
Results
Behavioral Data
Relative to the HC group, MDD participants were significantly less accurate in their estimation of the number of times they saw the green balls (i.e., frequency of wins) over the course of the 180 trial task (F(1,45) = 7.65, p < 0.01, η2 = 0.15), on average providing an underestimate of the actual number of wins (MDD Mean=86.44; SD=14.52; HC Mean=89.79; SD=3.39). There were no significant associations between green ball (i.e., reward) count accuracy and either the LPP (r = -.07; p = .67), FRN (r = -.13; p = .38), theta power (r = .11; p = .47) or delta power (r = -23; p = .11).
Only 5 participants (9.8%) provided responses indicating that they were aware of the contingencies (e.g., “I associated it [the 90:10 pattern] with the green ball [i.e., reward trials] and liked the curves”). The remainder of the participants attributed their preferences to the physical characteristics of the pattern (e.g., “I like the curves on it…it’s more eye-catching.”) or could not provide a reason for their preference (e.g., “I don’t know”). However, contrary to our hypotheses, there were no significant differences between the MDD and HC group in preferences for the most frequently rewarded (“90:10”) patterns (F(1,47) = 0.18, p = 0.68; range for HC: 0 – 20; mean (SD) = 10.44 (5.76); range for MDD: 0 – 19; mean (SD) = 10.42 (5.65)) or least frequently reward (“10:90”) patterns (F(1,47) = 1.17, p = 0.29; range for HC: 0 – 19; mean (SD) = 10.48 (5.08); range for MDD: 0 – 16; mean (SD) = 8.65 (4.26)).
Although the purpose of the conditioning procedure was exclusively for the collection of behavioral data on conditioned pattern preferences at the end of the task, it is possible that ERPs to reward and/or loss feedback are moderated by background patterns (i.e., 90:10, 50:50 or 10:90 pattern). Moreover, it is also possible that any effect of background pattern on ERPs is moderated by whether the trials occur earlier vs. later in the task. As a result, we examined whether there were differences in ERPs as a function of background patterns, as well as time (i.e., first half versus second half of task). There were no significant Pattern (i.e., 90:10, 50:50 vs. 10:90) or Pattern x Half (i.e., first vs. second half of task) effects in predicting ERPs in either the timeframe of the FRN (all Fs < 1.46; ps > .24) or the LPP (all Fs < .59; ps > .56), nor did these model terms interact with Group x Condition effects in predicting ERPs (all Fs <1.46; ps > .24). Accordingly, analyses focused on overall effects of Condition (rewards vs. losses) and Group (MDD vs. HC) x Condition interactions on ERPs.
Feedback-Related Negativity (FRN)
Time-Domain
Consistent with previous studies, the FRN was more negative to monetary losses relative to wins (see Table 1). The wins versus loss comparison was significant for both the HC, t(24) = 5.14, p < 0.001 (see Figure 2A, left panel), and MDD, t(25) = 6.80, p < 0.001 (see Figure 2A, right panel) adolescents. There was a significantly larger FRN difference wave in the MDD relative to the HC group, F(1,47) = 4.11, p = 0.048, d = 0.42. There were no significant group differences when examining the FRN to wins, F(1,47) = 0.88, p = 0.35, d = 0.28, or losses, F(1,47) = 0.001, p = 0.98, d = 0.08, separately.
Table 1.
Within-group comparisons for EEG/ERP variables
| Healthy Controls (n = 25) | Major Depressive Disorder (n = 26) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variables | Win vs Loss t-value | Cohen’s d | Win vs Loss t-value | Cohen’s d | ||
| Time-Domain FRN | 5.14 | ** | 1.05 | 6.80 | ** | 1.34 |
| Theta-FRN | -1.38 | -0.30 | -3.42 | ** | -0.78 | |
| Delta-FRN | 2.84 | ** | 0.63 | 2.72 | * | 0.54 |
| LPP | 4.14 | ** | 0.83 | -0.55 | -0.11 | |
Note: FRN = Feedback-Related Negativity; LPP = Late Positive Potential.
p < 0.05;
p < 0.01
Figure 2.
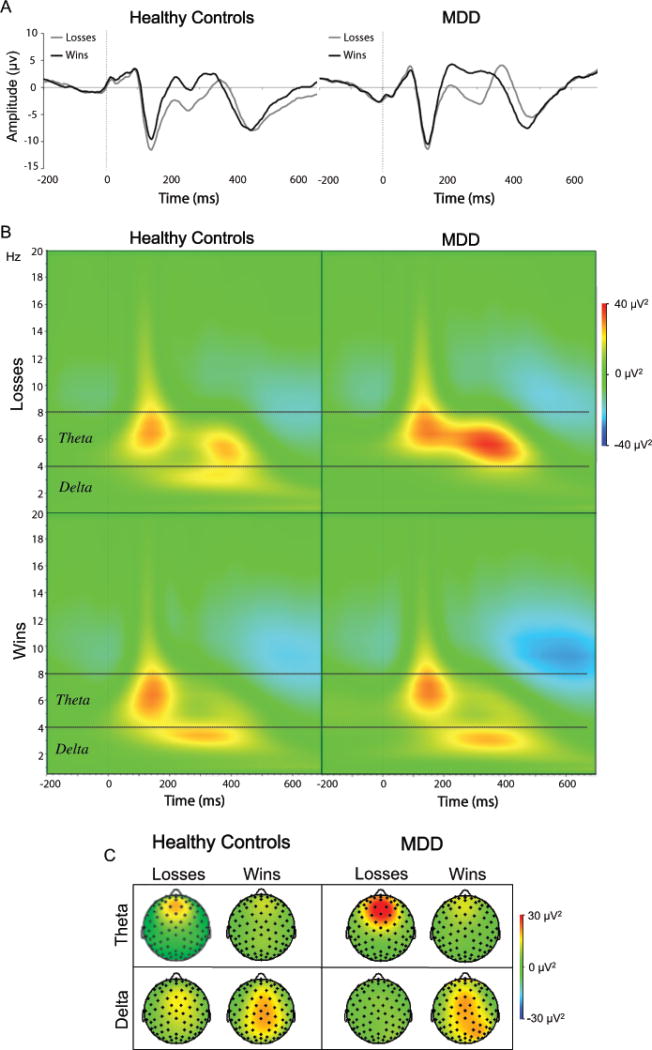
(A) Event-related potentials elicited by monetary losses (gray) and wins (black) for healthy (left panel) and depressed (right panel) participants shown in the time-domain (i.e., prior to time-frequency decomposition) at electrode FCz. (B) Time-frequency plots for losses (top panels) and wins (bottom panels) at electrode FCz for both groups. (C) Scalp distributions for theta power (top panel) and delta power (bottom panel) at 300 ms for both groups and conditions (for each figure healthy participants are shown in the left panel and depressed participants in the right panel).
Time-Frequency Decomposition
Theta power was significantly greater to losses than wins for the MDD group, t(25) = -3.42, p = 0.002 (Figure 2B), but not the HC group (t(24) = -1.38, p = 0.18 (Figure 2B & Table 1), and the Group x Condition interaction was significant, F(1,47) = 4.11, p = 0.048, η2 = 0.08. Between-group simple effects revealed significantly greater theta power to losses for MDD relative to HC participants, F(1,47) = 4.29, p = 0.044, d = 0.53, but no group difference in theta power to wins F(1,47) = 0.15, p = 0.70, d = 0.13.
Conversely, delta power was increased for wins relative to losses in both the HC, t(24) = 2.84, p = 0.009 (Figure 2B) and MDD, t(25) = 2.72, p = 0.012 (Figure 2B) groups (see Table 1). The Group x Condition interaction for delta power was not significant, F(1,47) = 1.91, p = 0.17, η2 = 0.04, and similarly, between-group differences in delta power to wins, F(1,47) = 1.26, p = 0.27, d = 0.21 and losses, F(1,47) = 0.36, p = 0.55, d =.38, were also not significant. In sum, time-frequency findings suggest that the significant group difference in FRN amplitude was driven by loss-related theta activity (rather than reward-related delta activity).
Both theta (r = -0.34, p = 0.016) and delta (r = 0.29, p = 0.036) power difference scores (loss minus wins) were significantly correlated with FRN difference scores (loss minus wins) in the full sample. The correlation between theta and delta differences scores was also significant (r = -0.41, p = 0.003).
Late Positive Potential (LPP)
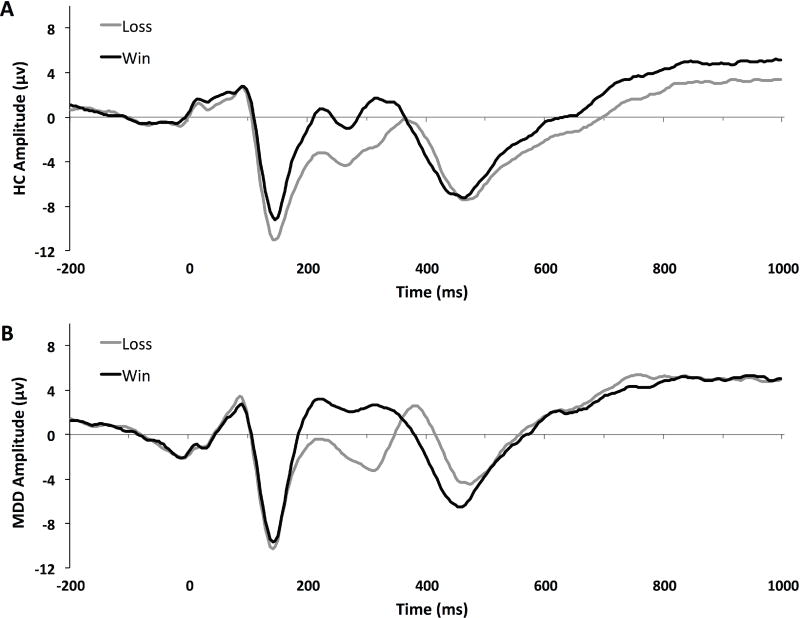
There was a significant Group x Condition interaction for the LPP, F(1,47) = 8.07, p = 0.007, η2 = 0.15. As hypothesized, between-group simple effects revealed that the LPP to losses was significantly larger (more positive) among MDD relative to HC participants, F(1,47) = 4.78, p = 0.034, d = 0.65. There was no significant group difference in the LPP to wins, F(1,47) = 0.53, p = 0.47, d = 0.17. Within-group simple effects revealed that the LPP was significantly larger for wins than losses among HC participants, F(1,47) = 13.53, p < 0.001 (Figure 3, top panel), but there was no such difference in the MDD group, F(1,47) = 0.21, p = 0.65 (Figure 3, bottom panel; Table 1). Notably, 76% (19/25) of HC participants had larger LPPs to wins than loss (binomial P(19/25) = 0.005) vs. only 34.6% (9/26) of the MDD participants (binomial P(9/26) < 0.05; Chi squared = 8.82, p < 0.05).
Figure 3.
Late positive potential (LPP) for healthy (A) and depressed (B) participants in response to wins and losses. The LPP (600–1000 ms) was averaged across electrode sites Fz, FCz, and Cz.
Rumination and the LPP
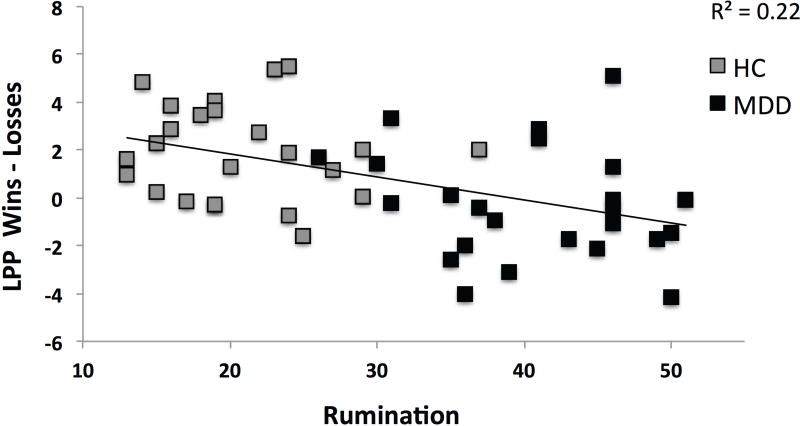
Across groups, the LPP difference score (wins minus losses) was negatively associated with self-reported rumination (r = -0.47; p < 0.001), such that individuals with higher levels of rumination exhibited a larger LPP to losses than wins (Figure 4). When examining the LPP to each feedback condition separately, higher rumination was significantly associated with a larger LPP to losses (r = 0.35; p = 0.015) but not wins (r = 0.10; p = 0.50), and these two correlations were significantly different (z = 3.07, p = .001) (Meng et al., 1992). In spite of the fact that BDI-II and rumination scores were highly correlated (r =.87; p < .001), the association between the LPP difference score and rumination remained significant when controlling for total BDI-II depression scores (pr = -0.33; p = 0.024). However, when controlling for BDI-II scores, the association between rumination and the LPP specifically to losses (pr = 0.10; p = 0.49) or wins (pr = -0.08; p = 0.61) was not significant. There was no significant association between rumination and the FRN (r = -0.05; p = 0.76; theta-FRN: r = 0.17; p = 0.25; delta-FRN: r = -0.05; p = 0.72).2
Figure 4.
Scatterplot displaying the significant association between rumination and the LPP (wins minus losses; r = -.47; p < .001) across groups.
Discussion
Depression in adolescents has been linked to both hypersensitivity to negative stimuli (Silk et al., 2013) and blunted responsiveness to reward-related stimuli (Forbes & Dahl, 2012). In the present study, we focused on two well-established ERP components to investigate neural abnormalities to incentive stimuli in depressed adolescents: the first linked to initial processing of negative versus rewarding outcomes (the FRN) and the second associated with relatively later, elaborative processing of motivationally salient stimuli (the LPP). In addition, capitalizing on recent findings among healthy controls (Foti et al., 2015; Bernat et al., 2015), we used time-frequency decomposition analyses to probe the putative role of delta and theta oscillations in incentive-related abnormalities in depressed adolescents.
FRN Abnormalities in Depression
The MDD participants exhibited significantly larger FRN amplitudes relative to HC participants. The FRN has traditionally been described as an ERP component sensitive to negative outcomes (i.e., observed as a relatively larger negative deflection in the waveform in response to negative than rewarding outcomes; Heldmann et al., 2008; Holroyd and Coles, 2002; Holroyd et al., 2003). However, others have argued that the FRN is more accurately conceptualized as a positive deflection in the ERP waveform that is larger for rewards than losses (i.e., a reward positivity; see Proudfit, 2015). These different conceptualizations of the FRN shape interpretations of observed differences between depressed and healthy participants (e.g., Cavanagh et al., 2011; Liu et al., 2014; Mueller et al., 2015; Tucker et al., 2003). Recent findings in healthy samples derived from time-frequency decomposition methods may help reconcile these seemingly conflicting views. Specifically, recent studies indicate that the FRN consists of both theta activity (which is more sensitive to negative outcomes) and delta activity (which is more sensitive to rewarding outcomes; Foti et al., 2015; Bernat et al., 2011; 2015). Whereas the latter studies were conducted in generally healthy, unselected undergraduate samples, the present study extends this work by examining theta and delta activity in both healthy and depressed participants. Paralleling the pattern of findings observed in prior studies, we found greater delta activity to monetary rewards than losses in both the healthy (Cohen’s d = .63) and depressed (d = .54) samples, and greater theta activity to losses than rewards in the depressed (d = -.78) but not the healthy (d = -.30) group. It is important to highlight that the current study employed a different reward task than that used in either the Foti et al. (2015) (i.e., the “Doors task”) or Bernat et al. (2011; 2015) (a modified version of Gehring & Willoughby’s (2002) gambling task) studies. The fact that the time-frequency findings converge across these studies using different reward paradigms, with each study indicating that the FRN is a composite of loss-related theta and reward-related delta activity, is noteworthy and strengthens confidence in these effects.
Depressed participants exhibited significantly greater theta activity to losses (d = .53) than healthy participants (no differences emerged for theta power to wins, nor for delta power to wins or losses), suggesting that group differences in FRN amplitude may be driven by loss-related theta activity (rather than reward-related delta activity). These theta findings may reflect neural hypersensitivity to negative feedback in depressed relative to healthy teens, which occurs at a relatively early stage of feedback processing (~300 ms). The FRN and theta findings (as well as the LPP results) remained significant even when excluding the 9 MDD participants who met criteria for a comorbid anxiety disorder (i.e., when comparing participants with “pure” MDD versus HC participants; see Footnote 2 for details). It should be highlighted that simply examining group differences in time-domain FRN amplitudes to wins versus losses separately did not differentiate depressed and healthy teens (perhaps due to the fact that both theta and delta power contribute to ERPs to wins and losses). Rather, it was only when time-frequency decomposition analyses were applied that we observed group differences in neural response to losses (specifically in the theta band), suggesting that time-frequency methods may be more sensitive probes of neural sensitivity to losses (and possibly also to rewards).
It is important to note that a variety of tasks elicit theta activity (with a similar midline frontal scalp distribution), including those involving the commission of errors (i.e., tasks eliciting the error-related negativity; ERN), stimulus-response conflict, novelty, and, as in the present study, negative feedback (Cavanagh & Frank, 2014; Cavanagh & Shackman, 2015). Some have argued that what is common across these tasks is the need to exert cognitive control (Cavanagh & Frank, 2014). Frontal midline theta elicited during these tasks, and likely generated from frontocingulate regions (e.g., ACC, medial prefrontal cortex), may signal the need to increase cognitive control and to adjust performance in an adaptive manner. Additional research is needed to clarify the functional significance and behavioral correlates of potentiated theta activity in depression. Cavanagh et al. (2011) found that both the time-domain FRN and theta power to incorrect feedback in a probabilistic reward learning task were associated with enhanced avoidance learning in depression, providing one mechanism through which neural hypersensitivity to negative feedback might manifest in maladaptive behavior.
Contrary to our hypothesis, we did not observe blunted delta activity to rewards among depressed participants. The latter finding is seemingly inconsistent with studies observing blunted ERPs to monetary rewards in depression, suggesting reduced reward sensitivity (e.g., Foti et al., 2014; Liu et al., 2014). However, and paralleling our findings, other FRN studies indicate that depressed individuals may be specifically characterized by hypersensitivity to negative outcomes (e.g., Cavanagh et al., 2011; Tucker et al., 2003; Mueller et al., 2015). Differences in samples and tasks used may help account for these inconsistent findings. Given the vast heterogeneity of MDD, it may be that certain depressed individuals exhibit reduced reward sensitivity whereas others are characterized by hypersensitivity to aversive outcomes (Goldstein, & Klein, 2014; Webb et al., 2016). This is further complicated by differences across studies in the paradigms used to probe sensitivity to rewards vs. negative outcomes, which may also influence ERP results. It will be important for future studies to tease apart the study and sample features that may account for different patterns of FRN findings.
It is also important to highlight that the abovementioned FRN findings are largely derived from standard, time-domain ERP analyses. The FRN is a composite of both loss-related theta and reward-related delta activity, two neural signals that share extensive temporal and spatial overlap and consequently cannot be isolated using standard ERP analyses. However, these signals can be parsed via time-frequency decomposition (Foti et al., 2015). To our knowledge the present study represents the first time-frequency investigation of reward-related delta abnormalities in clinical depression. Although, among a non-clinical, unselected sample of undergraduates, Foti et al. (2015) did find that higher self-reported depressive symptoms were associated with blunted delta response to gains versus losses. Differences in sample (all adolescent females in the current study) or task may help account for our pattern of delta findings.
LPP Abnormalities in Depression and their Link to Rumination
Our hypotheses regarding the LPP were supported. A Group x Condition interaction indicated that healthy teens exhibited a larger (more positive) LPP to wins than losses, relative to depressed youth. Indeed, the majority (76%) of healthy adolescents had a larger LPP to wins than losses. In contrast, nearly two-thirds (65.4%) of depressed teens had a larger LPP to losses than wins. Consistent with our hypothesis, a larger LPP to losses than wins was associated with greater trait rumination. Highlighting the specificity of this relationship, the LPP-rumination association remained significant even when controlling for overall depression and was specific to LPP to losses rather than wins. Moreover, only the LPP and not the FRN correlated with rumination, which is consistent with the notion that the FRN reflects initial feedback processing, whereas the LPP is an index of sustained, elaborative processing more characteristic of rumination. In further support of the LPP-rumination link, a recent study in unselected undergraduates found that experimentally induced rumination resulted in an enhanced LPP to negative images (Lewis et al., 2015). Future research should examine whether LPP abnormalities among depressed individuals normalize, at least in part, through the use of emotion regulation strategies such as cognitive reappraisal. With regards to treatment relevance, cognitive behavioral therapy (CBT), an empirically supported treatment for depressed adolescents (Auerbach, Webb, & Stewart, 2016; Webb, Auerbach & DeRubeis 2012), directly targets depressogenic cognitive patterns, including rumination, and focuses on the acquisition of cognitive reappraisal skills. This raises the question of whether CBT may normalize LPP abnormalities in depression, and whether these changes in part mediate depressive symptom improvement. In addition, further research is needed to determine whether CBT also modulates abnormalities in early ERP components such as the FRN (including abnormalities in theta and/or delta power) or only influences later ERP components such as the LPP.
Another important area for future research is whether abnormalities in certain ERPs predict elevated risk of depression relapse following treatment. For example, are those individuals with relatively elevated theta activity to losses and/or blunted activity to rewards at post-treatment at elevated risk of relapse (even if depressive symptoms have remitted)? Relatedly, although they did not investigate depression relapse, Bress et al. (2013) found that a blunted FRN to monetary rewards (but not losses) prospectively predicted future depression severity in non-depressed adolescent females. Longitudinal studies are required to test whether certain ERP or time-frequency variables predict depression relapse risk following treatment. To the extent that hypotheses are supported they may have important clinical implications regarding which individuals require additional or alternative treatment.
Limitations
There were several limitations to the present study. First, given gender differences in depression and to reduce heterogeneity in our ERP data we only recruited female adolescents. However, this decision precluded us from exploring gender differences in time-domain and time-frequency measures. Second, the present data are cross-sectional and focus on a sample of adolescent participants in a current major depressive episode. Thus, it is unclear whether the observed ERP abnormalities are correlates or consequences of depression, or if they serve as trait-like risk factors that predict the onset of depression. Future studies conducting similar time-frequency analyses in at-risk samples (e.g., children of depressed mothers) or in participants who have a history of depression, but are currently in remission, are needed. Third, although there were no significant associations between green ball (i.e., reward) count accuracy and ERPs, the working memory component of this particular gambling paradigm may have had some influence on ERPs, complicating comparisons with other gambling paradigms that do not involve this feature. It is also important to note that although we observed significant between-group differences in working memory capacity (i.e., keeping count of the frequency of wins), there were no differences in conditioned pattern preferences. Thus, it may be that this feature of the task is not a sensitive probe of appetitive conditioning, at least in healthy adolescent females, who did not display the expected preference for the more frequently rewarded pattern. Fourth, 7 participants in the MDD group were taking SSRI medications. Although there were no significant differences between medicated and unmedicated participants in ERP components, it remains possible that medication could impact ERPs, as well as the generalizability of results. These limitations notwithstanding, the current findings highlight the utility of employing time-frequency analyses to examine neural abnormalities in depression and provide novel evidence that theta-linked neural hypersensitivity to losses accounts for FRN abnormalities in depressed adolescents. In addition, the LPP to negative feedback may be a useful probe of the tendency to engage in depressive rumination.
Acknowledgments
Supported by NIMH grants F32 MH099801 (awarded to CAW) and R01 MH068376 (awarded to DAP). Dr. Webb and Auerbach were additionally supported by NIMH K23 MH108752 and K23 MH097786, respectively. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
There were two outliers within these ball count data (z = 5.01; z = 4.18). These two data points were excluded from the analyses.
The FRN, theta and LPP findings remained statistically significant when only including participants with “pure” MDD (i.e., no comorbidities). As noted in the Methods section, 9 MDD participants met criteria for a comorbid anxiety diagnosis (all GAD). When removing these 9 subjects, the group difference in FRN amplitude remained significant between the remaining MDD sample and the HC sample, F(1,38) = 8.85, p = .005; d = .67. Similarly, the Group x Condition interaction for theta activity remained significant (F(1,38) = 5.11, p = .030; η2 = 0.12), as well as the group difference in theta activity to losses (F(1,38) = 6.56, p = .015; d = .59). Finally, the Group x Condition interaction for the LPP remained significant (F(1,38) = 8.13, p = .007; η2 = 0.18), as well as the group difference in LPP to losses (F(1,38) = 6.66, p = .014; d = .85) and the association between the LPP to losses and rumination (r = .42; p = .007).
Financial Disclosures
Over the past 3 years, Dr. Pizzagalli has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Otsuka America Pharmaceutical, and Pfizer for activities unrelated to the current research. All other authors report no biomedical financial interests.
References
- Auerbach RP, Bondy E, Stanton CH, Webb CA, Shankman SA, Pizzagalli DA. Self-referential processing in adolescents: Stability of behavioral and ERP markers. Psychophysiology. 2016;53(9):1398–1406. doi: 10.1111/psyp.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach RP, Stanton CH, Proudfit GH, Pizzagalli DA. Self-referential processing in depressed adolescents: A high-density event-related potential study. Journal of abnormal psychology. 2015;124(2):233–245. doi: 10.1037/abn0000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach RP, Webb CA, Stewart JG. Cognitive Behavior Therapy for Depressed Adolescents: A Practical Guide to Management and Treatment. Routledge; 2016. [Google Scholar]
- Balodis IM, Lockwood KP, Magrys SA, Olmstead MC. Preference conditioning in healthy individuals: Correlates with hazardous drinking. Alcoholism: Clinical and Experimental Research. 2010;34(6):1006–1012. doi: 10.1111/j.1530-0277.2010.01175.x. [DOI] [PubMed] [Google Scholar]
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. American Journal of Psychiatry. 2008 doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Nelson LD, Baskin-Sommers AR. Time-frequency theta and delta measures index separable components of feedback processing in a gambling task. Psychophysiology. 2015;52(5):626–637. doi: 10.1111/psyp.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat EM, Nelson LD, Steele VR, Gehring WJ, Patrick CJ. Externalizing psychopathology and gain–loss feedback in a simulated gambling task: Dissociable components of brain response revealed by time-frequency analysis. Journal of abnormal psychology. 2011;120(2):352–364. doi: 10.1037/a0022124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams BR, van Duijvenvoorde AC, Peper JS, Crone EA. Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. The Journal of Neuroscience. 2015;35(18):7226–7238. doi: 10.1523/JNEUROSCI.4764-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress JN, Foti D, Kotov R, Klein DN, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50(1):74–81. doi: 10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- Bress JN, Meyer A, Hajcak G. Differentiating anxiety and depression in children and adolescents: Evidence from event-related brain potentials. Journal of Clinical Child & Adolescent Psychology. 2015;44(2):238–249. doi: 10.1080/15374416.2013.814544. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Richards HJ, Helps SK, Chronaki G, Bamford S, Sonuga-Barke EJ. An electrophysiological monetary incentive delay (e-MID) task: a way to decompose the different components of neural response to positive and negative monetary reinforcement. Journal of neuroscience methods. 2012;209(1):40–49. doi: 10.1016/j.jneumeth.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. Neuroimage. 2011;57(4):1608–1616. doi: 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124(1):111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Bismark AJ, Frank MJ, Allen JJ. Larger error signals in major depression are associated with better avoidance learning. Front Psychol. 2011;331:1–6. doi: 10.3389/fpsyg.2011.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends in cognitive sciences. 2014;18(8):414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Shackman AJ. Frontal midline theta reflects anxiety and cognitive control: meta-analytic evidence. Journal of Physiology-Paris. 2015;109(1):3–15. doi: 10.1016/j.jphysparis.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano-Vazquez L, Allen JJ. Theta lingua franca: A common mid-frontal substrate for action monitoring processes. Psychophysiology. 2012;49(2):220–238. doi: 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX. Analyzing neural time series data: theory and practice. MIT Press; 2014. [Google Scholar]
- Cohen MX, Cavanagh JF, Slagter HA. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: Temporospatial principal components analysis and source localization of the feedback negativity: Commentary. Human brain mapping. 2011;32(12):2270–2271. doi: 10.1002/hbm.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SM, Andrade A, Johnsrude IS. Learning to like: a role for human orbitofrontal cortex in conditioned reward. The Journal of neuroscience. 2005;25(10):2733–2740. doi: 10.1523/JNEUROSCI.3360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52(2):95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Dennis TA, Hajcak G. The late positive potential: a neurophysiological marker for emotion regulation in children. Journal of Child Psychology and Psychiatry. 2009;50(11):1373–1383. doi: 10.1111/j.1469-7610.2009.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience. 2011;12(8):467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Fischler I, Bradley M. Event-related potential studies of language and emotion: words, phrases, and task effects. Progress in Brain Research. 2006;156:185–203. doi: 10.1016/S0079-6123(06)56009-1. [DOI] [PubMed] [Google Scholar]
- Fisher RA. On the probable error of a coefficient of correlation deduced from a small sample. Metron. 1921;1:3–32. [Google Scholar]
- Foti D, Carlson JM, Sauder CL, Proudfit GH. Reward dysfunction in major depression: Multimodal neuroimaging evidence for refining the melancholic phenotype. NeuroImage. 2014;101:50–58. doi: 10.1016/j.neuroimage.2014.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological psychology. 2009;81(1):1–8. doi: 10.1016/j.biopsycho.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology. 2009;46(3):521–530. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Bernat EM, Proudfit GH. Anterior cingulate activity to monetary loss and basal ganglia activity to monetary gain uniquely contribute to the feedback negativity. Clinical Neurophysiology. 2015;126(7):1338–1347. doi: 10.1016/j.clinph.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, Hajcak G. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: Temporospatial principal components analysis and source localization of the feedback negativity. Human brain mapping. 2011;32(12):2207–2216. doi: 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Goldstein BL, Klein DN. A review of selected candidate endophenotypes for depression. Clinical psychology review. 2014;34(5):417–427. doi: 10.1016/j.cpr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Dunning J, Foti D. Motivated and controlled attention to emotion: Time-course of the late positive potential. Clinical Neurophysiology. 2009;120:505–510. doi: 10.1016/j.clinph.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. It’s worse than you thought: The feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology. 2007;44(6):905–912. doi: 10.1111/j.1469-8986.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective & Behavioral Neuroscience. 2006;6:291–297. doi: 10.3758/cabn.6.4.291. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Young JF, Abela JR, Smolen A, Jenness JL, Gulley LD, Oppenheimer CW, et al. Depression from childhood into late adolescence: Influence of gender, development, genetic susceptibility, and peer stress. Journal of abnormal psychology. 2015;124(4):803–816. doi: 10.1037/abn0000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel EM, Kujawa A, Hajcak Proudfit G, Klein DN. Neural reactivity to monetary rewards and losses differentiates social from generalized anxiety in children. Journal of Child Psychology and Psychiatry. 2015;56(7):792–800. doi: 10.1111/jcpp.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler J, Herbert C, Winkler I, Junghofer M. Emotion and attention in visual word processing--An ERP study. Biological Psychology. 2009;80(1):75–83. doi: 10.1016/j.biopsycho.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Kujawa A, Proudfit GH, Klein DN. Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. Journal of abnormal psychology. 2014;123(2):287. doi: 10.1037/a0036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KL, Taubitz LE, Duke MW, Steuer EL, Larson CL. State rumination enhances elaborative processing of negative material as evidenced by the late positive potential. Emotion. 2015;15(6):687. doi: 10.1037/emo0000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WH, Wang LZ, Shang HR, Shen Y, Li Z, Cheung EF, Chan RC. The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia. 2014;53:213–220. doi: 10.1016/j.neuropsychologia.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Meng XL, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psychological bulletin. 1992;111(1):172. [Google Scholar]
- Mueller EM, Pechtel P, Cohen AL, Douglas SR, Pizzagalli DA. Potentiated processing of negative feedback in depression is attenuated by anhedonia. Depression and anxiety. 2015;32(4):296–305. doi: 10.1002/da.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Harrington H, Caspi A, Kim-Cohen J, Goldberg D, Gregory AM, Poulton R. Depression and generalized anxiety disorder: cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Archives of general psychiatry. 2007;64(6):651–660. doi: 10.1001/archpsyc.64.6.651. [DOI] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Schroder HS, Donellan MB, Yeung N. On the relationship between anxiety and error monitoring: A meta-analysis and conceptual framework. Frontiers in Human Neuroscience. 2013;7:1–19. doi: 10.3389/fnhum.2013.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clinical psychology review. 2008;28(8):1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor M, Bradley M, Löw A, Versace F, Moltó J, Lang P. Affective picture perception: emotion, context, and the late positive potential. Brain research. 2008;1189:145–151. doi: 10.1016/j.brainres.2007.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and clinical neurophysiology. 1989;72(2):184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Schupp H, Cuthbert B, Bradley M, Cacioppo J, Ito T, Lang P. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37(02):257–261. [PubMed] [Google Scholar]
- Shestyuk AY, Deldin PJ. Automatic and strategic representation of the self in major depression: trait and state abnormalities. American Journal of Psychiatry. 2010 doi: 10.1176/appi.ajp.2009.06091444. [DOI] [PubMed] [Google Scholar]
- Simons RF. The way of our errors: theme and variations. Psychophysiology. 2010;47(1):1–14. doi: 10.1111/j.1469-8986.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- Steinberg L. The neural underpinnings of adolescent risk-taking: The roles of reward-seeking, impulse control, and peers. In: Oettingen G, Gollwitzer PM, editors. Self-regulation in adolescence. Cambridge: Cambridge University Press; 2015. pp. 173–192. [Google Scholar]
- Tacikowski P, Nowicka A. Allocation of attention to self-name and self-face: An ERP study. Biological Psychology. 2010;84(2):318–324. doi: 10.1016/j.biopsycho.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Takács Á, Kóbor A, Janacsek K, Honbolygó F, Csépe V, Németh D. High trait anxiety is associated with attenuated feedback-related negativity in risky decision making. Neuroscience letters. 2015;600:188–192. doi: 10.1016/j.neulet.2015.06.022. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. Advances in prospect-theory: Cumulative representation of uncertainty. Journal of Risk and Uncertainty. 1992;5:297–323. [Google Scholar]
- Waszczuk M, Zavos H, Gregory A, Eley T. The phenotypic and genetic structure of depression and anxiety disorder symptoms in childhood, adolescencs and young adulthood. JAMA Psychiatry. 2014;71:905–916. doi: 10.1001/jamapsychiatry.2014.655. [DOI] [PubMed] [Google Scholar]
- Watkins ER, Nolen-Hoeksema S. A habit-goal framework of depressive rumination. Journal of Abnormal Psychology. 2014;123(1):24–34. doi: 10.1037/a0035540. [DOI] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. Journal of abnormal psychology. 2005;114(4):522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Olvet DM, Hajcak G. Increased error-related brain activity in generalized anxiety disorder. Biological psychology. 2010;85(3):472–480. doi: 10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Kotov R, Proudfit GH. Neural indicators of error processing in generalized anxiety disorder, obsessive-compulsive disorder, and major depressive disorder. Journal of abnormal psychology. 2015;124(1):172–185. doi: 10.1037/abn0000019. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Meyer A, Hale Rude E, Perlman G, Kotov R, Klein DN, Hajcak G. Error-related negativity (ERN) and sustained threat: Conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology. 2016;53(3):372–385. doi: 10.1111/psyp.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CA, Auerbach RP, DeRubeis RJ. Processes of change in CBT of adolescent depression: Review and recommendations. Journal of Clinical Child and Adolescent Psychology. 2012;41(5):654–665. doi: 10.1080/15374416.2012.704842. [DOI] [PubMed] [Google Scholar]
- Webb CA, Dillon DG, Pechtel P, Goer FK, Murray L, Huys QJ, Kurian BT. Neural Correlates of Three Promising Endophenotypes of Depression: Evidence from the EMBARC Study. Neuropsychopharmacology. 2016;41(2):454–463. doi: 10.1038/npp.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]