Abstract
Pediatric cardiac arrest (CA) often leads to poor neurologic outcomes, including deficits in learning and memory. The only approved treatment for CA is therapeutic hypothermia, although its utility in the pediatric population remains unclear. This study analyzed the effect of mild therapeutic hypothermia after CA in juvenile mice on hippocampal neuronal injury and the cellular model of learning and memory, termed long-term potentiation (LTP). Juvenile mice were subjected to cardiac arrest and cardiopulmonary resuscitation (CA/CPR) followed by normothermia (37 °C) and hypothermia (30 °C, 32 °C). Histological injury of hippocampal CA1 neurons was performed 3 days after resuscitation using hematoxylin and eosin (H&E) staining. Field excitatory post-synaptic potentials (fEPSPs) were recorded from acute hippocampal slices 7 days after CA/CPR to determine LTP. Synaptic function was impaired 7 days after CA/CPR. Mice exposed to hypothermia showed equivalent neuroprotection, but exhibited sexually dimorphic protection against ischemia-induced impairment of LTP. Hypothermia (32 °C) protects synaptic plasticity more effectively in females, with males requiring a deeper level of hypothermia (30 °C) for equivalent protection. In conclusion, male and female juvenile mice exhibit equivalent neuronal injury following CA/CPR and hypothermia protects both males and females. We made the surprising finding that juvenile mice have a sexually dimorphic response to mild therapeutic hypothermia protection of synaptic function, where males may need a deeper level of hypothermia for equivalent synaptic protection.
Keywords: hypothermia, cardiac arrest, global ischemia, pediatric, synaptic plasticity, synaptic function
Cardiac arrest (CA) is an important and tragic event that has high morbidity across all ages. It is estimated that 600,000 adults and 16,000 children per year suffer CA in the United States (Sirbaugh et al., 1999; Michiels et al., 2013), leading to significant global ischemia and neurologic injury in those who survive. For reasons that are unclear, boys are at greater risk of CA than girls (Sirbaugh et al., 1999). The sequelae of pediatric CA result in a lifetime of dependency for all aspects of care (Sirbaugh et al., 1999; Moler et al., 2011; Michiels et al., 2013). A great deal of research has focused on improving these outcomes through efforts such as neuroprotection and improved rates of return of spontaneous circulation (ROSC). To date, therapeutic hypothermia is the only therapy shown to be effective in increasing survival and improving neurologic outcome and has become the standard of care in adults and neonates less than 6 h old (Arrich et al., 2012; Jacobs et al., 2013). However, a recent study of hypothermia in children with out-of-hospital CA showed no effect on survival with good neurobehavioral outcome (Moler et al., 2015). Differences in the etiology and pathophysiology may exist among different age groups and neuroprotective strategies following CA in neonates and adults may not be generalizable to children. In fact, little experimental data exist to assess the differences in brain injury following CA in adults versus children. We recently established a juvenile model of cardiac arrest and cardiopulmonary resuscitation (CA/CPR) (Deng et al., 2014) to begin addressing these differences. We found that there is similar neuronal injury between juvenile and adult male mice and that hypothermia decreased neuronal death (Deng et al., 2014). The current study is designed to assess the impact of hypothermia on neuronal injury and synaptic functional recovery in the young brain following CA/CPR.
Despite much effort, the field of cerebral ischemia has been plagued by lack of translatable strategies, even though there has been much promise in the laboratory in delivering neuroprotection (Herson and Traystman, 2014). This highlights the importance of moving beyond focusing on neuronal protection and, in turn, identifying approaches to improve functional recovery following cerebral ischemia. The ability of neurons to undergo synaptic plasticity (long-term potentiation; LTP) is recognized as an innate measure of function and is a widely accepted cellular model for learning and memory (Bliss and Collingridge, 1993; Neves et al., 2008). Hippocampal CA1 pyramidal neurons have been well studied in synaptic plasticity and are particularly susceptible to ischemic injury (Schmidt-Kastner and Freund, 1991), making these neurons useful targets in studying functional injury after ischemia. Ischemia-induced impairment of LTP is well established in adult models of ischemia, and many have implicated the loss of N-methyl-D-aspartate (NMDA) receptor expression and function (Zhang et al., 1997; Liu et al., 2010), though this remains an open question (Orfila et al., 2014). Surprisingly, little is known about the effect of ischemia on synaptic plasticity in the young brain or the effects of hypothermia on synaptic function following ischemia (Miyamoto et al., 2000). Here, we present the first data showing that a hypothermia strategy can preserve synaptic function after juvenile CA/CPR with sex-specific efficacy.
EXPERIMENTAL PROCEDURES
Experimental animals
All experimental protocols were approved by the Institutional Animal Care and Use Committee and conformed to the National Institutes of Health guidelines for care and use of animals. Male and female C57Bl/6 20–25-day-old (P20-25) mice (Charles River Laboratory) were used for this study. P20-25 mice are prepubertal mice and reported to be equivalent to 2–4-year-old children (Semple et al., 2013) and all analysis was completed by day 32, equivalent to less than 11-year-old children (Semple et al., 2013). These mice were weaned and not with dam at the time of experiment. The mice were housed in a standard 12-h light and 12-h dark cycle and had free access to food and water. Mice were randomly assigned to experimental groups and the investigator was blinded. A total of 66 mice were used for this study. The average age at CA/CPR was 23.2±0.2 day old (n=51). The average age for mice that were sacrificed for histology was 26.6±0.2 days old (n=26) and the average age for mice sacrificed for electrophysiology was 29.5±0.3 days old (n=22). Three mice were removed from the study due to premature death after CA/CPR (1 male in 37 °C group, 1 female in 32 °C group and 1 male in the 32 °C group). One additional mouse was removed in the male 32 °C group due to lack of usable signals. Age matched controls for electrophysiology averaged 28.8±0.5 days old (n=15).
CA/CPR
CA in juvenile mice was performed as previously described (Deng et al., 2014). Briefly, mice were anesthetized using 3% isoflurane and maintained with 1.5–2% isoflurane in 30% fraction of inspired oxygen (FiO2) via face mask. Body temperature was maintained at 37 °C using a heat lamp and heating pad while being monitored with temperature probes placed into the left ear canal and rectum. For drug administration, a PE-10 catheter was inserted into the right internal jugular vein and flushed with heparinized 0.9% normal saline solution. Animals were endotracheally intubated using a 24G intravenous catheter and connected to a mouse ventilator (Minivent, Hugo Sachs Elektronik, March-Hugstetten, Germany) set to a respiratory rate of 160 breaths per minute. Cardiac function was monitored throughout the experiment with EKG. CA was induced by injection of 30 μL of 0.5 M KCl via the jugular catheter and confirmed by asystole on electrocardiography and absence of spontaneous breathing. The endotracheal tube was disconnected from the ventilator during CA and no spontaneous breathing was observed. During this time anesthesia was not being delivered. Body warming was ceased 1 min prior to CA. During CA the pericranial temperature was maintained at 37.5±0.5 °C by using a water-filled coil. Body temperature was allowed to fall spontaneously to 35 °C. Resuscitation was begun 8 min after the initiation of CA by slow injection of 0.2–0.5 mL of epinephrine (16 μg epinephrine/mL 0.9% saline), chest compressions at a rate of approximately 300 min−1 and resumption of ventilation with 100% FiO2 at a rate of 210 breaths/min. Chest compressions were stopped upon ROSC, defined as electrical evidence of cardiac contractions. If ROSC was not achieved within 3 min of CPR initiation, resuscitation was stopped and the animal was excluded from the study. Five minutes following ROSC, FiO2 was decreased to 50%. When the spontaneous respiratory rate was 30 breaths/min, the ventilator was adjusted to 150 breaths/min and when the animals had at least 60 spontaneous breaths/min, the endotracheal tube was removed. Temperature probes and intravascular catheters were removed and the surgical wounds were closed.
Following ROSC, animals in the normothermia group were rewarmed to reach 37 °C by using a heating lamp and pad at a rate of 0.3–0.5 °C per minute and maintained at 37 °C throughout the recovery. Animals in the hypothermia groups were allowed to have their body temperatures fall spontaneously to 32 °C or 30°C and maintained for 30 min after CA/CPR when they were rewarmed to 37 °C as described above.
Mice were weighed and a health assessment score was calculated for each mouse daily for three days after CA/CPR by a blinded observer. The graded scoring systems ranged from 0 to 2, 0 to 3, or 0 to 5 depending on the behavior assessed, with 0 indicating no deficit and the upper limit indicating the most impaired. The behaviors assessed included consciousness (0–3), interaction (0–2), ability to grab wire top (0–2), motor function (0–5), and activity (0–2) (Allen et al., 2011; Deng et al., 2014). Scores in each category were summated to generate an overall health assessment score.
Hematoxylin & eosin staining
Three days after CA/CPR, animals were anesthetized with 3% isoflurane and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde. Brains were removed and post-fixed with paraformaldehyde and embedded in paraffin. 6-μm coronal sections were prepared and stained with hematoxylin and eosin (H&E). The CA1 area of the hippocampus was analyzed at three different regions (100 μm apart), beginning 1.5 mm posterior to Bregma. Nonviable neurons were determined by pyknotic nuclei and hypereosinophilic cytoplasm and the percentage of nonviable neurons was calculated for each hippocampal region (average of 3 levels per region). The investigator was blinded to treatment before analyzing neuronal damage. For cell density analysis, images were captured at 400× for each section and the number of nonviable and live cells per area was analyzed using ImageJ software. For statistical analysis, each n represents an individual mouse.
Acute hippocampal slice preparation
Hippocampal slices were prepared at 7 days after recovery from CA/CPR from juvenile mice (P20-25 at time of CA/CPR, P27-32 at time of sacrifice for slice preparation) or age-matched controls. Animals were anesthetized with 3% isoflurane in an O2-enriched chamber. Mice were transcardially perfused with ice-cold (2–5 °C) oxygenated (95% O2/5% CO2) artificial cerebral spinal fluid (aCSF) for 2 min prior to decapitation. The brains were then extracted and placed in the same aCSF. The aCSF was composed of the following (in mmol/L): 126 NaCl, 2.5 KCl, 25 NaHCO3, 1.3 NaH2PO4, 2.5 CaCl2, 1.2 MgCl2 and 12 glucose (Orfila et al., 2014). Transverse hippocampal slices (300 μm thick) were cut with a Vibratome 1200 (Leica) and transferred to a holding chamber containing aCSF for at least 1 h before recording.
Electrophysiology
Synaptically evoked field potentials were recorded from hippocampal CA1 slices that were placed on a temperature controlled (31±0.5 °C) interface chamber perfused with aCSF at a rate of 1.5 ml/min. Field excitatory post-synaptic potentials (fEPSP) were produced by stimulating the Schaffer collaterals and recording in the stratum radiatum of the CA1 region. The fEPSPs were adjusted to 50% of the maximum slope and test pulses were evoked every 20 s. Paired-pulse responses were recorded using a 50-ms interpulse interval (20 Hz) and expressed as a ratio of the slopes of the second pulse over the first pulse. Maximum slope of fEPSP was plotted against stimulus intensity (0–50 mA). Only slices in which the I/O curve fell within this range were used for this analysis. A 20-min stable baseline was established before delivering a theta burst stimulation (TBS) train of four pulses delivered at 100 Hz in 30-ms bursts repeated 10 times with 200-ms interburst intervals (Orfila et al., 2014). Following TBS, the fEPSP was recorded for 60 min. Analog fEPSPs were amplified (1000×) and filtered through a pre-amplifier (Model LP511 AC, Grass Instruments) at 1.0 kHz, digitized at 10 kHz and stored on a computer for later off-line analysis (Clampfit 10.4, Axon Instruments). The derivative (dV/dT) of the initial fEPSP slope was measured. The averaged 10-min slope from 50–60 min after TBS was divided by the average of the 10-min baseline prior to TBS to determine the amount of potentiation (Orfila et al., 2014). For time course graphs, normalized fEPSP slope values were averaged and plotted as the percent change from baseline. For statistical analysis, each n represents an individual hippocampal slice and no more than 2n were from an individual animal. A minimum of 4 animals was used for each electrophysiology experimental group to assure biologic variability.
Decay kinetics of the fEPSP were performed by averaging 10 min of recorded fEPSP prior to TBS stimulation and the 10–90% decay slope was fitted by single exponentials to obtain tau (τ). To analyze the effect of NMDA blockade on fEPSP decay time, control slices were recorded in the presence and absence of D-2-amino-5-phosphonopentanoate (AP5, 50 μM, Tocris).
Statistical analysis
All data are presented as mean±SEM. Using Bartlett’s test for equal variance, we found that our data had a normal distribution; therefore statistical analysis was performed using Student’s t-test for two-group comparisons and a one-way ANOVA with Holm-Sidek post hoc test for comparison of multiple groups. The effect of AP5 on kinetics of fEPSP decay was analyzed by paired t-test. Differences were considered statistically significant at p<0.05.
RESULTS
Global ischemia impairs synaptic plasticity following juvenile CA/CPR
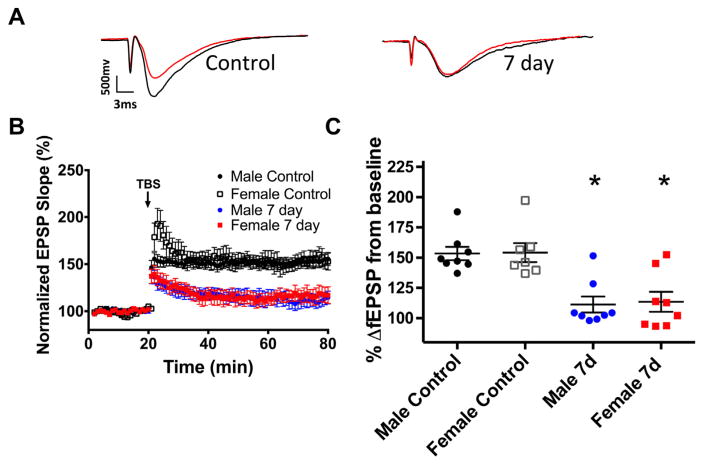
To determine the effect of global ischemia induced by CA/CPR in juvenile mice on synaptically evoked LTP, extracellular field recordings were performed in acute hippocampal slices prepared 7 days following CA/CPR and compared with sham-operated control mice. Juvenile mice were subjected to 8 min of normothermic (37 °C) CA followed by resuscitation. In control slices from juvenile mice, a brief TBS (40 pulse, 100 Hz) resulted in LTP that increased fEPSP slope to 153 ±5.5% of baseline (set at 100%) in males (n=8, Fig. 1) and 154±7.8% of baseline in females (n=7, Fig. 1) after 60 min. In contrast, recordings obtained 7 days after CA/CPR demonstrated impairment in LTP in both male (111±8.9%; n=8) and female animals (113±8.2%; n=8; both p<0.05 compared to respective controls; Fig. 1).
Fig. 1.
Ischemia impairs synaptic plasticity in juvenile mice. (A) Example fEPSP traces from control mice and mice 7 days after CA where red indicates pre-TBS trace and black indicates post-TBS trace. (B) Time course of fEPSP slope (mean±SEM) from control male mice (black circle), control female mice (white square), male (blue circle) and female (red square) 7 days after CA/CPR. Arrow indicates timing of theta-burst stimulation (40 pulses). (C) Quantification of change in fEPSP slope after 60 min following TBS normalized to baseline, set to 100%. *p<0.05 compared with control mice. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
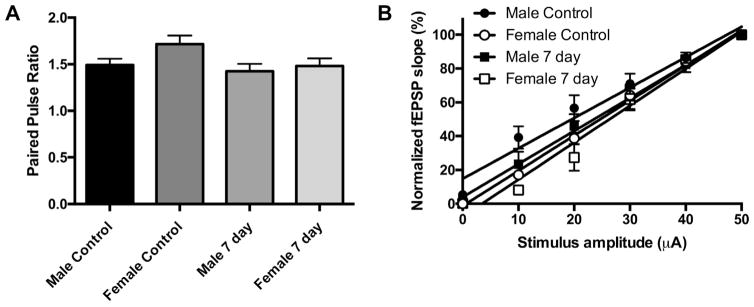
To test whether the impairment of LTP after juvenile CA/CPR was pre- or post-synaptic, we examined the paired-pulse ratio (PPR), a measure of the pre-synaptic transmitter release probability (Debanne et al., 1996; Sudhof, 2004). Paired-pulse responses were recorded from CA1 pyramidal cells in stratum radiatum using a 50-ms (20 Hz) interpulse interval applied to the Schaffer collateral pathway. Fig. 2A shows that no differences in PPR were observed in control juvenile mice or mice 7 days after CA/CPR in either sex (control male 1.49 ±0.11 [n=7]; control female 1.72±0.12 [n=7]; 7-day male 1.42±0.11 [n=8]; 7-day female 1.48 ±0.11 [n=8]; p=0.08), suggesting that the impairment in LTP seen at this time point is likely not due to a decrease in pre-synaptic probability of release.
Fig. 2.
Juvenile CA/CPR does not affect probability of release or intrinsic excitability. (A) Quantification of the paired-pulse ratio of the control and 7 days after CA/CPR in each sex. (B) Input–output curve showing normalized fEPSP slope plotted against stimulus intensity (fEPSP slope is normalized to the maximum of each recording). Data are presented as mean±SEM.
Input–output functions of fEPSPs versus stimulation amplitude were established at the beginning of each recording prior to TBS to examine CA3 axonal intrinsic excitability and synaptic transmission. The slope of each recording was measured individually and compared to control slices (Fig. 2B). Using linear regression, no difference in the input–output slope was observed between controls and mice 7 days after CA/CPR in either sex (control male 1.80±0.12 [n=5]; control female 2.08±0.07 [n=5]; 7-day male 1.95±0.12 [n=5]; 7-day female 2.18±0.12 [n=4], p=0.10).
Hypothermia protects against neuronal injury in juvenile CA/CPR
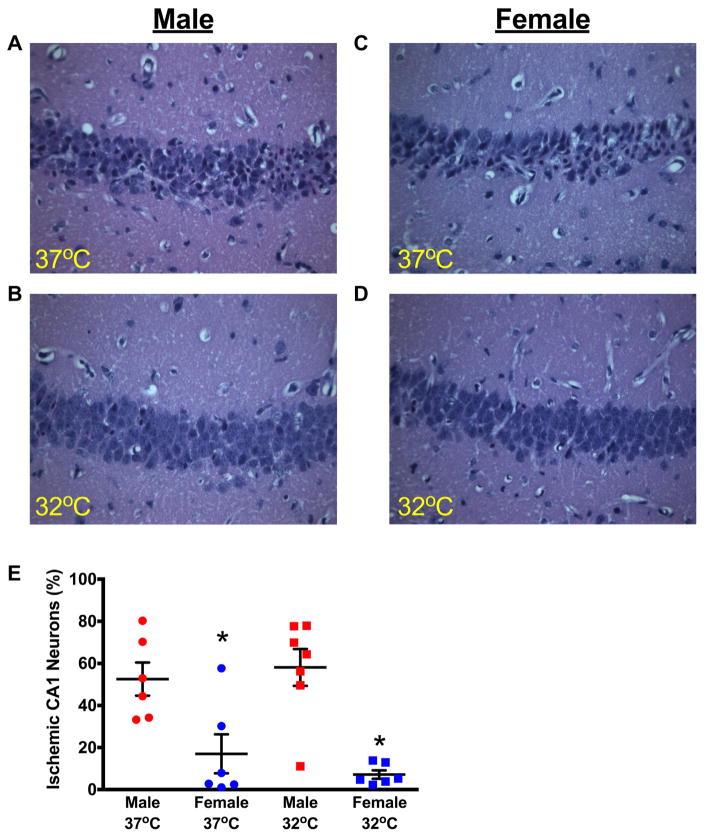
To compare the vulnerability of juvenile neurons to global ischemia, hippocampal damage was analyzed in male and female juvenile mice (P20-25). We have previously described that our CA/CPR model results in delayed neuronal death, with ischemic damage of hippocampal neurons peaking 3 days after CPR, no additional cell death was observed at the 7-day time point after CA/CPR (Deng et al., 2014). Based on this previous analysis, we quantified CA1 hippocampal injury by H&E staining at 3 days after CA/CPR. These experiments revealed equivalent hippocampal CA1 injury in both sexes; 52±8% in male mice (n=6) and 58±10% (n=7) in female mice (Fig. 3). We tested whether mild hypothermia was neuroprotective in our juvenile CA model. Mild hypothermia (32 ±0.5 °C) was initiated immediately after resuscitation and maintained for 30 min. Hippocampal CA1 damage was reduced following hypothermia (17±9% in males [n=6] and 7.0±3% in females [n=6], p<0.05) and was not different between sexes (p=0.87, Fig. 3).
Fig. 3.
Mild therapeutic hypothermia significantly reduces CA1 neuronal injury in juvenile mice. Representative photomicrographs of hippocampal CA1 neurons from male (A) and female (B) juvenile mice exposed to normothermic cardiac arrest at 37 °C and cardiac arrest followed by 30 min of mild hypothermia (32 °C) in male (C) and female (D) mice. (E) Quantification of ischemic CA1 neurons 3 days after CA/CPR (mean±SEM), indicating a significant reduction in neuronal injury in mice treated with post-arrest hypothermia. *p<0.05 compared to normothermia.
Mice were observed closely during this time and parameters of CA/CPR and health were measured. Body weight was not different between males and females and total time of ischemia was not different between groups. Survival and health assessment scores were not different between sexes, but overall health assessment did improve temporally (Table 1).
Table 1.
Body weight, cardiac arrest parameters, survival rate and general health assessment
| CA 37 °C
|
CA 32 °C
|
|||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| N | 7 | 6 | 7 | 6 |
| Body Weight (BW, g) | 10.7 ± 0.7 | 9.7 ± 0.7 | 11.1 ± 0.7 | 10.8 ± 0.8 |
| Total ischemia time (s) | 573 ± 10 | 577 ± 5 | 555 ± 12 | 561 ± 9 |
| Epinephrine (μg/g BW) | 0.51 ± 0.06 | 0.59 ± 0.03 | 0.44 ± 0.09 | 0.50 ± 0.08 |
| Epinephrine (mL) | 0.34 ± 0.05 | 0.35 ± 0.02 | 0.28 ± 0.04 | 0.32 ± 0.03 |
| Survival (%) | 86 | 100 | 100 | 100 |
| Health assessment score | ||||
| POD 1* | 1.3 ± 0.2** | 2.1 ± 0.3 | 0.7 ± 0.2** | 1.3 ± 0.3 |
| POD 2* | 0.3 ± 0.2 | 0.4 ± 0.2 | 0.3 ± 0.2 | 0.2 ± 0.2 |
| POD 3 | 0.0 ± 0.0 | 0.2 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Total ischemia represents time from induction of cardiac arrest to successful resuscitation. Postoperative day (POD) refers to day following CA/CPR. BW, body weight.
Indicates p<0.05 compared to corresponding sex and temperature group.
Indicates p<0.05 male 37 °C group compared to male 32 °C group.
Hypothermia protects against impairment of synaptic plasticity
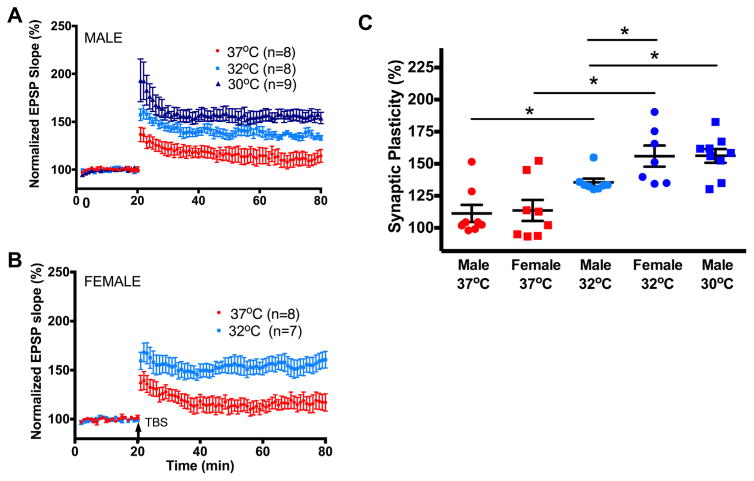
We next determined whether the neuroprotection provided by hypothermia shown above correlated with the rescue of synaptic function as measured by synaptic plasticity. Juvenile mice were exposed to mild hypothermia (32±0.5 °C) for 30 min after CA/CPR followed by rewarming and hippocampal slices were collected 7 days after resuscitation for recording, where we saw impairment in synaptic function as described above. By testing at this time point, we were assured to be beyond the time of maximal hippocampal cell death (3 days, as shown above and described previously (Deng et al., 2014)). Hypothermia preserved synaptic function in male mice (135±2%, n=8, p<0.05, Fig. 4) and female mice (156±8%, n=7, p<0.05), compared to 7-day normothermic mice (baseline set to 100%). Interestingly, the preservation in female mice was greater than the preservation in male mice (p<0.05). Together, these data demonstrate that CA/CPR-induced impairment of synaptic plasticity is prevented by mild hypothermia.
Fig. 4.
Mild therapeutic hypothermia preserves synaptic plasticity in a sex-specific manner. (A) Time course of fEPSP slope (mean±SEM) from juvenile male mice 7 days following CA/CPR recovered at normothermia (37 °C, red), 32 °C (light blue) and 30 °C (dark blue). Arrow indicates timing of theta-burst stimulation (40 pulses, 100 Hz). (B) Time course of fEPSP slope (mean±SEM) from juvenile female mice 7 days following CA/CPR recovered at normothermia (37 °C, red) and 32 °C (light blue). Arrow indicates timing of theta-burst stimulation (40 pulses, 100 Hz). (C) Quantification of change in fEPSP slope after 60 min following TBS normalized to baseline, set to 100%. *p<0.05 compared with control mice. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We next tested the hypothesis that a deeper level of hypothermia will provide further protection in male mice. Male mice were maintained at 30±0.5 °C for 30 min after resuscitation and then rewarmed and allowed to recover. Histology was not performed as mild hypothermia (32 °C) provided equivalent neuroprotection (shown above). These mice had enhanced LTP compared to the animals at 32 °C (156±5.3%, n=9, p<0.05), but were not different from the females at 32 °C (Fig. 4). Females were not tested at 30 °C as synaptic function was back to pre-ischemic control levels.
Lack of role of NMDA receptors in impairment and recovery of synaptic function
It has previously been suggested that impairment of synaptic plasticity after ischemia is due to changes in NMDA receptor function (Zhang et al., 1997; Liu et al., 2010). To determine whether the relative expression of functional NMDA receptors was altered by CA/CPR-induced ischemia, we analyzed the decay phase of the fEPSP (Stone et al., 2012). Consistent with the presence of functional NMDA receptors under control conditions, application of NMDA-receptor antagonist D-2-amino-5-phosphonopentanoate (AP5, 50 μM) resulted in an increase in the decay, changing the tau from 6.88 ±0.6 ms in aCSF to 6.32±0.6 ms in AP5 (p<0.05 by paired t-test). We next compared the decay kinetics of sham mice and mice 7 days after CA/CPR with and without hypothermia and found there were no differences in any of the experimental conditions (Table 2), suggesting that the impairment in synaptic function after CA/CPR, and preservation of function by hypothermia, is independent of NMDA receptor activity.
Table 2.
Decay kinetics of fEPSPs indicate that there is no change in NMDA kinetics to account for impairment or recovery of synaptic function
| Control (τ, ms) | 7 day 37 °C (τ, ms) | 7 day 32 °C (τ, ms) | |
|---|---|---|---|
| Male (n) | 7.11 ± 0.9 (6) | 7.97 ± 0.8 (5) | 6.80 ± 0.5 (6) |
| Female (n) | 7.33 ± 0.4 (6) | 7.18 ± 1.0 (6) | 6.88 ± 0.7 (7) |
p>0.05 for all groups within the same sex by ANOVA, numbers in parentheses indicate N.
DISCUSSION
Our results show that global ischemia produced by CA and resuscitation in our novel juvenile mouse model produces impaired synaptic plasticity up to a week after injury. Our data verify that a therapeutic hypothermia strategy can decrease neuronal death after CA, and we provide the first evidence that hypothermia preserves synaptic function after global ischemia. We further show a sex difference in functional protection related to the depth of hypothermia, with males requiring a deeper level of hypothermia for the same synaptic protection provided in females.
Hypothermia is well established to provide neuroprotection in animal models (Drury et al., 2014) and is the standard of care for clinical therapy following CA in adults and perinatal asphyxiation in neonates (Arrich et al., 2012; Jacobs et al., 2013). Our data indicate that hypothermia reduces neuronal death and protects against the loss of synaptic plasticity, likely indicating protection of function in the ischemia-sensitive regions of the hippocampus, in our model of juvenile CA/CPR. Our previous data indicate that maximal hippocampal cell death occurs 3 days after CA (Allen et al., 2011; Nakayama et al., 2013; Deng et al., 2014), however it remains possible that hypothermia delays neuronal injury. Nonetheless, we observe functional protection (LTP) at time points well beyond this 3-day time point, indicating that there is sustained functional preservation due to hypothermia in juvenile animals. It has been suggested previously that understanding the physiologic consequences of brain injury, rather than only relying on decreased neuronal death, is an important measure of damage and recovery potential following injury (Ivanco and Greenough, 2000; Carmichael, 2012). The sensitivity of LTP may provide a more subtle measure to identify mechanisms of therapeutic recovery over the lower fidelity measures of histology and neurobehavior (Ivanco and Greenough, 2000). Indeed, our data showing differences in response to therapeutic hypothermia would not have been evident by examining histology alone, as our data indicate no differences in neuroprotection between sexes. Thus, by investigating synaptic function after hypothermia, our data indicate that the young brain may be more sensitive to therapeutic hypothermia at the physiologic level.
The observation that CA/CPR results in the loss of synaptic plasticity (LTP) in CA1 synapses is consistent with extensive literature that global ischemia causes CA1 injury and adds to the literature that LTP is impaired during the first week after global ischemic injury (Mori et al., 1998; Dai et al., 2007; Orfila et al., 2014). Likely consistent with the well-documented protective effects of hypothermia, learning tasks have been shown to be preserved in hypothermic animals following global ischemia (Colbourne and Corbett, 1995; Wagner et al., 2002). We are the first to examine synaptic plasticity following therapeutic hypothermia after global ischemia. Given that learning induces LTP in behavior studies (Bliss and Collingridge, 1993; Bliss et al., 2006; Cooke and Bliss, 2006; Pastalkova et al., 2006; Whitlock et al., 2006), our data are consistent with prior observation that hypothermia preserves learning tasks after global ischemia (Colbourne and Corbett, 1995; Wagner et al., 2002; Smith et al., 2015). The impairment in LTP likely correlates with multiple observations that memory deficits are present following human CA (Grubb et al., 1996; Drysdale et al., 2000; O’Reilly et al., 2003; Sulzgruber et al., 2014), and animal ischemia (Kofler et al., 2004; Allen et al., 2011).
LTP is well known to be post-synaptic (Malenka, 1991; Malenka and Bear, 2004) and our findings are consistent with these previous data in showing that there is no effect of CA/CPR on paired-pulse stimulation or input–output excitation curves (Fig. 2). Further, LTP at the CA1 synapse is induced by influx of Ca2 + through synaptic NMDA receptors, activating the calcium-dependent protein kinase CaMKII and ultimately increased synaptic strength (for a review see (Malenka and Bear, 2004)). Thus, it is not surprising that the effect of ischemia on NMDA receptor function has been the focus of several studies, suggesting that ischemia-induced loss of LTP is due to changes in NMDA expression and function (Zhang et al., 1997; Liu et al., 2010). However, we recently observed that global ischemia does not alter NMDA receptor expression or function in adult mice (Orfila et al., 2014). The data presented here are consistent with these data, suggesting that CA/CPR-induced ischemia in the juvenile mouse does not alter the NMDA receptor component of fEPSPs recorded from CA1 neurons. We used a relatively novel approach to assess the effect of ischemia on NMDA receptor function, analyzing the kinetics of fEPSPs. It is well established that NMDA receptors are responsible for a slow phase of synaptic current (Lester et al., 1990) and it was recently established that blocking NMDA receptors with the NMDA-receptor antagonist AP5 induces a faster decay time in fEPSPs (Stone et al., 2012). Therefore, we demonstrate here that we are able to detect functional NMDA receptors in our fEPSP recordings by analyzing fEPSP decay kinetics. As our data suggest that NMDA receptor function is unchanged by CA/CPR or post-ischemic hypothermia by this analysis, future studies could further elucidate the role of NMDA receptors as well as downstream signaling of the NMDA receptor such as increased CaMKII activity, PSD-95 or other synaptic molecules implicated in mediating synaptic plasticity.
While it was somewhat surprising that a relatively short duration of hypothermia provided such robust protection against neuronal death and loss of function, we believe this can be an important tool for future mechanistic studies. This may be particularly important in light of recent human studies showing lack of effect of hypothermia after out-of-hospital CA in children (Moler et al., 2015), suggesting a need for a better understanding through basic science. Further, it was also somewhat unexpected that there was a difference in the response to hypothermia in male and female juvenile mice, though questions regarding the depth of hypothermia have recently emerged from human trials (Nielsen et al., 2013) and previous reports have suggested sexually dimorphic responses in some behavior tasks in neonatal hypoxia–ischemia models (Burnsed et al., 2015; Smith et al., 2015). Lee and colleagues found that exposing neonatal male rats to 30 °C or 33 °C for varying lengths of time did not further reduce the loss of residual brain volume or motor behavior tasks one month after a model of neonatal hypoxic injury (Lee et al., 2010). Others have tested different hypothermia temperature targets and some have found that there was no further neuroprotection (Weinrauch et al., 1992; Colbourne and Corbett, 1995), though some have found additional protective effect at 31 °C versus 34 °C in a neonatal rat model (Yager et al., 1993). Further, hypothermia in a similar neonatal model in mice has been observed to provide neuroprotection (as measured by MRI) and improved behavioral testing in males out to 20 days after ischemic injury, whereas the effect on females was variable and transient (Burnsed et al., 2015). A major difference between our findings and previous studies is the age of the rodents at the time of ischemia, as well as the mechanism of injury that is incurred. In previous work, a neonatal-equivalent rodent is used following unilateral ischemia, whereas our model represents a juvenile animal following global ischemia. With these important differences in mind, much work remains in understanding the differences in response to hypothermia between males and females in juvenile animals, particularly over the long term. In addition, there are likely other protective effects of hypothermia beside neuroprotection that account for the preservation of synaptic function including neural, vascular or inflammatory events that have been extensively studied (Drury et al., 2014). Further, it is possible the cognitive decline in people and animals following an ischemic event may be explained by the combination of neuronal cell death commonly observed in CA1 neurons as well as impaired synaptic function in surviving neurons.
CONCLUSIONS
The current study provides the first evidence that hypothermia preserves synaptic plasticity after global ischemia, but there may be a sex-specific influence on this effect. While it remains possible that this is a species-specific finding, recent clinical controversies regarding the role for hypothermia after CA in humans (Arrich et al., 2012; Jacobs et al., 2013; Moler et al., 2015) highlight the need for better mechanistic understanding through basic science. By demonstrating the increased sensitivity to physiologic changes in studying synaptic plasticity, we purport that future studies evaluating therapeutic agents should include this technique as a functional outcome and that evaluating treatments between sexes is paramount. Our unique model of juvenile CA can be used to evaluate a cellular model of learning and memory that may provide a tool to discover new therapeutic approaches in the treatment of global ischemia in children.
Acknowledgments
FINANCIAL SUPPORT
NIH NS046072, NIH NS092645, NIH T32 HD 007186.
Abbreviations
- aCSF
artificial cerebral spinal fluid
- AP5
D-2-amino-5- phosphonopentanoate
- CA
cardiac arrest
- CA/CPR
cardiac arrest and cardiopulmonary resuscitation
- fEPSPs
field excitatory post-synaptic potentials
- FiO2
fraction of inspired oxygen
- H&E
hematoxylin and eosin
- LTP
long-term potentiation
- NMDA
N-methyl-D-aspartate
- PPR
paired-pulse ratio
- ROSC
return of spontaneous circulation
- TBS
theta burst stimulation
References
- Allen D, Nakayama S, Kuroiwa M, Nakano T, Palmateer J, Kosaka Y, Ballesteros C, Watanabe M, Bond CT, Lujan R, Maylie J, Adelman JP, Herson PS. SK2 channels are neuroprotective for ischemia-induced neuronal cell death. J Cerebral Blood Flow Metabol. 2011;31:2302–2312. doi: 10.1038/jcbfm.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrich J, Holzer M, Havel C, Mullner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. The Cochrane database of systematic reviews. 2012;9:CD004128. doi: 10.1002/14651858.CD004128.pub3. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL, Laroche S. ZAP and ZIP, a story to forget. Science. 2006;313:1058–1059. doi: 10.1126/science.1132538. [DOI] [PubMed] [Google Scholar]
- Burnsed JC, Chavez-Valdez R, Hossain MS, Kesavan K, Martin LJ, Zhang J, Northington FJ. Hypoxia-ischemia and therapeutic hypothermia in the neonatal mouse brain–a longitudinal study. PLoS ONE. 2015;10:e0118889. doi: 10.1371/journal.pone.0118889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST. Brain excitability in stroke: the yin and yang of stroke progression. Arch Neurol. 2012;69:161–167. doi: 10.1001/archneurol.2011.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourne F, Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci. 1995;15:7250–7260. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SF, Bliss T. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- Dai X, Chen L, Sokabe M. Neurosteroid estradiol rescues ischemia-induced deficit in the long-term potentiation of rat hippocampal CA1 neurons. Neuropharmacology. 2007;52:1124–1138. doi: 10.1016/j.neuropharm.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol. 1996;491(Pt 1):163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G, Yonchek JC, Quillinan N, Strnad FA, Exo J, Herson PS, Traystman RJ. J Neurosci Methods. Vol. 222. Elsevier B.V; 2014. A novel mouse model of pediatric cardiac arrest and cardiopulmonary resuscitation reveals age-dependent neuronal sensitivities to ischemic injury; pp. 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury PP, Gunn ER, Bennet L, Gunn AJ. Mechanisms of hypothermic neuroprotection. Clin Perinatol. 2014;41:161–175. doi: 10.1016/j.clp.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Drysdale EE, Grubb NR, Fox KA, O’Carroll RE. Chronicity of memory impairment in long-term out-of-hospital cardiac arrest survivors. Resuscitation. 2000;47:27–32. doi: 10.1016/s0300-9572(00)00194-5. [DOI] [PubMed] [Google Scholar]
- Grubb NR, O’Carroll R, Cobbe SM, Sirel J, Fox KA. Chronic memory impairment after cardiac arrest outside hospital. BMJ. 1996;313:143–146. doi: 10.1136/bmj.313.7050.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herson PS, Traystman RJ. Animal models of stroke: translational potential at present and in 2050. Future Neurol. 2014;9:541–551. doi: 10.2217/fnl.14.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanco TL, Greenough WT. Physiological consequences of morphologically detectable synaptic plasticity: potential uses for examining recovery following damage. Neuropharmacology. 2000;39:765–776. doi: 10.1016/s0028-3908(00)00004-6. [DOI] [PubMed] [Google Scholar]
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. The Cochrane database of systematic reviews. 2013;1:CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler J, Hattori K, Sawada M, DeVries AC, Martin LJ, Hurn PD, Traystman RJ. Histopathological and behavioral characterization of a novel model of cardiac arrest and cardiopulmonary resuscitation in mice. J Neurosci Methods. 2004;136:33–44. doi: 10.1016/j.jneumeth.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Lee BS, Woo C-W, Kim S-T, Kim K-S. Long-term neuroprotective effect of postischemic hypothermia in a neonatal rat model of severe hypoxic ischemic encephalopathy: a comparative study on the duration and depth of hypothermia. Pediatr Res. 2010;68:303–308. doi: 10.1203/PDR.0b013e3181ef3007. [DOI] [PubMed] [Google Scholar]
- Lester RA, Clements JD, Westbrook GL, Jahr CE. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990;346:565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhao W, Xu T, Pei D, Peng Y. Alterations of NMDA receptor subunits NR1, NR2A and NR2B mRNA expression and their relationship to apoptosis following transient forebrain ischemia. Brain Res. 2010;1361:133–139. doi: 10.1016/j.brainres.2010.09.035. [DOI] [PubMed] [Google Scholar]
- Malenka RC. The role of postsynaptic calcium in the induction of long-term potentiation. Mol Neurobiol. 1991;5:289–295. doi: 10.1007/BF02935552. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Michiels EA, Dumas F, Quan L, Selby L, Copass M, Rea T. Long-term outcomes following pediatric out-of-hospital cardiac arrest. Pediatr Crit Care Med. 2013;14:755–760. doi: 10.1097/PCC.0b013e31829763e2. [DOI] [PubMed] [Google Scholar]
- Miyamoto O, Nakamura T, Yamagami S, Negi T, Tokuda M, Matsui H, Itano T. Depression of long term potentiation in gerbil hippocampus following postischemic hypothermia. Brain Res. 2000;873:168–172. doi: 10.1016/s0006-8993(00)02521-x. [DOI] [PubMed] [Google Scholar]
- Moler FW, Donaldson AE, Meert K, Brilli RJ, Nadkarni V, Shaffner DH, Schleien CL, Clark RSB, Dalton HJ, Statler K, Tieves KS, Hackbarth R, Pretzlaff R, van der Jagt EW, Pineda J, Hernan L, Dean JM Network PECAR. Multicenter cohort study of out-of- hospital pediatric cardiac arrest. Crit Care Med. 2011;39:141–149. doi: 10.1097/CCM.0b013e3181fa3c17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moler FW, Silverstein FS, Holubkov R, Slomine BS, Christensen JR, Nadkarni VM, Meert KL, Clark AE, Browning B, Pemberton VL, Page K, Shankaran S, Hutchison JS, Newth CJ, Bennett KS, Berger JT, Topjian A, Pineda JA, Koch JD, Schleien CL, Dalton HJ, Ofori-Amanfo G, Goodman DM, Fink EL, McQuillen P, Zimmerman JJ, Thomas NJ, van der Jagt EW, Porter MB, Meyer MT, Harrison R, Pham N, Schwarz AJ, Nowak JE, Alten J, Wheeler DS, Bhalala US, Lidsky K, Lloyd E, Mathur M, Shah S, Wu T, Theodorou AA, Sanders RC, Jr, Dean JM, Investigators TT. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med. 2015;372:1898–1908. doi: 10.1056/NEJMoa1411480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Yoshioka M, Suda N, Togashi H, Matsumoto M, Ueno K, Saito H. An incomplete cerebral ischemia produced a delayed dysfunction in the rat hippocampal system. Brain Res. 1998;795:221–226. doi: 10.1016/s0006-8993(98)00295-9. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Vest R, Traystman RJ, Herson PS. J Mol Neurosci. Vol. 51. Springer; US: 2013. Sexually dimorphic response of TRPM2 inhibition following cardiac arrest-induced global cerebral ischemia in mice; pp. 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Cooke SF, Bliss TVP. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wise MP, Aneman A, Al-Subaie N, Boesgaard S, Bro-Jeppesen J, Brunetti I, Bugge JF, Hingston CD, Juffermans NP, Koopmans M, Kober L, Langorgen J, Lilja G, Moller JE, Rundgren M, Rylander C, Smid O, Werer C, Winkel P, Friberg H, Investigators TTMT. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- O’Reilly SM, Grubb NR, O’Carroll RE. In-hospital cardiac arrest leads to chronic memory impairment. Resuscitation. 2003;58:73–79. doi: 10.1016/s0300-9572(03)00114-x. [DOI] [PubMed] [Google Scholar]
- Orfila JE, Shimizu K, Garske AK, Deng G, Maylie J, Traystman RJ, Quillinan N, Adelman JP, Herson PS. Increasing small conductance Ca(2+) -activated potassium channel activity reverses ischemia-induced impairment of long-term potentiation. Eur J Neurosci. 2014;40:3179–3188. doi: 10.1111/ejn.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbaugh PE, Pepe PE, Shook JE, Kimball KT, Goldman MJ, Ward MA, Mann DM. A prospective, population-based study of the demographics, epidemiology, management, and outcome of out-of-hospital pediatric cardiopulmonary arrest. Ann Emerg Med. 1999;33:174–184. doi: 10.1016/s0196-0644(99)70391-4. [DOI] [PubMed] [Google Scholar]
- Smith AL, Garbus H, Rosenkrantz TS, Fitch RH. Sex differences in behavioral outcomes following temperature modulation during induced neonatal hypoxic ischemic injury in rats. Brain Sci. 2015;5:220–240. doi: 10.3390/brainsci5020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone E, Hoffman K, Kavanaugh M. Identifying neurotransmitter spill-over in hippocampal field recordings. Math Biosci. 2012;240:169–186. doi: 10.1016/j.mbs.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Sulzgruber P, Kliegel A, Wandaller C, Uray T, Losert H, Laggner AN, Sterz F, Kliegel M. Survivors of cardiac arrest with good neurological outcome show considerable impairments of memory functioning. Resuscitation. 2014 doi: 10.1016/j.resuscitation.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Wagner BP, Nedelcu J, Martin E. Delayed postischemic hypothermia improves long-term behavioral outcome after cerebral hypoxia-ischemia in neonatal rats. Pediatr Res. 2002;51:354–360. doi: 10.1203/00006450-200203000-00015. [DOI] [PubMed] [Google Scholar]
- Weinrauch V, Safar P, Tisherman S, Kuboyama K, Radovsky A. Beneficial effect of mild hypothermia and detrimental effect of deep hypothermia after cardiac arrest in dogs. Stroke. 1992;23:1454–1462. doi: 10.1161/01.str.23.10.1454. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Yager J, Towfighi J, Vannucci RC. Influence of mild hypothermia on hypoxic-ischemic brain damage in the immature rat. Pediatr Res. 1993;34:525–529. doi: 10.1203/00006450-199310000-00029. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hsu JC, Takagi N, Gurd JW, Wallace MC, Eubanks JH. Transient global ischemia alters NMDA receptor expression in rat hippocampus: correlation with decreased immunoreactive protein levels of the NR2A/2B subunits, and an altered NMDA receptor functionality. J Neurochem. 1997;69:1983–1994. doi: 10.1046/j.1471-4159.1997.69051983.x. [DOI] [PubMed] [Google Scholar]