Abstract
Atypical responses to salient information are a candidate endophenotype for both autism and psychosis spectrum disorders. The present study investigated the costs and benefits of such atypicalities for saliency-based selection in a large cohort of neurotypical adults in whom both autism and psychosis expressions were assessed. Two experiments found that autism tendencies and psychosis proneness interactively modulated the cost incurred in the presence of a task-irrelevant salient distractor. Specifically, expressions of autism and psychosis had opposing effects on responses to salient information such that the benefits associated with high expressions for autism offset costs associated with high expressions for psychosis. The opposing influences observed on saliency cost may be driven by distinct attentional mechanisms that are differentially affected by expressions for autism and psychosis.
Key words: interindividual differences, salience, schizotypy, autism, selective attention, cognitive control
Introduction
In both clinical and nonclinical participants, expressions of autism and psychotic spectrum disorders (ASD and PSD, respectively) are associated with differences in attentional processing.1 Although these are often seen as deficits, there is also some evidence of autism-related benefits on some tasks.2,3 As such, it is far from clear whether autism and psychosis expressions yield these effects for the same reasons, not least because it is uncommon for these expressions to be assessed in the same participants.4 Although ASD and PSD have been formally conceptualized as distinct disorders since the 1970s,5 recent theoretical and empirical evidence, however, highlights the need to assess both autism and psychosis expressions in tandem as they might be additive or even interact.6–8 This is based on increasing evidence suggesting that ASD and PSD, and particularly schizophrenia, share multiple phenotypes (eg, attentional and social functioning difficulties) and risk factors (eg, genetic and neurodevelopmental),7,9,10 and can co-occur at both the diagnostic and trait levels within the individual.11–13 Here, we investigate the effect of autism traits and positive psychosis expressions on saliency-based selection in a large cohort of neurotypical adults. The assessment of positive psychotic expressions rather than the general construct of psychotic experiences (which includes both positive and negative symptoms) is based on evidence showing that autism traits and positive psychotic expressions constitute end points of a single continuum in the nonclinical population14–16 and that negative symptoms do not reliably discriminate between ASD and PSD.17 Salience-based selection is a key attentional mechanism associated with the ability to bias attention toward (or away from) salient information.18 Although atypical responses to salient information are considered a candidate endophenotype for both ASD19 and PSD,20 understanding how healthy variations in the expression of autism and psychosis affect attentional processing can facilitate our understanding of clinical autism and psychosis and their interaction.
Research in PSD and the broader spectrum of PSD expressions in healthy participants has consistently shown increased processing cost in the presence of salient distractor stimuli.15,21–24 For example, in a global-local processing paradigm where participants were required to judge whether a pair of compound stimuli (global forms composed of local forms) were identical or not, there was a significant slowing in participants with schizophrenia, particularly in the presence of a salient distractor at the local level.22 Similarly, neurotypicals with high positive schizotypy scores had more difficulty filtering out the nonrelevant salient stimulus (the more complex figure) when they were required to detect an embedded figure.15
In contrast, research in ASD and the broader spectrum of ASD traits in neurotypical participants finds evidence of both positive and negative effects on the ability to ignore distracting salient information.3,25,26 For example, compared with typically developing children, children with ASD have been shown to be more resistant to both nonsocial (eg, oddballs)2 and social (eg, faces) distractors3 and that the degree of interference produced by the distractor appears to negatively correlate with the severity of autistic traits.3 Notably, there are also reports of negative effects of ASD on information processing in the presence of salient distractors.26–28 For the present purposes, the critical point is that although handling of salient information may be atypical in ASD, the effects are not necessarily negative.
The evidence described above opens the possibility that expressions of ASD and PSD might each have effects on salience processing. However, since ASD and PSD can co-occur at both the diagnostic and trait levels within the individual,4,8,11 it is important to determine the relative impact of disorder-specific expressions on phenotypes within an individual. This question has significant implications for the individual’s treatment and prognosis as well as the nature of the relationship between ASD and PSD. Despite evidence of saliency-related effects in both ASD and PSD, no previous studies of salience effects have directly compared the two conditions or the effect of their co-occurrence at either the trait or diagnostic levels.
One approach to evaluating the effect of ASD-PSD co-occurrence on the suppression (or filtering) of salient information is by examining the association of psychosis proneness and autistic tendencies among nonclinical populations. This approach draws on the notion that both autistic29 and psychotic tendencies30–32 exist on a continuum, ranging from typicality to disorder, and has the advantage of eliminating the confounding effects of active symptomatology, medication, or chronicity.32 We therefore investigated the effect of autistic tendencies and psychosis proneness on the cost associated with the processing of information in the presence of competing salient information in a large sample of nonclinical adults. More specifically, we examined how autistic and psychotic tendencies affected the processing of two competing sources of information where one set of information was more prominent (ie, more readily available for processing) but irrelevant and the other was relevant but less prominent.
To this end, saliency was examined in two separate experiments. The first was Mevorach et al.’s33 variant of Navon’s classic global-local task,34 and the second was a novel face-scene perception task. The first task assesses overall local and global biases, selective attention, and saliency suppression in the context of compound letters (see Method section). The second task expands on the first in that it also enabled us to test for attentional/perceptual biases to socially relevant stimuli (ie, faces) as well as whether the effects were perceptual or attentional, which is made possible by the inclusion of neutral displays that do not require attention selection or filtering. In addition, the use of two tasks allows us to assess the generalizability of the effects of autism tendencies and psychosis proneness on the suppression/filtering of competing salient information. Autistic tendencies and positive psychotic experiences were respectively assessed with the Autism Spectrum Quotient29 and the Community Assessment of Psychic Experiences Questionnaire.35 These are well-validated questionnaires that have been used extensively in the general population. Although the focus of the current study was on saliency, we also tested for the effect of autism and psychosis traits on differences in information processing as a function of level (ie, local vs global), target (face vs scene), and congruency (congruent vs incongruent information), given research suggesting that information processing related to these factors also varies in both autism and psychosis.15,36,37
Based on the findings from existing literature, we predicted that higher levels of psychosis proneness would increase the burden of information processing in the presence of salient distracting stimuli. Although the literature regarding the affect of autism traits is less clear, we further predicted that any increased cost associated with autism tendencies would vary depending on the level of co-occurring psychosis proneness and thus may help resolve some of the discrepancy within autism research. The effect of their co-occurrence would lead to one of the following effects: (1) co-occurrence would result in a nonadditive effect perhaps due to a ceiling effect, or a dominance effect where the effect is mainly driven by level of psychosis; (2) co-occurrence would result in an additive effect leading to greater interference by the competing salient distractor; or (3) co-occurrence would result in a subadditive effect where the cost would be reduced in the presence of both conditions, perhaps through some canceling out effect whereby saliency suppression is contrastingly modulated by autism and psychosis tendencies. The latter scenario is conceivable if traits for autism and psychosis have opposite effects on distraction by salient but irrelevant information3,15,21 and would be consistent with the suggestion that ASD and PSD exert diametric effects on cognition and behavior.16
Method
Participants
Data were collected from 202 healthy university students (43 males, 159 females; mean age = 21.45, SD = 4.33). Participants self-reported that they have no history of psychiatric illness, epilepsy, neurological disorders or brain injury, and current or past alcohol and/or substance abuse problems. The University of Birmingham Research Ethics Committee approved the study, and written informed consent was obtained from all participants.
Measures and Materials
Psychosis proneness was assessed using a 20-item positive scale of the Community Assessment of Psychic Experiences (CAPEp) questionnaire,35 and autism tendencies were assessed using the Autism Spectrum Quotient (AQ).29 The internal consistency in this study of CAPEp (Cronbach’s α = .84) and AQ (Cronbach’s α = .82) was very good. Both CAPEp and AQ have been, respectively, shown to be sensitive to the screening of individuals with first episode psychosis38 and ASD,29 which undescores their sensitivity to the spectrum of expressions from typicality to disorder. Depressive symptomatology was assessed using The Center for Epidemiologic Studies Depression Scale-Revised (CESD-R).39 The internal consistency in this study is high (Cronbach’s α =.91). Depressive symptoms are measured because they are frequent clinical features in both ASD40 and PSD41 and may affect performance on cognitive tasks.42 Higher scores on AQ, CAPEp, and CESD-R reflect higher levels of symptom expressions.
The Global-Local Task.
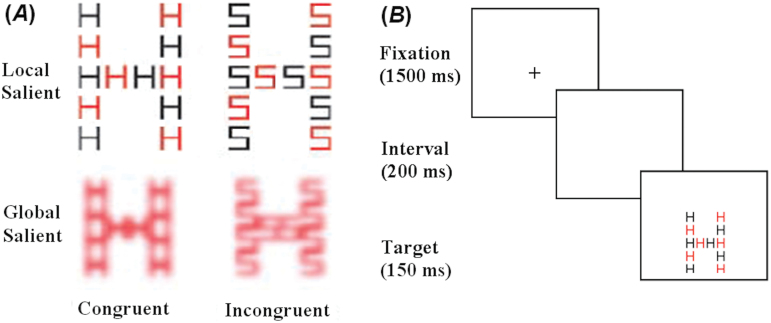
In this task, participants were required to identify the global letter of the compound figure made up of small letters (an S or an H) or the local letter of the compound figure (an S or an H) while ignoring information on the other level. As can be seen from figure 1A below, saliency was manipulated in two ways: local saliency was achieved by using alternating colors for the local elements (to break grouping) and global saliency was achieved by blurring the local elements. As such, participants were asked to detect the local or the global letter under four conditions: identifying the global letter when the global level is more salient, identifying the global letter when the local level is more salient, identifying the local letter when the local level is more salient, and identifying the local letter when the global level is more salient. Participant pressed one of the two keys on the keyboard: ‘K’ and ‘M’, which were respectively labeled ‘S’ and ‘H’ (see supplementary material, for further details).
Fig. 1.
(A) Example of stimuli for the global-local task (Original stimuli were presented against a black background). (B) Typical trial display sequence.
The Face-Scene Perception Task.
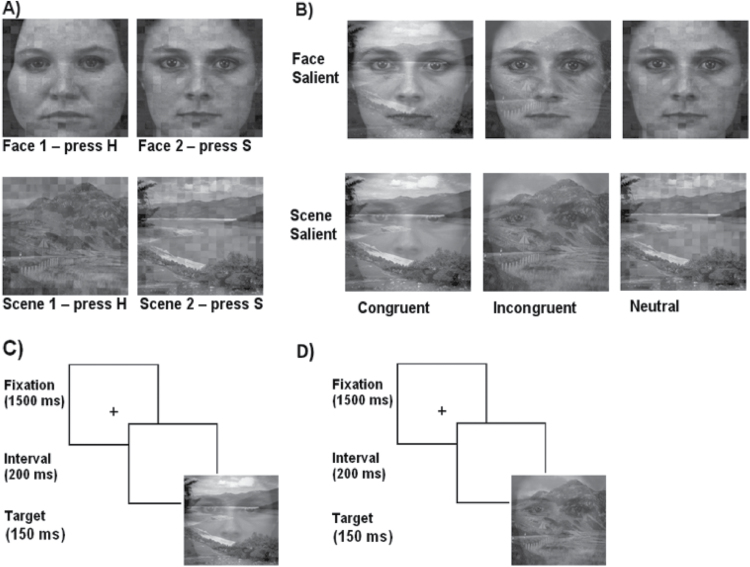
In this task, participants were required to either detect a face or a scene. To be consistent with the response keys from the global-local task, the two faces and two scenes were associated with the same response keys on the keyboard: ‘K’ and ‘M’, relabeled ‘S’ and ‘H’. Accordingly, participants were required to associate the scene or the face with the corresponding letter (H or S; see figure 2A). A sheet depicting these associations was placed in front of the participant while performing the task, should the participant need a reminder as to which face/scene was associated with which letter. These faces and scenes were superimposed onto each other (figure 2B) to manipulate saliency and congruency. In the neutral condition, the face (or the scene) was presented together with a scrambled version of the scene (or the face). The superimposed combinations used a manipulation of the face or scene contrast at a percentage ratio of 70:30. Thus, for more salient face displays, the face was presented at 70% contrast and the scene (or scrambled scene) was presented at 30% contrast. For more salient scene displays, these values were reversed. Congruency and incongruency were achieved by superimposing faces and scenes that were associated with the same letter (ie, no response conflict) or different letters (ie, response conflict), respectively (see supplementary material, for details).
Fig. 2.
(A) Stimuli of the face-scene perception task. (B) Faces and scenes in salient/nonsalient, congruent/incongruent, and neutral conditions. (C) Typical trial display sequence for congruent stimuli, ie, where the face and the scene are associated with the same letter (S-S; no response conflict). (D) Typical trial display sequence for incongruent stimuli, ie, where the face and the scene are associated with different letters (S-H, response conflict).
Analytic Approach
For each task, we computed inverse efficiency scores (reaction time (RT)/proportion correct) for each cell of the design for each participant. The use of efficiency scores, which is particularly recommended for tasks in which overall accuracy is over 80%, allows us to incorporate both RT and accuracy into a single measure42 and to be consistent with previous studies using similar paradigms.18,44 In our study, overall accuracy was 96.5% in the global-local task, and 92.31% in the face-scene task. Overall performance on the global-local task was assessed using a 2×2 × 2 repeated measures ANOVA, with level (global vs local), saliency (global salient vs local salient), and congruency (congruent vs incongruent) as within-subject factors. Overall performance on the face-scene task was assessed using a 2×2 × 3 repeated measures ANOVA, with target (face vs scene), saliency (face salient vs scene salient), and congruency (congruent vs incongruent vs neutral) as within-subject factors.
Next, and to minimize the potential influence of outliers,45 we used generalized linear models to assess the association of individual differences of autism and positive psychotic expressions and their interaction on the cost associated with each factor in the global-local and face-scene tasks, viz saliency cost, level/target difference, and congruency interference. In both tasks, saliency cost was computed as the difference in performance between conditions when the nontarget is the salient aspect of the display (ie, distractor salient) and when the target is the salient aspect of the display (ie, target salient, hence, Distractor salient − Target salient). In the global-local task (figure 1), level difference was computed as the difference between conditions when the target is presented at the local level and when the target is presented at the global level (ie, Local − Global), and congruency interference as the difference between conditions when the target is incongruent with the other element of the display and conditions when the target is congruent with the other element of the display (ie, Incongruent − Congruent).
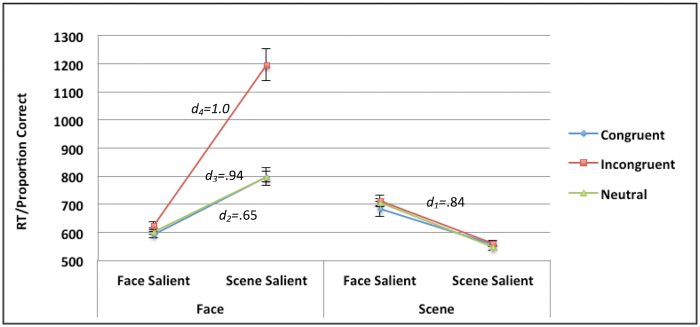
In the face-scene task (figure 2), target difference was computed as the difference between conditions identifying faces and conditions identifying scenes (ie, Face − Scene) and congruency interference as the difference between conditions containing a response conflict between the target and the competing element of the display and conditions in which there is no response conflict (ie, Incongruent/Neutral − Congruent/Neutral). We also tested the effect of autism tendencies and psychosis proneness on performance in the neutral-only condition to see whether they also explain simple effects of perception.
Where appropriate, group comparisons were conducted using t tests, and correlation analyses were conducted using spearman’s ρ.
Results
Two hundred and two participants completed both the global-local and the face-scene tasks. Due to data exclusion, the sample composition in both tasks differed slightly. Table 1 summarizes the sample characteristics in each of the two tasks.
Table 1.
Sample Characteristics in the Global-Local and Face-Scene Tasks
| Task | Global-Local Task (N = 196)a | Face-Scene Task (N = 197)b |
|---|---|---|
| Gender (male/female) | 41/155 | 42/155 |
| Age | 21.40±4.36 | 21.45±4.35 |
| CAPEp | 27.25±5.06 | 27.25±4.98 |
| AQ | 16.25±6.29 | 16.25±6.29 |
| CESD-R | 12.47±11.13 | 12.47±11.13 |
Note: AQ, Autism Spectrum Quotient; CAPEp, positive scale of the Community Assessment of Psychic Experiences; CESD-R, Center for Epidemiologic Studies Depression Scale-Revised.
aSix participants were excluded. The data of one participant were excluded for a program failure and five additional for not following instructions, ie, detecting global when the task was to detect local and vice versa.
bFive participants were removed from the analysis for failing to follow task instructions, ie, responding to faces rather than to scenes or vice versa.
In both the global-local and face-scene tasks, significant Spearman’s ρ correlations were observed between AQ and CAPEp (r = .31, p < .001; r = .32, p < .001), AQ and CESD-R (r = .38, p < .001; r = .38, p<.001), and between CESD-R and CAPEp (r = .36, p < .001; r = .38, p < .001). There were no associations between age and either AQ or CAPEp (−.07 < rs < .07, all ps > .34; −.06 < rs < .08, all ps > .26). Age was negatively correlated with CESD-R (r = −.16, p = .024; r = −.15, p = .036). There were no differences between male and female participants on any of these measures except for age where female (M ± SD = 20.92±4.08; 20.94±4.08) were younger than male (M ± SD = 23.22±4.93; 23.31±4.85) participants (t = 2.76; p = .008; t = 2.90; p = .005).
The Global-Local Task
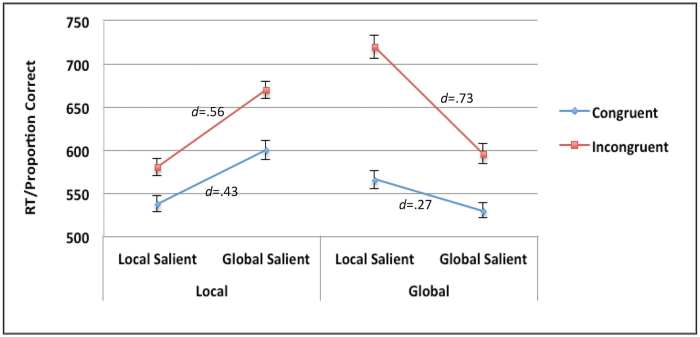
Figure 3 shows the results of the 2×2 × 2 repeated measures ANOVA of the participants’ performance on the task, suggesting that the presence of a salient distractor has a measurable effect on processing cost (see supplementary material, for complete analytic details).
Fig. 3.
Overall performance on the global-local task as a function of level (local vs global), saliency (local salience vs global saliency) and congruency (congruent vs incongruent). Effect sizes (Cohen’s d) represent the magnitude of the cost associated with the shift from target salient to distractor salient in the congruent and incongruent conditions (all ps < .001). Bars represent standard errors of the mean.
The Effect of Autism Tendencies and Psychosis Proneness on Level Difference, Congruency Interference, and Saliency Cost.
First, Spearman’s ρ indicated no correlations between age or CESD-R scores on any of the dependent variables (ie, level difference, congruency interference, and saliency cost) except for a negative association between age and congruency interference (r = −.17, p = .015). There were also no significant correlations among the dependent measures or differences as a function of gender. Each dependent measure was analyzed in separate regression models with the AQ scores, CAPEp scores, and their interaction (AQ × CAPEp) as predictors. Since only age was associated with congruency interference, the model estimating congruency interference was carried out while also controlling for age.46 The regression models for the level difference ( = 2.59, p = .46) and congruency interference ( = 3.44, p = .49) were nonsignificant. The model estimating saliency cost was significant ( =11.03, p = .012; R2 = .055), with parameter estimates showing significant positive association between saliency cost and the CAPEp scores and at a positive trend with the AQ scores The interaction term of the AQ scores × CAPEp scores was negatively associated with saliency cost Importantly, the change in the variance explained by the model due to the inclusion of the interaction was significant (∆R2 = .026; F = 5.24, p = .023) or, representing 47.27% of the total variance explained by the model.
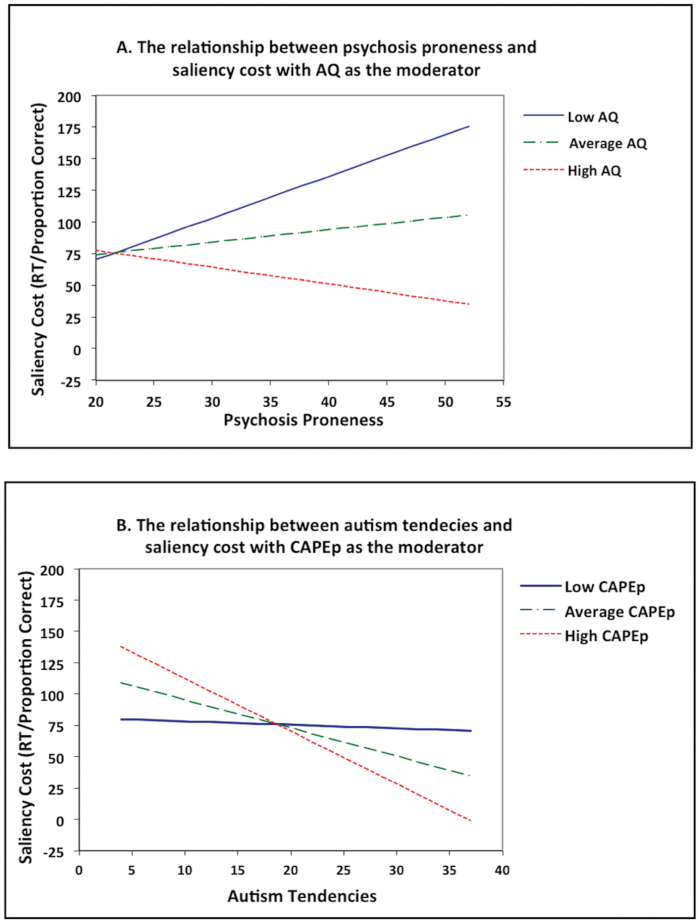
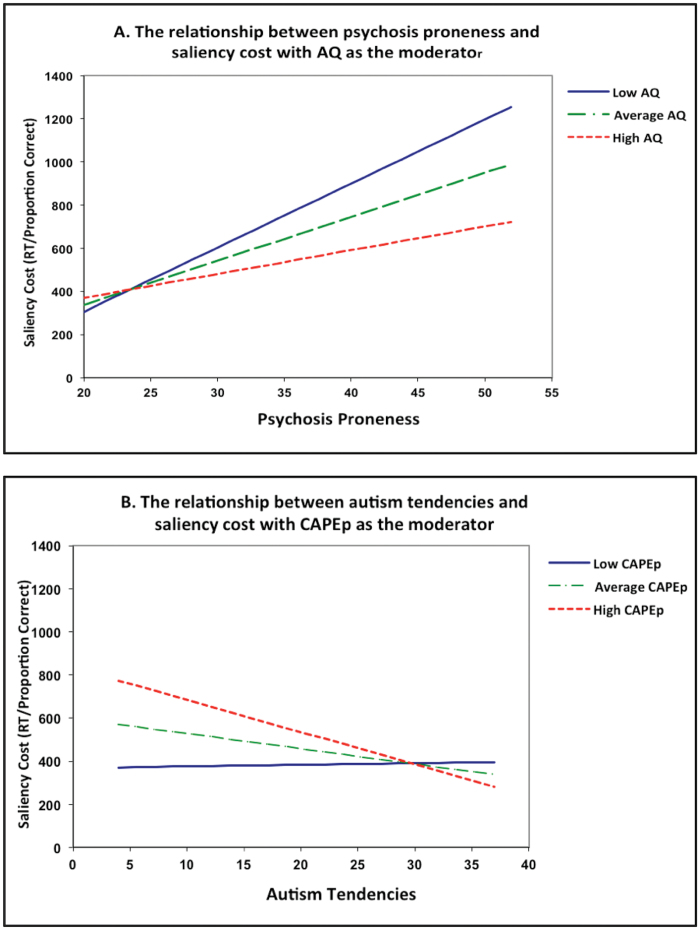
To investigate the interaction, we first visualized, by plots of simple regression lines, the effect of one predictor on saliency cost at the participants’ mean score, 1 SD below the participants’ mean score, and 1 SD above the participants’ mean score of the other predictor using MODPROBE for SPSS.47 Figure 4A visualizes the association between psychosis and saliency cost at low AQ (AQ = 9.96), average AQ (AQ = 16.25), and high AQ (AQ = 22.54), and figure 4B visualizes the association between autism tendencies and saliency cost at low CAPEp (CAPEp = 22.19), average CAPEp (CAPEp = 27.25), and high CAPEp (CAPEp = 32.31). To identify the region/values of the moderator variable where the predictor has a significant effect on saliency cost, we used the Johnson-Neyman method using MODPROBE for SPSS.47 This method provides a “high-resolution picture” of the interaction by estimating the value(s) of AQ, at which CAPEp (or vice versa) has a significant effect on saliency cost. This is established by identifying the precise value(s) along the continuum of the moderator for which the regression slopes of the predictor is estimated to be significantly different from zero. According to this analysis, increasing psychosis proneness (figure 4A) is associated with significantly increasing saliency cost in individuals scoring below 9 on the AQ, but with significantly reduced cost in individuals scoring above 29 on the AQ. Conversely, increasing autism tendencies were only associated with a significant reduction in saliency costs in individuals scoring above 26 (average or high) on the CAPEp (figure 4B).
Fig. 4.
(A) Visualizes the association between psychosis proneness and saliency cost by plots of simple regression lines at low (−1 SD), average, and high (+1 SD) AQ scores as moderators. (B) Visualizes the association between autism tendencies and saliency cost by plots of simple regression lines at low (−1 SD), average, and high CAPEp scores (+1 SD) as moderators. Overall, saliency cost is increased with increasing psychosis proneness, except when the AQ scores are high (figure 4A) and decreased with increasing autism tendencies, especially when the CAPEp scores are average and above (figure 4B). AQ = Autism Spectrum Quotient; CAPEp = positive scale of the Community Assessment of Psychic Experiences.
The Face-Scene Perception Task
Figure 5 shows the results of the 2×2 × 3 repeated measures ANOVA of the participants’ performance on the task, suggesting that the presence of a salient distractor has a measurable effect on target identification (see supplementary material, for complete analytic details).
Fig. 5.
Overall performance on the face-scene perception task as a function of target (face vs scene), saliency (face salient vs scene salient), and congruency (congruent vs incongruent vs. neutral). Effect sizes (Cohen’s d) represent the magnitude of the cost associated with the shift from target salient to distractor salient (all ps < .001). d1 represents the effect size of overall saliency cost in the scene condition (no effect for congruency). In the face condition, d2 and d3 represent the effect sizes of saliency cost in the congruent and neutral conditions, respectively. The effect size of saliency cost in the incongruent condition is represented by d4. Bars represent standard errors of the mean.
The Effect of Autism Tendencies and Psychosis Proneness on Target Difference, Congruency Interference, and Saliency Cost.
First, Spearman’s ρ revealed no correlations between any of the dependent variables (ie, target difference, congruency interference, and saliency cost) with age or CESD-R scores. There were also no differences between male or female participants on any of these measures. There were, however, significant associations between target difference and congruency interference (r = .24, p = .001), target difference and saliency cost (r = .50, p < .001), and congruency interference and saliency cost (r = .40, p < .001). Accordingly, each dependent measure was entered into separate regression models with the AQ scores, CAPEp scores, and their interaction (AQ × CAPEp) as predictors while controlling for the other two dependent measures. Since age, gender, and CESD-R scores were not associated with any of the dependent measures, they were not included as covariates in the regression models.46
The overall models for target difference (controlling for saliency cost and congruency interference; = 4.42, p = .22) and congruency interference (controlling for saliency cost and target difference; = 3.71, p = .30) were nonsignificant, suggesting that neither target selection nor the presence or absence of response conflict was affected by interindividual differences on autism or psychosis. In contrast, the regression model estimating saliency cost (controlling for target difference and congruency interference) was significant ( = 18.94, p < .001, R2 = .092), with parameter estimates showing significant association between saliency cost and CAPEp scores (β (SE)=23.47 (9.97), = 10.56, p = .001) and at a trend with the AQ scores (β (SE) = 17.90 (7.22), = 3.22, p = .073). The interaction term of AQ scores × CAPEp scores was also significant but negatively associated with saliency cost (β (SE) = −.79 (.37), = 4.52, p = .034). Importantly, the change in the variance explained by the model due to the inclusion of the interaction was significant (∆R2 = .021; F = 4.43, p = .037), representing 22.8% of the total variance explained by the model.
We probed the interaction following the same analysis we applied in the global-local task. Figure 6A visualizes the interaction between psychosis and saliency cost by plots of simple regression lines at low AQ (AQ = 9.95), average AQ (AQ = 16.25), and high AQ (AQ = 22.54), and figure 6B visualizes the interaction between autism tendencies and saliency cost at low CAPEp (CAPEp = 22.27), average CAPEp (CAPEp = 27.25), and high CAPEp (CAPEp = 32.23). According to the Johnson-Neyman method, increasing psychosis proneness was associated with significantly increased saliency cost (ie, p < .05) only in individuals scoring below 23 (low or average) on the AQ (figure 6A). Conversely, increasing autism tendencies were only associated with decreasing saliency costs in individuals scoring above 27 (average or high) on the CAPEp scale (figure 6B).
Fig. 6.
(A) Visualizes the association between psychosis proneness and saliency cost by plots of simple regression lines with low (−1 SD), average, and high (+1 SD) AQ scores as moderators. (B) Visualizes the association between autism tendencies and saliency cost by plots of simple regression lines with low (−1 SD), average, and high CAPEp scores (+1 SD) as moderators. Overall, saliency cost is increased with increasing psychosis proneness (figure 6A) and decreased with increasing autism tendencies, especially when CAPEp score are average and above (figure 6B). AQ = Autism Spectrum Quotient; CAPEp = positive scale of the Community Assessment of Psychic Experiences.
The Effect of Autism Tendencies and Psychosis Proneness on Performance During the Neutral Condition.
We investigated the association of autism, psychosis, and their interaction on the cost incurred during the neutral condition. This is in order only to see whether they also explain simple effects of perception as in the neutral displays there is no need for attention selection or filtering. Although the omnibus test of the overall model approached significance ( = 7.09, p = .07), all the parameter estimates were clearly nonsignificant (all ps > .18).
The Effect of Autism Attentional Traits and Psychosis Proneness on Saliency Cost
The AQ includes two subscales that reflect attentional abilities, namely “attention to details” and “attention switching,” where higher scores reflect increased focused of attention. To test the specific effects of these AQ traits and their interactive effect with CAPEp on saliency cost, we conducted an exploratory analysis whereby saliency cost was estimated as a function of CAPEp, attention switching and attention to details, and their two-way interactions with CAPEp.
For the global-local task, the regression analysis revealed an overall significant model ( = 22.69, p < .001, R2 = .064), with parameter estimates showing significant positive association between saliency cost and CAPEp (β (±SE) = 8.48 (3.54), = 5.74, p = .017) and a significant negative association of saliency cost with the interaction term of attention switching × CAPEp (β (±SE) = −.83 (.42), = 3.95, p = .047). No other main effects or interactions were observed.
Similar results were obtained in the face-scene task. Specifically, the overall model was significant (, = 12.91, p = .012, R2 = .109), with parameter estimates showing positive significant association between saliency cost and CAPEp (β (±SE) = 16.57 (7.70), = 4.64, p = .031) and attention switching (β (±SE) = 53.42 (25.53), = 4.38, p = .036). In addition, there was a significant negative association of saliency cost with the interaction term of attention switching x CAPEp (β (±SE) = −2.29 (.91), = 6.29, p = .012). No other main effects or interactions were observed.
Discussion
The results of two experiments provide converging evidence suggesting that autism tendencies and psychosis proneness interactively reduce the cost incurred in the presence of salient distractors, but these interact such that the effect of the expression of one condition depends on the relative expression of the other condition. Specifically, higher psychosis proneness was generally associated with higher saliency cost, though when autism tendencies were also high this effect was nonsignificant (in the face-scene task) or even reversed (in the global-local task). Conversely, higher autism tendencies were generally associated with lower saliency cost, though in both tasks this effect was absent for participants with low psychosis proneness (figures 4 and 6). Importantly, autism and psychosis expressions had no effects on other task variables (ie, level/target or congruency), suggesting that this interactive effect is specific to salience suppression and that the effect appears specific to attentional rather than perceptual abilities. These findings confirm that the filtering out of salient information is sensitive to the presence of subthreshold clinical traits for ASD and PSD in healthy adults and highlight the importance of testing whether the processing of salient information in ASD or PSD is moderated by the relative expression of their co-occurring phenotypes.
However, what mechanism(s) might account for the interactive effect of autism and positive psychotic expressions on saliency cost reduction? An important clue comes from our findings showing that the interactive effect of autism traits with psychosis proneness on saliency cost reduction is driven by increased focused of attention, as measured by the attention switching subscale of the AQ.29 Indeed, a common feature in accounts of attention is the presence of complementary but potentially competing processes for maintaining a focus of attention and for switching attention.48,49 These processes have been respectively associated with the coordinated action of the top-down (dorsal) and bottom-up (ventral) frontoparietal networks that play a prominent role in the processing of salient information.48 Intriguingly, existing evidence indicates that these processes may be differentially affected by ASD and PSD, such that individuals with ASD show increased focus of attention15,28 and that individuals with PSD (and specifically those with positive symptom schizophrenia) show overswitching.50 By considering these different attentional styles/tendencies, and the interactive neural networks with which they are associated (ie, the dorsal and ventral attentional systems), it becomes apparent how overswitching and increased focused of attention can compensate for one another, particularly when the expressions of both autism and psychosis are high.
Another, but related, attentional mechanism that might account for the observed interactive effect on saliency cost is proactive and reactive attentional control.51 In proactive control, individuals bias attention by maintaining goal-relevant information and preventing interference in an anticipatory manner before the onset of the stimulus. Thus, proactive control can be called upon to effectively ignore salient distractors. In reactive control, individuals respond “online” to interference after the onset of the stimulus, and thus their responses to salient distractors appear to operate reflexively. Within the context of our tasks, participants must attend to task-relevant information and filter out salient but task-irrelevant distracting information, which suggests that these tasks may require proactive attentional control. In fact, previous research has shown that, in the global-local task used here, participants tend to rely on proactive processes preceding stimulus onset which appears to be modulated by the left posterior parietal cortex.33 Thus, the fact that higher autism tendencies were generally associated with lower saliency cost may be due to a preferential bias toward proactive suppression in autism, which may be induced by a general failure in engaging the ventral attentional system.52 Conversely, there is evidence suggesting that individuals with schizophrenia preferentially rely on reactive suppression,53 which could explain why psychosis proneness drives saliency cost in our tasks that require proactive processes. In this regard, a plausible prediction is that the use of reactive tasks would yield the reverse results—increased saliency cost in autism and decreased in psychosis. Taken together, our results could be accounted for by supposing that the effects of autism tendencies and psychosis proneness on salient distractors are respectively associated with preferential reliance on top-down (proactive) and bottom-up (reactive) modulation. Future research comparing performance on paradigms that tap reactive and proactive processes is important to confirm these predictions.
Furthermore, it is interesting to note that the anterior insula, which is an important hub of the salience network, appears to have a causal role in the initiation of cognitive control systems,54 including the default mode network and the central executive networks.55 Thus, if autism and psychosis are preferentially associated with proactive and reactive modes of cognitive/attentional control, it may not be serendipitous that a recent structural MRI study56 found that individuals with ASD have smaller gray matter volume in the anterior insula compared with those with schizophrenia in whom insular gray matter volume correlated positively with hallucinatory behavior. This opponency is consistent with evidence at the system level within the salience network whereby schizophrenia is associated with decreased functional connectivity57 and autism with increased functional connectivity.18 Thus, this is further evidence that the observed interaction of autism and psychosis expressions on saliency is intimately associated with interactive networks and structures that modulate the processing of salient information and that appear sensitive to the level autism and psychosis expressions.
Methodologically, although both tasks offer converging evidence on the interactive effect of autism tendencies and psychosis proneness on saliency cost, the face-scene perception task appears more sensitive in that it explains almost twice the variance afforded by the global-local task (R2 = .092 vs .055, respectively). In addition, the inclusion of the neutral condition in the face-scene task provides an important insight as to whether the effects are due to attentional or perceptual processes.
Our study is the first to observe that co-occurring autistic tendencies and psychosis proneness exert interactive influences on saliency, which we suggest is possibly driven by contrasting attentional mechanisms that may be differentially affected by traits for autism and psychosis, and by implication in ASD and PSD. Our findings further suggest that previous reports assessing saliency as a function of one trait without assessing the other need to be viewed with some caution. This is because unmeasured differences in the proportion of one trait in a sample that was intended to look at the other trait might adversely affect results and thus might account for some inconsistencies in previous literature. However, although no gender effect was observed, some caution regarding the generalizability of our findings is warranted, given that our sample consisted largely of female participants. In addition, although it is obvious that replications of our findings within the clinical populations are needed before any definitive conclusion can be made about how autism and psychosis impact one another, our findings may nonetheless have clinical implications. First, they imply that phenotypic variation in individuals diagnosed with either condition are likely to be a reflection of the relative expression of one disorder vis-à-vis the other and raise the intriguing possibility that saliency-related abnormalities may be attenuated in individuals with comorbid ASD and PSD. Second, by showing that the effect of subthreshold clinical expressions on saliency cost follow a similar trend to those observed in the clinical populations, we provide evidence that the risk of the disorder may, at least in part, be mediated by variation in the processing of salient information. Finally, our findings can inform cognitive attentional control-based interventions58 in that they identify areas of strength and weaknesses of information processing as a function of the relative expression of autism and psychosis. In sum, our approach of simultaneously assessing autism and psychosis expressions is potentially a useful framework for the development of behavioral interventions in both ASD and PSD as well as to understand the relationship between ASD and PSD and their concurrent effect on outcome and behavior.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Crespi B, Go MC. Diametrical diseases reflect evolutionary-genetic tradeoffs: evidence from psychiatry, neurology, rheumatology, oncology and immunology. Evol Med Public Health. 2015;2015:216–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blaser E, Eglington L, Carter AS, Kaldy Z. Pupillometry reveals a mechanism for the Autism Spectrum Disorder (ASD) advantage in visual tasks. Sci Rep. 2014;4:4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riby DM, Brown PH, Jones N, Hanley M. Brief report: faces cause less distraction in autism. J Autism Dev Disord. 2012;42:634–639. [DOI] [PubMed] [Google Scholar]
- 4. Chisholm K, Lin A, Abu-Akel A, Wood SJ. The association between autism and schizophrenia spectrum disorders: a review of eight alternate models of co-occurrence. Neurosci Biobehav Rev. 2015;55:173–183. [DOI] [PubMed] [Google Scholar]
- 5. Kolvin I. Studies in the childhood psychoses. I. Diagnostic criteria and classification. Br J Psychiatry. 1971;118:381–384. [DOI] [PubMed] [Google Scholar]
- 6. Abu-Akel A, Wood SJ, Hansen PC, Apperly IA. Perspective-taking abilities in the balance between autism tendencies and psychosis proneness. Proc Biol Sci. 2015;282:20150563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sasson NJ, Pinkham AE, Carpenter KL, Belger A. The benefit of directly comparing autism and schizophrenia for revealing mechanisms of social cognitive impairment. J Neurodev Disord. 2011;3:87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hommer RE, Swedo SE. Schizophrenia and autism-related disorders. Schizophr Bull. 2015;41:313–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crespi B, Stead P, Elliot M. Evolution in health and medicine Sackler colloquium: comparative genomics of autism and schizophrenia. Proc Natl Acad Sci USA. 2010;107(Suppl 1):1736–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sullivan PF, Magnusson C, Reichenberg A, et al. Family history of schizophrenia and bipolar disorder as risk factors for autism. Arch Gen Psychiatry. 2012;69:1099–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hofvander B, Delorme R, Chaste P, et al. Psychiatric and psychosocial problems in adults with normal-intelligence autism spectrum disorders. BMC Psychiatry. 2009;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sheitman BB, Kraus JE, Bodfish JW, Carmel H. Are the negative symptoms of schizophrenia consistent with an autistic spectrum illness? Schizophr Res. 2004;69:119–120. [DOI] [PubMed] [Google Scholar]
- 13. Solomon M, Olsen E, Niendam T, et al. From lumping to splitting and back again: atypical social and language development in individuals with clinical-high-risk for psychosis, first episode schizophrenia, and autism spectrum disorders. Schizophr Res. 2011;131:146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dinsdale NL, Hurd PL, Wakabayashi A, Elliot M, Crespi BJ. How are autism and schizotypy related? Evidence from a non-clinical population. PLoS One. 2013;8:e63316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Russell-Smith SN, Maybery MT, Bayliss DM. Are the autism and positive schizotypy spectra diametrically opposed in local versus global processing? J Autism Dev Disord. 2010;40:968–977. [DOI] [PubMed] [Google Scholar]
- 16. Crespi B, Badcock C. Psychosis and autism as diametrical disorders of the social brain. Behav Brain Sci. 2008;31:241–261; discussion 261–320. [DOI] [PubMed] [Google Scholar]
- 17. Spek AA, Wouters SGM. Autism and schizophrenia in high functioning adults: behavioural differences and overlap. Res Autism Spect Disord. 2010;4:709–717. [Google Scholar]
- 18. Mevorach C, Humphreys GW, Shalev L. Opposite biases in salience-based selection for the left and right posterior parietal cortex. Nature Neurosci. 2006;9:740–742. [DOI] [PubMed] [Google Scholar]
- 19. Uddin LQ, Supekar K, Lynch CJ, et al. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 2013;70:869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. [DOI] [PubMed] [Google Scholar]
- 21. Hahn B, Robinson BM, Kaiser ST, et al. Failure of schizophrenia patients to overcome salient distractors during working memory encoding. Biol Psychiatry. 2010;68:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poirel N, Brazo P, Turbelin MR, et al. Meaningfulness and global-local processing in schizophrenia. Neuropsychologia. 2010;48:3062–3068. [DOI] [PubMed] [Google Scholar]
- 23. Morris R, Griffiths O, Le Pelley ME, Weickert TW. Attention to irrelevant cues is related to positive symptoms in schizophrenia. Schizophr Bull. 2013;39:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roiser JP, Stephan KE, den Ouden HE, Barnes TR, Friston KJ, Joyce EM. Do patients with schizophrenia exhibit aberrant salience? Psychol Med. 2009;39:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ploog BO. Stimulus overselectivity four decades later: a review of the literature and its implications for current research in autism spectrum disorder. J Autism Dev Disord. 2010;40:1332–1349. [DOI] [PubMed] [Google Scholar]
- 26. Leader G, Loughnane A, McMoreland C, Reed P. The effect of stimulus salience on over-selectivity. J Autism Dev Disord. 2009;39:330–338. [DOI] [PubMed] [Google Scholar]
- 27. Becchio C, Mari M, Castiello U. Perception of shadows in children with autism spectrum disorders. PLoS One. 2010;5:e10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Belmonte MK, Gomot M, Baron-Cohen S. Visual attention in autism families: ‘unaffected’ sibs share atypical frontal activation. J Child Psychol Psychiatry. 2010;51:259–276. [DOI] [PubMed] [Google Scholar]
- 29. Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001;31:5–17. [DOI] [PubMed] [Google Scholar]
- 30. Allardyce J, Suppes T, Van Os J. Dimensions and the psychosis phenotype. Int J Methods Psychiatr Res. 2007;16(Suppl 1):S34–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Claridge G. Schizotypy: Implications for Illness and Health. Oxford, UK: OUP; 1997. [Google Scholar]
- 32. Ettinger U, Mohr C, Gooding DC, et al. Cognition and brain function in schizotypy: a selective review. Schizophr Bull. 2015;41(Suppl 2):S417–S426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mevorach C, Humphreys GW, Shalev L. Reflexive and preparatory selection and suppression of salient information in the right and left posterior parietal cortex. J Cogn Neurosci. 2009;21:1204–1214. [DOI] [PubMed] [Google Scholar]
- 34. Navon D. Forest before trees: the precedence of global features in visual perception. Cogn Psychol. 1977;9:353–383. [Google Scholar]
- 35. Stefanis NC, Hanssen M, Smirnis NK, et al. Evidence that three dimensions of psychosis have a distribution in the general population. Psychol Med. 2002;32:347–358. [DOI] [PubMed] [Google Scholar]
- 36. Koldewyn K, Jiang YV, Weigelt S, Kanwisher N. Global/local processing in autism: not a disability, but a disinclination. J Autism Dev Disord. 2013;43:2329–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McCabe KL, Melville JL, Rich D, et al. Divergent patterns of social cognition performance in autism and 22q11.2 deletion syndrome (22q11DS). J Autism Dev Disord. 2013;43:1926–1934. [DOI] [PubMed] [Google Scholar]
- 38. Boonstra N, Wunderink L, Sytema S, Wiersma D. Improving detection of first-episode psychosis by mental health-care services using a self-report questionnaire. Early Interv Psychiatry. 2009;3:289–295. [DOI] [PubMed] [Google Scholar]
- 39. Eaton WW, Smith C, Ybarra M, Muntaner C, Tien A. Center for Epidemiologic Studies Depression Scale: review and revision (CESD and CESD-R) In: Maruish ME, ed. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment: Instruments for Adults. Vol 3 3rd ed. Mahwah, NJ: Lawrence Erlbaum; 2004:363–377. [Google Scholar]
- 40. Stewart ME, Barnard L, Pearson J, Hasan R, O’Brien G. Presentation of depression in autism and Asperger syndrome: a review. Autism. 2006;10:103–116. [DOI] [PubMed] [Google Scholar]
- 41. Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35:383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jones NP, Siegle GJ, Muelly ER, Haggerty A, Ghinassi F. Poor performance on cognitive tasks in depression: Doing too much or not enough? Cogn Affect Behav Neurosci. 2010;10:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Townsend JT, Ashby FG. Stochastic Modeling of Elementary Psychological Processes. Cambridge, UK: Cambridge University Press; 1983. [Google Scholar]
- 44. Mevorach C, Shalev L, Allen HA, Humphreys GW. The left intraparietal sulcus modulates the selection of low salient stimuli. J Cogn Neurosci. 2009;21:303–315. [DOI] [PubMed] [Google Scholar]
- 45. Moore B, Natarajan B. A compressive sensing based analysis of anomalies in generalized linear models. Commun Stat Theory Methods. 2015;44:2705–2719. [Google Scholar]
- 46. Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48. [DOI] [PubMed] [Google Scholar]
- 47. Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav Res Methods. 2009;41:924–936. [DOI] [PubMed] [Google Scholar]
- 48. Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Rev Neurosci. 2002;3:201–215. [DOI] [PubMed] [Google Scholar]
- 50. Yogev H, Sirota P, Gutman Y, Hadar U. Latent inhibition and overswitching in schizophrenia. Schizophr Bull 2004;30:713–726. [DOI] [PubMed] [Google Scholar]
- 51. Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn Sci. 2012;16:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Keehn B, Nair A, Lincoln AJ, Townsend J, Muller RA. Under-reactive but easily distracted: An fMRI investigation of attentional capture in autism spectrum disorder. Dev Cogn Neurosci. 2016;17:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lesh TA, Westphal AJ, Niendam TA, et al. Proactive and reactive cognitive control and dorsolateral prefrontal cortex dysfunction in first episode schizophrenia. NeuroImage Clin 2013;2:590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eckert MA, Menon V, Walczak A, et al. At the heart of the ventral attention system: the right anterior insula. Hum Brain Mapp. 2009;30:2530–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Radeloff D, Ciaramidaro A, Siniatchkin M, et al. Structural alterations of the social brain: a comparison between schizophrenia and autism. PLoS One. 2014;9:e106539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Orliac F, Naveau M, Joliot M, et al. Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr Res. 2013;148:74–80. [DOI] [PubMed] [Google Scholar]
- 58. Edwards BG, Barch DM, Braver TS. Improving prefrontal cortex function in schizophrenia through focused training of cognitive control. Front Hum Neurosci. 2010;4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.