Abstract
Background
Xuanwei district in Yunnan Province has the highest incidence of lung cancer in China, especially among non‐smoking women. Cruciferous vegetables can reduce lung cancer risk by prompting a protective mechanism against respiratory tract inflammation caused by air pollution, and are rich in sulforaphane, which can induce changes in gene expression. We investigated the effect of sulforaphane‐induced apoptosis in Xuanwei lung adenocarcinoma cell line (XWCL‐05) to explore the value of sulforaphane in lung cancer prevention and treatment.
Methods
Cell growth inhibition was determined by methyl thiazolyl tetrazolium assay; cell morphology and apoptosis were observed under transmission electron microscope; cell cycle and apoptosis rates were detected using flow cytometry; B‐cell lymphoma 2 (Bcl‐2) and Bcl‐2‐like protein 4 (Bax) messenger RNA expression were determined by quantitative PCR; and p53, p73, p53 upregulated modulator of apoptosis (PUMA), Bax, Bcl‐2, and caspase‐9 protein expression were detected by Western blotting.
Results
Sulforaphane inhibited XWLC‐05 cell growth with inhibitory concentration (IC)50 of 4.04, 3.38, and 3.02 μg/mL at 24, 48, and 72 hours, respectively. Sulforaphane affected the XWLC‐05 cell cycle as cells accumulated in the G2/M phase. The proportion of apoptotic cells observed was 27.6%. Compared with the control, the sulforaphane group showed decreased Bcl‐2 and p53 expression, and significantly increased p73, PUMA, Bax, and caspase‐9 protein expression (P < 0.05).
Conclusion
Sulforaphane induces Xuanwei lung adenocarcinoma cell apoptosis. Its possible mechanism may involve the upregulation of p73 expression and its effector target genes PUMA and Bax in lung cancer cells, downregulation of the anti‐apoptotic gene B cl ‐2, and activation of caspase‐9. It may also involve downregulation of the mutant p53 protein.
Keywords: Apoptosis, lung neoplasm, sulforaphane, Xuanwei, XWLC‐05
Introduction
Lung cancer is the leading cause of malignant tumor‐related death. It is estimated that 650 000 people in China are diagnosed with lung cancer every year, and that about 530 000 people die of the disease.1, 2 Most patients are diagnosed at a late stage, and the average life expectancy of lung cancer patients without treatment is 7.15 months, with a five‐year survival rate of 16.1%.3, 4 Xuanwei district in Yunnan Province has the highest lung cancer incidence in China. The district has a population of 1.46 million, 96% of which are agricultural workers. The mortality rate of lung cancer in Xuanwei between 1973 and 1979 (men 27.75 per 100 000; women 24.50 per 100 000) was higher than the national average (men 7.17 per 100 000; women 3.69 per 100 000).5 Xuanwei women do not have high rates of smoking, but do have one of the highest rates of lung cancer among non‐smoking women in the world. In 1987, women in Laibin town had a lung cancer incidence rate as high as 400 per 100 000, 20 times the national average.6 Epidemiological studies have found that lung cancer incidence in Xuanwei is closely related to the high indoor emissions of bituminous coal combustion from stoves.7 Since the 1980s, the local government has taken active measures to help residents replace their stoves to reduce indoor air pollution, but after 30 years, the data show that lung cancer mortality in Xuanwei continues to increase (83.28 per 100 000 in 2004–2005; 30.83 per 100 000 national average.).5
Cruciferous vegetables have been shown to significantly reduce the risk of lung cancer.8 This protection is independent of the smoking factor. Cruciferous vegetables also prompt a protective mechanism against respiratory tract inflammation caused by air pollution, and are rich in glucosinolate, which can be hydrolyzed into sulforaphane by myrosinase contained in the plant or by human colonic microbes.9, 10 Sulforaphane can induce changes in gene expression, especially in the differential expression of genes. Genes regulated by sulforaphane mainly include those involved in the metabolism of exogenous substances, antioxidation, cell cycle regulation, apoptosis, and stress reaction.11 The chemopreventive effect of sulforaphane has thus been widely noted.
In order to investigate the preventive effect and treatment value of sulforaphane in Xuanwei lung cancer and to provide a theoretical basis for new approaches to lung cancer prevention, we used the Xuanwei lung adenocarcinoma cell line (XWLC‐05) to conduct in vitro experiments to assess the effect of sulforaphane on proliferation and apoptosis in lung cancer cells.
Methods
Materials
Cell lines
The Institute of Oncology of Yunnan Provincial Tumor Hospital Human provided XWLC‐05 and immortal human bronchial epithelial cell line (BEAS‐2B).
Experimental drugs
Sulforaphane (Shaanxi Parnell Bio‐Technology, Shanxi, China, batch number PB0140526), purity ≥98%, stored at −20°C, and cisplatin (DDP; Luoxin Pharmaceutical Co., Ltd., Shandong, China, batch number 1106103) were used in this study. The drugs were diluted with physiological saline before use.
Main reagents and instruments
The following main reagents and instruments were utilized for this study: fetal bovine serum (FBS), culture medium RPMI 1640, culture medium high glucose Dulbecco's modified Eagle medium (HDMEM), trypsin, phosphate buffer solution (PBS), antibodies (Hyclone, Logan, UT, USA); methyl thiazolyl tetrazolium (MTT), dimethyl sulfoxide (DMSO), propidium iodide (PI), the TUNEL apoptosis kit, Trizol lysate, rabbit anti‐human monoclonal antibodies against p53, p73, p53 upregulated modulator of apoptosis (PUMA), B‐cell lymphoma 2 (Bcl‐2), Bcl‐2‐like protein 4 (Bax), caspase‐9 and secondary antibodies (Signalway Antibody, College Park, MD, USA); primer synthesis, a RevertAid First Strand cDNA Synthesis Kit, SYBR Green master mix, electrochemiluminescence detection reagent, high speed refrigerated centrifuge (Thermo Scientific, Waltham, MA, USA); radioimmunoprecipitation assay protein lysate, KODAK X‐OMAT BT film (Solarbio Life Sciences, Beijing, China); polyvinylidene difluoride membrane (Millipore, Temecula, CA, USA); transmission electron microscopy (JEOL, Akishima, Tokyo, Japan, model JEM‐1011); flow cytometry (Beckman, Indianapolis, IN, USA, model EPICS ALTRA 8 CLR); quantitative (q)PCR (ABI, Oyster Bay, NY, USA); gradient PCR, a vertical electrophoresis apparatus and film transfer device, horizontal electrophoresis apparatus (Bio‐Rad, Hercules, CA, USA); and a gel imaging system (Syngene, Frederick, MD, USA).
Methods
Cell culture and passage
XWLC‐05 and BEAS‐2B cells were respectively cultured in RPMI 1640 and HDMEM medium containing 10% FBS and maintained at 37°C and 5% CO2 in an incubator. At 70% confluency, cells were passaged using 0.25% trypsin.
Cell growth inhibition
XWLC‐05 and BEAS‐2B cells in the logarithmic growth phase were adjusted to a suspension concentration of 5 × 105/mL using complete medium. Aliquots of 5 × 103cells were seeded into a 96‐well plate, with 100 μL of suspension in each well. The following day, sulforaphane was added at concentrations of 0.5, 1, 2, 3, 4, and 5 μg/mL to the experimental groups, and appropriate controls were included. MTT experiments were performed 24, 48, and 72 hours later. MTT solution (20 μL/well) was added and cells were incubated for four hours. After removal of supernatant, DMSO was added at 150 μL/well and the plate was shaken for 10 minutes. Absorbance (A) was measured at 490 nm in an enzyme‐linked immunosorbent detector, and the inhibitory concentration (IC)50 rate was calculated for each group. Cell growth inhibition rate = (1 − treatment group A values/control group A values) × 100%. IgIC50 = Xm – I (P−(3 − Pm − Pn)/4) (Xm: Ig maximum dose, I:Ig (maximum dose/associated dose); P = the sum of positive reaction rates, Pm = maximum positive reaction rate; and Pn = minimum positive reaction rate).
Observation of cell apoptosis under transmission electron microscopy
XWLC‐05 and BEAS‐2B cells were grown to adherence for 24 hours. Medium was replaced with complete medium containing 1 μg/mL of sulforaphane, followed by culturing for another 48 hours. Cells were collected in an agar centrifuge tube and 4% paraformaldehyde was added for fixation. After washing, dehydrating, saturating, embedding, and ultrathin slicing, apoptosis was observed using a transmission electron microscope.
Detecting cell cycle changes by flow cytometry
XWLC‐05 cells in the logarithmic growth phase were digested into single‐cell suspension. Aliquots of 5 × 106 cells/well were seeded into a six‐well plate at 2 mL suspension in each well. Following 24 hours of culture, complete medium containing 1 μg/mL of sulforaphane was added, and cells were cultured for a further 48 hours while blank control groups were established. Cells were digested and collected, and 5 mL of 70% pre‐cooled ethanol was added for fixation. After washing, centrifugation, and cell resuspension, cells were treated with PI for 20 minutes, protected from light. The percentage of cells in G1/G0, S, and G2/M phases was measured by flow cytometry at 488 nm excitation and 525 nm emitting wavelength.
Detection of apoptosis by flow cytometry using TUNEL assay
XWLC‐05 cells were cultured in six‐well plates and treated with 1 μg/mL sulforaphane, as described previously. After digestion, collection, washing, and centrifugation, cells were resuspended in PBS. Cell concentration was adjusted to 1 × 106/mL, and cells were added to 1.5 mL tubes with 200 μL of 4% paraformaldehyde after washing and centrifugation. Cells were then incubated for 30 minutes at 4°C, 200 μL of 70% alcohol was added after washing and centrifugation, and then cells were incubated for a further 30 minutes at −20°C. Next, 30 μL of terminal deoxynucleotidyl transferase reaction solution or negative control solution was added after washing and removing the supernatant, and cells were incubated for 1 hour at 37°C. After washing and centrifugation, 20 μL of RNAse and 200 μL Triton were added, and cells were incubated for 15 minutes at 37°C. Finally, cells were treated with 1 mL PI dye for 20 minutes, protected from light, and flow cytometry was performed within 24 hours. The percentages of apoptotic (labeled with fluorescein isothiocyanate) and dead (non‐fluorescein isothiocyanate labeled, hypodiploid position) cells were calculated, and the phase in which apoptotic cells were found was evaluated.
Evaluation of B‐cell lymphoma 2 (Bcl‐2)/ Bcl‐2‐like protein 4 (Bax) messenger (m)RNA expression by real‐time fluorescent quantitative PCR
XWLC‐05 cells in the logarithmic growth phase were digested into a single‐cell suspension, and 5 × 106 cells/well were seeded into six‐well plates with 2 mL suspension per well. After 24 hours of culture, the medium was replaced with complete culture medium containing 1 μg/mL of sulforaphane or 1 μg/mL cisplatin (DDP) and cells were cultured for 72 hours. Cells were harvested and centrifuged, lysed using Trizol, shaken after the addition of chloroform, and centrifuged. Isopropanol was added to the aqueous phase, incubated at 4°C, and centrifuged. The precipitate was washed twice with 75% ethanol. The air‐dried precipitate was then dissolved with the RNase‐free water to obtain RNA. Next, 3‐μL aliquots of RNA solution were placed into 0.5 mL RNA enzyme removal tubes.
RNA was reverse transcribed to cDNA using a RevertAid First Strand cDNA Synthesis kit. The following primers were designed for qPCR: Bcl‐2 R: GAGCAGAGTCTTCAGAGA, F: TGCCTTTGTGGAACTGTA; Bax F: AAGAAGCTGAGCGAGTGT, R: GGCGGCAATCATCCTCTG; glyceraldehyde 3‐phosphate dehydrogenase: R: GCTGTTGTCATACTTCTC, F: AAAGGGTCATCATCTCTG. The following were added in order to each tube: 12.5 μL of 2×SYBR Green Master Mix, 0.5 μL upstream primer, 0.5 μL downstream primer, 1 μL cDNA template, and 10.5 μL nuclease‐free PCR water to achieve a final reaction solution of 25 μL. Mixtures were added to the wells of a 96‐well PCR plate, which was placed into an ABI 7300 cycler. The following PCR cycle reaction conditions were used: pre‐denaturation for 10 minutes at 95°C; 40 cycles of denaturation for 15 seconds at 95°C and annealing/extension for 30 seconds at 60°C; and melting curve analysis for 15 seconds at 95°C, 30 seconds at 60°C, and 15 seconds at 95°C. Finally, cycle threshold (Ct) value analysis was performed.
Determination of p53, p73, p53 upregulated modulator of apoptosis (PUMA), Bax, Bcl‐2, and caspase‐9 protein expression by Western blotting
XWLC‐05 cells were cultured in six‐well plates, as described previously, and treated with 1 μg/mL of sulforaphane or DDP for 48 or 72 hours. After washing in cold PBS, lysis buffer was added, and the contents of the well were collected into 1.5 mL tubes using a cell scraper and centrifuged at 12 000 rpm for 30 minutes at 4°C. Supernatant was aliquoted into 1.5 mL centrifuge tubes and stored at −20°C until use. For Western blotting, protein concentration was determined to calculate sample loading volume, and loading buffer was added to each sample before denaturation in boiling water for 15 minutes. Following electrophoresis, proteins were transferred to a polyvinylidene difluoride membrane, which was placed in 5% skimmed milk. After washing, primary antibody was added and membranes were incubated at 4°C overnight. Incubation with secondary antibody was performed for two hours at room temperature, membranes were washed, and substrate luminescence and film exposure were performed immediately.
Statistical analysis
SPSS version 18.0 (IBM Corp., Armonk, NY, USA) was used for data analysis, Data with normal distribution were expressed as mean ± standard deviation. For data with\skewed distribution, the central tendency was described using the median and dispersion was described using the interquartile range. The F test was used to test the homogeneity of variance. Data that conformed with the homogeneity of variance and normal distribution were subjected to t‐tests. Data characterized by inhomogeneity of variance, skewed distribution, and small sample size were subject to a non‐parametric rank‐sum test. P values <0.05 were considered statistically significant.
Results
Inhibitory effect of sulforaphane on Xuanwei lung adenocarcinoma cell line (XWLC‐05) cell growth
Proliferation was inhibited in XWLC‐05 cells at sulforaphane concentrations of 0.5, 1, 2, 3, 4, and 5 μg/mL. Inhibition gradually increased with increasing sulforaphane concentration (Fig 1). IC50 was 4.04, 3.38, and 3.02 μg/mL at the time points of 24, 48, and 72 hours, respectively.
Figure 1.
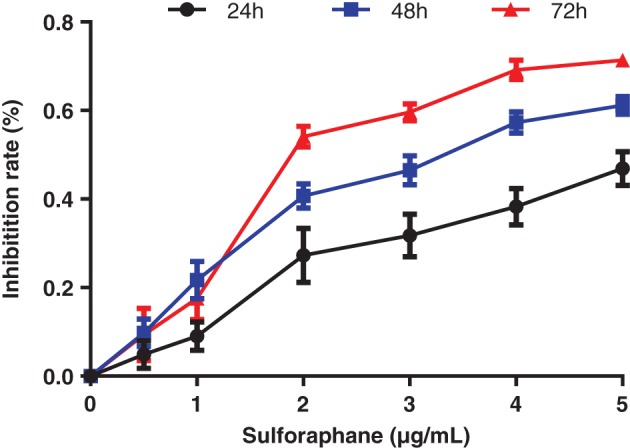
Inhibition of Xuanwei lung adenocarcinoma cell line cell proliferation by sulforaphane at various concentrations.
Effect of sulforaphane on XWLC‐05 cell cycle
Changes in cell cycle were detected by PI using flow cytometry. After 1 μg/mL of sulforaphane was applied to XWLC‐05 cells for 48 hours, the percentage of cells in the G0/G1 phase decreased while those in the G2/M phase increased. No significant difference was observed, however, in cells in the S phase compared with the control, while the differences mentioned above were statistically significant (P < 0.05; Fig 2).
Figure 2.
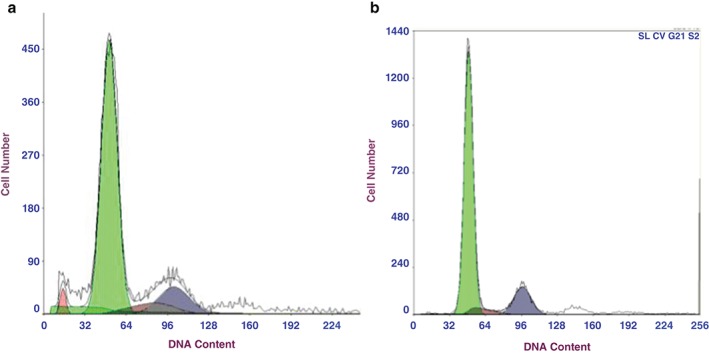
Cell cycle phase distribution of Xuanwei lung adenocarcinoma cell line cells by sulforaphane (1 μg/mL, 48 hours). (a ) Sulforaphane, and (b) control groups.
Apoptosis in XWLC‐05 cells induced by sulforaphane
Transmission electron microscopy
Early‐stage apoptosis was observed in XWLC‐05 cells 48 hours after treatment with 1 μg/mL sulforaphane. Nuclear chromatin was concentrated at the boundary of the nuclear membrane. As apoptosis progressed, the nuclei shrank and formed irregular shapes. Gas bubbles appeared in the condensed cytoplasm, and cell membranes burst, producing further bubbles. As shown in Figure 3, apoptotic bodies and apoptosis were observed. BEAS‐2B cells were treated with the same concentration of sulforaphane, but nuclei and cell membranes remained intact and apoptosis was not observed.
Figure 3.
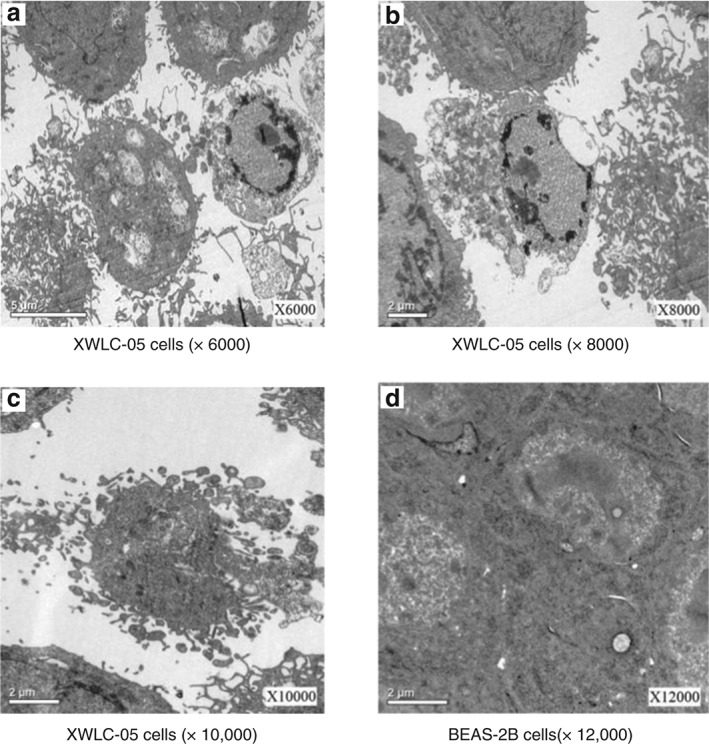
Cell morphology following sulforaphane treatment (1 μg/mL, 48 hours) under transmission electron microscopy. (a) Xuanwei lung adenocarcinoma cell line (XWCL‐05) cells (×6000), (b) XWLC‐05 cells (×8000), (c) XWLC‐05 cells (×10 000), and (d) bronchial epithelial (BEAS‐2B) cells (×12 000).
Flow cytometry using TUNEL assay
In XWLC‐05 cells treated for 48 hours with 1 μg/mL sulforaphane, apoptosis at a rate of 27.6% was observed by flow cytometry. This effect was statistically different from the control group (P < 0.05; Fig 4, Table 1).
Figure 4.
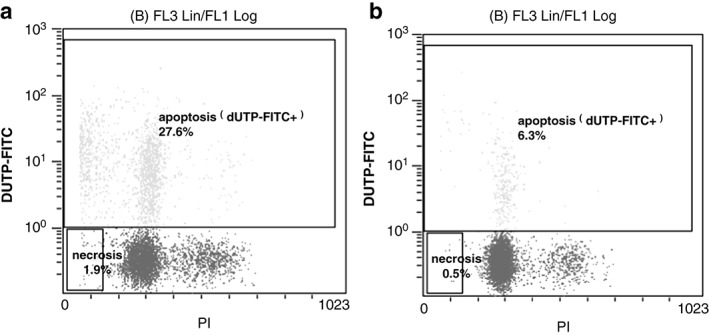
Apoptosis rate in Xuanwei lung adenocarcinoma cell line cells following sulforaphane treatment (1 μg/mL, 48 hours). (a) Sulforaphane, and (b) control groups. FITC, fluorescein isothiocyanate.
Table 1.
Xuanwei lung adenocarcinoma cell line cell apoptosis following sulforaphane treatment (1 μg/mL, 48 h)
| Group | Apoptosis and cell death rates (n = 9) | |
|---|---|---|
| Apoptosis rate (%) | Cell death rate (%) | |
| Sulforaphane | 27.68 ± 1.11* | 2.00 ± 0.19* |
| Control | 6.01 ± 0.52 | 0.50 ± 0.12 |
Versus control, P < 0.05.
Sulforaphane downregulated Bcl‐2 mRNA expression and upregulated Bax mRNA expression
Based on the calibration samples in the control group, differential gene expression among the experimental groups was analyzed using qPCR (2−ΔΔCt). Analysis was repeated three times per sample, and the average Ct value was used to calculate ΔCt, ΔΔCt, and 2−ΔΔCt, where ΔCt = detected gene Ct–reference gene Ct; and ΔΔCt = ΔCt (test sample) − ΔCt (calibration sample). The 2−ΔΔCt value represents relative gene expression in test samples compared with calibration samples. The results are shown in Table 2. Compared with the control group, Bcl‐2 expression in the sulforaphane group was significantly decreased, while Bax was significantly increased (P < 0.05). Similarly, the Bcl‐2 messenger (m)RNA expression level also decreased significantly, while that of Bax mRNA increased significantly in the DDP group (P < 0.05). Compared with the DDP group, Bax and Bcl‐2 expression levels in the sulforaphane group did not show significant differences (P > 0.05).
Table 2.
mRNA expression of Bcl‐2 and Bax in Xuanwei lung adenocarcinoma cell line cells (72 hours)
| Group | 2−ΔΔCt value (n = 9) | |
|---|---|---|
| Bcl‐2 mRNA | Bax mRNA | |
| Sulforaphane | 0.71 (0.67, 0.76)* | 1.65 (1.59, 1.73)* |
| DDP | 0.68 (0.61, 0.76)* | 1.55 (1.39, 1.71)* |
| Control | 1 | 1 |
Versus control, P < 0.05.
Bcl‐2, B‐cell lymphoma 2; DDP, cisplatin; mRNA, messenger RNA.
Sulforaphane downregulated Bcl‐2 and p53 protein expression
Compared with the control, Bcl‐2 and p53 protein expression in the sulforaphane group was significantly decreased (P < 0.05); the change was even more obvious 72 hours after treatment. Bcl‐2 protein expression in the DDP group decreased significantly (P < 0.05), but there were no significant changes in p53 protein expression (P > 0.05). Compared with the DDP group, no significant differences in Bcl‐2 expression were exhibited (P > 0.05), while p53 protein expression was significantly lower in the sulforaphane group (P < 0.05; Fig 5).
Figure 5.
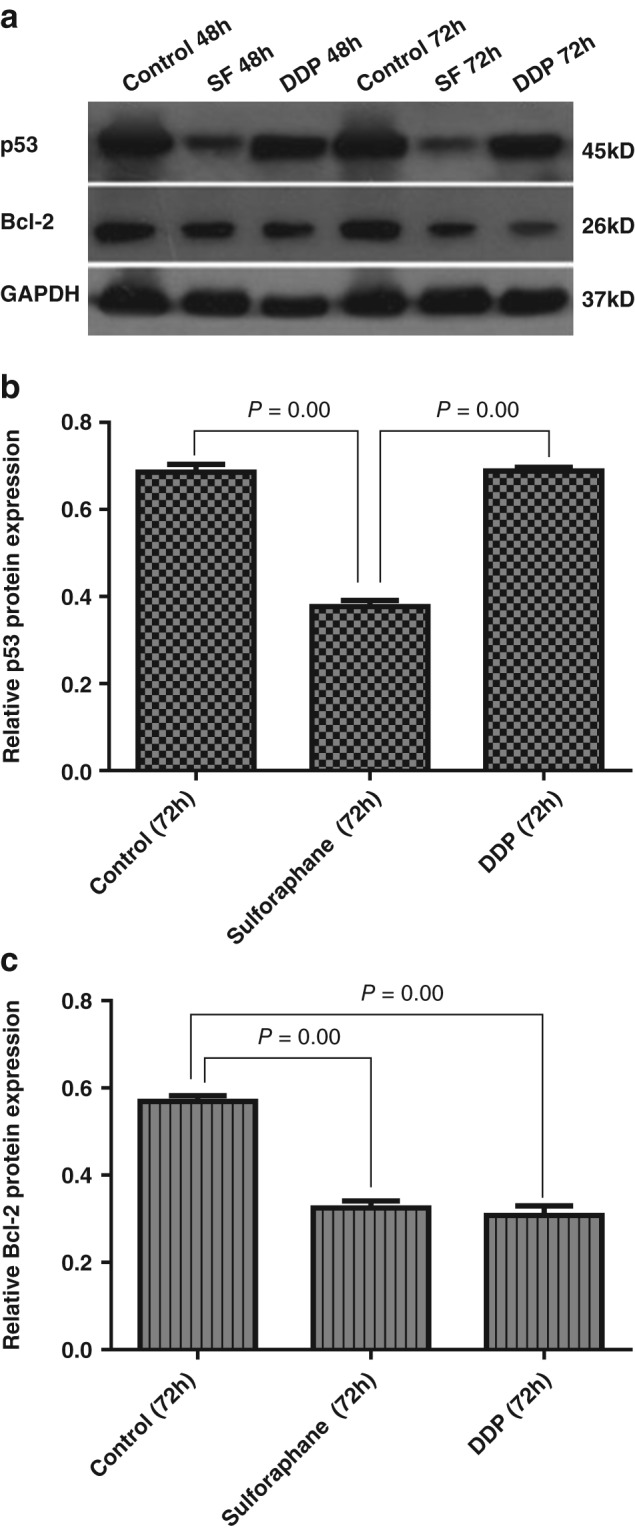
p53 and B‐cell lymphoma 2 (Bcl‐2) protein expression in Xuanwei lung adenocarcinoma cell line cells. (a) Western blot assay of Bcl‐2 and p53. Relative protein expression of (b) p53 and (c) Bcl‐2.DDP, cisplatin; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; SF, sulforaphane.
Sulforaphane upregulated p73, PUMA, Bax, and caspase‐9 protein expression
Compared with the control, the sulforaphane group showed significantly increased expression levels of p73, PUMA, Bax, and caspase‐9 proteins (P < 0.05), particularly at 72 hours (Fig 6).
Figure 6.
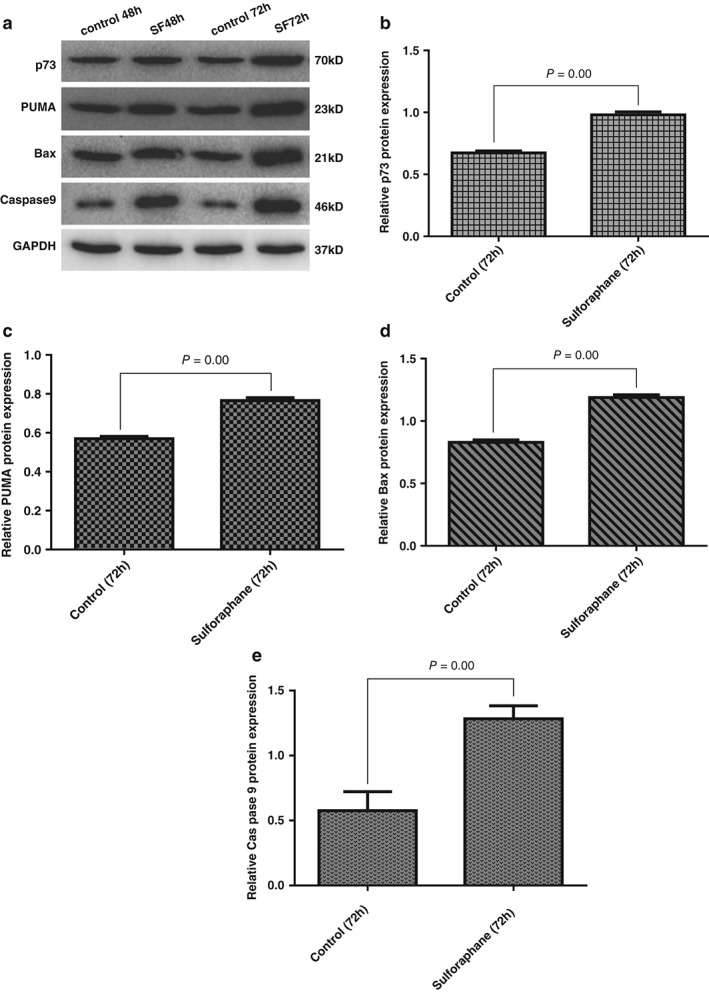
Expression of p73, p53 upregulated modulator of apoptosis (PUMA), Bax and caspase‐9 proteins in Xuanwei lung adenocarcinoma cell line cells. (a) Western blot assay of p73, PUMA, Bax, and caspase‐9. Relative protein expression of (b) p73, (c) PUMA, (d) Bax, and (e) caspase‐9. SF, sulforaphane.
Discussion
Apoptosis is an important mechanism for regulating cell proliferation under physiological and pathological conditions. It also plays a key role in the occurrence and development of tumors. Apoptosis control in malignant cells is usually abnormal, allowing cells to escape cell death. Inducing apoptosis in malignant cells is thus an important objective in research for cancer prevention and control. XWLC‐05, a Xuanwei lung adenocarcinoma cell line, was established in 2007 to represent lung cancer found in women in Xuanwei, Yunnan, and is ideal in an in vitro experimental model for studying Xuanwei lung cancer prevention and control.12 This study showed that sulforaphane inhibits the proliferation of and induces apoptosis in XWLC‐05 cells, similar to results from other studies on the growth inhibition effects of sulforaphane in colon, cervical, lung, prostate, and bladder cancers.13, 14, 15, 16, 17 Sulforaphane at a specific concentration (1 μg/mL) inhibited the proliferation of XWLC‐05 cells but had no obvious effect on the structure and proliferation of normal (BEAS‐2B) cells.
B‐cell lymphoma 2 overexpression is commonly observed in lung cancer. A meta‐analysis indicated approximately 76 and 35% positive Bcl‐2 protein expression in tissue samples from small cell and non‐small cell lung cancer patients, respectively.18 Studies have shown a positive Bcl‐2 expression rate of 78.16% in Xuanwei lung cancer patients, higher than in patients from mining regions and other areas in Yunnan Province (37.73 and 47.74%, respectively).19 Overexpression of anti‐apoptotic Bcl‐2 protein often leads to resistance to apoptosis and treatment in many tumor cell types. Downregulation of Bcl‐2 is thus a strategy for treating malignant tumors. Studies have found that sulforaphane can downregulate Bcl‐2 and Bcl‐xL proteins in cervical cancer HeLa cells and hepatocellular carcinoma HepG2 cells, and can upregulate pro‐apoptotic protein Bax, activate caspase‐3 protein, and degrade/cleave poly‐ADP ribose polymerase, thereby inducing apoptosis.15 Here, we showed that sulforaphane can downregulate Bcl‐2 protein in Xuanwei lung adenocarcinoma cells. Bcl‐2 is an important regulating factor in the mitochondrial apoptosis pathway. Similar concentrations of sulforaphane and the chemotherapeutic drug DDP showed an equal effect in downregulating Bcl‐2.
The p53 protein is a transcriptional product of the p53 gene. Wild‐type p53 (wt p53) is an important tumor suppressor gene; its transcriptional protein is only activated under cell stress or damage. Through upregulation of pro‐apoptotic genes, such as Bax, it prevents cells with destructive genetic information from entering into the cell cycle and thereby induces apoptosis, inhibiting the occurrence of cancer. Mutation type p53 gene (mt p53) exists in more than 50% of tumors and its protein is often expressed at a high level without influence from environmental stimuli. Mt p53 cannot promote cell growth, apoptosis, or DNA repair, and thus changes from a cancer suppressor gene to a cancer‐inducing oncogene.20 The spatial conformation of mt p53 is changed, with a prolonged half‐life, and can be detected in tissue and cells, while the wt p53 protein is generally undetectable as its half‐life is less than 20 minutes. Mt p53 protein has a negative regulatory effect on wt p53 protein; it inhibits the function of the wt p53 protein, leading to inefficient activation of apoptosis and promotion of tumor development. Therefore, destroying the stable expression of mt p53 protein is a molecular target for cancer prevention and treatment. Our study found that sulforaphane significantly induced apoptosis in Xuanwei lung adenocarcinoma cells while significantly lowering p53 protein expression. DDP had no significant effect on p53 protein expression in these cells, possibly because it is a non‐specific broad‐spectrum anti‐tumor drug that inhibits DNA replication in cancer cells, while the type of p53 protein downregulated by sulforaphane is mt p53 protein. The mutation rate of the p53 gene is about 60–70, 50–70, and 75% in lung squamous carcinoma, lung adenocarcinoma, and small cell lung cancer, respectively.21 p53 gene mutation is typically observed in cells from lung cancer patients from Xuanwei, at a mutation rate significantly higher than that of lung cancer patients in other regions of Yunnan and Guangdong Provinces.22, 23 Previous studies also found that the benzyl isothiocyanate and phenethyl isothiocyanate isomers of sulforaphane could dependently transcribe and selectively deplete mt p53 protein levels without losing wt p53 protein functionality.24 The mechanism behind this effect may be that nitrogen = carbon = sulfur‐containing phenethyl isothiocyanate compounds are highly electrophilic and susceptible to reaction with sulfur, nitrogen, and oxygen in amino acids. At cellular and molecular levels, covalent binding to intracellular molecules leads to changes in spatial conformation, thus consuming mt p53 protein.
As a member of the p53 transcription factor family, p73 has become one the most extensively studied proteins since its discovery in 1997. The p73 gene shares homologous structures and functions with the p53 gene, and has similar cell functions, such as the binding and transcriptional activation of p53‐responsive genes, and the induction of cell apoptosis and cell cycle arrest. Research has shown that the p73 gene is rarely mutated in human cancer (<1%) and the functions of p53 and p73 overlap. This indicates that p73 may play a role in tumor cell apoptosis by substituting p53 in the case of p53 mutation or deactivation.25 This study showed that p73 protein expression increased during the induction of apoptosis by sulforaphane in Xuanwei lung cancer cells. p53 upregulated modulator of apoptosis (PUMA) is an apoptosis‐regulatory factor directly transcriptionally activated by p73, which is capable of transmitting various apoptotic signals to important media in intracellular mitochondria. The effector target gene PUMA and the downstream pro‐apoptotic gene Bax of p73 were analyzed in this study. The expression levels of both genes were upregulated. Melino et al. found that the kinematics of p73 in regulating cell apoptosis corresponded to mitochondrial translocation but did not completely match the increased level of Bax protein, indicating that PUMA can also promote cell apoptosis through other pathways.26 Thus, we conducted further investigation of Bcl‐2 protein expression, and the results show that Bcl‐2 expression significantly decreased while that of its corresponding apoptotic protease caspase‐9 significantly increased. Our study revealed that p73 promoted the translocation of Bax via PUMA, triggered cell apoptosis in the mitochondrial pathways, weakened the blocking effect of the anti‐apoptotic Bcl‐2 protein in mitochondria, activated caspase, and caused the apoptosis of lung cancer Xuanwei cells.27
In summary, sulforaphane exerts apoptotic effects on Xuanwei lung adenocarcinoma cells, with few toxic or side effects. Its possible mechanism of action may involve the upregulation of p73 and its effector target genes PUMA and Bax in lung cancer cells, downregulation of anti‐apoptotic Bcl‐2, activation of the apoptotic protease caspase‐9, and promotion of cell apoptosis. The pro‐apoptotic effect may also be associated with the downregulation of mutant p53 protein. The results of this study represent a preliminary theoretical basis for new approaches to prevent lung cancer in Xuanwei.
Disclosure
No authors report any conflict of interest.
Supporting information
Table S1 Inhibition rate of Xuanwei lung adenocarcinoma (XWLC)‐05 cell proliferation by sulforaphane at various concentrations.
Acknowledgments
This project was funded by the Basic Research Joint Project of Yunnan Provincial Science and Technology Department and Kunming Medical University (2014FZ032).
References
- 1. Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res 2015; 27: 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 3. Wao H, Mhaskar R, Kumar A, Miladinovic B, Djulbegovic B. Survival of patients with non‐small cell lung cancer without treatment: A systematic review and meta‐analysis. Syst Rev 2013; 2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zeng H, Zheng R, Guo Y et al. Cancer survival in China, 2003–2005: A population‐based study. Int J Cancer 2015; 136: 1921–30. [DOI] [PubMed] [Google Scholar]
- 5. Xiao Y, Shao Y, Yu X, Zhou G. The epidemic status and risk factors of lung cancer in Xuanwei City, Yunnan Province, China. Front Med 2012; 6: 388–94. [DOI] [PubMed] [Google Scholar]
- 6. Mumford JL, He XZ, Chapman RS et al. Lung cancer and indoor air pollution in Xuan Wei, China. Science 1987; 235: 217–20. [DOI] [PubMed] [Google Scholar]
- 7. Barone‐Adesi F, Chapman RS, Silverman DT et al. Risk of lung cancer associated with domestic use of coal in Xuanwei, China: Retrospective cohort study. BMJ 2012; 345: e5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lam TK, Gallicchio L, Lindsley K et al. Cruciferous vegetable consumption and lung cancer risk: A systematic review. Cancer Epidemiol Biomarkers Prev 2009; 18: 184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riedl MA, Saxon A, Diaz‐Sanchez D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin Immunol 2009; 130: 244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: Metabolism and excretion in humans. Cancer Epidemiol Biomarkers Prev 2001; 10: 501–8. [PubMed] [Google Scholar]
- 11. Cramer JM, Jeffery EH. Sulforaphane absorption and excretion following ingestion of a semi‐purified broccoli powder rich in glucoraphanin and broccoli sprouts in healthy men. Nutr Cancer 2011; 63: 196–201. [DOI] [PubMed] [Google Scholar]
- 12. Yan FC, Wang QQ, Ruan YH, Ma LJ, Jia JT, Jin KW. [Establishment and biological characteristics of lung cancer cell line XWLC‐05.] Chin J Canc 2007; 26: 21–5. (In Chinese.) [PubMed] [Google Scholar]
- 13. Constantinescu S, Hecht K, Sobotzki N et al. Transcriptomic responses of cancerous and noncancerous human colon cells to sulforaphane and selenium. Chem Res Toxicol 2014; 27: 377–86. [DOI] [PubMed] [Google Scholar]
- 14. Rudolf E, Andelová H, Cervinka M. Activation of several concurrent proapoptic pathways by sulforaphane in human colon cancer cells SW620. Food Chem Toxicol 2009; 47: 2366–73. [DOI] [PubMed] [Google Scholar]
- 15. Park SY, Kim GY, Bae SJ, Yoo YH, Choi YH. Induction of apoptosis by isothiocyanate sulforaphane in human cervical carcinoma HeLa and hepatocarcinoma HepG2 cells through activation of caspase‐3. Oncol Rep 2007; 18: 181–7. [PubMed] [Google Scholar]
- 16. Cho SD, Li G, Hu H et al. Involvement of c‐Jun N‐terminal kinase in G2/M arrest and caspase‐mediated apoptosis induced by sulforaphane in DU145 prostate cancer cells. Nutr Cancer 2005; 52: 213–24. [DOI] [PubMed] [Google Scholar]
- 17. Park HS, Han MH, Kim GY et al. Sulforaphane induces reactive oxygen species‐mediated mitotic arrest and subsequent apoptosis in human bladder cancer 5637 cells. Food Chem Toxicol 2014; 64: 157–65. [DOI] [PubMed] [Google Scholar]
- 18. Martin B, Paesmans M, Berghmans T et al. Role of Bcl‐2 as a prognostic factor for survival in lung cancer: A systematic review of the literature with meta‐analysis. Br J Cancer 2003; 89: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao J, Jin KW. [Bcl ‐2 and Bax in lung cancer in Gejiu and Xuanwei of Yunnan.] J Kunming Med Coll 2003: 1–65. (In Chinese.) [Google Scholar]
- 20. Gui SQ, Zhang JH, Luo Y. Research progress on stabilization mechanism of mutant p53 protein expression. Tumor 2014; 34: 673–7. [Google Scholar]
- 21. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008; 359: 1367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang YP, Jin KW, Gao Q, Li B. [p53 mutation in lung cancer in Gejiu and Xuanwei regions of Yunnan Province.] Hua Xi Yi Ke Da Xue Xue Bao 2001; 32: 361–4. (In Chinese.) [PubMed] [Google Scholar]
- 23. Yu XJ, Yang MJ, Zhou B et al. Characterization of somatic mutations in air pollution‐related lung cancer. EBioMedicine 2015; 2: 583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang X, Di Pasqua AJ, Govind S et al. Selective depletion of mutant p53 by cancer chemopreventive isothiocyanates and their structure‐activity relationships. J Med Chem 2011; 54: 809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dötsch V, Bernassola F, Coutandin D, Candi E, Melino G. p63 and p73, the ancestors of p53. Cold Spring Harb Perspect Biol 2010; 2 (9): a004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Melino G, Bernassola F, Ranalli M et al. p73 induces apoptosis via PUMA transactivation and Bax mitochondrial translocation. J Biol Chem 2004; 279: 8076–83. [DOI] [PubMed] [Google Scholar]
- 27. Yoon MK, Ha JH, Lee MS, Chi SW. Structure and apoptotic function of p73. BMB Rep 2015; 48: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Inhibition rate of Xuanwei lung adenocarcinoma (XWLC)‐05 cell proliferation by sulforaphane at various concentrations.


