Abstract
Articular cartilage is an avascular, alymphatic, and aneural system with very low regeneration potential because of its limited capacity for self-repair. Mesenchymal stem cells (MSCs) are the preferred choice for cell-based therapies. Glycogen synthase kinase 3 (GSK-3) inhibitors are compounds that can induce the Wnt signaling pathway, which is involved in chondrogenesis and cartilage development. Here, we investigated the influence of lithium chloride (LiCl) and SB216763 synergistically with TGF-β3 on chondrogenic differentiation in human mesenchymal stem cells derived from Wharton’s jelly tissue (hWJ-MSCs). hWJ-MSCs were cultured and chondrogenic differentiation was induced in monolayer and pellet experiments using chondrogenic medium, chondrogenic medium supplemented with LiCl, or SB216763 for 4 weeks. After in vitro differentiation, cultured cells were examined for the expression of Sox9, ACAN, Col2a1, and β-catenin markers. Glycosaminoglycan (GAG) accumulation was also examined by Alcian blue staining. The results indicated that SB216763 was more effective than LiCl as evidenced by a higher up-regulation of the expression of cartilage-specific markers, including Sox9, ACAN, Col2a1 as well as GAG accumulation. Moreover, collagen type II expression was strongly observed in cells cultured in the chondrogenic medium + SB216763 as evidenced by western blot analysis. Both treatments appeared to mediate the Wnt signaling pathway by up-regulating β-catenin gene expression. Further analyses showed that all treatments suppressed the progression of chondrocyte hypertrophy, determined by decreased expression of Col10a1 and Runx2. These results indicate that LiCl and SB216763 are potential candidates for further in vivo therapeutic trials and would be of great importance for cartilage regeneration.
Introduction
Articular cartilage is a highly specialized connective tissue of the synovial joints. Chondrocytes are specialized cells in this tissue responsible for the generation of the extracellular matrix (ECM) and the maintenance of the tissue function. Generally, articular cartilage injuries cannot self-repair due to the lack of vascular, lymphatic, or nervous systems [1].
An alternative approach of cartilage preservation and repair that uses stem cell-based therapies such as mesenchymal stem cells (MSCs) was recently developed. MSCs can be isolated from the bone marrow [2], adipose tissue [3], dental pulp [4], umbilical cord blood [5], and Wharton’s jelly tissue [6]. Several sources of MSCs exhibit different properties of stemness, expansion capacity, and multilineage differentiation [7,8]. Wharton’s jelly tissue (WJ) is an alternative source of MSCs, which show properties similar to MSCs from other sources. It is a rich source of primitive cells [6,9]. In addition, WJ-MSCs have greater proliferation rates, expansion potential, and differentiation potential than other adult MSCs [10]. Thus, WJ-MSCs have been considered a source of candidate cells and present therapeutic potential for cartilage regeneration.
Members of the transforming growth factor-beta (TGF-β) superfamily are the most crucial inducers of chondrogenic differentiation in MSCs such as transforming growth factor-beta (TGF-β) and bone morphogenetic proteins (BMPs) [11]. The Wnt signaling pathway is also involved in chondrogenesis and cartilage development [12]. Canonical Wnt signaling is mediated by β-catenin, which accumulates in the cytoplasm and then translocates to the nucleus. β-catenin forms a complex with DNA-binding T-cell factors (TCFs) to activate the transcription of target genes. The β-catenin signaling pathway often crosstalks with other signaling pathways to modulate chondrogenesis [13–18]. However, the Wnt/β-catenin signaling pathway plays a crucial role in the hypertrophic maturation of chondrocytes during the endochondral ossification process [19]. Another key regulator of the Wnt signaling pathway is glycogen synthase kinase 3 (GSK-3) that mediates β-catenin phosphorylation [20]. GSK-3 inhibition promotes the accumulation of β-catenin and complex with co-transcription factors, LEFs/TCFs, to promote transcription [21,22]. Lithium chloride (LiCl) or SB216763 has the potential to be inhibitor of GSK-3, which inactivates the phosphorylation of β-catenin to initiates the Wnt signaling pathway [23,24]. LiCl was the first GSK-3 inhibitor to be discovered and has been used in the treatment of bipolar disorder [23]. SB216763 is a synthetic small molecule that highly ATP competitive to inhibit the GSK-3 [24].
In this study, we investigated the influence of LiCl and SB216763, which act synergistically with TGF-β3 on chondrogenic differentiation in hWJ-MSCs by monolayer and pellet cultures experiments. The differentiated cells were characterized by GAG analysis, immunofluorescent staining, western blot, and gene expressions analysis.
Materials and Methods
Chemicals and reagents
All chemicals and reagents were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA), unless otherwise indicated.
Human articular cartilage preparation
This study was approved by the Ethics Committee for Researches Involving Human Subjects, Suranaree University of Technology. Human articular cartilage (n = 1) was obtained from a patient undergoing total knee replacement for osteoarthritis at the Suranaree University of Technology Hospital, Nakhon Ratchasima, Thailand after the patient had signed the informed consent. Human articular cartilage was used as a control in the experiments.
Isolation and expansion of hWJ-MSCs
The human umbilical cord (n = 1) was collected from Suranaree University of Technology Hospital, Nakhon Ratchasima after patient’s informed consent was obtained. MSCs were isolated from the umbilical cord using a tissue explant procedure as previously described [25]. Briefly, the umbilical cord was washed in sterile PBS and cut lengthwise to open the gelatinous (Wharton’s jelly; WJ) tissue. The vessels were excised and diced into small fragments (about 3 × 3 mm). Then, WJ tissues were plated onto 6-well tissue culture plate (SPL life sciences, Gyeonggi-do, Korea) and then carefully covered with 1 mL of culture medium comprised of alpha modification of Eagle’s medium (α-MEM) supplemented with 100 units/mL penicillin, 100 μg/mL streptomycin and 10% of fetal bovine serum (FBS, Life Technologies Inc. Gibco-BRL Division, Grand Island, NY, USA). Culture cells were then incubated at 37°C in a humidified atmosphere of 5% CO2 in air for 7–10 days. Medium was replaced every 2 days and, when visible fibroblast-like cells were observed, then tissue explants were removed. The cells were expanded until passage 3 (P3), then the cells were either directly used for experiments or cryopreserved with 10% dimethyl sulfoxide (DMSO, Calbiochem, San Diego, CA, USA) and stored in liquid nitrogen.
Immunophenotyping
hWJ-MSCs at passage 5 were cultured onto 4-well tissue culture dishes (Nunc, Roskilde, Denmark) in the growth medium until reaching 70% confluence. Cells were fixed by 4% paraformaldehyde (PFA) for 30 min. Nonspecific binding was blocked by 10% normal goat serum. Primary antibodies were raise against CD34 (BD biosciences, San Jose, CA, USA), CD73 (Millipore, Massachusetts, USA), CD90 (Santa Cruz Biotechnology, Dallas, TX, USA), and CD105 (Santa Cruz Biotechnology) at 4°C overnight. Cells were incubated with secondary antibodies, Alexa fluor® 488 goat anti-mouse IgG (Invitrogen, Carlsbad, CA, USA) or Alexa fluor® 488 goat anti-rabbit IgG (Invitrogen). Nuclei were stained with 4, 6-diamino-2-phenylindole (DAPI, Millipore) and observed under a fluorescence microscope (Nikon Eclipse Ti-S, Japan).
Multipotency assays
hWJ-MSCs were cultured at the final density of approximately 2 × 104 cells/cm2 in 6-well culture plates coated with 0.1% gelatin.
hWJ-MSCs were induced to osteogenic differentiation by culture in the culture medium with reduced FBS to 5% and supplemented with 100 nM dexamethasone, 0.2 mM L-ascorbate-2-phosphate, and 10 mM β-glycerophosphate. The medium was subsequently replaced every 2 days for 3 weeks. Calcium deposits from the cells were then visualized by Alizarin Red staining.
To induce adipogenic differentiation, hWJ-MSCs were cultured in the culture medium with reduced FBS to 5% and supplemented with 10 μg/mL insulin, 100 μM indomethacin, 1 μM dexamethasone, 0.5 mM isobutylmethylxanthine (IBMX). IBMX was removed from this medium after 1 week of culture. The medium was subsequently replaced every 2 days for 3 weeks. Cells were then stained with Oil Red O to observe oil droplets.
To induce chondrogenic differentiation, hWJ-MSCs were cultured in a completed chondrogenic medium consisting of culture medium with reduced FBS to 2% and supplemented with 1% Insulin-Transferrin-Selenium-Ethanolamine (ITS-X, Invitrogen), 50 μg/mL ascorbate-2-posphate, 40 μg/mL L-proline, 100 μg/mL sodium pyruvate, 100 nM dexamethasone, and 10 ng/mL of TGF-β3 (Prospec, East Brunswick, NJ, USA). The medium was replaced every 2 days for 3 weeks. GAG production was assessed by Alcian blue 8× staining.
Cytotoxicity test
One thousand hWJ-MSCs were re-plated and cultured in 96-well culture plates (SPL life sciences) in the culture medium for 6 h to allow attachment. Next, LiCl or SB216763 cytotoxicity was assessed by adding either LiCl or SB216763 to the culture medium at concentrations of 0, 5, 10, and 20 mM or 0, 1, 2.5, and 5 μM, respectively. All cultures were maintained for 72 h at 37°C in a humidified atmosphere of 5% CO2 in air. The effects of LiCl and SB216763 on cell viability were quantified by the MTT assay. Briefly, culture medium was replaced by 5 mg/mL MTT solution (Invitrogen) in culture medium and cells were incubated for 2 h. DMSO (Calbiochem) was then added and incubated at 37°C for 10 min. The absorbance was measured at 540 nm. (Microplate reader Sunrise, TECAN, Austria)
Chemical induction of chondrogenic differentiation
For monolayer cultures, hWJ-MSCs were cultured and chondrogenic differentiation was induced as mentioned previously. The experiments were performed by dividing the cells into 4 groups. Each group of cells was cultured in culture medium with reduced FBS to 2% (Control), chondrogenic medium, chondrogenic medium supplemented with 5 mM LiCl or 1 μM SB216763. For pellet cultures, 2.5 × 105 hWJ-MSCs were centrifuged at 3000 rpm for 5 min in a 15-mL conical tube (Corning, Corning, NY, USA) to form pellets [24] and then incubated at 37°C in a humidified atmosphere of 5% CO2 in air. After 24 h, the pellets were cultured in 4 different media as mentioned above. The tubes were incubated with loosened cap at 37°C in a humidified atmosphere of 5% CO2 in air. The medium was replaced every 3 days.
GAG analysis
Cell monolayer and pellets from the differentiation experiments were fixed with 4% PFA for 30 min at room temperature. Cell pellets were embedded in Cryostat embedding medium (Pink, Killik, Italy) and frozen on dry ice. The embedded samples were cut at 10-μm thickness with a cryostat microtome (CM2850, Hestion, Australia) and placed in the center of a coated slide. Monolayer expanded cells and pellet section samples were examined for GAG accumulation by staining with Alcian blue 8× for 30 min.
Immunofluorescence staining
Monolayer expanded cells and pellet sections were blocked and permeabilized for 1 h at 37°C with 5% bovine serum albumin (BSA), 5% normal goat serum, and 0.1% triton-X100. For collagen type II and X staining, samples were predigested with 1 mg/mL pepsin and 0.2% hyaluronidase, respectively. Anti-human type II collagen (clone 6B3, Chemicon) and anti-human type X collagen (Calbiochem) primary antibodies were incubated overnight. Samples were incubated for 2 h with the respective secondary antibodies. Then, samples were stained with DAPI and observed under a fluorescence microscope.
Gene expression analysis
After 28 days of induction, total RNA was isolated from the cells by total RNA extraction kit (RBC Real Genomics, RBC Bioscience, Taipei, Taiwan) according to the manufacturer’s instructions. Then, RNA was reverse-transcribed in the presence of oligo-dT primer for complementary DNA (cDNA) synthesis by iScript™ Reverse Transcription Supermix for RT-qPCR (BioRad, Hercules, CA, USA). The expression of several genes was assessed by using Light Cycler® 480 (Roche Diagnostics, Basel, Switzerland) and KAPA SYBR-Green PCR Master mix (Applied Biosystems, Carlsbad, CA, USA). The primers used are shown in Table 1. Melting curve analysis was undertaken to determine the specificity of the primers. Gene expression was normalized to the reference gene GAPDH and calculated as the relative expression compared to control cells. The qPCR analyses were performed three times.
Table 1. Primers used for qPCR analysis.
| Gene | Primer Sequences (5’→3’) | Annealing Temperature | Product Size (bp) | References |
|---|---|---|---|---|
| ACAN | F:ACTTCCGCTGGTCAGATGGA | 63 | 111 | [26] |
| R:TCTCGTGCCAGATCATCACC | ||||
| Sox9 | F:GGCAAGCTCTGGAGACTTCTG | 59 | 207 | [26] |
| R:CTGCAGCGCCTTGAAGATG | ||||
| Col2a1 | F:GAGACAGCATGACGCCGAG | 62 | 67 | NM_001844.4 |
| R:GCGGATGCTCTCAATCTGGT | ||||
| Col10a1 | F:CCCTCTTGTTAGTGCCAACC | 62 | 155 | [27] |
| R:AGATTCCAGTCCTTGGGTCA | ||||
| Runx2 | F:ATACCGAGTGACTTTAGGGATGC | 62 | 131 | [27] |
| R:AGTGAGGGTGGAGGGAAGAAG | ||||
| β-catenin | F:AATGCTTGGTTCACCAGTG | 62 | 176 | [17] |
| R:GGCAGTCTGTCGTAATAGCC | ||||
| GAPDH | F:TGCCCCCGACCGTCTAC | 60 | 110 | [28] |
| R:ATGCGGTTCCAGCCTATCTG |
Western blot analysis
Total protein was extracted from the samples 4 weeks post-induction by using lysis buffer containing 10% sodium dodecyl sulfate (SDS, Affymetrix Inc, Santa Clara, CA, USA), 0.1 M dithiothreitol (DTT, Invitrogen), 1% glycerol, 1.2% urea, and 1 M Tris-HCl pH 7.4 and complete protease inhibitor. The total protein concentration was determined by using the Bradford assay [29]. Twenty micrograms of total protein were separated on 10% SDS-PAGE, followed by electro-transfer to nitrocellulose membrane (BioRad). The membranes were exposed to blocking buffer (5% skim milk in PBS with 0.1%Tween-20 (PBST)) and then incubated with either anti-human type II collagen (dilution 1:1,000, clone 6B3, Chemicon) or anti-human β-actin (dilution 1:1,000, Millipore). Membranes were incubated with (goat anti-rabbit or -mouse) secondary antibody conjugated to alkaline phosphatase (dilution 1:20,000) and were then developed by using 5-Bromo-4-chloro-3-indolyl phosphate/Nitro blue tetrazolium (SIGMA FAST™ BCIP/NBT).
Statistical analysis
All experiments were repeated three times. Statistical analyses were performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as mean ± standard deviation of independent experiments. Statistical difference was analyzed using one-way analysis of variance (ANOVA) with Tukey’s HSD Post Hoc Test. P-value <0.05 was considered significant.
Results
Isolation and characterization of hWJ-MSCs
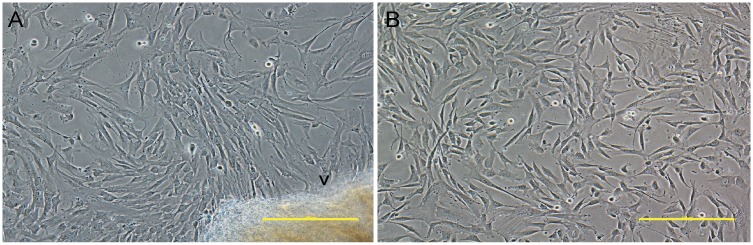
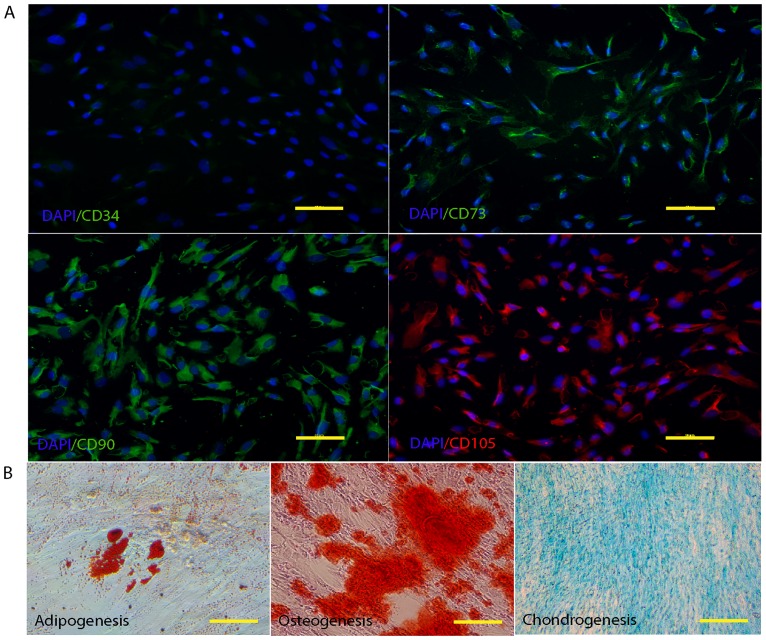
MSCs were isolated from WJ tissue of the human umbilical cord. Primary MSCs derived from WJ tissue were adherent, spindle shape, and fibroblast-like cells. The outgrowths of the cells were observed after 5–7 days of culture (Fig 1A). When the cells reached 80% confluence (Fig 1B), they were harvested and expanded for further usage. Based on the MSC properties using standard criteria for the identification of MSCs [28], hWJ-MSCs were positive for the MSC markers, CD73, CD90, and CD105, and negative for the hematopoietic marker, CD34 (Fig 2A). In addition, hWJ-MSCs were induced toward adipocytes, osteoblasts, and chondrocytes to confirm their capacity for MSC differentiation. As shown in Fig 2B, the lipid droplets formation was demonstrated by Oil Red O staining. Calcium deposit was visualized by Alizarin Red. Moreover, the accumulation of GAGs was examined by Alcian blue staining.
Fig 1. Morphology of hWJ-MSCs with a typical fibroblast-like morphology.
(A) Phase contrast images of hWJ-MSCs expanded from Wharton’s jelly tissue (arrow) and (B) hWJ-MSCs at 80% confluences. Scale bar = 10 μm.
Fig 2. Characterization of hWJ-MSCs.
(A) Immunophenotype of MSCs, immunofluorescent micrographs staining expression of MSC markers (CD73, 90, and 105), Nuclei were counterstains with DAPI (blue). Cells were negative for hematopoietic marker (CD34). Scale bar = 20 μm. (B) Differentiation of hWJ-MSCs to mesodermal linage cells. The cells were induced to undergo adipogenic, osteogenic, and condrogenic differentiation.
Effect of LiCl and SB216763 on hWJ-MSC viability
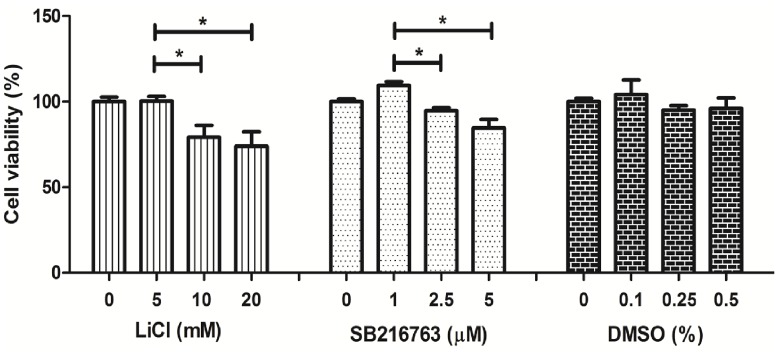
The viability of hWJ-MSCs was determined after culture in the presence of different concentrations of LiCl and SB216763 for 3 days using MTT assay. Culture medium without LiCl and SB216763 was used as control. Data are expressed as percent (%) normalized to the control condition without chemical supplementation. For the vehicle control, the cells were treated with DMSO (0.1–0.5%). The results indicated that DMSO did not decrease hWJ-MSC viability as shown in Fig 3. However, LiCl and SB216763 presented cytotoxic effects. In the presence of LiCl, the viability of hWJ-MSCs was reduced in a dose-dependent manner. In the presence of 5 mM LiCl, cell viability was 100.3 ± 2.73% which was significantly higher than that in the presence of 10 and 20 mM LiCl (79.17 ± 7.00%, 73.96 ± 8.5%, respectively). These results indicated that high LiCl concentrations, 10 and 20 mM, could induce hWJ-MSC death. In the presence of SB216763, the cell viability significantly decreased in a dose-dependent manner. In the presence of 1 μM SB216763, cell viability was 109.38 ± 2.36%, which was significantly higher than that in the presence of 2.5 and 5 μM SB216763 (95.64 ± 1.79% and, 84.52 ± 5.14%, respectively) (Fig 3). Hence, for further experiments, 5 mM of LiCl and 1 μM SB216763 were selected to study the effects of LiCl and SB216763 on chondrogenic differentiation.
Fig 3. The toxicity effect of LiCl and SB216763 on hWJ-MSC viability.
hWJ-MSCs were cultured with 0–20 mM LiCl (A), 0–5 μM SB216763 (B), or 0–0.5% DMSO (C), for 72 hrs in 96-well plate. Then, the viability was detected by MTT assay. **DMSO without chemical was use as vehicle control. **Data were exposed as mean ± SD. *P<0.05.
Effect of LiCl and SB216763 on chondrogenic differentiation
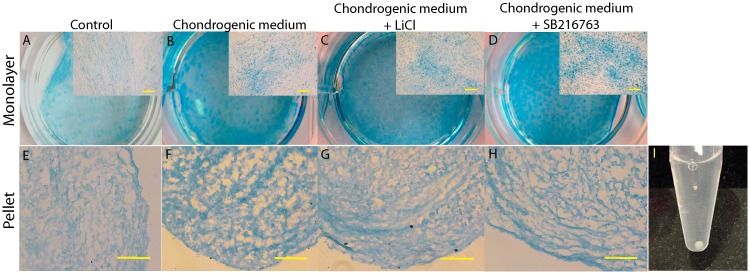
Chondrogenic differentiation of hWJ-MSCs was confirmed by Alcian blue staining for GAG matrix synthesis that is an important ECM component of the cartilage tissue. hWJ-MSCs were cultured in different culture media, including chondrogenic medium and chondrogenic medium supplemented with LiCl or SB216763. Monolayer and pellet cultures were grown in the medium. After 2 weeks of monolayer experiment, positive staining with Alcian blue was identified in all treatment groups except for the control group (Fig 4A–4D). Strong staining was observed in chondrogenic medium + SB216763, a weaker blue staining was observed in cells cultured in chondrogenic medium + LiCl and chondrogenic medium alone. For pellet experiments, after 4 weeks, the morphology has a white shiny cartilage-like appearance (Fig 4I). We further stained the pellet sections with Alcian blue (Fig 4E–4H). Alcian blue staining of GAGs was observed in all treatment groups, except for the control group.
Fig 4. Accumulation of GAGs was stained by alcian blue.
(A-D) Photographs of monolayer expanded cells cultured for 2 weeks. Scale bar = 10 μm. (E-H) Pellets culture at 4 weeks after differentiation. Scale bar = 20 μm. (I) Morphology of pellet culture at 4 weeks after differentiation.
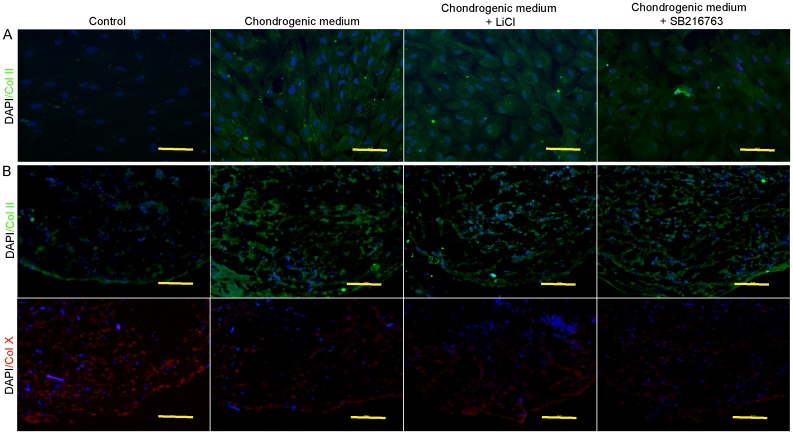
To further analyze chondrogenic differentiation, we performed immunofluorescence staining for a cartilage specific marker, collagen type II (Fig 5). In monolayer experiments, an increase in the expression of collagen type II was detected in the treatment groups, in which cells develop a dense filamentous matrix network. This was not observed in the control group (Fig 5A). We also observed a substantial number of collagen type II positive cells in the pellet experiments (Fig 5B). Interestingly, collagen type II expression clearly increased in the treatment groups. However, in the control group, no tissue-like morphology was observed and cells were negative for collagen type II. Collagen type II expression was confirmed by western blot analysis. As shown in Fig 6, collagen type II expression was stronger in cells cultured in the chondrogenic medium + SB216763 than in cells cultured in the chondrogenic medium + LiCl and chondrogenic medium alone.
Fig 5. Immunofluorescent staining of cartilage specific type collagen.
(A) immunofluorescent staining for collagen type II in monolayer expanded cultured on 3 weeks after differentiation. Scale bar = 20 μm. (B) Collagen type II and X expressions in pellet experiment on 4 weeks after differentiation. Scale bar = 20 μm.
Fig 6. The collagen type II protein after 4 weeks of inductions examined by western blot analysis.
β-actin was used as an internal control.
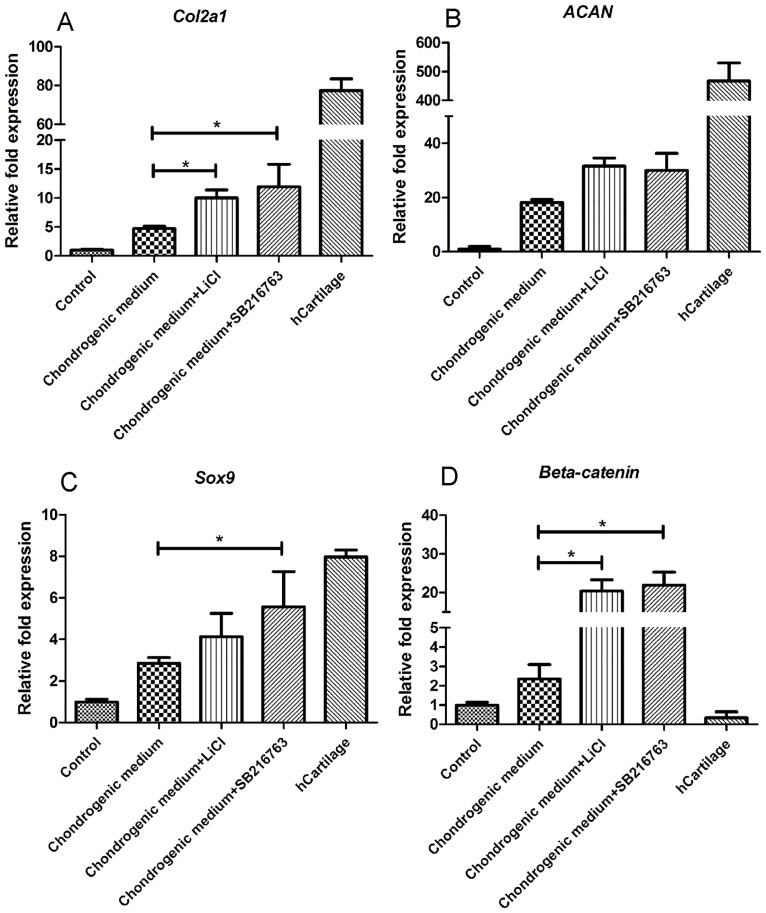
In addition, the expression of chondrogenic genes (Col2a1, ACAN, and Sox9) was investigated. Gene expression was normalized to GAPDH and calculated as the relative expression compared to the control group. Col2a1 expression significantly increased in cells cultured in the chondrogenic medium + SB216763 (12-fold) and chondrogenic medium + LiCl (10-fold) groups compared to cells cultured in the chondrogenic medium alone (4-fold) (Fig 7A). No significant difference was observed between the chondrogenic medium + SB216763 group and the chondrogenic medium + LiCl group. ACAN expression increased in both groups (chondrogenic medium + SB216763 and chondrogenic medium + LiCl) and reached 30-and 31-fold when compared to the control group (Fig 7B), respectively. However, no significant difference was observed when compared to cells cultured in the chondrogenic medium alone (18-fold). In the chondrogenic medium + SB216763 group, Sox9 expression increased by 5-fold and was significantly different than that in the chondrogenic medium alone group (2-fold), while that of the chondrogenic medium + LiCl group was not significantly different (4-fold) than that of the control group (Fig 7C).
Fig 7. qPCR analysis for chondrogenic gene expressions after 4 weeks of inductions.
(A) Col2a1, (B) ACAN, (C) Sox9 and (D) β-catenin. Gene expression was normalized to coresponding GAPDH and calculated by relative expression compared to control cells. The experiments were perfromed three times. **Data were expressed as mean±SD, *P<0.05.
Effect of LiCl and SB216763 on the Wnt signaling pathway
We next examined the expression of members of the Wnt/β-catenin signaling pathway. β-catenin expression increased in the chondrogenic medium + SB216763 and chondrogenic medium + LiCl groups, reaching 22-and 20-fold that of the control group, respectively (Fig 7D). In both groups, β-catenin expression was significantly higher than that of cells cultured in the chondrogenic medium alone (2-fold). These results indicated that LiCl and SB216763 are able to induce the Wnt signaling pathway by increasing β-catenin expression.
LiCl and SB216763 treatments suppressed the progression of chondrocyte hypertrophy
We also investigated the effects of LiCl and SB216763 on chondrocyte hypertrophy markers. The pellet sections were stained with anti-human collagen type X, a marker of hypertrophic chondrocytes developing from the osteogenic lineage. Cells in the control group developed normally to the osteogenic linage as evidence by strong collagen type X expression. However, in the treatment groups, positive staining was not or only slightly observed in the pellet sections (Fig 5B).
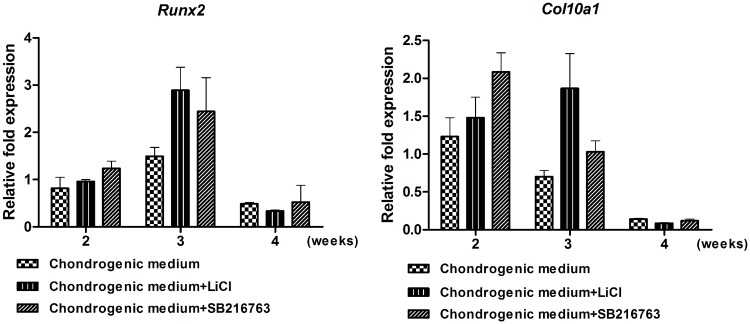
In addition, qPCR was used to examine the expression of markers of the development of chondrocytes derived from hWJ-MSCs to the hypertrophic state. We collected samples at 2, 3, and 4 weeks after differentiation to evaluate Runx2 and Col10a1 expression. We observed a modest and transient enhancement in Col10a1 and Runx2 mRNA levels, which was more evident in chondrogenic medium + SB216763 and chondrogenic medium + LiCl groups after 2 weeks of induction. At later time-points of differentiation (4 weeks of induction), Col10a1 and Runx2 expression decreased in all treatment groups (Fig 8A and 8B). These results indicated that LiCl and SB216763 did not induce hypertrophic differentiation of hWJ-MSCs, while promoting chondrogenesis.
Fig 8. Expression level of hypertrophic marker genes (A) Col10a1, and (B) Runx2 was quantified by qPCR after 2, 3, and 4 weeks of inductions.
Gene expression was normalized to coresponding GAPDH and calculated by relative expression compared to control cells. Data were expressed as mean±SD, The expperiments were perfromed three times.
Discussion
Degenerative articular cartilage remains one major problem worldwide, especially for the elder. Regeneration requires chondrocytes to repair the erosion of the ECM and improved cartilage repair. The use of chondrogenically differentiated MSCs has been proposed as a therapeutic strategy for cartilage regeneration [30]. Here, we investigated the influence of LiCl and SB216763 synergistically with TGF-β3 on chondrogenic differentiation of hWJ-MSCs.
The Wnt signaling pathway modulates chondrogenesis and cartilage development [12] by regulating chondrocyte proliferation and differentiation, and by maintaining the cell phenotype [31]. GSK-3 inhibition by LiCl or SB216763 promotes β-catenin accumulation and induces the Wnt signaling pathway [23,24]. β-catenin accumulation activates transcription in conjunction with co-transcription factors, LEFs/TCFs [32]. It was previously reported that LiCl (5–10 mM) can effectively induce the canonical Wnt signaling pathway and mediate cell differentiation of MSCs [16,17,33] and articular chondrocytes [34]. SB216763 has been shown to regulate cell proliferation and survival and to induce the transcription of β-catenin-dependent genes [24].
Several studies reported that the crosstalk between Wnt signaling and other signaling pathways can modulate chondrogenesis. Fischer and colleagues demonstrated that Wnt3A in combination with BMP-2 can enhance chondrogenesis in mMSCs [13]. Narcisi and colleagues also reported that Wnt3A in combination with FGF-2 supported the long-term expansion and enhanced chondrogenic potential in MSCs [18]. Eslaminejad and colleagues reported that combination of TGF-β with LiCl or SB216763 induced chondrogenic differentiation, demonstrated by an increase in the expression of Sox9, ACAN, and Col2a1. Proteoglycan levels were also evaluated during chondrogenic differentiation of MSCs from the bone marrow [17]. Our results showed that treatment of hWJ-MSCs with LiCl or SB216763 synergistically with TGF-β3 to induce chondrogenic differentiation up-regulated the expression of cartilage-specific markers, including ACAN, Col2a1, and Sox9 as well as GAG accumulation in the monolayer and pellet experiments. Western blot analysis revealed that the production of collagen type II was increased. However, the results of this study came from only one patient. Samples from different patient donors might have different efficiency, proliferation capacity, and potential for differentiation [35]. Since MSCs from umbilical cords are easy to isolate and has expansion potential. It would be a choice candidate for cell-based therapies in the future. This study reveals the mechanisms by which TGF-β3 affects the Wnt/β-catenin signaling pathway, promoting the chondrogenic differentiation of hWJ-MSCs. TGF-β3 is known as a major inducer promoting chondrogenic differentiation by inducing the downstream phosphorylation of Smad2/3, which directly leads to the induction of chondrogenesis due to the stabilization of the Sox9 transcription complex by Smad2/3 [36,37]. TGF-β can independently or cooperatively regulate LEF/TCF target genes in the Wnt signaling pathway and these pathways can synergistically activate target genes [38]. Another report showed that β-catenin signaling induces transcriptional activity and promotes chondrogenic differentiation in a Sox9-dependent manner [39]. Sox9 plays an important role directly regulating the expression of the cartilage genes, Col2a1 and ACAN, during chondrogenesis [40].
The Wnt/β-catenin signaling pathway plays a crucial role in the progression of chondrocyte hypertrophy. Our results showed that LiCl or SB216763 treatment suppressed the progression of chondrocyte hypertrophy as evidenced by decreased expression of Col10a1 and Runx2 markers. These results are in agreement with a study from Yang and colleagues showing that the continuous co-activation of two signaling pathways inhibited chondrocyte hypertrophy by suppressing the expression of Col10a1, Runx2, and alkaline phosphatase, and did not lead to ossified tissue in vivo [16]. However, Kawata and colleagues showed that activation of the Wnt/β-catenin signaling pathway in chondrocytes by Wnt3a or SB216763 inhibits GSK-3 phosphorylation and decreased the expression of ACAN and Col2a1, while increasing the expression of Col10a1 and MMP-13 [19]. In this study, cells were not cultured with any TGF-β supplements. The supplementation of any TGF-β stimulates the early chondrogenic differentiation but they inhibit the terminal differentiation of chondrocytes. Mueller and colleagues reported that TGF-β3 having the highest chondrogenic potential of all isoforms, and their action results in rapid cell differentiation [41].
Prolonged activation of Wnt signaling pathway promotes chondrocyte maturation. This study showed that cultured cells with GSK-3 inhibitors (Wnt agonist) increased the expression of β-catenin to induce the Wnt signaling pathway, while the cartilage chondrocytes from the patient expressed a very low level. It might be not improved homeostasis to repair tissue. In previously study showed that Wnt signaling pathway are involved in supporting repair processes by maintaining a stem cell pool and specifying cell fates in other organs [39,42], it would be a similar function in the tissue repair after transplantation.
Both LiCl and SB216763 act as GSK-3 inhibitors, thereby initiating the Wnt signaling pathway. LiCl is a chemical compound that has already been approved as a therapeutic and used for the treatment of patients with bipolar disorder [43]. Our results showed that the expression of collagen type II was strongly increased in the chondrogenic medium + SB216763 as evidence by western blot analysis. It also up-regulated the expression of several genes, including ACAN, Col2a1, and Sox9. SB216763 was more effective than LiCl treatment, as previously reported [17]. SB216763 is a synthetic small molecule that can rapidly diffuse across cell membranes, reach intracellular sites of action, and specifically target the signaling pathway [44].
In conclusion, GSK-3 inhibitors (LiCl and SB216763) can induce the Wnt signaling pathway and promote chondrogenic differentiation of hWJ-MSCs in the presence of TGF-β3, without inducing chondrocyte hypertrophy. These results indicate that LiCl and SB216763 are choice candidates for further in vivo therapeutic trials and would be of great importance for cartilage regeneration.
Acknowledgments
This work was supported by Suranaree University of Technology (SUT), the Office of the Higher Education Commission under NRU project of Thailand, Thailand Research Fund. The authors would like to thank Mr. Wachira. Panta and Ms. Sujitra. Khampang (Embryo Technology and Stem Cell Research Center) for their technical assistance. Dr. Imsoonthornruksa was supported by SUT postgraduate research fellowships.
Data Availability
All relevant data are within the paper.
Funding Statement
This work received financial support from the Bangkok Stem Cell Co., Ltd. Nakhon Pathom, Thailand and Suranaree University of Technology (SUT), the Office of the Higher Education Commission under NRU project of Thailand, Thailand Research Fund. Dr. Imsoonthornruksa was supported by SUT postgraduate research fellowships. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fox AS, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009; 1: 461–468. 10.1177/1941738109350438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284(5411): 143–147. [DOI] [PubMed] [Google Scholar]
- 3.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tis Eng. 2001; 7: 211–228. [DOI] [PubMed] [Google Scholar]
- 4.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000; 97: 13625–13630. 10.1073/pnas.240309797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phuc P, Nhung T, Loan D, Chung D, Ngoc P. Differentiating of banked human umbilical cord blood-derived mesenchymal stem cells into insulin-secreting cells. In Vitro Cell Dev Biol Anim. 2011; 47(1): 54–63. 10.1007/s11626-010-9356-5 [DOI] [PubMed] [Google Scholar]
- 6.Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, et al. Mesenchymal stem cells in the wharton's jelly of the human umbilical cord. Stem cells. 2004; 22(7): 1330–1337. 10.1634/stemcells.2004-0013 [DOI] [PubMed] [Google Scholar]
- 7.Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells. Exp Hematol. 2000; 28: 707–715. [DOI] [PubMed] [Google Scholar]
- 8.Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006; 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Troyer DL, Weiss ML. Concise review: Wharton's jelly-derived cells are a primitive stromal cell population. Stem cells. 2008; 26: 591–599. 10.1634/stemcells.2007-0439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fong CY, Chak LL, Biswas A, Tan JH, Gauthaman K, Chan WK, et al. Human Wharton's jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev. 2011; 7: 1–16. 10.1007/s12015-010-9166-x [DOI] [PubMed] [Google Scholar]
- 11.Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, et al. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun. 2004; 320: 914–919. 10.1016/j.bbrc.2004.06.029 [DOI] [PubMed] [Google Scholar]
- 12.Chun JS, Oh H, Yang S, Park M. Wnt signaling in cartilage development and degeneration. BMB Rep. 2008; 41: 485–494. [DOI] [PubMed] [Google Scholar]
- 13.Fischer L, Boland G, Tuan RS. Wnt-3A enhances bone morphogenetic protein-2-mediated chondrogenesis of murine C3H10T1/2 mesenchymal cells. J Biol Chem. 2002; 277: 30870–30878. 10.1074/jbc.M109330200 [DOI] [PubMed] [Google Scholar]
- 14.Tuli R, Tuli S, Nandi S, Huang X, Manner PA, Hozack WJ, et al. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling crosstalk. J Biol Chem. 2003; 278: 41227–41236. 10.1074/jbc.M305312200 [DOI] [PubMed] [Google Scholar]
- 15.Kirton JP, Crofts NJ, George SJ, Brennan K, Canfield AE. Wnt/β-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes potential relevance to vascular disease?. Circ Res. 2007; 101: 581–589. 10.1161/CIRCRESAHA.107.156372 [DOI] [PubMed] [Google Scholar]
- 16.Yang Z, Zou Y, Guo XM, Tan HS, Denslin V, Yeow CH, et al. Temporal activation of β-catenin signaling in the chondrogenic process of mesenchymal stem cells affects the phenotype of the cartilage generated. Stem Cells Dev. 2012; 21: 1966–1976. 10.1089/scd.2011.0376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eslaminejad MB, Karimi N, Shahhoseini M. Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells treated by GSK-3 inhibitors. Histochem Cell Biol. 2013; 140: 623–633. 10.1007/s00418-013-1121-x [DOI] [PubMed] [Google Scholar]
- 18.Narcisi R, Cleary MA, Brama PA, Hoogduijn MJ, Tüysüz N, ten Berge D, et al. Long-Term Expansion, Enhanced Chondrogenic Potential, and Suppression of Endochondral Ossification of Adult Human MSCs via WNT Signaling Modulation. Stem Cell Reports. 2015; 4: 459–472. 10.1016/j.stemcr.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawata K, Kubota S, Eguchi T, Moritani NH, Shimo T, Kondo S, et al. Role of the lowdensity lipoprotein receptor-related protein-1 in regulation of chondrocyte differentiation. J Cell Physiol. 2010; 222: 138–148. 10.1002/jcp.21930 [DOI] [PubMed] [Google Scholar]
- 20.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009; 17: 9–26. 10.1016/j.devcel.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Jope RS. Is glycogen synthase kinase-3 a central modulator in mood regulation?. Neuropsychopharmacol. 2010; 35(11): 2143–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuang DM, Wang Z, Chiu CT. GSK-3 as a target for lithium-induced neuroprotection against excitotoxicity in neuronal cultures and animal models of ischemic stroke. Front Mol Neurosci. 2011; 4: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996; 93: 8455–8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000; 7: 793–803. [DOI] [PubMed] [Google Scholar]
- 25.Petsa A, Gargani S, Felesakis A, Grigoriadis N, Grigoriadis I. Effectiveness of protocol for the isolation of Wharton’s Jelly stem cells in large-scale applications. In Vitro Cell Dev Biol Anim. 2009; 45: 573–586. 10.1007/s11626-009-9227-0 [DOI] [PubMed] [Google Scholar]
- 26.Peran M, Ruiz S, Kwiatkowski W, Marchal JA, Yang SL, Aranega A, et al. Activin/BMP2 chimeric ligands direct adipose-derived stem cells to chondrogenic differentiation. Stem Cell Res. 2013; 10: 464–476. 10.1016/j.scr.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 27.Karl A, Olbrich N, Pfeifer C, Berner A, Zellner J, Kujat R, et al. Thyroid hormone-induced hypertrophy in mesenchymal stem cell chondrogenesis is mediated by bone morphogenetic protein-4. Tissue Eng Part A. 2014; 20: 178–188. 10.1089/ten.TEA.2013.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasuike S, Ido A, Uto H, Moriuchi A, Tahara Y, Numata M, et al. Hepatocyte growth factor accelerates the proliferation of hepatic oval cells and possibly promotes the differentiation in a 2-acetylaminofluorene/partial hepatectomy model in rats. J Gastroenterol Hepatol. 2005; 20: 1753–1761. 10.1111/j.1440-1746.2005.03922.x [DOI] [PubMed] [Google Scholar]
- 29.Bradford MM. The Environment and Disease: Association or Causation?. Proc R Soc Med. 1965; 58(5): 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung C, Burdick JA. Engineering cartilage tissue. Adv Drug Deliv Rev. 2008; 60: 243–262. 10.1016/j.addr.2007.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasuhara R, Ohta Y, Yuasa T, Kondo N, Hoang T, Addya S, et al. Roles of β-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab Invest. 2011; 91: 1739–1752. 10.1038/labinvest.2011.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002; 296: 1644–1646. 10.1126/science.1071549 [DOI] [PubMed] [Google Scholar]
- 33.Zhu Z, Yin J, Guan J, Hu B, Niu X, Jin D, et al. Lithium stimulates human bone marrow derived mesenchymal stem cell proliferation through GSK-3bdependent b-catenin/Wnt pathway activation. FEBS Journal. 2014; 281: 5371–5389. 10.1111/febs.13081 [DOI] [PubMed] [Google Scholar]
- 34.Krase A, Abedian R, Steck E, Hurschler C, Richter W. BMP activation and Wnt-signalling affect biochemistry and functional biomechanical properties of cartilage tissue engineering constructs. Osteoarthritis Cartilage. 2014; 22: 284–292. 10.1016/j.joca.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 35.Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G, et al. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008; 9:60 10.1186/1471-2121-9-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furumatsu T, Ozaki T, Asahara H. Smad3 activates the Sox9-dependent transcriptionn chromatin. Int J Biochem Cell Biol. 2009; 41: 1198–1204. 10.1016/j.biocel.2008.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amano K, Hata K, Muramatsu S, Wakabayashi M, Takigawa Y, Ono K, et al. Arid5a cooperates with Sox9 to stimulate chondrocyte-specific transcription. Mol Biol Cell. 2011; 22: 1300–1311. 10.1091/mbc.E10-07-0566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Labbé E, Letamendia A, Attisano L. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and wnt pathways. Proc Natl Acad Sci U S A. 2000; 97: 8358–8363. 10.1073/pnas.150152697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yano F, Kugimiya F, Ohba S, Ikeda T, Chikuda H, Ogasawara T, et al. Canonical Wnt signaling pathway promotes chondrocyte differentiation in a Sox9-dependent manner. Biochem Biophys Res Commun. 2005; 333: 1300–1308. 10.1016/j.bbrc.2005.06.041 [DOI] [PubMed] [Google Scholar]
- 40.Akiyama H, Chaboissier MC, Martin JF, Schedl A, Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002; 16: 2813–2828. 10.1101/gad.1017802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mueller MB, Fischer M, Zellner J, Berner A, Dienstknecht T, Prantl L, et al. Hypertrophy in mesenchymal stem cell chondrogenesis: effect of TGF-beta isoforms and chondrogenic conditioning. Cells Tissues Organs. 2010; 192:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Developmental Cell. 2005; 8: 727–738. 10.1016/j.devcel.2005.02.013 [DOI] [PubMed] [Google Scholar]
- 43.Dubovsky SL. Treatment of Bipolar Depression. Psychiatr Clin North Am. 2005; 28: 349–370. 10.1016/j.psc.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 44.Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer. 2006; 6: 714–727. 10.1038/nrc1913 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.