Abstract
Pulsatillae radix is a conventional traditional Chinese medicine (TCM) with common name Baitouweng, and has notable effects on inflammation and dysentery. Pulsatilla chinensis (Bge.) Regel is the only source plant of Baitouweng recorded in Chinese Pharmacopoeia, but its adulteration often occurs in the market that possibly affects medicinal efficacy and safety. We have established an internal transcribed spacer 2 (ITS2) barcode library based on 105 plant samples from 12 Pulsatilla species and 10 common adulterants. Results indicate that ITS2 barcoding can accurately distinguish Pulsatilla species from their adulterants. Pulsatilla chinensis can be discriminated from 11 congeneric species by two stable single nucleotide polymorphisms (SNPs) in the ITS2 region. Additionally, a quick specific PCR-RFLP identification assay based on the ITS2 barcode was developed. Using specific primers ITS2/PR1 combined with restriction enzyme Bgl I, Pu. chinensis can rapidly be differentiated from other species via simple and low-cost test procedures. Furthermore, 30 commercial Baitouweng products were tested and only two products were derived from authentic Pu. chinensis. Thus, these two molecular approaches provide practical tools for quick identification of commercial Baitouweng products and can help ensure the safe use of this TCM product.
In the last decade, traditional medicine has had a global expansion and is no longer viewed as merely a health care in developing countries. The World Health Organization estimates that 70–80% of the population in developed countries has used complementary and alternative medicine to manage their health1. The huge increase in demand for herbal remedies in recent years demonstrates that herbal medicines are perceived to be as natural and safe. However, fraudulent labeling, counterfeit production, substitution, and adulteration of traditional medicines are becoming increasingly severe, leading to global concern that these activities threaten consumer safety in the absence of more stringent and effective regulation for quality control. TCM herbal materials procured from markets are always in the form of dried or powdered plant parts; therefore, their accurate identification by conventional taxonomy are difficult. Many DNA based methods have been developed for identifying species, which are not dependent on morphological identification by plant specialists2. RAPD (random amplified polymorphic DNA), SSR (simple sequence repeat), ISSR (Inter-SSR), AFLP (amplified fragment length polymorphisms) and RFLP (restriction fragment length polymorphism) were the early molecular marker identification methods that have been widely used for identifying species3,4. However, these methods involved time intensive, and expensive laboratory procedures, making them unsuitable for rapid species identification. DNA barcoding is a technology that uses short and standardized DNA sequences to identify species5,6. In 2003, the mitochondrial gene cytochrome c oxidase subunit 1 (COI), was proposed as a DNA barcode marker for the discrimination of animal species7,8. Since then, DNA barcoding has aroused great attention and research interest for practical applications9,10,11. The Plant Working Group in botany, the consortium for the barcodes of life (CBOL), recommended that the plastid DNA regions matK and rbcL act as the core barcodes for land plants12, to be supplemented with the plastid psbA-trnH and the nuclear ribosomal internal transcribed spacer (ITS)13. Chen et al. reported that the second internal transcribed spacer (ITS2) of the nuclear ribosomal DNA had better species discriminating power and suggested it as the standard DNA barcode to identify medicinal plants14,15. DNA barcoding can achieve rapid, accurate identification of species from raw materials and has become a reliable molecular tool for authenticating medicinal plants and their adulterants2,4,16,17. Meanwhile, several DNA barcoding databases were established and published online, such as the Barcode of Life Data System (http://www.boldsystems.org)6, DNA barcoding system for herbal materials (http://www.tcmbarcode.cn/en)2, and organism-specific databases for Lepidoptera (http://www.lepbarcoding.org/) and fish (http://www.fishbol.org/). These barcoding systems provide open barcode databases and standard protocols for species identification. In recent years, a lot of quick identification methods based on DNA barcode markers were developed, such as Allele-specific PCR18, Real-time PCR19, High Resolution Melting (HRM) analysis20, PCR-RFLP21, and etc. These methods have simpler test procedures and shorter time-consuming than conventional methods that were more convenient for rapid identification.
Pulsatillae radix, Baitouweng (Chinese vernacular name), is well known as a commonly used traditional Chinese medicines (TCM) because of its notable medicinal uses in heat-clearing, blood cooling and detoxification22. Recent, chemical studies have found that Baitouweng contains anemonin, triterpenoid saponins, pulsatillic acids, flavonoids, daucosterols, and etc.23,24,25,26. Pharmacological research indicated that it had anti-amoebic, antibacterial27, and antitumor effects28,29. However, Baitouweng is an easily confused TCM and its adulteration often occurs in the markets where it is sold to consumers. Pulsatilla chinensis (Bge.) Regel of the Ranunculaceae family is the only plant source of Pulsatillae radix recorded officially in the Chinese Pharmacopoeia22. In the Flora of China, Pulsatilla is regarded as a separate genus differ from Anemone because of morphology on the achenes30. But molecular phylogenetic studies have shown that Pulsatilla is nested within Anemone31, and Pulsatilla is treated as a clade of Anemone, e.g. the TROPICOS (www.tropicos.org/Name/27104112) and the Flora of North American (http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=101733). Thus, Pu. chinensis and Anemone chinensis actually represent the same species. Here, we continue to follow use of the name Pu. chinensis out of deference to current usage in TCM. There are about 33 Pulsatilla species in the world, mainly distributed in Asia, Europe and North America, and 11 Pulsatilla species are widely distributed in China throughout the provinces of Yunnan, Sichuan, Qinghai, Shaanxi, Henan, Heilongjiang and Nei Mongol30. Besides Pu. chinensis, Pu. cernua (Thunb.) Bercht. et Opiz., Pu. turczaninovii Kryl. et Serg., Pu. ambigua Turcz. ex Pritz., Pu. dahurica (Fisch. ex DC.) Spreng., and Pu. campanella Fisch. ex Regel have also been referenced as the plant source of medicinal Baitouweng in some Chinese herbal books32. In addition, vegetative morphology similar to that of Pu. chinensis is found not only in some closely related species (e.g. Anemone hupehensis Lem.), but also in considerably more distantly related ones like Potentilla chinensis Ser. (recorded as “Huangzhou Baitouweng”) or Po.discolor Bge. (recorded as “Lanling Baitouweng”), both of which are in the Rosaceae33. Similarly, due to similar appearances of medicinal parts, dry roots from Rhaponticum uniflorum (L.) DC. (Compositae) and Platycodon grandiflorus (Jacq.) A. DC. (Campanulaceae) have also been mistaken for, and sold as Baitouweng. Theses misuse practices of Baitouweng will not only decrease the pharmacological efficacy but also compromise the medicinal safety, which must be avoided as much as possible. Baitouweng products in the market are in form of pieces or powders and can not be identified by plant morphological characteristics. So far, the main methods for distinguishing them are based on microscopic characteristics34,35 and physicochemical analysis36,37, but still difficult to determine specific species. There have been a few molecular studies focused on the phylogenetic relationships of Ranunculaceae based on DNA markers31,38. Sun et al. used the ITS region to distinguish a new Pulsatilla species, Pu. tongkangensis, from other Pulsatilla species39. However, research on distinguishing medicinal Pu. chinensis from its adulterants is still limited. Therefore, an accurate and convenient identification tool to distinguish authentic medicinal Pu. chinensis (Baitouweng) from its adulterants is absolutely essential.
The aim of this study was to explore effective and quick approaches to discriminate the genuine origin of medicinal Baitouweng, Pu. chinensis, from its adulterants based on the ITS2 region, and then use these tools to authenticate commercial medicinal Baitouweng products for ensuring the quality of raw materials in the market.
Results and Discussion
The ITS2 library of Pu. chinensis and its adulterants
Establishment of a DNA barcode database is the precursor for molecular identification of species. To extend the existing ITS2 database, 84 fresh plant samples representing 12 Pulsatilla species and 10 adulterant species were collected from different localities in China (see Supplementary Table S1). All these species were identified by taxonomic experts according to the Flora of China (http://foc.eflora.cn/) and deposited in the herbarium of the Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences (IMD). PCR results showed that the ITS2 region of all 84 samples were successfully amplified by universal primers ITS2F/ITS3R and sequenced well. By removing the flanking 5.8S and 28S rRNA gene sequences, 84 ITS2 sequences were obtained and deposited in GenBank under accession numbers KR611721–86, KT969417-31, FJ980329, FJ980329, and FJ980346. To include more genotypes of the sampled species, 21 ITS2 sequences were downloaded from GenBank (see Supplementary Table S1). Because some downloaded sequences were partial, seven nucleotides at the 3′ terminus after sequence alignment were cut off to keep all sequences consistent. Thus, the ITS2 barcode library of Pu. chinensis and its common adulterants was successfully established, containing 105 reference ITS2 sequences from 12 Pulsatilla species and 10 commonly confused species. Sequence analysis showed that the length of the ITS2 sequences ranged from 204 bp to 255 bp, the average GC contents ranged from 49.8% to 67.0%, the number of variable sites ranged from 0 to 20 (Table 1), and the average intraspecific K2P distance of ITS2 in each species varied from 0 to 0.046 (see Supplementary Table S2). This established library expanded the ITS2 barcodes of Pu. chinensis and its common adulterants in our existing DNA barcoding database for herbal materials2 and was necessary for identifying the origin species of medicinal Baitouweng.
Table 1. Characteristics of the ITS2 sequences in the reference database.
| Specie | Sample number | Sequence length (bp) | Average GC content (%) | Variable sites |
|---|---|---|---|---|
| Pulsatilla chinensis | 25 | 211 | 64.5 | 4 |
| Pu. cernua | 9 | 212 | 64.0 | 1 |
| Pu. campanella | 8 | 212 | 64.0 | 9 |
| Pu. millefolium | 1 | 212 | — | — |
| Pu. tongkanensis | 4 | 212 | 64.6 | 0 |
| Pu. violacea | 3 | 11 | 64.6 | 3 |
| Pu. dahurica | 2 | 212 | 64.0 | 8 |
| Pu. albana | 1 | 211 | 64.9 | — |
| Pu. halleri | 1 | 210 | 65.2 | — |
| Pu. rubra | 1 | 212 | 65.6 | — |
| Pu. sp. | 1 | 212 | 65.2 | — |
| Pu. turczaninovii | 1 | 212 | 64.2 | — |
| Potentilla chinensis | 10 | 204–206 | 64.3 | 20 |
| Po. discolor | 8 | 204 | 63.2 | 3 |
| Rhaponticum uniflorum | 10 | 216 | 57.5 | 4 |
| Platycodon grandiflorus | 6 | 255 | 63.9 | 0 |
| Anemone hupehensis | 4 | 204–205 | 67.0 | 2 |
| Ajuga decumbens | 4 | 221 | 63.9 | 1 |
| Leontopodium leontopodioides | 2 | 216 | 51.6 | 0 |
| Duhaldea cappa | 2 | 223 | 51.0 | 0 |
| Gerbera piloselloides | 1 | 222 | 59.0 | — |
| Gnaphalium affine | 1 | 217 | 49.8 | — |
| All species | 105 | 204–255 | 61.9 | — |
Discrimination of Pu. chinensis and its adulterants using ITS2 barcoding
ITS2 sequences from 25 individuals of Pu. chinensis were obtained in this study. Sequence analysis showed that 25 ITS2 sequences had the same length of 211 bp and the average GC content was 64.5% (Table 1). There were four variable sites (17 bp, 63 bp, 125 bp, and 169 bp) in the ITS2 sequences that segregated as five sequence haplotypes (H): H1 (B1-B13, B15-16), H2 (B20, B22-25), H3 (B17-19), H4 (B14) and H5 (B21) (see Supplementary Fig. S1). Among them, H1 contains 15 sequences, indicating it may be the major ITS2 haplotype of Pu. chinensis. Moreover, the Kimura 2-parameter (K2P) intraspecific distances among them had a mean of 0.006, ranging from 0 to 0.019 (Table 2).
Table 2. The intra- and inter-specific K2P distance of ITS2 sequences.
| Species | KP2 distances of the ITS2 | ||
|---|---|---|---|
| Minimum | Maximum | Mean | |
| Pulsatilla chinensis & Pu. chinensis | 0 | 0.019 | 0.006 |
| Pu. chinensis & other 11 Pulsatilla species | 0.005 | 0.060 | 0.020 |
| Pu. chinensis & adulterants of other genera | 0.113 | 0.755 | 0.595 |
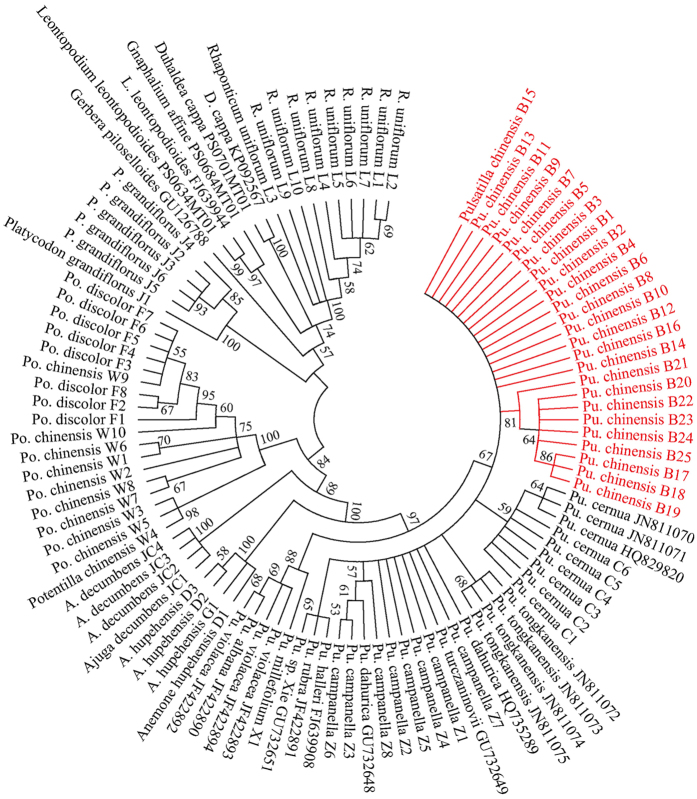
Nearest genetic distance, BLAST and neighbor-joining (NJ) tree methods were used to evaluate the identification capability of the ITS2 barcode15,40. Firstly, we estimated discrimination power of the ITS2 barcode between Pu. chinensis and 10 adulterants from other genera. Sequence divergences showed that the K2P interspecific distances between Pu. chinensis and these species ranged from 0.113 to 0.755 (Table 2). Thus, the minimum interspecific distance 0.113 was much more than the maximum intraspecific distance of Pu. chinensis (0.019), indicating that Pu. chinensis can easily be distinguished from these 10 adulterants based on the ITS2 sequences by K2P distance method. NJ tree result also showed that Pu. chinensis clustered into one clade with congeneric species and can discriminate from other included taxa (Fig. 1). Moreover, our results also exhibited that Anemone hupehensis clustered into one clade with a bootstrap value of 100, and had the closest relationship to Pulsatilla species (Fig. 1), and the interspecific distance between them was 0.104. R. uniflorum, P. grandiflorum, Ajuga decumbens, Leontopodium leontopodioides, Duhaldea cappa, Gerbera piloselloides, and Gnaphalium affine also represented monophyletic groups, but Po. chinensis and Po. discolor clustered into the same clade with a bootstrap value of 100 (Fig. 1), implying that the ITS2 sequences in these two species were very similar. However, these 10 known adulterants of medicinal Baitouweng can be easily and accurately be discriminated from Pu. chinensis using the ITS2 barcode.
Figure 1. Phylogenetic NJ tree of Pulsatilla chinensis and its adulterants constructed with the ITS2 sequences.
The bootstrap scores (1000 replicates) are shown (≥50%) for each branch. All voucher numbers are from sample codes in this study and GenBank accession numbers.
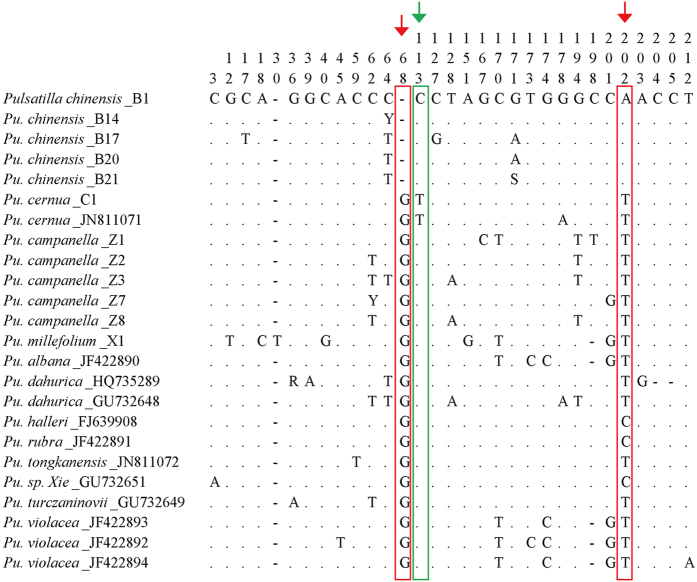
Secondly, we analyzed identification ability of the ITS2 barcode between Pu. chinensis and 11 congeneric species. The ITS2 length 12 Pulsatilla species ranged from 210 bp to 212 bp and the average GC content was 64.6% (Table 1). The K2P distance analysis indicated that the minimum interspecific distance between Pu. chinensis and 11 congeneric species (0.005) was less than the maximum intraspecific distance of Pu. chinensis (0.019) (Table 2). The NJ tree showed that 12 Pulsatilla species were clustered into one clade with a bootstrap value of 97 (Fig. 1). These results demonstrated that the 12 Pulsatilla species shared similar ITS2 sequences and neither K2P genetic distance nor NJ tree analysis could distinguish them well. Searching the chromosome numbers tabulated in The Chromosome Counts Database (CCDB)41 showed that most of the examined Pulsatilla species were diploids (n = 8), that probably why the ITS2 sequences shows little variation. Furthermore, by comparing different ITS2 haplotypes from the 12 Pulsatilla species, 32 single nucleotide polymorphism (SNP) sites were found in them, and two SNP sites were distinctive in five ITS2 haplotypes of Pu. chinensis, which were a G nucleotide deletion at position 68 and an A nucleotide variation at position 202 (Fig. 2). Thus, although there was a high similarity among ITS2 sequences of Pulsatilla species, the two specific and stable SNP sites can still effectively discriminate Pu. chinensis from the sampled congeneric species. Meanwhile, SNP analysis of the ITS2 region detected one specific SNP site at position 113 with a T nucleotide variation in Pu. cernua (Fig. 2), which provides a fast molecular identification method for that species.
Figure 2. SNPs analysis of Pulsatilla species in the ITS2 sequences. Samples represent different ITS2 haplotypes of each Pulsatilla species.
The number represents the position of variable sites (bp); dots (.) represents the nucleotide identical to the first line; dash (−) represents a nucleotide indel; Y represents T/C; S represents C/G; R represents A/G. Red boxes and arrows indicate SNPs in Pulsatilla chinensis, and green boxes and arrow indicate SNPs in Pu. cernua.
In summary, ITS2 barcoding can distinguish Pu. chinensis from its known adulterants. However, there are still challenges for distinguishing closely related species based on DNA barcoding42. In this study, some Pulsatilla species and two Potentilla species (Po. chinensis and Po. discolor) could not be discriminated well by the ITS2 barcode (Fig. 1). The poor resolution of ITS2 and other barcodes were also reported in the genus Crataegus43. Thus, more work is necessary to expand the reference plant data set, and screening of more supplementary DNA loci to identify specific barcodes for difficult genera.
Authentication of commercial Baitouweng products through ITS2 barcoding
A total of 30 Baitouweng products in the market were surveyed in this study to confirm the identification efficiency of ITS2 barcoding. These products were purchased in medicinal herb markets and stores in the cities of Baoding, Shijiazhuang, Nanning, Beijing, Anguo, Yulin, and Bozhou in China and numbered as Y1 to Y30. They were in the forms of dry root pieces, leaves, or powder, with no morphologically recognizable features that could allow species identification (Fig. 3). Our results showed that ITS2 sequences of 30 commercial products were all successfully amplified by the universal primers ITS2F/3R. Among them, ITS2 sequences of Y1 to Y29 were obtained by directly sequencing the purified PCR products. Direct sequencing of Y30 failed because of double sequence peaks, implying that Y30 might be a mixture containing several taxa. Eventually, 17 ITS2 sequences were obtained from Y30 by using the TA cloning method. Sequence alignments showed that these sequences divided into two types, nine sequences were 216 bp in length and the other eight ones were 210 bp in length. Therefore, all ITS2 sequences from 30 commercial products were finally obtained. ITS2 length ranged from 211 bp to 255 bp (Table 3), suggesting that Y1 to Y30 were not just derived from one species.
Figure 3. Photos of commercial Baitouweng products.
Y1 to Y30 represent sample codes of commercial products. Scale bar, 1 cm.
Table 3. Authentication of commercial Baitouweng products.
| Sample codes | Products forms | ITS2 barcoding |
specific PCR | PCR-RFLP | ||
|---|---|---|---|---|---|---|
| Sequence length (bp) | Blast identified organism | Maximum identity (%) | ||||
| Y1 | root | 211 | Pulsatilla chinensis | 100 | + | + |
| Y2 | root | 212 | Pu. campanella | 99.5 | + | − |
| Y3 | root | 212 | Pu. cernua | 100 | + | − |
| Y4 | root | 212 | Pu. cernua | 100 | + | − |
| Y5 | root | 211 | Pu. chinensis | 99.5 | + | + |
| Y6 | root | 212 | Pu. dahurica | 99.5 | + | − |
| Y7 | root | 212 | Pu. cernua | 100 | + | − |
| Y8 | root | 212 | Pu. cernua | 100 | + | − |
| Y9 | root | 212 | Pu. cernua | 100 | + | − |
| Y10 | root | 212 | Pu. cernua | 100 | + | − |
| Y11 | root | 212 | Pu. dahurica | 99.5 | + | − |
| Y12 | root and leaf | 204 | Potentilla chinensis | 100 | − | N/A |
| Y13 | root and leaf | 204 | Po. chinensis | 100 | − | N/A |
| Y14 | root and leaf | 204 | Po. chinensis/ Po. nivea | 98.1 | − | N/A |
| Y15 | root and leaf | 204 | Po. chinensis | 100 | − | N/A |
| Y16 | root and leaf | 204 | Po. chinensis | 100 | − | N/A |
| Y17 | root | 204 | Po. chinensis | 99.0 | − | N/A |
| Y18 | root | 204 | Po. chinensis/ Po. discolor | 100 | − | N/A |
| Y19 | root and leaf | 204 | Po. chinensis/ Po. discolor | 100 | − | N/A |
| Y20 | root | 216 | Rhaponticum uniflorum | 100 | − | N/A |
| Y21 | root | 216 | R. uniflorum | 100 | − | N/A |
| Y22 | root | 216 | R. uniflorum | 100 | − | N/A |
| Y23 | root | 204 | Anemone hupehensis | 100 | − | N/A |
| Y24 | root | 205 | A.hupehensis | 100 | − | N/A |
| Y25 | root | 255 | Platycodon grandiflorus | 100 | − | N/A |
| Y26 | root | 212 | Pu. cernua | 100 | + | − |
| Y27 | root | 212 | Pu. cernua | 100 | + | − |
| Y28 | root | 212 | Pu. cernua | 100 | + | − |
| Y29 | root | 224 | Gossypium hirsutum/ G. barbadense | 100 | − | N/A |
| Y30 | powder | 216 | R. uniflorum | 98.2–100 | − | N/A |
| 210 | Atragalus genus | 99.1–99.5 | ||||
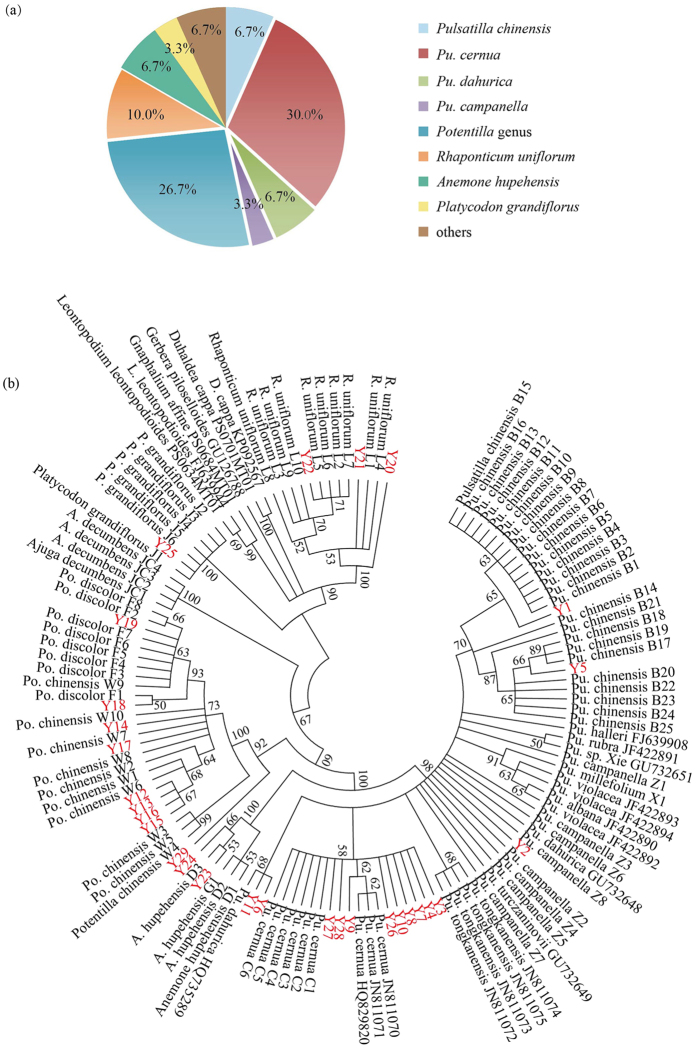
BLAST and NJ tree methods were used to authenticate commercial Baitouweng products. All ITS2 sequences were aligned using the BLAST algorithm to search for the highest sequence similarity amongst samples to identify the species based on the established ITS2 library of Pu. chinensis and its adulterants, and online databases (http://www.tcmbarcode.cn/en/, and http://blast.ncbi.nlm.nih.gov/Blast.cgi). The results indicated Y1 and Y5 shared 100% and 99.5% homology with Pu. chinensis, respectively (Table 3), and had the two identical SNP sites as Pu. chinensis (see Supplementary Fig. S2). Thus, Y1 and Y5 can be authenticated as Pu. chinensis. Nine products (Y3, Y4, Y7, Y8, Y9, Y10, Y26, Y28 and Y29) shared 100% identity with Pu. cernua and contained the same SNP site at position 113 (see Supplementary Fig. S2), thus were identified as Pu. cernua. Three products had the most similarity with Pu. dahurica (Y6 and Y11) and Pu. campanella (Y2) (Table 3). In addition, eight products were likely derived from the genus Potentilla, sharing 98.1–100% sequence similarity with Po. chinensis, Po. nivea, and Po. Discolor. Seven products were likely derived from R. uniflorum (3), A. hupehensis (2), P. grandiflorus (1), and Gossypium species (1). For product of Y30, nine sequences shared 98.2% to 100% homology with R. uniflorum and the other eight sequences shared 99.1 to 99.5% similarity with Astragalus species, indicating that Y30 was mixed with at least two species. In summary, two Baitouweng products (6.7%) were derived from Pu. chinensis, 12 from congeneric species, and 16 from other species (Fig. 4a). NJ tree analysis was consistent with the BLAST result (Fig. 4b).
Figure 4. Authentication of commercial Baitouweng products by ITS2 barcoding.
(a) Species diversity of commercial Baitouweng products. (b) Phylogenetic NJ tree of voucher species and commercial products based on the ITS2 sequences. The bootstrap scores (1000 replicates) are shown (≥50%) for each branch. Y1 to Y30 represent sample codes of commercial products, others are sample codes of voucher samples and GenBank accession numbers.
Our market survey revealed that there were a number of adulterants of medicinal Baitouweng in the market, causing serious potential health risks for users. These adulterants were mainly divided into two types. Type one are substitutes from congeneric species. Our study showed 12 Baitouweng products were from congeners of Pu. chinensis. Nine products (30.0%) were from Pu. cernua, implying that Pu. cernua holds a considerable share as a substitute plant source of Baitouweng on the Chinese market. Pu. cernua is mainly located in Heilongjiang, Jilin, Liaoning, and Nei Mongol within China, but also occurs in Japan, Korea, and Russia. Although it was not stipulated in Chinese Pharmacopoeia, it was noted as plant origin of medicinal Baitouweng in “Chinese Herb Medicine”32. Thus, these congeneric substitutes may have similar medicinal efficacy with Baitouweng, and merit further testing. The second type of adulterants that we detected in medicinal market samples were contaminants from unrelated species. Baitouweng is an inexpensive, but easily confused herb. Plants with white hairs near the root are always misidentified as the origin plant. Therefore, contaminants are mainly due to misidentification, and not through intentional adulteration. Our finding indicated that 16 Baitouweng products (53.3%) belonged to this type, including Po. chinensis, R. uniflorum, A. hupehensis, and P. grandiflorus. Among them, Potentilla species might be the most common contaminant of medicinal Baitouweng in the market. Po. chinensis is also a commonly used TCM with Chinese vernacular name Weilingcai22 and is often be reported as adulterant of Baitouweng33,35. Additionally, plants from the Gossypium and Astragalus were also detected in this study. Therefore, ITS2 barcoding can effectively authenticate commercial Baitouweng products and its adulterants, aiding in solving the security issues of medicinal Baitouweng in the market.
DNA barcoding is becoming a helpful method for authenticating bioingredients in commercial herbal product. Newmaster et al. reported contamination and substitution in North American herbal products using a tiered DNA barcoding approach (rbcL + ITS2)44. Bruni et al. used the rbcL and psbA-trnH regions to identify the taxonomic composition of honey in multiflower honey, and detected one toxic plant in one sample45. Stoeckle et al. also identified some unlisted ingredients in herbal teas46. Xin et al. distinguished super food Lycium barbarum from its closely related species via ITS2 barcoding47. For authenticating commercial Pu. chinensis and its adulterants, choosing an ideal DNA barcode is very important. As our result, the ITS2 region is a suitable one for this goal. Firstly, the ITS2 barcode is much shorter than other candidate barcodes and can easily be amplified even for some degraded DNA samples15. The manufacturing process often degrades the crude form of Baitouweng. In this study, the ITS2 region was 100% amplified and successfully sequenced for all fresh samples and commercial products. Secondly, the ITS2 barcode has a high identification power48,49, and has already identified many medicinal materials successfully47,50,51,52. Our study indicated that using the ITS2 barcode could accurately authenticate genuine medicinal Baitouweng of Pu. chinensis from its adulterants. A similar result was reported that this region enabled a molecular discrimination of Pu. patens and Pu. vernalis53. Therefore, ITS2 barcoding is an effective tool for authenticating commercial Baitouweng products from its adulterants. But for mixed herbal products, obtaining ITS2 sequences by TA cloning is still laborious and time-consuming. Next-Generation Sequencing (NGS) represents a new vision to detect the organic ingredients in mixed samples, such as tablets, powders, capsules and pills54,55,56, and will provide an efficient and cost-effective mean to detect species composition of mixed TCM products. Targeting both DNA and characteristic components by DNA sequencing and chromatographic analysis will be a better integrative approach for authenticating TCM products, especially for standardized extracts with degraded DNA57.
Quick identification of Pu. chinensis via specific PCR-RFLP assay based on the ITS2 region
A distinctive restriction site of Bgl I (GCCNNNNNGGC) in the ITS2 region of Pu. chinensis was selected by SNP analysis (Fig. 5a); thus, a PCR-RFLP assay was developed for quick identification of Pu. chinensis. We designed a specific PCR primer pair ITS2F/PR1 and replaced the thirteenth G in PR1 with T to avoid another Bgl I site in Pulsatilla species (Fig. 5a), thus PCR amplicons by ITS2/PR1 will have no Bgl I site in Pulsatilla species except Pu. chinensis. Then, four Pulsatilla species and two closely related species (Cimicifuga simplex Wormsk and Clematis armandii Franch) and other four adulterant species of Baitouweng were selected to test this assay. PCR results indicated that the DNA were of good quality for PCR amplification because the ITS2 regions were all successfully amplified by ITS2F/ITS3R. Moreover, ITS2/PR1 were able to successfully amplify approximately 292 bp products from four Pulsatilla species but no PCR product were present in other species (Fig. 5b), indicating ITS2/PR1 is highly specific for Pulsatilla species and can significantly discriminate them from other species. Bgl I digestion results showed that only two PCR amplicons from Pu. chinensis were cleaved into two expected fragments of approximately 168 bp and 124 bp lengths, others could not be digested any more (Fig. 5c). Although one PCR product of Pu. chinensis was not digested completely, that might be due to the small PCR fragment. However, this did not affect the identification result. Therefore, using the specific PCR-RFLP primers ITS2/PR1 combined with restriction enzyme Bgl I can not only identify Pulsatilla species from others, but also authenticate Pu. chinensis from congeneric species. In addition, 30 commercial Baitouweng products were also examined by the specific PCR-RFLP assay. The identification results were consistent with ITS2 barcoding results discussed previously (Table 3), indicating that this assay was suitable for market samples.
Figure 5. Specific PCR-RFLP assay of Pulsatilla chinensis and its adulterants.
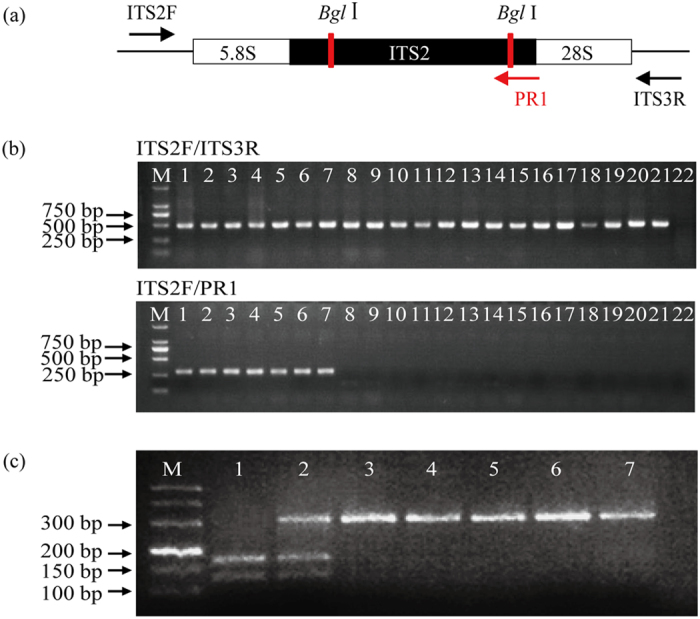
(a) Specific primers for Pulsatilla species and Bgl I sites within the ITS2 region of Pu. chinensis. (b) PCR products by primers ITS2F/ITS3R and ITS2/PR1. Lane 1–22: Pu. chinensis (1–2), Pu. cernua (3–4), Pu. campanella (5–6), Pu. millefolium (7), Anemone hupehensis (8–9), Cimicifuga simplex (10–11), Clematis armandii (12–13), Potentilla chinensis (14–15), Po. discolor (16–17), Rhaponticum uniflorum (18–19), Platycodon grandiflorus (20–21) and water control (22). Lane M, DL2000 DNA marker. (c) Bgl I restriction digest pattern of PCR products of Pulsatilla species. Lane 1–7: Pu. chinensis (1–2), Pu. cernua (3–4), Pu. campanella (5–6) and Pu. millefolium (7). Lane M, DL500 DNA marker. Full-length gels are presented in Supplementary Figure S3.
In conclusion, we presented two molecular approaches for identifying medicinal Pu. chinensis from its adulterants. ITS2 barcoding can accurately identify Pu. chinensis and its adulterants by DNA sequencing, thereby providing the sequence basis for species identification. The specific PCR-RFLP assay based on the ITS2 region can rapidly identify authenticate the genuine plant origin Pu. chinensis of Baitouweng from others without DNA sequencing. Although specific PCR-RFLP assay cannot identify the certain species of adulterants, it offers a faster, simpler, and less expensive manner for identifying commercial Baitouweng products and more convenient for market survey.
Methods
Materials and taxon sampling
A total of 84 voucher samples derived from 13 species were collected from the provinces of Henan, Shaanxi, Hubei, Jilin, Beijing, Guangxi, Shandong, Chongqing, Anhui and Yunnan in China. These include: Pu. chinensis, Pu. cernua, Pu. campanella, Pu. millefolium, Po. chinensis, Po. discolor, R. uniflorum, P. grandiflorus, A. hupehensis, A. decumbens, L. leontopodioides, D. cappa, and G. affine (see Supplementary Table S1). All corresponding voucher samples are deposited in the herbarium of the Institute of Medicinal Plant Development, Chinese Academy of Medicinal Sciences (IMD). 21 sequences were downloaded from GenBank, the accession numbers being JN811072, JN811073, JN811074, JN811075, HQ829820, JN811070, JN811071, JF422892, JF422893, JF422894, JF422890, HQ735289, GU732648, FJ639908, JF422891, GU732651, GU732649, HQ440207, FJ639944, GU126788, and KP092567. Additionally, 30 medicinal Baitouweng products were purchased from different drug stores and markets in China, and one sample from each product was randomly selected to test.
ITS2 barcoding: DNA extraction, PCR amplification, sequencing and data analysis
All fresh samples and incomplete dried commercial products were dried with silica gel. 30 mg of each sample was powdered for 2 min at 50 Hz in a tissue grinder (Sceintz-48, China). Total genomic DNA was isolated using the Plant Genomic DNA Kit (Tiangen Biotech Co., China). The ITS2 region was amplified using the published universal primers ITS2F (5′-ATGCGATACTTGGTGTGAAT-3′) and ITS3R (5′-GACGCTTCTCCAGACTACAAT-3′)15, 25 μL PCR reaction volume containing <100 ng of genomic DNA, 2X Taq PCR MasterMix (Aidlab Biotechnologies Co., China), 1 μL of each primer (2.5 μM, synthesized by Sangon Co., China), and distilled-deionized water. PCR conditions were 1 cycle of 95 °C for 3 min, 35 cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 40 s followed by 1 cycle of 72 °C for 10 min. PCR products (6 μL of each) were examined on 1.2% agarose gel electrophoresis in 1 × TAE buffer for 20 minutes at 120 V. Purified PCR products were sequenced bidirectionally with PCR primers on an ABI-3730 sequencer (Applied Biosystem, USA). For TA cloning, purified PCR products were cloned into pMD18T vector, transformed into E. coli DH5αcompetent cells, and then screened positive monoclonies and sequenced as previously described.
Sequences were assembled using Codoncode aligner v. 4.2.4. The complete ITS2 sequences were annotated based on the Hidden Markov Model (HMM)58. All ITS2 sequences were deposited in GenBank and DNA barcoding system for identifying herbal medicine (http://www.tcmbarcode.cn/en/). Then, seven nucleotides at the 3′terminus were cut off after sequence alignment to keep all sequences consistent. Sequence alignment was performed by ClustalW in MEGA5.1. The intra- and inter-specific distances were calculated using the K2P model59 and the phylogenetic tree was constructed using the NJ method in MEGA5.160. DNA barcoding database for herbal materials (http://www.tcmbarcode.cn/en/) and nucleotide database in National Center for Biotechnology Information (NCBI) (http://blast.ncbi.nlm.nih.gov/Blast.cgi) were used as sources for complementary ITS2 data for species identification.
Specific PCR-RFLP assay
PCR amplification was carried out with ITS2F and PR1 (5′-TGGGTCATCTTCTCCCAGC-3′ in a final volume of 25 μL as described before. The PCR conditions were 1 cycle of 95 °C for 3 min, 35 cycles of 94 °C for 30 se, 62 °C for 20 s, and 72 °C for 20 s followed by 1 cycle of 72 °C for 10 min. PCR products (6 μL of each) were visualized by 2% agarose gel electrophoresis in 1 × TAE buffer for 20 minutes at 120 V.
Restriction enzymes were predicted by Vector NTI 10.3.0 (Invitrogen, Carlsbad, CA, USA) and Bgl I was selected as the best. Digestion was performed by incubating 15 μL of PCR product with 10 U of Bgl I (NEB) and 2.5 μL 10 × buffer in a final volume of 25 μL at 37 °C for 1 h, and then restriction fragments were separated by 3% agarose gel electrophoresis in 1 × TAE buffer for 30 min at 120 V.
Additional Information
How to cite this article: Shi, Y. et al. Rapidly discriminate commercial medicinal Pulsatilla chinensis (Bge.) Regel from its adulterants using ITS2 barcoding and specific PCR-RFLP assay. Sci. Rep. 7, 40000; doi: 10.1038/srep40000 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This study was supported by the Major Scientific and Technological Special Project for “Significant New Drugs Creation” (2014ZX09304307; 2014ZX09201021008) and the National Natural Science Foundation of China (81603248; 81403046). We thank Dianyun Hou, Zhigang Hu, Xia Liu, Jingjing Zhang and other collaborators for collecting plant samples.
Footnotes
Author Contributions S.L.C., W.S., and Y.H.S. designed the study. Y.H.S., M.M.Z., H.Y., P.Y., T.Y.X. and B.L. corrected the samples and carried out the experiments. Y.H.S. analyzed the data. Y.H.S., W.S. and S.L.C. drafted the manuscript with assistance from all the authors.
References
- World Health Organization. WHO Traditional Medicine Strategy 2002–2005 Ch. 2 http://apps.who.int/iris/bitstream/10665/67163/1/WHO_EDM_TRM_2002.1_eng.pdf (2002) (Date of access:24/11/2016).
- Chen S. L. et al. A renaissance in herbal medicine identification: from morphology to DNA. Biotechnol. Adv. 32, 1237–1244 (2014). [DOI] [PubMed] [Google Scholar]
- Pereira F., Carneiro J. & Amorim A. Identification of species with DNA-based technology: current progress and challenges. Recent Pat. DNA Gene Seq. 2, 187–199 (2008). [DOI] [PubMed] [Google Scholar]
- Heubl G. New aspects of DNA-based authentication of Chinese medicinal plants by molecular biological techniques. Planta Med. 76, 1963–1974 (2010). [DOI] [PubMed] [Google Scholar]
- Kress W. J. et al. Use of DNA barcodes to identify flowering plants. Proc. Natl. Acad. Sci. USA 102, 8369–8374 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnasingham S. & Hebert P. D. BOLD: The Barcode of Life Data System (http://www.barcodinglife.org). Mol. Ecol. Notes 7, 355–364 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert P. D., Ratnasingham S. & deWaard J. R. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc. Biol. Sci. 270, S96–99 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert P. D. N., Cywinska A., Ball S. L. & DeWaard J. R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 270, 313–321 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. E. DNA barcoding and the renaissance of taxonomy. Proc. Natl. Acad. Sci. USA 104, 4775–4776 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajibabaei M., Singer G. A. C., Hebert P. D. N. & Hickey D. A. DNA barcoding: how it complements taxonomy, molecular phylogenetics and population genetics. Trends Genet. 23, 167–172 (2007). [DOI] [PubMed] [Google Scholar]
- Marshall E. Will DNA barcodes breathe life into classification. Science 307, 1037 (2005). [DOI] [PubMed] [Google Scholar]
- Hollingsworth P. M. et al. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 106, 12794–12797 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. Z. et al. Comparative analysis of a large dataset indicates that internal transcribed spacer (ITS) should be incorporated into the core barcode for seed plants. Proc. Natl. Acad. Sci. USA 108, 19641–19646 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H. et al. Use of ITS2 Region as the Universal DNA Barcode for Plants and Animals. PloS ONE 5, e13102, doi: 10.1371/journal.pone.0013102 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. L. et al. Validation of the ITS2 Region as a Novel DNA Barcode for Identifying Medicinal Plant Species. PloS ONE 5, e8613, doi: 10.1371/journal.pone.0008613 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. W. et al. Plant DNA barcoding: from gene to genome. Biol. Rev. Camb. Philos. Soc. 90, 157–166 (2015). [DOI] [PubMed] [Google Scholar]
- Li D. Z. et al. Plant DNA barcoding in China. J. Syst. Evol. 49, 165–168 (2011). [Google Scholar]
- Wang H. et al. Molecular authentication of Panax ginseng and ginseng products using robust SNP markers in ribosomal external transcribed spacer region. J. Pharm. Biomed. Anal. 55, 972–976 (2011). [DOI] [PubMed] [Google Scholar]
- Palle-Reisch M., Cichna-Markl M. & Hochegger R. Development and validation of a duplex real-time PCR assay for the simultaneous detection of three mustard species (Sinapis alba, Brassica nigra and Brassica juncea) in food. Food Chem. 153, 66–73 (2014). [DOI] [PubMed] [Google Scholar]
- Osathanunkul M. et al. Refining DNA Barcoding Coupled High Resolution Melting for Discrimination of 12 Closely Related Croton Species. PLoS ONE 10, e0138888, doi: 10.1371/journal.pone.0138888 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. et al. DNA Barcode-Based PCR-RFLP and Diagnostic PCR for Authentication of Jinqian Baihua She (Bungarus Parvus). Evid. Based Complement Alternat. Med. 2015, 402820, doi: 10.1155/2015/402820 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China Part I (eds Zhao Y. Y. et al.) 104, 214 (China Medical Science Press, 2015). [Google Scholar]
- Liu Q. et al. Concise synthesis of two natural triterpenoid saponins, oleanolic acid derivatives isolated from the roots of Pulsatilla chinensis. Carbohydr. Res. 344, 1276–1281 (2009). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. Validated rapid resolution LC-ESI-MS/MS method for simultaneous determination of five pulchinenosides from Pulsatilla chinensis (Bunge) Regel in rat plasma: application to pharmacokinetics and bioavailability studies. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 942–943, 141–150 (2013). [DOI] [PubMed] [Google Scholar]
- Liu J. Y. et al. Saponins with neuroprotective effects from the roots of Pulsatilla cernua. Molecules 17, 5520–5531 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H. Q. et al. Effect of anemonin on NO, ET-1 and ICAM-1 production in rat intestinal microvascular endothelial cells. J. Ethnopharmacol. 104, 362–366 (2006). [DOI] [PubMed] [Google Scholar]
- Ye W., Zhang Q., Hsiao W. W., Zhao S. & Che C. T. New lupane glycosides from Pulsatilla chinensis. Planta Med. 68, 183–186 (2002). [DOI] [PubMed] [Google Scholar]
- Xu Q. M. et al. Antitumor activity of Pulsatilla chinensis (Bunge) Regel saponins in human liver tumor 7402 cells in vitro and in vivo. Phytomedicine. 19, 293–300 (2012). [DOI] [PubMed] [Google Scholar]
- Liu T. et al. Immunopontentiating and antitumor activities of a polysaccharide from Pulsatilla chinensis (Bunge) Regel. Int. J. Biol. Macromol. 54, 225–229 (2013). [DOI] [PubMed] [Google Scholar]
- Wang W. C. & Bartholomew B. Pulsatilla Miller http://flora.huh.harvard.edu/china/PDF/PDF06/PULSATILLA.pdf (2001) (Date of access: 24/11/2016).
- Hoot S. B., Meyer K. M. & Manning J. C. Phylogeny and reclassification of Anemone (Ranunculaceae), with an emphasis on austral species. Syst. Bot. 37, 139–152 (2012). [Google Scholar]
- State Administration of Traditional Chinese Medicine. Zhong Hua Ben Cao (eds Song L. R. et al.) Vol. 3, 239–241 (Shanghai Science and Technology Press, 1999). [Google Scholar]
- Wei Y. H. Herbal research and identification of Baitouweng and its homonyms. Xinjiang J. Trad. Chi. Med. 25, 73–75 (2007). [Google Scholar]
- Wang S. Y., Zhang M. & Wang Z. T. Microscopic identification of homonym drugs of “Baitouweng” by digital imaging technique. Acta. Pharm. Sinica 39, 797–802 (2004). [PubMed] [Google Scholar]
- Liu B. Q., Liu Y. & Chen Q. Identification of Pulsatillae radix and its adulterants. Shizhen J. Trad. Chin. Med. Res. 16, 130–131 (2005). [Google Scholar]
- Li H., Li H., Hao N., Xu Y. & Piao Z. Study on HPLC fingerprint characteristics and chemotaxonomy of Pulsatilla medicinal plants. China J. Chin. Mater. Med. 36, 1478–1482 (2011). [PubMed] [Google Scholar]
- Tang B., Yang Y., Huang C., Luo Z. & Li C. HPLC fingerprint spectra for discrimination of spurious breed of radix Pulsatillae. Chin. J. Pharm. 44, 281–286 (2013). [Google Scholar]
- Horandl E. et al. Phylogenetic relationships and evolutionary traits in Ranunculus s.l. (Ranunculaceae) inferred from ITS sequence analysis. Mol. Phylogenet. Evol. 36, 305–327 (2005). [DOI] [PubMed] [Google Scholar]
- Sun Y. L., Park W. G., Oh H. K. & Hong S. K. Genetic diversity and hybridization of Pulsatilla tongkangensis based on the nrDNA ITS region sequence. Biologia 69, 24–31 (2014). [Google Scholar]
- Ross H. A., Murugan S. & Li W. L. Testing the reliability of genetic methods of species identification via simulation. Syst. Biol. 57, 216–230 (2008). [DOI] [PubMed] [Google Scholar]
- Rice A. et al. The Chromosome Counts Database (CCDB) - a community resource of plant chromosome numbers. New Phytol. 206, 19–26 (2015). [DOI] [PubMed] [Google Scholar]
- Hollingsworth P. M., Graham S. W. & Little D. P. Choosing and using a plant DNA barcode. PLoS ONE 6, e19254, doi: 10.1371/journal.pone.0019254 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrei M. et al. DNA barcodes from four loci provide poor resolution of taxonomic groups in the genus Crataegus. AoB. Plants 7, plv045, doi: 10.1093/aobpla/plv045 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmaster S. G., Grguric M., Shanmughanandhan D., Ramalingam S. & Ragupathy S. DNA barcoding detects contamination and substitution in North American herbal products. BMC Med. 11, 222; doi: 10.1186/1741-7015-11-222 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bruni I. et al. A DNA barcoding approach to identify plant species in multiflower honey. Food Chem. 170, 308–315 (2015). [DOI] [PubMed] [Google Scholar]
- Stoeckle M. Y. et al. Commercial teas highlight plant DNA barcode identification successes and obstacles. Sci. Rep. 1, 42, doi: 10.1038/srep00042 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin T. et al. Survey of commercial Rhodiola products revealed species diversity and potential safety issues. Sci. Rep. 5, 8337, doi: 10.1038/srep08337 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou D. Y. et al. Stability and Accuracy Assessment of Identification of Traditional Chinese Materia Medica Using DNA Barcoding: A Case Study on Flos Lonicerae Japonicae. Biomed. Res. Int. 2013, 549037, doi: 10.1155/2013/549037 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin T. Y. et al. Stability and accuracy of the identification of Notopterygii Rhizoma et Radix using the ITS/ITS2 barcodes. Acta. Pharm. Sinica 47, 1098–1105 (2012). [PubMed] [Google Scholar]
- Yao H. et al. Identification of Dendrobium species by a candidate DNA barcode sequence: the chloroplast psbA-trnH intergenic region. Planta Med. 75, 667–669 (2009). [DOI] [PubMed] [Google Scholar]
- Pang X., Song J., Xu H. & Yao H. Using ITS2 barcode to identify ephedrae herba. China J. Chin. Mater. Med. 37, 1118–1121 (2012). [PubMed] [Google Scholar]
- Chen X. et al. A fast SNP identification and analysis of intraspecific variation in the medicinal Panax species based on DNA barcoding. Gene 530, 39–43 (2013). [DOI] [PubMed] [Google Scholar]
- Szczecinska M. & Sawicki J. Genomic Resources of Three Pulsatilla Species Reveal Evolutionary Hotspots, Species-Specific Sites and Variable Plastid Structure in the Family Ranunculaceae. Int. J. Mol. Sci. 16, 22258–22279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan M. L. et al. Deep sequencing of plant and animal DNA contained within traditional Chinese medicines reveals legality issues and health safety concerns. PLoS Genet. 8, e1002657, doi: 10.1371/journal.pgen.1002657 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soon W. W., Hariharan M. & Snyder M. P. High-throughput sequencing for biology and medicine. Mol. Syst. Biol. 9, 640, doi: 10.1038/msb.2012.61 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X. W. et al. Biological ingredient analysis of traditional Chinese medicine preparation based on high-throughput sequencing: the story for Liuwei Dihuang Wan. Sci. Rep. 4, 5147, doi: 10.1038/srep05147 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova N. V., Kuzmina M. L., Braukmann T. W., Borisenko A. V. & Zakharov E. V. Authentication of Herbal Supplements Using Next-Generation Sequencing. PloS ONE 11, e0156426, doi: 10.1371/journal.pone.0156426 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A. et al. 5.8 S-28S rRNA interaction and HMM-based ITS2 annotation. Gene 430, 50–57 (2009). [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16, 111–120 (1980). [DOI] [PubMed] [Google Scholar]
- Tamura K. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.