Abstract
Objectives
Sustained release of small molecule hydrophilic drugs at high doses remains difficult to achieve from electrospun fibers and limits their use in clinical applications. Here we investigate tunable release of several water-soluble anti-HIV drugs from electrospun fibers fabricated with blends of two biodegradable polyesters.
Methods
Drug-loaded fibers were fabricated by electrospinning using ratios of PCL and PLGA. Fiber morphology was imaged using SEM, and DSC was used to measure thermal properties. HPLC was used to measure drug loading and release from fibers. Cytotoxicity and antiviral activity of drug-loaded fibers were measured in an in vitro cell culture assay.
Results
We show programmable release of hydrophilic antiretroviral drugs loaded up to 40 wt%. Incremental tuning of highly-loaded drug fibers within 24 hours or >30 days was achieved by controlling the ratio of PCL and PLGA. Fiber compositions containing higher PCL content yielded greater burst release whereas fibers with higher PLGA content resulted in greater sustained release kinetics. We also demonstrated that our drug-loaded fibers are safe and can sustain inhibition of HIV in vitro.
Conclusions
These data suggest that we were able to overcome current limitations associated with sustained release of small hydrophilic drugs at clinically relevant doses. We expect that our system represents an effective strategy to sustain delivery of water-soluble molecules that will benefit a variety of biomedical applications.
Keywords: Programmable release, HIV, high loading, tenofovir, electrospinning
Introduction
Electrospun polymeric fibers are a well-established technology with many uses across diverse biomedical applications (1-4). The ability to electrospin various polymers and fabricate fibers with high drug content makes the platform particularly attractive for drug delivery (5,6,11). For example, the high surface area to volume ratio of fibers facilitates rapid drug dissolution and promotes tissue absorption (6,9,10). In addition, a variety of natural and synthetic polymers are amenable to electrospinning and can be selected for their compatibility with physiochemically diverse agents required in various therapies and treatments (1,10). The use of electrospun fibers as a topical microbicide for HIV inhibition has only been recently investigated and presents a promising alternative to existing vaginal drug delivery systems (5,6,11). Studies have already shown the ability to electrospin fibers that can provide rapid release of antiretroviral compounds loaded at high drug content (5,6,11). However, the design constraints to achieve sustained release from electrospun fibers, specifically for the inhibition of HIV and other clinical applications requiring long-acting drug delivery, remains unknown.
Sustained release from electrospun fibers has been demonstrated but is primarily limited to examples using hydrophobic small molecule drugs or large proteins. In these cases, modest drug release leverages properties of the agents such as their poor dissolution and preferential partitioning into hydrophobic polymers, or large molecular diffusivities (16-17). Low drug loading is also typical of many fiber-based systems demonstrating sustained release (18, 21). Using superhydrophobic polymeric fibers, Yohe and Grinstaff (2012) proposed a mechanism in which air displacement within the bulk matrix and subsequent water penetration into individual fibers facilitates >60 day sustained release of SN-38, a hydrophobic small molecule anticancer drug loaded at 1 wt% (18). Small molecule drugs that are water-soluble and show low potency, and are therefore required at high dosing, are an important category of antiviral and antibacterial compounds being considered for microbicides. However, the ability to realize sustained release of this class of molecules from electrospun fibers has proven difficult due to the dual challenges associated with polymer-drug compatibility as well as high drug dissolution rates and large molecular diffusivity (19). Core-shell fibers fabricated by coaxial electrospinning have shown some success for sustaining the release of small molecule hydrophilic drugs at high drug loading (20), but coaxial electrospinning is a more complicated process that is limited to certain polymers and solvent systems and is not scalable with existing commercial instruments. While there have been examples of sustained release of water-soluble small molecules from fibers (21-22), their loading or programmability limit potential applications (23-24). Therefore, tunable sustained water-soluble drug release from uniaxial matrix fibers formulated at high drug loading remains to be an important area of investigation for many clinical applications employing electrospun fibers.
Biodegradable polyesters and their blends have extensive use in the development of sustained release drug delivery systems (25-27). FDA-approved and biodegradable polyester blends of poly-(lactic-co-glycolic) acid (PLGA), poly-caprolactone (PCL), and poly-lactic acid (PLA) are of particular interest due to their ability to achieve polymer chemistries from physical mixtures rather than de novo synthesis. Lao and Peppas (2008) investigated the release of paclitaxel, a hydrophobic drug formulated at 1 wt% in solvent-cast films of PCL and PLGA (28). Their results showed that paclitaxel release was faster from pure PCL compared to pure PLGA, and that blending the two polymers produced intermediate drug release kinetics. The observed release kinetics of paclitaxel from the PCL/PLGA blends was predicted by a heuristic model that assumed partitioning and release of the drug from phase-separated PCL- or PLGA-rich domains (28). Based on these observations, we examined the generalizability of these same blended polyesters to sustain drug release of a water-soluble small molecule drug from electrospun fibers loaded at 10-40 wt%. We hypothesized that drug release would be faster from pure PCL compared to pure PLGA and that a blend of the two polymers would yield intermediate release kinetics.
To test this hypothesis, we developed a uniaxial electrospun fiber system for the controlled release of tenofovir (TFV), a small molecule hydrophilic nucleotide reverse transcriptase inhibitor. Here we show that TFV loaded at 10-40 wt% in fibers that combine PCL and PLGA in specific ratios can tune drug release within 24 hours or >30 days. Both the drug loading and the PCL content influenced the magnitude of the burst release phase, whereas the sustained release phase was dominated by the PLGA content. In contrast to Yohe et al. (18), our equilibrium water contents studies show rapid water penetration of the porous bulk mesh, which is likely due to the high TFV drug content, and suggests that controlled surface wetting is not a dominant mechanism for sustaining TFV release. Based on the solution compositions and process of electrospinning, thermal analysis indicates that PCL and PLGA polymer chains are miscible and interact in the finished solid fibers, which may influence water penetration and TFV release from individual fibers. We propose that PCL polymer chains disrupt the glassy state of PLGA chains to facilitate faster TFV release. We also demonstrate that the PCL/PLGA fiber system can be used to load and sustain the release of other water-soluble antiretroviral drugs such as raltegravir (RAL), maraviroc (MVC), and azidothymidine (AZT). Antiviral fibers based on PCL/PLGA compositions demonstrate safety in vitro and sustained HIV inhibition. Our results extend the versatility of the electrospinning platform to be designed for sustained release microbicides, and may have important implications for other biomedical applications.
Materials and methods
Materials
Poly (D, L-lactic acid-co-glycolic) acid (PLGA) with a 50:50 LA:GA ratio with an acid end cap and molecular weight (Mw) average of 100,000 Da was purchased from PolySciTech® (West Lafayette, IN, USA). Polycaprolactone (PCL) with a molecular weight (Mw) average of 80,000 Da was purchased from Sigma Aldrich (St. Louis, MO, USA). Tenofovir was provided by CONRAD (Arlington, VA, USA). Maraviroc (MVC) and raltegravir (RAL) were purchased from UW pharmacy and purified in house. Tenofovir disoproxil fumarate (TDF) was purchased through the NIH AIDS Research & Reference Reagent Program (Germantown, MD, USA). Solvents dimethyl sulfoxide (DMSO) and hexafluoroisopropanol (HFIP) were purchased from VWR® (Radnor, PA, USA) and Oakwood Laboratories (Wayne County, MI, USA). Dulbecco's phosphate buffered saline (DPBS) was purchased from Mediatech Inc. (Manassas, VA, USA). Citric acid was purchased from Sigma Aldrich (St. Louis, MO, USA) and sodium chloride (NaCl) was purchased from Avantor Performance Materials (Central Valley, PA, USA). HPLC grade Acetonitrile, trifluoroacetic acid, HPLC grade water, and potassium phosphate monobasic were purchased from Fisher Scientific (Pittsburgh, PA, USA).
Cell and virus
HIV-1 BaL is a CCR5 tropic virus. It was obtained from the NIH AIDS Research & Reference Reagent Program (Germantown, MD, USA). Virus stock was propagated in PM-1 cells and titrated in both PM-1 and TZM-bL cell lines. TZM-bL cell line (JC53-BL) was obtained from the NIH AIDS Research & Reference Reagent Program. TZM-bL cells are a genetically engineered HeLa cell clone that expresses CD4, CXCR4, and CCR5 and contains Tat-responsive reporter genes for firefly luciferase (Luc) and Escherichia coli β-galactosidase under regulatory control of an HIV-1 long terminal repeat, permitting sensitive and accurate measurements of HIV infection. The TZM-bL cell line was maintained in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100U penicillin ml−1, 100 mg streptomycin ml−1 and 2 mM L-glutamine and 25 mM HEPES. PM-1 and CEMx174 T cell lines were also kindly provided by the NIH AIDS Research & Reference Reagent Program and grown in RPMI1640 medium supplemented as above. All cell lines were incubated at 37°C in a humidified 5% CO2 air environment.
Preparation of drug-loaded PCL/PLGA electrospun fibers
TFV loaded PCL/PLGA fibers were fabricated through uniaxial electrospinning. Briefly, PCL and/or PLGA were dissolved in HFIP (15% (w/v)). PCL/PLGA content was varied to create a range of blends (w/w): 100:0, 80:20, 60:40, 40:60, 20:80, and 0:100. Then, drug was dissolved in polymer/solvent solution (10-40% (w/w) = wt%). AZT, MVC, and RAL-loaded PCL/PLGA fibers were also fabricated through uniaxial electrospinning. PCL/PLGA content was varied to create a range of blends (w/w): 80:20, 20:80. 10:90, and 5:95. Then, drugs were dissolved in HFIP-polymer solution (15% (w/w)). All solutions were extruded from a 5 mL glass syringe, through a 21G blunt-end needle at 50 μl/min, using a NE-1000 programmable single syringe pump (Farmingdale, NY, USA). Solutions were pumped in the presence of an electric field ranging in strength from 0.65 kV/cm to 1.5 kV/cm and collected on a grounded metal plate at a distance of 10 cm. Resulting fiber mats were dried and stored in a vacuum desiccator until used for analysis.
Scanning Electron Microscopy
The morphologies of drug-loaded PCL/PLGA fibers were characterized using scanning electron microscopy (SEM). Circular punches were cut from fibrous mat and placed on SEM stub using conductive carbon tape. Samples were then sputter coated with Gold/Palladium for 90 seconds using an SPI sputter-coater (West Chester, PA, USA) at 80-100 mTorr. Micrographs were acquired using FEI Sirion SEM system (UW, NanoTech User Facility).
Differential Scanning Calorimetry
Thermal analysis of drug-loaded PCL/PLGA electrospun fibers was performed using differential scanning calorimetry with a TA Auto Q20 DSC instrument (New Castle, DE, USA). Electrospun fiber samples (3-7 mg) were placed in sealed T-Zero aluminum pans and headed from 30°C to 310°C at a rate of 10°C/min. All thermograms were analyzed using TA Instruments Universal Analysis (v. 4.7A).
In vitro drug release from PCL/PLGA electrospun fibers and drug loading
In vitro release of TFV, AZT, and RAL from PCL/PLGA electrospun fibers was carried out in 1X DPBS (pH ~7.2-7.4) in sink conditions (Volume 10-fold above solubility limit of drug). In vitro release of MVC from PCL/PLGA electrospun fibers was carried out in 154 mM Citrate Buffer (pH ~ 4.0) in sink conditions. In vitro release of TDF from PCL/PLGA electrospun fibers was carried out in distilled H2O, supplemented NaCl, in sink conditions. Briefly, ~1.25 cm2 fiber punches were cut, weighed (~3-10 mg), and placed in 40 mL of media. All samples were incubated in a shaker at 37°C and 200 rpm. At predetermined time points, 200 μL samples were removed for HPLC analysis and replaced with fresh media to maintain sink conditions. Curve fitting was performed on time points <60% release using the power law equation below:
Mt represents the amount of drug released at time (t), Mtotal is the total amount of drug released from the fiber sample, k (slope parameter) and n (power law expression) are parameters that are calculated using regression (44). Temperature dependent in vitro release of TFV from PCL/PLGA electrospun fibers was carried out with the same conditions as listed above except for the temperature of incubation. Low and high temperature release was accomplished by placing samples on rotator in a 4°C refrigerator and incubator set to 50°C, respectively.
Encapsulation efficiency was defined as the total amount of drug associated with the fibers (entrapped and surface localized drug) relative to the theoretical drug loading. To assess drug encapsulation efficiency of TFV loaded PCL/PLGA fibers, 3-6 mg samples were cut and dissolved in 5-15 mL of DMSO, in 20 mL glass scintillation vials. Then, 200 μL samples were extracted, filtered, and placed in HPLC vials. Drug content was quantified using a Shimadzu Prominence LC20AD UV-HPLC system equipped with a Phenomenex Luna 153 C18 column (5 μm, 250 × 4.6 mm) and LCSolutions software. The mobile phase used for TFV was 72% H2O supplemented with 0.045% trifluoroacetic acid and 28% acetonitrile supplemented with 0.036% trifluoroacetic acid. The TFV method parameters used were: 30°C column temperature, 1 mL/min flow rate, 10 minute run time, 20 μL sample injection volume and UV/vis detection at 259 nm. TFV content was quantified by comparing to a standard curve of TFV dissolved in DMSO (1 μg/mL – 100 μg/mL). TFV, TDF, and AZT (although AZT was detected at 265 nm with a 10 μL sample injection volume) release were analyzed using the TFV HPLC method described above. Two methods were used for MVC. The first method was used to assess drug loading in DMSO and the second was used to assess MVC release. The mobile phase for the first method was 26% acetonitrile and 74% 50 mM KH2PO4 in H2O. The first MVC method parameters were: 44°C column temperature, 1 mL/min flow rate, 15 minute run time, 20 μL sample injection volume and UV/vis detection at 193nm. The mobile phase used for the second MVC method was 40% acetonitrile and 60% 0.01 M KH2PO4 in H2O. The second MVC method parameters were: 25°C column temperature, 1 mL/min flow rate, 10 minute run time, 20 μL sample injection volume and UV/vis detection at 193 nm. RAL release was analyzed using a mobile phase of 55% acetonitrile and 45% 25 mM KH2PO4 in H2O. The RAL method parameters used were: 35°C column temperature, 0.9 mL/min flow rate, 10 minute run time, 20 μL sample injection volume and UV/vis detection at 300 nm. Drug release in vitro was quantified by comparing to a standard curve of each drug dissolved in the same buffer used for release (1 μg/mL – 100 μg/mL), using previously describe methods (39, 45, 46). Drug loading (encapsulation efficiency) was quantified by comparing to a standard curve of each drug dissolved in DMSO (1 μg/mL – 100 μg/mL). Supplementary figure 1 shows chromatograms and standard curves for TFV, AZT, RAL, and MVC standards in DMSO used to validate the quantitative HPLC methods described above.
Percent change in water content and percent polymer mass loss
To test the degree of hydration and polymer mass loss of TFV-loaded PCL/PLGA electrospun fibers, samples were weighed, pre mass (Mpre) and placed in ~40 mL DPBS and incubated in a shaker at 37°C. At predetermined time points, fiber samples were removed from DPBS. Samples were pressed lightly with Kimwipes to remove surface liquid and were then weighed to determine a wet mass (Mwet). Samples were then dried at room temperature for at least 72 hours and reweighed to determine a dry mass (Mdry). Dry weights were measured multiple times over several hours to ensure complete drying and a stable weight measurement. Percent change in water content (% CH2O) and polymer mass loss (% MLpolymer) were calculated as shown below:
Prior to percent polymer mass loss calculation, the amount of total drug loss (DLtotal) to release was measured by taking 200 μL samples and analyzed using HPLC methods described above. Neat PCL/PLGA fibers were also assessed for percent change in water content as a means of evaluating the influence of the hydrophilic drug on sample hydration.
Antiviral activity and cytotoxicity
A 40-60 mg piece of TFV-loaded PCL/PLGA fiber was suspended in DPBS in conical centrifuge tube and incubated at 37°C on an orbital shaker. Supernatant was collected at different time points and serial dilution was made for antiviral assay. The antiviral activity of TFV-loaded fiber was assessed based on a reduction in luciferase reporter gene expression after infection of TZM-bL cells with HIV-1 BaL. Briefly, TZM-bL cells were seeded at a concentration of 1×104 per well in 96-well microplate. The following day, TZM-bL cells were incubated with various concentrations of fiber extract at 37°C for one hour prior to virus exposure. Then cell free HIV-1 BaL(200TCID50) was added to the cultures and incubated for 48 hours. Untreated wells were used as control. The Promega™ Luciferase Assay System (Promega™, Cat#: E4030) was used to determine luciferase expression. Antiviral activity was expressed as an IC50 value, which is the sample concentration giving 50% of relative luminescence units (RLUs) compared with those of virus control after subtraction of background RLUs (GraphPad Prism v5.0). TZM-bL cells were cultured in 96-well plates in the absence or presence of various concentrations of fiber extract preparation. After three days in culture, cell viability was assessed using CellTiter-Blue® Cell Viability Assay (Promega) following manufacturer's recommended procedures. Briefly, cells were incubated for four hours with 20 μl/well of CellTiter-Blue®Reagent and fluorescence was recorded at 560/590 nm.
Statistical Analysis
Statistical analyses were carried out using one-way ANOVA and significance level was set at p < 0.05. All analysis was done in GraphPad Prism v.5 for Mac OS X (GraphPad Software, San Diego CA, USA).
Results/Discussion
Electrospinning of anti-HIV biodegradable fibers loaded with tenofovir
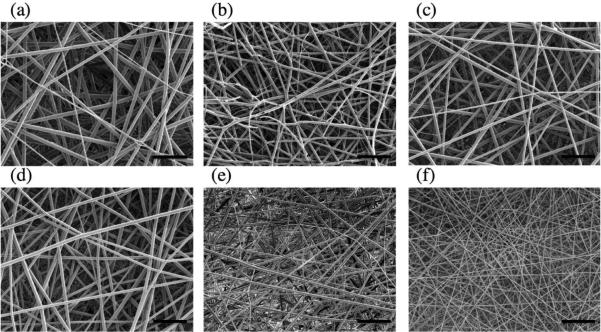
TFV was electrospun at 15 wt% loading into fibers comprised of PCL and PLGA in ratios that varied each polymer between 20-100% in the final fiber formulations (Table 1). Measured drug loading indicated an encapsulation efficiency of 100% for all fiber formulations (Supplementary Table 1). SEM micrographs showed that fabric meshes were free of defects and that the fibers were continuous and had a smooth morphology (Figure 1). Fibers had a measured diameter of 1.0-1.6 μm irrespective of the specific polymer compositions and showed similar thickness, basis weight, density and porosity (Supplementary Table 1).
Summary of thermal characteristics and curve fitting parameters of PCL/PLGA fibers.
| Formulation | Thermal Characteristics | Power Law Paramaters1 | |||||
|---|---|---|---|---|---|---|---|
| Tm (°C)2 | ΔH (J/g)3 | Tg (°C)4 | CTFV (%)5 | CPCL (%)5 | k | n | |
| PCL Blank | 57.5 | 57.2 | n.d. | n.d. | 49.1 | n/a | n/a |
| PLGA Blank | n.d. | n.d. | 53.8 | n.d. | n.d. | n/a | n/a |
| PCL + 15 wt% TFV | 58.8 | 61.2 | n.d. | 1.7 | 66.2 | n/a | n/a |
| PCL/PLGA 80:20 + 15 wt% TFV | 58.2 | 43.0 | n.d. | 1.4 | 35.9 | n/a | n/a |
| PCL/PLGA 60:40 + 15 wt% TFV | 57.4 | 33.5 | n.d. | 1.8 | 24.2 | 26.6 | 0.19 |
| PCL/PLGA 40:60 + 15 wt% TFV | 57.4 | 22.0 | n.d. | 1.6 | 10.2 | 26.7 | 0.29 |
| PCL/PLGA 20:80 + 15 wt% TFV | 56.3 | 0.5 | 45.1 | 0.9 | n.d. | 12.1 | 0.29 |
| PLGA + 15 wt% TFV | n.d. | n.d. | 46.3 | 1.6 | n.d. | 5.0 | 0.38 |
Power law parameters derived from power law equation: Mt/Mtotal = ktn, where k and n describe the characteristics of macromolecular network and release mechanism, respectively.36,40,41
Tm = Melting temperature of PCL in final fiber formulation.
ΔH = Enthalpy of PCL melting peak. Value listed is the integration under the curve of PCL melting peak.
Tg = Glass transition temperature of PLGA in final fiber formulation.
Relative TFV and PCL crystallinity calculated by integrating area under the melting curve (ΔH) of each and comparing to pure TFV powder and PCL pellets, respectively. Crystallinity values were also calculated relative to the mass content of each component in the final fiber formulation.
Figure 1.
Representative scanning electron micrographs showing consistent fiber morphology across PCL:PLGA ratios: (a) PCL, (b) 80:20, (c) 60:40, (d) 40:60, (e) 20:80, and (f) PLGA. The scale bar in each micrograph represents 20μm.
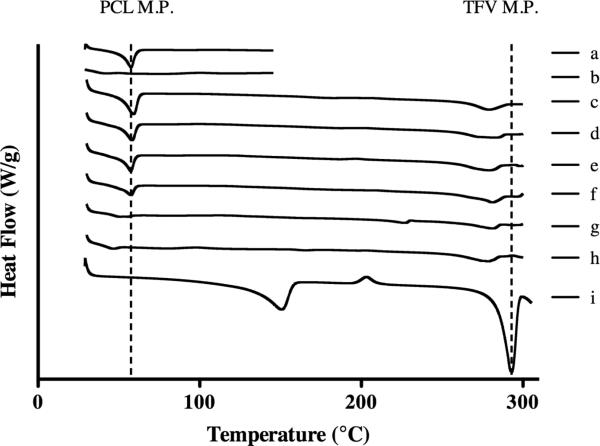
We used differential scanning calorimetry (DSC) to probe the physical state of the drug and polymer in finished fibers of different PCL/PLGA compositions. In particular, we looked at the thermal characteristics of the semi-crystalline PCL, amorphous PLGA, and TFV in the finished fibers to determine the compatibility of each of the constituents as well as their influence on the bulk polymer properties (Figure 2). We only detected extremely low levels of crystalline TFV in solid fiber dispersions of PCL, PLGA or their combinations (Table 1). The absence of significant crystalline drug indicates high compatibility with the polymers during and after electrospinning. The relative crystallinity of pure PCL fibers was calculated to be 49.1%, which is consistent with values reported for PCL (33). Polymer blends showed that a 20% increase in the amount of PLGA led to an average decrease of >40% in PCL crystallinity relative to its mass content in the final fiber compositions (Table 1). The result suggests miscibility between the two polymers, and may arise from polymer chains of PLGA disrupting PCL crystalline domains and decreasing its area under the melting peak (42). Changes in the individual polymer Tg upon blending can also provide information about miscibility and phase separation (38). However, because of the low Tg of PCL (reported Tg −60°C)(33) and because the Tg of PLGA was masked by the Tm of PCL, we were unable to use this measure to further confirm polymer miscibility. However, the uniform macroscopic morphology and the change in relative PCL crystallinity in the blended polyester fibers collectively support that PCL and PLGA are miscible in our system. This finding is in contrast to the work of Lao and Peppas (28), which may be a result of the chosen solvent and the process of fabricating electrospun fibers compared to solvent-cast films from the same PCL and PLGA polymers.
Figure 2.
Differential Scanning Calorimetry of PCL/PLGA electrospun fibers and TFV powder. Dashed lines show melting points of PCL (~60°C) and TFV (293°C). There were two other peaks seen on the TFV powder trace: the first is a broad peak right of 100°C which shows water released from the monohydrate form of TFV and a broad peak around 210°C which displays the recrystallization of TFV. All blends displayed negligible TFV crystallinity demonstrating compatibility with fiber system: (a) PCL blank, (b) PLGA blank, (c) PCL, (d) PCL/PLGA 80:20, (e) PCL/PLGA 60:40, (f), PCL/PLGA 40:60, (g) PCL/PLGA 20:80, (h) PLGA, (i) TFV powder
Tuning the sustained release of tenofovir using polyester blends
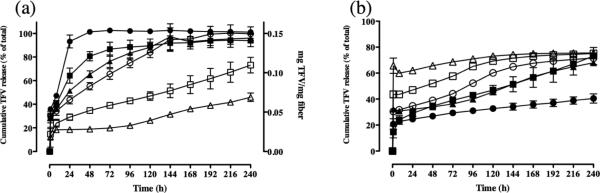
We next investigated the influence that different PCL/PLGA polymer compositions would have on drug release kinetics based on the physical properties of the polymers and TFV in the finished fibers. We observed that pure PCL fibers (100:0 PCL:PLGA) released all their TFV content (100%) after 24 hours, which was approximately five times greater than the percentage of TFV released from pure PLGA fibers (0:100 PCL:PLGA) (Figure 3a). In contrast, pure PLGA fibers showed only 20% release of TFV in the first 24 hours followed by an apparent linear release rate of ~2% per day over the 10 day experiment. This pseudo-linear phase of TFV release from pure PLGA is expected to be a result of drug diffusion out of the polymer network, which depends on the available free volume for drug dissolution. We assumed that polymer degradation did not influence TFV release since PCL and PLGA can be considered to have low swellabillity in water and these polymers degrade over a period of time that is much longer (>1 month) than the drug release experiments conducted in these studies (10 days) (25, 33).
Figure 3.
(a) 15 wt% TFV-loaded blended polyester fibers incrementally tune drug release. (Filled circles) PCL, (Filled squares) PCL/PLGA 80:20, (Filled triangles) PCL/PLGA 60:40, (Open circles) PCL/PLGA 40:60, (Open squares) PCL/PLGA 20:80, and (Open Triangles) PLGA. Results display that higher PCL content yields burst release and higher PLGA content yields sustained release. TFV release was quantified using HPLC. (b) Polyester fiber blends (PCL/PLGA 20:80 fibers) show incremental burst release of TFV as drug loading is increased, during in vitro release. (Filled circles) 10 wt% TFV, (Filled squares) 15 wt% TFV, (Filled triangles) 20 wt% TFV, (Open circles) 25% TFV, (Open squares) 30 wt% TFV, and (Open triangles) 40 wt% TFV. Results display that the initial loading of TFV had an impact on the burst release phase of TFV. However, subsequent release of TFV over 10 days followed similar trends to PCL/PLGA 20:80 fibers loaded with 15 wt% TFV.
By changing the PLGA to PCL ratio, we achieved incremental tuning of TFV drug release. In general, 15 wt% TFV-loaded fiber compositions containing higher PCL content resulted in a greater fraction of burst release whereas fibers with higher PLGA content yielded more sustained release kinetics (Figure 3a). All fiber compositions burst released 15-35% of TFV within the first hour. We observed that TFV release increased by 10-25% with every 20% increase in PCL content during the early stage pseudo-linear kinetics (24-72 hours). For fiber compositions comprised of 20-40% PCL, TFV burst release phase increased by only modest amounts compared to release from pure PLGA (20-30%). However, the rate of drug release during the pseudo-linear release phase for these same formulations compared to pure PLGA increased by almost 6-10 fold. As a consequence, PCL:PLGA 40:60 fibers completely released their full TFV content (100%) after 6 days, whereas PCL:PLGA 20:80 and pure PLGA showed only 75% and 40% TFV release after 10 days, respectively. In summary, the polyester blends allowed us to tune complete TFV (100%) release within 24 hours and out to 30 days (by extrapolating the pseudo-linear release results from our data out to the time that 100% of the drug loading would be depleted). Interestingly, the magnitude of burst release in blended fibers was typically less than predicted if equal partitioning into PCL content occurred across blends. We fit our release kinetics to a power law equation and determined the slope factor (k) and the power law exponent (n), which describe characteristics of the macromolecular network and release mechanism, respectively (Table 1) (39,43,44). Our analysis showed that there was a significant positive and negative correlation between relative PCL crystallinity and the slope factor (k) (R = 0.85, p < 0.05) and power law exponent (n) (R = −0.90, p < 0.05), respectively.
We also studied the effect of drug loading on the incremental release profile of TFV from PCL/PLGA 20:80 fiber compositions. TFV content was varied between 10-40 wt% in this fiber composition and drug release was measured over 10 days. We observed that every 10% increase in TFV loading resulted in 15-20% increase in the magnitude of burst release from the fibers. However, the subsequent release over the remaining 10 days appeared to show similar rates of TFV release of ~3-7% per day (Figure 3b). The result is consistent with other previously published results showing that higher loadings usually result in either crystalline drug or otherwise drug that is not encapsulated within the fiber (31). Therefore, it is expected that excess drug would crystallize or localize at the surface and burst, while the original amount of drug would be trapped within the fiber and exhibit similar release compared to fibers with lower drug loadings.
Several factors play important roles in governing drug release kinetics from matrix drug delivery systems. These factors include the drug molecular weight, diffusion path length, and drug physical state, solubility and dissolution rate (30-31). Our results are consistent with the first two of three phases of drug release that have been described previously for pure PLGA and PCL systems (25,28). The pseudo-linear phase shows faster TFV release kinetics for fiber compositions that are PCL-dominated, which may suggest greater free volume for drug dissolution and is consistent with the fact that PCL chains are in a rubbery state due to its low Tg value. PLGA chains have a higher Tg, which results in a glassier polymer that restricts the mobility of water and TFV diffusion.
Programmable release of other water-soluble antiretroviral drugs from PCL/PLGA fibers
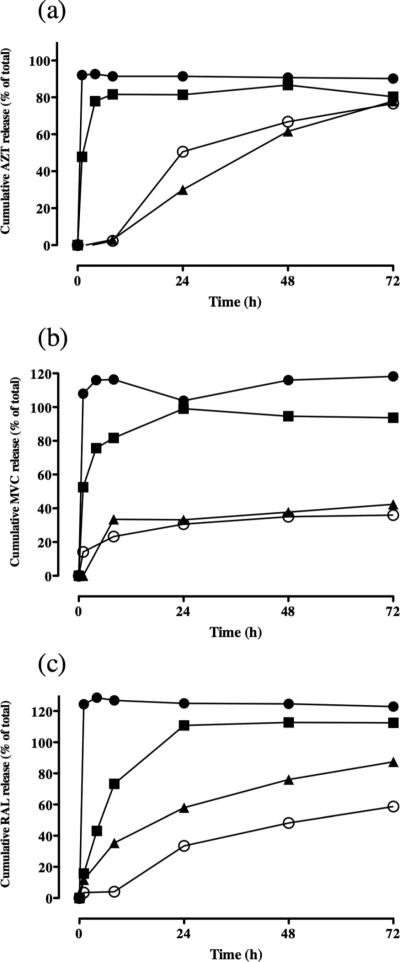
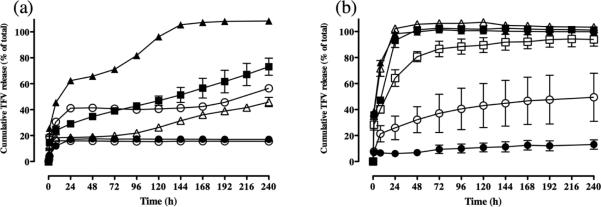
The PCL/PLGA fiber system was tested with other highly water-soluble antiretroviral drugs to assess the generalizability of the platform with structurally different small molecule compounds. Using a PCL and PLGA ratio of 80:20 and 20:80, we formulated fibers encapsulating azidothymidine (AZT), maraviroc (MVC) and raltegravir (RAL). These three ARV drugs have a similar molecular weight to TFV, but have important physiochemical differences including ionization state, functional groups, and solubility. PCL:PLGA 80:20 and 20:80 fibers loaded with AZT-, MVC-, and RAL showed similar size, morphology, drug loading and density compared to equivalent fiber formulations of TFV (Supplementary Table 1). However, release of these water-soluble ARV drugs compared to TFV did not show the same dependency on the PCL and PLGA ratio (Figure 4). Only after increasing the PLGA proportion to 90-95% (10:90 and 5:95 PCL/PLGA) did we achieve significant dampening of the release of AZT, MVC and RAL. For example, at 24 hours, release of all three drugs decreased by 40-60% when increasing the PLGA content of the fibers >90%. Fibers with >90% PLGA content also sustained release of all three drugs past 24 hours and did not show total drug release by 72 hours. Based on these data, we concluded that the PCL/PLGA blends could sustain the release of structurally diverse water-soluble drugs, but only when PCL content was limited to <10%.
Figure 4.
Polyester fiber blends incrementally tune release of other antiretroviral drugs over narrower range of blends. (a) Release of 15 wt% azidothymidine from (Filled circles) PCL/PLGA 80:20, (Filled squares) PCL/PLGA 20:80, (Filled triangles) PCL/PLGA 10:90, and (Open circles) PCL/PLGA 5:95. (b) Release of 15 wt% maraviroc from (Filled circles) PCL/PLGA 80:20, (Filled squares) PCL/PLGA 20:80, (Filled triangles) PCL/PLGA 10:90, and (Open circles) PCL/PLGA 5:95. (c) Release of 15 wt% raltegravir from (Filled circles) PCL/PLGA 80:20, (Filled squares) PCL/PLGA 20:80, (Filled triangles) PCL/PLGA 10:90, and (Open circles) PCL/PLGA 5:95.
Effect of water content, polymer mass loss and chain mobility on TFV release from PCL/PLGA blends
Because dissolution of other small molecule drugs did not exhibit the same programmed release behavior as TFV, we hypothesized that interactions between TFV and PCL/PLGA facilitates tuning of its release kinetics. Several experiments were conducted to investigate the primary factors that contribute to TFV release from PCL/PLGA fibers. It has been reported that in PLGA systems, diffusion through water-filled pores, diffusion through the polymer, and erosion are potential mechanisms of release (26). Furthermore, other processes such as polymer chain mobility, hydrophobicity, and molecular weight also influence the previously mentioned release mechanisms (26).
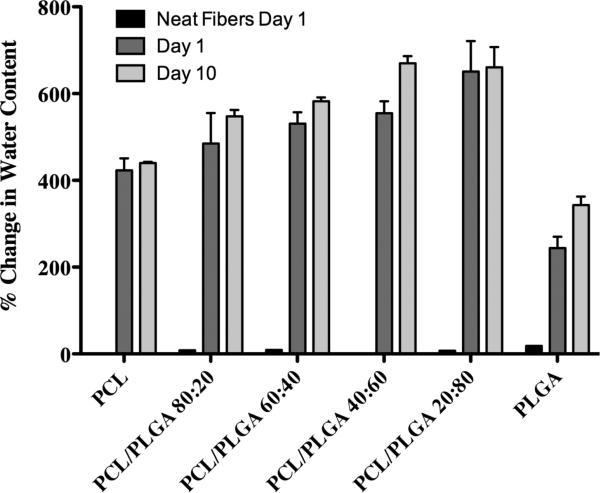
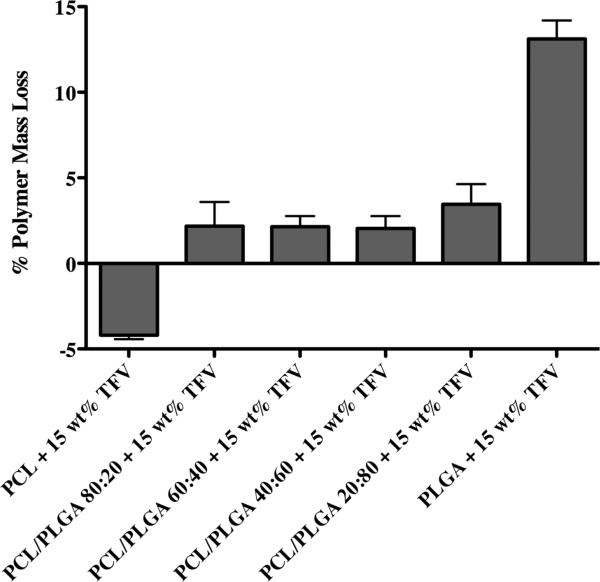
The equilibrium water content of our electrospun fibers was measured to determine the influence of swelling and hydration on TFV release from PCL/PLGA blended fibers. We hypothesized that if wetting and hydration were the primary process governing release then fibers with higher PCL content would show increased water absorption compared to fibers with higher PLGA content. We observed that neat fiber meshes exhibited a maximum of 18% increase in water content while TFV-loaded fiber meshes showed a minimum of ~225% increase in water content irrespective of the PCL and PLGA content (Figure 5). This result suggests that TFV content, which is equal amongst the blends, drives rapid water penetration into the porous fiber network. We observed that water content reached rapid equilibrium within one day and no significant increase in water content was observed at 10 days. However, TFV release for most blends continued out past 7 days. In contrast to Yohe et al. (18), who observed that controlled surface wetting from superhydrophobic polymer fibers could facilitate long-term drug release, we expect that differences in water penetration into the polymer network modulates sustained TFV release. That is, differences in release between PCL-dominated and PLGA-dominated fibers may come from water penetrating PCL polymer chains more rapidly compared to PLGA. In fact, analysis of water content between pure PCL and PLGA showed that PCL had a statistically significant increase in percent water content on day one (423 +− 27.5%) compared to PLGA (244 +− 26.3%). However, there was not a significant difference between the two pure polymers at day 10. This, coupled with the lack of difference in percent water content across blends, suggests that hydration of the mesh network alone does not fully explain the dampened release in PLGA-dominated fibers compared to PCL-dominated fibers over 10 days of release. Polymer mass loss was also evaluated after 10 days of release to investigate the influence of degradation and erosion on TFV release. Results showed minimal mass loss for all PCL/PLGA fibers except for pure PLGA (Figure 6). As expected, erosion is considered a very slow process especially with high molecular weight polymers (26).
Figure 5.
Percent change in water content analysis of PCL/PLGA electrospun fiber blends after Day 1 and Day 10 in D-PBS. Neat fibers group displays water content of fibers without loaded drug. Day 1 and Day 10 groups include 15 wt% TFV loading for each fiber formulation displayed. Analysis shows that higher PCL content does not yield increase in water content. TFV is the main source of swelling in polymer system.
Figure 6.
Analysis of polymer fiber mass loss after 10 day drug dissolution study. All fiber formulations displayed include 15 wt% TFV loading. Results show minimal mass loss for all PCL/PLGA fibers except for pure PLGA. Pure PLGA shows approximately 13% mass loss. Erosion may play a minor role in release of TFV, but is not the main mechanism of release as it occurs much slower than other processes.
Chain mobility and flexibility is a unique property of different polymers and is described by the polymer glass transition (Tg) temperature (36,43). At 37°C we would expect PCL (Tg = −60°C) and PLGA (Tg = of 46°C) chains to have high and low chain mobility, respectively. We hypothesized that as long as the PCL and PLGA do not phase separate then increasing amounts of PCL to pure PLGA could add flexibility to the bulk polymer, which in turn could facilitate improved drug diffusion and faster release. We expected that pure PLGA fibers would exhibit slower release at 4°C and faster release at 50°C due to the chain mobility predicted by the polymer's Tg. The maximum change in diffusivity of the drug at these different temperatures was calculated to be only ~11% (37). Dissolution of TFV from polymer blends was tested at 4°C and 50°C with all other conditions matched to our previous 37°C release experiments (Figure 3a). We observed a ≤10% change in the magnitude of TFV burst release from PLGA fibers when changing the release temperature from 37°C to either 4°C or 50°C, which would be consistent with diffusion of surface localized TFV. In contrast, TFV release at 240 hours was reduced by ~30% at 4°C and increased by 15-20% at 50°C (Figure 7a). For pure PCL fibers, we expected slower release at 4°C as the experimental temperature approaches the polymer's Tg whereas faster release was expected at 50°C. In fact, TFV release decreased by >70% at all time points past 24 hours at 4°C (Figure 7b). When the temperature was elevated to 50°C, we observed a ~30% increase in TFV release at 8 hours, which supports improved diffusion of TFV from PCL polymer chains. These data suggest that at physiologically relevant temperatures (37°C), PCL chains are in a rubbery state and more mobile thereby facilitating faster diffusional release of TFV compared to PLGA, which is more glassy and immobile.
Figure 7.
Polyester fiber blends dampen and quicken release of TFV at low and high temperatures, respectively. (a) (Filled circles) PCL/PLGA 20:80 at 4°C, (Filled squares) PCL/PLGA 20:80 at 37°C (Filled triangles) PCL/PLGA 20:80 at 50°C, (Open circles) PLGA at 4°C, (Open squares) PLGA at 37°C, and (Open triangles) PLGA at 50°C. (b) (Filled circles) PCL at 4°C, (Filled squares) PCL at 37°C (Filled triangles) PCL at 50°C, (Open circles) PCL/PLGA 80:20 at 4°C, (Open squares) PCL/PLGA 80:20 at 37°C, and (Open triangles) PCL/PLGA 80:20 at 50°C. Results show the influence of the polymer glass transition temperature (PCL: −60°C, PLGA: 43°C) on amount of free volume for diffusion of drug. At 4°C PCL and PLGA have less free volume for diffusion and therefore display slower release overall compared to 37°C. At 50°C, the temperature of the study is above both polymer Tg values and therefore has significantly more free volume for diffusion. Change of drug diffusivity in solution was estimated to be a maximum of ~11% at tested temperatures. The change in drug diffusivity in solution is much lower than the change drug release at the tested temperatures.
Effect of polymer-drug interaction on TFV release from PCL/PLGA fibers
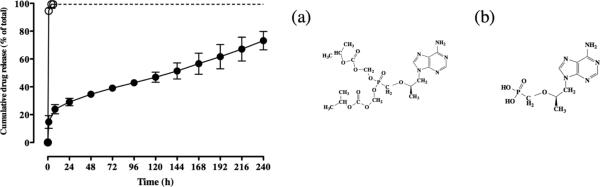
Since the phosphonic acid functional group is unique to TFV and absent in the other small molecule drugs, we investigated whether its unique release kinetics could be attributed to hydrogen bonding with the carbonyl ester and acid end groups on the polyester backbone (34). At the low solution pH used for electrospinning (pH ~2), we hypothesized that the protonated phosphonic acid may shows preferential hydrogen bonding to the PLGA backbone due to the two-fold greater mole fraction of carbonyl esters and acid end groups compared to PCL. To evaluate this interaction, we electrospun tenofovir disoproxil fumarate (TDF), a prodrug of TFV in which the phosphonic acid hydrogen bond donors are replaced with methyl isopropyl carbonate protecting groups. The TDF prodrug was electrospun in a 20:80 PCL:PLGA blend and the release kinetics were compared to TFV. TDF release was only carried out to 6 hours because the protecting groups begin to hydrolyze in solution at later time points. Figure 8 shows that within 6 hours ~100% TDF is released compared to only ~20% release of TFV. TFV and TDF are structurally identical except for the presence of the methyl isopropyl carbonate protecting groups on the prodrug. These data suggest that the presence of hydrogen donating groups on the phosphonic acid of TFV may be important in stabilizing and inhibiting its release from polyester fibers. Direct evidence implicating hydrogen bonding between the phosphonic acid of TFV and the polyester backbone would need to be tested with specific adenosine analogs that only varied this functional group. The comparison of TFV and TDF release kinetics demonstrate a possible explanation for difference in release seen with TFV and the other small hydrophilic antiretroviral drugs evaluated in our study.
Figure 8.
In vitro release profiles of adenosine analogs in electrospun PCL/PLGA 20:80 fibers over 10 days (D-PBS sink conditions, at 37°C). (a)(Open circles) 15 wt% tenofovir disoproxil fumarate, (b) (Filled circles) 15 wt% tenofovir. Results suggest that hydrogen bonding from the hydroxyl groups on the phosphonic group of TFV contribute to the programmable release of TFV from PCL/PLGA fibers. Dashed line extends 6 hour time point of release for TDF to highlight level of release (no data taken).
In vitro cytotoxicity and viral activity of PCL/PLGA electrospun fibers
TFV-loaded PCL/PLGA were tested for cytotoxicity and antiviral activity in vitro using TZM-bL cells. Four PCL/PLGA blends were used for testing: 100:0 PCL:PLGA, 80:20 PCL:PLGA, 20:80 PCL:PLGA, and 0:100 PCL:PLGA. Cell viability remained at approximately 100% for all tested polymer concentrations indicating that the biodegradable polymers used for the electrospun fiber platform are not cytotoxic to cells in vitro (Supplemental Figure 2). Viral activity was assessed to determine the ability of PCL/PLGA fibers to sustain release of TFV and inhibit HIV. IC50 values at each time point were used to calculate percent release and then compared to conventional dissolution of TFV in vitro (Supplemental Figure 3). The release of TFV from each of the PCL/PLGA blends, as calculated from viral inhibition, followed expected release as analyzed by HPLC. The result supports the programmable release of TFV from PCL/PLGA fibers and also indicates that TFV retains it bioactivity throughout the electrospinning process.
Conclusions
A programmable controlled release platform for TFV was developed using PCL/PLGA electrospun nanofibers. By varying the ratio of PCL and PLGA in the system it was possible to achieve burst release within 24 hours (PCL) or sustain release up to and past 10 days (PLGA). The ability to modulate the release of a small hydrophilic antiretroviral drug from hydrophobic polymers represents an important stride forward when considering sustained release drug delivery systems. We also investigated potential factors that drive sustained release of TFV from PCL/PLGA fibers. Our data suggests that fiber mesh hydration and polymer mass loss do not significantly contribute to the programmable release profile over the 10 day experiment. Assessment of PCL and PLGA chain mobility showed that at physiologically relevant temperatures, PCL chains were mobile leading compared to PLGA polymer chains. Finally, we determined sustained release of TFV could be influenced by interactions between functional groups on TFV and the polyester backbone. Furthermore, the developed meshes have shown biocompatibility as well as sustained inhibition of HIV in vitro. It has also been shown that PCL/PLGA electrospun fibers can be used to modulate the release of other antiretroviral drugs, although over a narrower range of blends. These results indicate our uniaxial electrospun fiber system is able to overcome limitations associated with sustained delivery of small hydrophilic molecules from hydrophobic materials, which could benefit numerous biomedical applications in the field of drug delivery.
Supplementary Material
Acknowledgements
The authors thank the UW-NNIN for assistance with SEM imaging, and T. Kuykendall (UW MSE) for assistance with DSC. Raltegravir and maraviroc samples were purified from their pharmaceutical formulations by M. Ebner, A. Bever and I. Suydam (Seattle University). This work was supported by grants to K.A.W. from the Bill and Melinda Gates Foundation (OPP11110945) and the National Institutes of Health (AI098648, AI112002).
Abbreviations
- SN-38
active metabolite of irinotecan
- PLGA
poly(lactic-co-glycolic) acid
- PCL
poly-caprolactone
- PLA
poly-lactic acid
- TFV
tenofovir
- RAL
raltegravir
- MVC
maraviroc
- AZT
azidothymidine
- ARV
antiretroviral
- TDF
tenofovir disoproxil fumarate
- Mt
amount of drug release at time (t)
- Mtotal
amount of total drug release from the sample
- k
power law slope parameter
- n
power law expression, release mechanism
- %CH2O
Percent change in water content
- Mpre
sample mass prior to analysis
- Mwet
wet sample mass
- Mdry
sample mass after drying
- %MLpolymer
polymer mass loss percentage
- DLtotal
total amount of drug loss
- HPLC
high performance liquid chromatography
- SEM
scanning electron microscopy
- DSC
differential scanning calorimetry
- DMSO
dimethyl sulfoxide
- HFIP
hexafluoroisopropanol
- ACN
acetonitrile
- TFA
trifluoroacetic acid
- DPBS
Dulbecco's phosphate buffered saline
- DMEM
Dulbecco's Modified Eagle's Medium
- FBS
fetal bovine serum
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- Tg
glass transition temperature
- Tm
melting temperature
- TZM-bL
HeLa cell line
- PM-1
T cell line
- CEMx174
T cell line
- TCID50
median tissue culture infective does
- IC50
half maximal inhibition concentration
- RLU
relative luminescence unit
References
- 1.Agarwal S, Wendorff JH, Greiner A. Use of electrospinning technique for biomedical applications. Polymer. 2008;49(26):5603–5621. [Google Scholar]
- 2.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J Biomed Mater Res, Part A. 2002;60(4):613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 3.Khil MS, Cha DI, Kim HY, Kim IS, Bhattarai N. Electrospun nanofibrous polyurethane membrane as wound dressing. J Biomed Mater Res, Part B. 2003;67B(2):675–679. doi: 10.1002/jbm.b.10058. [DOI] [PubMed] [Google Scholar]
- 4.Zeng J, Xu X, Chen X, Liang Q, Bian X, Yang L, et al. Biodegradable electrospun fibers for drug delivery. J Controlled Release. 2003;92(3):227–231. doi: 10.1016/s0168-3659(03)00372-9. [DOI] [PubMed] [Google Scholar]
- 5.Blakney AK, Krogstad EA, Jiang YH, Woodrow KA. Delivery of multipurpose prevention drug combinations from electrospun nanofibers using composite microarchitectures. Int J Nanomedicine. 2014;9:2967–2978. doi: 10.2147/IJN.S61664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ball C, Woodrow KA. Electrospun solid dispersions of maraviroc for rapid intravaginal preexposure prophylaxis of HIV. Antimicrob Agents Chemother. 2014;58(8):4855–4865. doi: 10.1128/AAC.02564-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang YZ, Venugopal J, Huang ZM, Lim CT, Ramakrishna S. Characterization of the surface biocompatibility of the electrospun PCL-Collagen nanofibers using fibroblasts. Biomacromolecules. 2005;6(5):2583–2589. doi: 10.1021/bm050314k. [DOI] [PubMed] [Google Scholar]
- 8.Gu SY, Wu QL, Ren J, Vancso GJ. Mechanical properties of a single electrospun fiber and its structures. Macromol Rapid Commun. 2005;26:716–720. [Google Scholar]
- 9.Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. Int J Nanomedicine. 2006;1(1):15–30. doi: 10.2147/nano.2006.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goh YF, Shakir I, Hussain R. Electrospun fibers for tissue engineering, drug delivery, and wound dressing. J Mater Sci. 2013;48(8):3027–3054. [Google Scholar]
- 11.Huang C, Soenen SJ, Gulck EV, Vanham G, Rejman J, Calenbergh SV, et al. Electrospun cellulose acetate phthalate fibers for semen induced anti-HIV vaginal drug delivery. Biomaterials. 2012;33(3):962–969. doi: 10.1016/j.biomaterials.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Akil A, Parniak MA, Dezzuitti CS, Moncla BJ, Cost MR, Li M, et al. Development and characterization of a vaginal film containing dapivirine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv Transl Res. 2011;1(3):209–222. doi: 10.1007/s13346-011-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson TJ, Gupta KM, Fabian J, Albright TH, Kiser PF. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur J Pharm Sci. 2010;39(4):203–212. doi: 10.1016/j.ejps.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Karim QA, Karim SSA, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damme LV, Corneli A, Ahmed K, Kawango A, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. New Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chew SY, Wen J, Yim EKF, Leong KW. Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules. 2005;6(4):2017–2024. doi: 10.1021/bm0501149. [DOI] [PubMed] [Google Scholar]
- 17.Xie J, Wang CH. Electrospun Micro- and nanofibers for sustained delivery of paclitaxel to treat C6 glioma in vitro. Pharm Res. 2006;23(8):1817–1825. doi: 10.1007/s11095-006-9036-z. [DOI] [PubMed] [Google Scholar]
- 18.Yohe ST, Colson YL, Grinstaff MW. Superhydrophobic materials for tunable drug release: using displacement of air to control delivery rates. J Am Chem Soc. 2012;134(4):2016–2019. doi: 10.1021/ja211148a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng J, Yang L, Liang Q, Zhang X, Guan H, Xu X, et al. Influence of the drug compatibility with polymer solution on the release kinetics of electropun fiber formulation. J Controlled Release. 2005;105(1-2):43–51. doi: 10.1016/j.jconrel.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Sohrabi A, Shaibani PM, Etayash H, Kaur K, Thundat T. Sustained drug release and antibacterial activity of ampicillin incorporated poly(methyl methacrylate). Polymer. 2013;54(11):2699–2705. [Google Scholar]
- 21.Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, et al. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J Controlled Release. 2004;98(1):47–56. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Hsu YH, Chen DW, Tai CD, Chou YC, Liu SJ, Ueng SW, et al. Biodegradable drug-eluting nanofiber-enveloping implants for sustained release of high bactericidal concentrations of vancomycin and ceftazidime: in vitro and in vivo studies. Int J Nanomedicine. 2014;9:4347–4355. doi: 10.2147/IJN.S66526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valarezo E, Tammaro L, Malagon O, Gonzalez S, Armijos C, Vittoria V. Fabrication and characterization of Poly(lactic acid)/Poly(e-caprolactone) blend electrospun fibers loaded with amoxicillin for tunable delivering. J Nanosci Nanotechnol. 2014;14:1–7. doi: 10.1166/jnn.2015.9726. [DOI] [PubMed] [Google Scholar]
- 24.Reise M, Wyrwa R, Muller U, Zylinski M, Volpel A, Schnabelrauch M, et al. Release of metronidazole from electrospun poly(L-lactide-co-D/L-lactide) fibers for local periodontitis treatment. Dent Mater. 2012;28(2):179–188. doi: 10.1016/j.dental.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel) 2011;3(3):1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fredenber S, Wahlgren M, Reslow M, Axelsson A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems-A review. Int J Pharm. 2011;415(1-2):34–52. doi: 10.1016/j.ijpharm.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 27.McDonald PF, Lyons JG, Geever LM Higginbotham CL. In vitro degradation and drug release from polymer blends based on poly(DL-lactide), poly(L-lactide-glycolide) and poly(ecaprolactone). J Mater Sci. 2010;45(5):1284–1292. [Google Scholar]
- 28.Lao L, Venkatraman S, Peppas N. Modeling of drug release from biodegradable polymer blends. Eur J Pharm Biopharm. 2008;70(3):796–803. doi: 10.1016/j.ejpb.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 29.Cui W, Li X, Zhu X, Yu G, Zhou S, Weng J. Investigation of drug release and matrix degradation of electrospun poly(DL-lactide) fibers with paracetanol inoculation. Biomacromolecules. 2006;7(5):1623–1629. doi: 10.1021/bm060057z. [DOI] [PubMed] [Google Scholar]
- 30.Langer RS, Wiser DL, et al. Medical Applications of Controlled Release. CRC Press, Inc.; Boca Raton: 1984. [Google Scholar]
- 31.Natu MV, de Sousa HC, Gil MH. Effects of drug solubility, state and loading on controlled release in bicomponent electrospun fibers. Int J Pharm. 2010;397(1-2):50–58. doi: 10.1016/j.ijpharm.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 32.Clark JT, Johnson TJ, Clark MR Nebeker JS, Fabian J, Tuitupou AL, et al. Quantitative evaluation of a hydrophilic matrix intravaginal ring for the sustained delivery of tenofovir. J Controlled Release. 2012;163(2):240–248. doi: 10.1016/j.jconrel.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 33.Labet M, Thielemans W. Synthesis of polycaprolactone: a review. Chem Soc Rev. 2009;38(12):3484–3504. doi: 10.1039/b820162p. [DOI] [PubMed] [Google Scholar]
- 34.Blasi P, Schoubben A, Giovagnoli S, Perioli L, Ricci M, Rossi C. Ketoprofen poly(lactideco-glycolide) physical interaction. AAPS PharmSciTech. 2007;8(2):E78–E85. doi: 10.1208/pt0802037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu Y, Kao W. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin Drug Deliv. 2010;7(4):429–444. doi: 10.1517/17425241003602259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyu S, Sparer R, Hobot C, Dang K. Adjusting drug diffusivity using miscible polymer blends. J Controlled Release. 2005;102(3):679–687. doi: 10.1016/j.jconrel.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Peppas NA, Brannon-Peppas L. Water diffusion sorption in amorphous macromolecular systems and foods. J Food Eng. 1994;22(1-4):189–210. [Google Scholar]
- 38.Tan LP, Hidayat A, Lao LL, Quah LF. Release of hydrophilic drug from biodegradable polymer blends. J Biomater Sci, Polym Ed. 2009;20(10):1381–1392. doi: 10.1163/092050609X12457418874260. [DOI] [PubMed] [Google Scholar]
- 39.Ball C, Krogstad EA, Chaowanachan T, Woodrow KA. Drug-eluting fibers for HIV-1 inhibition and contraception. PLoS One. 2012;7(11):1–14. doi: 10.1371/journal.pone.0049792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu D, Branford-White C, White K, Li XL, Zhu LM. Dissolution improvement of electrospun nanofiber-based solid dispersion for acetaminophen. AAPS PharmSciTech. 2010;11(2):809–817. doi: 10.1208/s12249-010-9438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen SC, Huang XB, Cai XM, Lu J, Yuan J, Shen J. The influence of fiber diameter of electrospun poly(lactic acid) on drug delivery. Fib Polym. 2012;13(9):1120–1125. [Google Scholar]
- 42.You Y, Youk JH, Lee SW, Byung-Moo M, Lee SJ, Park WH. Preparation of porous ultrafine PGA fibers via selective dissolution of electrospun PGA/PLA blend fibers. Mater Lett. 2006;60(6):757–760. [Google Scholar]
- 43.Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Del Rev. 2001;48(2-3):139–157. doi: 10.1016/s0169-409x(01)00112-0. [DOI] [PubMed] [Google Scholar]
- 44.Ritger PL, Peppas NA. A simple equation for the description of solute release I. fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Controlled Release. 1987;5(1):23–36. [PubMed] [Google Scholar]
- 45.Krogstad EA, Woodrow KA. Manufacturing scale-up of electrospun poly(vinyl alcohol) fibers containing tenofovir for vaginal delivery. Int J Pharm. 2014;475(1-2):282–291. doi: 10.1016/j.ijpharm.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Notari S, Tommasi C, Nicastri E, Bellagamba R, Tempestilli M, Pucillo LP, et al. Simultaneous determination of maraviroc and raltegravir in human plasma by HPLC-UV. IUBMB Life. 2009;61(4):470–475. doi: 10.1002/iub.181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.