Abstract
Precision medicine aims to individualize care by understanding differences in genetics, lifestyle, and environment. Pharmacogenomics and cancer genetics represent two promising areas for this approach. Pharmacogenomic tests have the potential to direct drug prescribing to increase safety and effectiveness because individuals vary on a genetic basis in their response to many drugs. Similarly, tests to identify people with an inherited cancer risk can guide prevention. For both, a few tests have entered clinical practice and more are under development. Implementation challenges include the limited evidence base available to guide clinical use and the lack of data from diverse populations. Accordingly, ongoing research should prioritize procedures that enhance the trustworthiness of clinical practice guidelines and create decision support for clinicians and patients that address their needs and accommodate flexibility. Each step involves choices with ethical implications.
Keywords: precision medicine, pharmacogenomics, cancer genetics, implementation science, clinical decision support
Precision medicine applications in pharmacogenomics and cancer prevention
The concept of precision medicine (see Glossary), defined as the use of genomics, lifestyle and environmental data to individualize health care, offers an unprecedented opportunity to improve clinical care [1, 2]. Before this approach can be implemented, however, evidence is needed to establish the benefits and potential risks of specific precision medicine interventions, and strategies must be developed to ensure effective uptake in health care settings. The steps taken to accomplish these goals involve choices with ethical implications. In this review, we use two prominent examples of precision medicine—pharmacogenomic tests to guide safe and effective use of drug therapy and tests for inherited cancer risk that can guide prevention—to explore the ethical challenges in the implementation of precision medicine.
Pharmacogenomics (PGX) represents one of the most promising healthcare applications to emerge from the Human Genome Project. Individuals vary in their response to many drugs and in their likelihood of developing adverse side effects. This interindividual variation can have important consequences in health care: in the case of drug nonresponders, effective treatment is delayed and patients are exposed to unnecessary treatment costs and risks; with regard to adverse effects, some individuals suffer serious and even life-threatening complications. Current research suggests that for many drugs a large proportion of interindividual variation in drug response is due to population variation in genes associated with drug uptake, distribution, target levels and intrinsic function, metabolism, and excretion [3]. Although robust evidence of clinical utility is currently available for only a few pharmacogenomic tests [4], active investigation of gene-drug interactions is underway, with the prospect of increasing numbers of PGX tests available to clinicians.
Testing for inherited cancer risk offers another important benefit of genomic research. Although the etiology of most cancer is multifactorial, several inherited cancer syndromes have been identified that result in high lifetime risks for specific cancers. Examples include hereditary breast ovarian cancer syndrome, conferring risk of breast and ovarian cancer [5], and Lynch Syndrome [6], conferring risk of colorectal cancer. Discovery of the genes associated with these cancer syndromes has enabled genetic testing to identify individuals at risk, who can then be offered a range of preventive interventions [7–9]. As with PGX, active on-going research is identifying additional genes associated with cancer risk, which will increase opportunities for this testing approach over time.
Unfortunately, the potential benefits of these scientific discoveries do not always reach the patients who need them. A recent workshop summary estimated that only 6% of eligible patients will benefit from a well-evidenced genomic test that identifies a treatable condition, because of failure to implement clinical testing in a consistent manner [10]. An emphasis on implementation science [11–13] is thus essential if the benefits of precision medicine are to be realized. Ethical challenges to achieving this goal fall into four categories: evidence gaps, clinician decision-making, patient needs, and health care disparities.
Addressing evidence limitations
What evidence is sufficient to introduce a new precision medicine test?
The evidence available to support a particular clinical innovation is rarely definitive. Even in the case of a large-scale clinical trial with positive results, clinicians and health care systems need to consider the degree to which the study population corresponds to the patients they serve. But in fact, relatively few new interventions are tested in clinical trials, and this approach is rarely utilized for new diagnostic tests. An exception is HLA-B*57:01 testing to prevent abacavir hypersensitivity reactions in patients taking the antiretroviral drug. In this case, a clinical trial was conducted that demonstrated the positive and negative predictive values (48% and 100%, respectively) of the test in patients considered for abacavir therapy in the treatment of HIV and prompted rapid uptake of HLA testing prior to abacavir use [14]. Yet lesser quality evidence, including observational data and mechanistic studies, may provide compelling data for benefit of a new technology such as PGX.
The first ethical challenge in the implementation of precision medicine is thus to determine when evidence has reached a sufficient level of certainty to warrant clinical introduction. In considering the available evidence, relevant factors include the scope of estimated benefit, existence of alternative treatments, nature and scope of potential harms, and the overall quality of evidence [15, 16]. As an example, TPMT testing to identify individuals who may have adverse hematological reactions to thiopurines has never been prospectively tested in a controlled trial. Yet the evidence for benefit is compelling: retrospective analysis of leukemia clinical trials demonstrated that TPMT genotype was a significant predictor of life-threatening hematological complications [17, 18]. This evidence has been considered sufficient to establish TPMT testing protocols for childhood leukemia treatment, limiting thiopurine doses for those with the risk genotype. Conversely CYP2C9 testing to identify individuals who require lower doses of warfarin remains controversial despite rigorous clinical trial data, because trial results have been conflicting as to whether testing provides a clinical benefit [19, 20]. A credible explanation for the discrepant findings has been offered (differences in ethnic composition and gene variation of the study populations) [21], but this hypothesis remains untested.
The ethical challenge in this context is to assure that judgments about the available evidence are made in a considered and appropriate manner, so that the resulting clinical practice guidelines can be justified. Key elements include assuring that evidence is collected and reviewed in a systematic and reproducible way; that the strength of clinical recommendations is tied to the level of evidence supporting them; and that the process includes full consideration of the views and preferences of all stakeholders. An Institute of Medicine report outlines the characteristics of trustworthy clinical practice guidelines, emphasizing these procedural issues, as well the importance of avoiding conflicts of interest among members of the guidelines committee [22].
Evaluation of evidence for clinical practice guidelines should take into account the context in which an intervention will be used. A number of system and patient variables may influence the utility and appropriate deployment of a test. For example, in the case of cancer genetics, the availability of screening services or other preventive measures will play a central role in the clinical utility of testing. Other factors such as health literacy (including, increasingly, proficiency in the use of information technology) and social support network quality may be critical to achieving desired clinical objectives, and may thus be important to address in a practice guideline.
Taking action when evidence is limited
If a health system or guidelines committee identifies a new test with high potential for benefit, yet with only limited evidence to support its use, a possible consideration would be the introduction of the test with a commitment to gather on-going evidence, e.g., to undertake a practical clinical trial (in which the test is introduced in some clinical settings while usual care is offered in other comparable settings, to allow a comparison of outcomes). This type of effectiveness-implementation study, recently described in a National Academy of Medicine workshop summary [23], may be an appropriate way to introduce promising new precision medicine tests, allowing innovation with a relatively low evidentiary threshold—but how low is too low? Clinicians and policymakers will need to weigh the potential benefit of the intervention against the possibility of unanticipated adverse outcomes. Hybrid approaches that study both effectiveness of the intervention and the process of implementation hold promise for the delivery of precision medicine, leveraging both implementation science and learning health care system approaches [24–26]. These approaches raise further questions about informed consent [27–30] and plans for de-implementation if the intervention performs poorly [31].
Supporting clinical decision-making
As new tests enter clinical practice, appropriate support of clinician and patient choice is an ethical concern. Rarely is there a single right way to use a test, and appropriate use of any intervention must be individualized to the patient’s circumstances, including co-morbidities and social factors contributing to quality of life. Given the evidence gaps that are common when tests are introduced into practice, clinicians and patients also need to consider what uncertainties they are most comfortable with and how those judgments should determine use of a new precision medicine intervention. Clinicians need information about the test, ideally in readily accessible formats that clarify the strength of the evidence supporting its use, potential harms, and alternatives. Implementation should also offer clinicians guidance to ensure that patients are making informed decisions based on the best evidence available—including recognition that the best evidence available may have significant gaps. This approach supports shared decision-making between clinician and patient.
An important development for supporting clinical innovation is the use of clinical decision support tools within the electronic health record (EHR). While offering support to clinicians, and potentially to patients, these tools also raise questions about the range of choices available and how they are constructed.
Examples of decision support tools currently being tested are Vanderbilt University’s PREDICT project, which aims to incorporate pharmacogenomic information into decision support deployed through their medical center’s EHR platform [32] and the DIGITizE project, which has created a template of decision support rules for patients with variants that affect their sensitivity to abacavir and azathioprine (http://www.nationalacademies.org/hmd/Activities/Research/GenomicBasedResearch/Innovation-Collaboratives/EHR.aspx). Both projects work with guidelines that were, at least partially, developed without the distinct choices of “electronic” translation in mind. For example, clinical practice guidelines should provide disambiguated guidance (“early onset” breast cancer should instead be “diagnosed at age 50 or earlier”), so that triggers can be deployed appropriately [33]. However, guidelines creators should also consider where electronic decision support should accommodate shared decision-making between patient and physician rather than force an artificially discrete choice. (For example, actions triggered by the tool might include the option for clinician overrides based on patient co-morbidities or preferences.) These technical considerations need to be specific so that adherence can be measured where appropriate but general enough that they can be adapted by diverse EHR systems. Failure to create clinical practice guidelines with a broader, and electronic, implementation view in mind may mean that smaller clinics, community-based clinics, and clinics in rural locales may not be able to incorporate the guidelines or decision support into their EHR systems. Because these clinics often serve populations more diverse than large academic medical centers, such a lacuna has the possibility of excluding those populations from the health benefits of genomic medicine.
Patient needs
Enhancing patient understanding of precision medicine and the intervention proposed
Patients are likely to need education on precision medicine options and alternatives so that they can make informed choices not only about a precision medicine test but also about the potential results and follow-up recommendations. Educational materials would likely need to cover the ideas behind precision medicine, as well as a realistic presentation of its patient impact, in addition to the more specific information about the particular precision medicine intervention under immediate consideration by the patient and clinician. These materials are likely to require adaptation to different patient audiences. For example, patients may vary in their awareness and acceptance of cancer prevention or other measures recommended as a result of a precision medicine test. Information aids will likely need to be developed for different media and using a range of examples and illustrations, so that they can be tailored to specific patient needs, including health literacy levels and acceptable formats. Failure to do so may inadvertently result in precision medicine bypassing population groups.
Supporting family communication and outreach
An additional issue arises when a precision medicine test identifies a risk that is shared among family members. For example, when Lynch Syndrome is diagnosed in a patient with colorectal cancer, the diagnosis has implications for the patient’s risk for recurrent cancer, but it also offers an important prevention opportunity for the patient’s family members [6]. First degree relatives have a 50% chance of inheriting the condition, and can benefit from preventive colon screening if they are aware of their risk. Clinicians and health care systems have an ethical duty to inform patients of this family risk, and to counsel them about contacting family members to inform them of the benefits of testing. How far this duty extends, however, is a matter of debate. Arguably the duty is assumed by the patient once he or she has been counseled about the importance of informing family members. Yet many patients are unlikely to be able to discharge this duty without assistance from health care systems.
Addressing health care disparities
An important consideration for precision medicine testing is that new tests may exacerbate health care disparities. Most genetic data are drawn from individuals of northern European ancestry [34, 35]. We know that the prevalence of genetic variants varies across populations, and in at least some cases, this provides an explanation for differences in drug response observed across populations [36–38].
The implications of this evidence deficit are significant—e.g., some have suggested that genotype-based warfarin prescribing should be considered only for European-ancestry patients due to lack of evidence in other populations [39]. African-Americans are already under-treated for atrial fibrillation and are at a higher risk of stroke. That coupled with research that focuses on white populations has led to a “treatment disparity” [40, 41]. A similar problem related to lack of evidence from diverse populations has to do with the likelihood of indeterminate results in cancer genetics. In the case of testing for hereditary breast and ovarian cancer, for example, minority women are more likely than women of European ancestry to receive a test result that indicates a “variant of unknown clinical significance (VUS)” [42]. This outcome reflects the fact that variants in the BRCA1 and BRCA2 genes are frequent, and when a new variant is identified it may be difficult to characterize as pathogenic versus normal, benign variation. Less research in non-white populations makes this result more common. To achieve more equitable benefits from precision medicine, research addressing this evidence gap should be prioritized.
Research on how to implement clinical innovation in small or rural clinics and hospitals is also crucial to establish access to the benefits of precision medicine for everyone. But it is easier to conduct research and implementation in academic medical centers and large health systems in which processes already in place may serve as templates for genomic medicine. By contrast, implementing genomic medicine in resource-poor or geographically remote clinics may require significant tailoring of the intervention for successful deployment, the development of new outcome measures, and the provision of any resources deemed “must haves” (e.g., access to genetic expertise, perhaps via telemedicine).
Concluding Remarks
The translation of pharmacogenomics and cancer susceptibility testing from bench to bedside involves choices along the way, each with ethical ramifications ranging from equitable distribution of the research supporting precision medicine and the benefits accruing from its clinical implementation to providing information to support clinician-patient shared decision-making (see Outstanding Questions). Because evidence is still evolving and is often limited, policymakers need to consider how and when to introduce precision medicine tests. Implementing promising new tests in a timely fashion may be beneficial if they are tied to the collection of data that reduce uncertainties about test outcomes over time. However, these approaches must anticipate that some new tests will fail to live up to expectations, requiring de-implementation. Attention to system and stakeholder needs is also important, including decision tools for providers and patients and implementation plans that take local capacities into account. These issues require ongoing consideration during intervention creation, implementation, and outcomes measurement—all undertaken while also looking for opportunities to improve process and health outcomes for all patients, including those who are historically underserved.
Outstanding Questions Box.
If a precision medicine test with limited evidence is used clinically as part of a study intended to determine its benefits, how and when should patients be informed? How should the health system prepare for the possibility that emerging data may fail to support the new test?
When should a PGX or cancer genetics test be considered standard of care? Should some tests be considered optional, with enough evidence to consider but reasons a patient might refuse?
How can health systems and third-party payers better support test availability and patient and clinician choice?
If testing is covered by insurance, how can health systems better provide follow-up interventions (e.g., increased monitoring), to ensure benefits of testing?
How should health systems assist patients to inform family members about shared genetic risks?
What measures should be taken to avoid disparities in access to precision medicine?
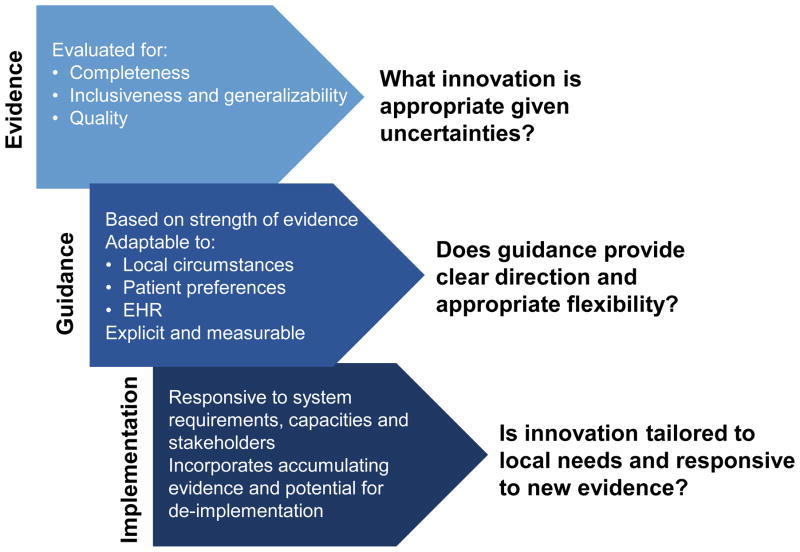
Figure 1.
Ethical Implications in Implementation
Ethical concerns in implementation: key factors impacting guidance creation through deployment.
Abbreviations used: Electronic Health Record (EHR)
Trends Box.
Pharmacogenomics and tests for inherited cancer risk are two promising areas of precision medicine.
Some genetic tests have entered clinical practice: TPMT and HLA tests to identify individuals at risk for adverse drug response to thiopurines and abacavir, respectively; BRCA1/2 and Lynch Syndrome tests to identify increased risk for specific cancers; and tests to diagnose rare genetic conditions. More tests are under development.
The evidence base for most genetic tests is limited and lacks data from diverse populations.
Trustworthy clinical practice guidelines require systematic evidence review; consideration of all stakeholder views; attention to conflicts of interest; and alignment of the strength of recommendations with the level of evidence available.
Promising new approaches to health care such as precision medicine have the potential to exacerbate health care disparities.
Acknowledgments
This work was supported by the National Human Genome Research Institute and the National Institute of General Medical Sciences of the National Institutes of Health under award numbers K01HG008180, P50 HG003374 and U01GM092676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary Box
- Implementation science
Implementation science (IS) focuses on the way individuals identify and overcome barriers associated with implementing new interventions. The Consolidated Framework for Implementation Research (CFIR), developed by health services researchers at the Veterans Administration, is an IS model that offers a way to identify what works as well as where and why it works in a manner that is transferable across health disciplines.
- Lynch Syndrome
Lynch Syndrome (LS) is an autosomal dominant, highly penetrant condition that confers a high lifetime risk of colorectal cancer (~80%), the third most common cancer in the United States; 2–4% of colorectal cancers can be attributed to LS. The Evaluation of Genomic Applications in Practice and Prevention working group has recommended universal screening of colorectal cancer tumors for LS since 2009.
- Pharmacogenomics
Pharmacogenomics (PGX) refers to the development of genetic tests to identify gene variants associated with drug response and the likelihood of adverse events.
- Precision medicine
Precision medicine (PM) represents an approach to individualizing health care based on an understanding of the genetic, lifestyle, and environmental factors contributing to a particular person’s health and health risks. A Precision Medicine Initiative launched by the National Institutes of Health will assemble a large research cohort to pursue research to develop PM interventions.
- Variant of unknown clinical significance
A variant of unknown clinical significance (VUS) refers to a gene variant that cannot be characterized as either pathogenic or benign. Many variants identified by current DNA sequencing techniques fall into this category. If a VUS is observed in multiple families, observation over time may clarify its clinical relevance. For rare VUS, resolution may be difficult.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Diane M Korngiebel, Dept. of Biomedical Informatics and Medical Education, University of Washington.
Kenneth E Thummel, Dept. of Pharmaceutics, University of Washington.
Wylie Burke, Dept. of Bioethics and Humanities, University of Washington.
References
- 1.Collins FS, Varmus H. A new initiative on precision medicine. The New England journal of medicine. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashley EA. The precision medicine initiative: a new national effort. Jama. 2015;313:2119–2120. doi: 10.1001/jama.2015.3595. [DOI] [PubMed] [Google Scholar]
- 3.Evans WE. Pharmacogenomics: marshalling the human genome to individualise drug therapy. Gut. 2003;52(Suppl 2):ii10–18. doi: 10.1136/gut.52.suppl_2.ii10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang B, et al. Clinical evidence supporting pharmacogenomic biomarker testing provided in US Food and Drug Administration drug labels. JAMA internal medicine. 2014;174:1938–1944. doi: 10.1001/jamainternmed.2014.5266. [DOI] [PubMed] [Google Scholar]
- 5.Desmond A, et al. Clinical Actionability of Multigene Panel Testing for Hereditary Breast and Ovarian Cancer Risk Assessment. JAMA oncology. 2015;1:943–951. doi: 10.1001/jamaoncol.2015.2690. [DOI] [PubMed] [Google Scholar]
- 6.Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genetics in medicine : official journal of the American College of Medical Genetics. 2009;11:35–41. doi: 10.1097/GIM.0b013e31818fa2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen SA, Leininger A. The genetic basis of Lynch syndrome and its implications for clinical practice and risk management. The application of clinical genetics. 2014;7:147–158. doi: 10.2147/TACG.S51483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall MJ, et al. Genetic testing for hereditary cancer predisposition: BRCA1/2, Lynch syndrome, and beyond. Gynecologic oncology. 2016;140:565–574. doi: 10.1016/j.ygyno.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pal T, Vadaparampil ST. Genetic risk assessments in individuals at high risk for inherited breast cancer in the breast oncology care setting. Cancer control : journal of the Moffitt Cancer Center. 2012;19:255–266. doi: 10.1177/107327481201900402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Applying an Implementation Science Approach to Genomic Medicine: Workshop Summary. National Academy of Sciences; 2016. [PubMed] [Google Scholar]
- 11.Fisher ES, et al. Implementation Science: A Potential Catalyst for Delivery System Reform. Jama. 2016;315:339–340. doi: 10.1001/jama.2015.17949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damschroder LJ, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implementation science : IS. 2009;4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirk MA, et al. A systematic review of the use of the Consolidated Framework for Implementation Research. Implementation science : IS. 2016;11:72. doi: 10.1186/s13012-016-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallal S, et al. HLA-B*5701 screening for hypersensitivity to abacavir. The New England journal of medicine. 2008;358:568–579. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 15.Gallego CJ, et al. Next-Generation Sequencing Panels for the Diagnosis of Colorectal Cancer and Polyposis Syndromes: A Cost-Effectiveness Analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:2084–2091. doi: 10.1200/JCO.2014.59.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basu A, et al. A Framework for Prioritizing Research Investments in Precision Medicine. Medical decision making : an international journal of the Society for Medical Decision Making. 2016;36:567–580. doi: 10.1177/0272989X15610780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Relling MV, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clinical pharmacology and therapeutics. 2011;89:387–391. doi: 10.1038/clpt.2010.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Relling MV, et al. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clinical pharmacology and therapeutics. 2013;93:324–325. doi: 10.1038/clpt.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimmel SE, et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. The New England journal of medicine. 2013;369:2283–2293. doi: 10.1056/NEJMoa1310669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pirmohamed M, et al. A randomized trial of genotype-guided dosing of warfarin. The New England journal of medicine. 2013;369:2294–2303. doi: 10.1056/NEJMoa1311386. [DOI] [PubMed] [Google Scholar]
- 21.Duconge J, et al. Why admixture matters in genetically-guided therapy: missed targets in the COAG and EU-PACT trials. Puerto Rico health sciences journal. 2015;34:175–177. [PMC free article] [PubMed] [Google Scholar]
- 22.Graham R, et al., editors. Clinical Practice Guidelines We Can Trust. 2011. 2011 by the National Academy of Sciences. [PubMed] [Google Scholar]
- 23.Applying an Implementation Science Approach to Genomic Medicine: Workshop Summary. 2016. [PubMed] [Google Scholar]
- 24.Chambers DA, et al. Convergence of Implementation Science, Precision Medicine, and the Learning Health Care System: A New Model for Biomedical Research. Jama. 2016;315:1941–1942. doi: 10.1001/jama.2016.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aronson SJ, Rehm HL. Building the foundation for genomics in precision medicine. Nature. 2015;526:336–342. doi: 10.1038/nature15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curran GM, et al. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Medical care. 2012;50:217–226. doi: 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiscella K, et al. Ethical oversight in quality improvement and quality improvement research: new approaches to promote a learning health care system. BMC medical ethics. 2015;16:63. doi: 10.1186/s12910-015-0056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grady C, Wendler D. Making the transition to a learning health care system. Commentary. The Hastings Center report. 2013;(Spec No):S32–33. doi: 10.1002/hast.137. [DOI] [PubMed] [Google Scholar]
- 29.Faden RR, et al. An ethics framework for a learning health care system: a departure from traditional research ethics and clinical ethics. The Hastings Center report. 2013;(Spec No):S16–27. doi: 10.1002/hast.134. [DOI] [PubMed] [Google Scholar]
- 30.Kelley M, et al. Patient Perspectives on the Learning Health System: The Importance of Trust and Shared Decision Making. The American journal of bioethics : AJOB. 2015;15:4–17. doi: 10.1080/15265161.2015.1062163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasad V, Ioannidis JP. Evidence-based de-implementation for contradicted, unproven, and aspiring healthcare practices. Implementation science : IS. 2014;9:1. doi: 10.1186/1748-5908-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pulley JM, et al. Operational implementation of prospective genotyping for personalized medicine: the design of the Vanderbilt PREDICT project. Clinical pharmacology and therapeutics. 2012;92:87–95. doi: 10.1038/clpt.2011.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biondich PG, et al. Collaboration between the medical informatics community and guideline authors: fostering HIT standard development that matters. AMIA ... Annual Symposium proceedings / AMIA Symposium. AMIA Symposium; 2006. pp. 36–40. [PMC free article] [PubMed] [Google Scholar]
- 34.Knerr S, et al. Inclusion of racial and ethnic minorities in genetic research: advance the spirit by changing the rules? The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2011;39:502–512. doi: 10.1111/j.1748-720X.2011.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perera MA, et al. Warfarin pharmacogenetics: an illustration of the importance of studies in minority populations. Clinical pharmacology and therapeutics. 2014;95:242–244. doi: 10.1038/clpt.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan SL, et al. Genetic diversity of variants involved in drug response and metabolism in Sri Lankan populations: implications for clinical implementation of pharmacogenomics. Pharmacogenetics and genomics. 2016;26:28–39. doi: 10.1097/FPC.0000000000000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng Q, et al. Validation of warfarin pharmacogenetic algorithms in 586 Han Chinese patients. Pharmacogenomics. 2015;16:1465–1474. doi: 10.2217/pgs.15.87. [DOI] [PubMed] [Google Scholar]
- 38.Abe J, et al. Analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis using the Japanese Adverse Drug Event Report database. Journal of pharmaceutical health care and sciences. 2016;2:14. doi: 10.1186/s40780-016-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson JA, Cavallari LH. Warfarin pharmacogenetics. Trends in cardiovascular medicine. 2015;25:33–41. doi: 10.1016/j.tcm.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akinboboye O. Use of oral anticoagulants in African-American and Caucasian patients with atrial fibrillation: is there a treatment disparity? Journal of multidisciplinary healthcare. 2015;8:217–228. doi: 10.2147/JMDH.S74529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golwala H, et al. Racial/ethnic differences in atrial fibrillation symptoms, treatment patterns, and outcomes: Insights from Outcomes Registry for Better Informed Treatment for Atrial Fibrillation Registry. American heart journal. 2016;174:29–36. doi: 10.1016/j.ahj.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 42.Hall MJ, et al. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer. 2009;115:2222–2233. doi: 10.1002/cncr.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]