Abstract
Viral myocarditis is a disease with a high morbidity and mortality. The pathogenesis of this disease remains poorly characterized, with components of both direct virus-mediated and secondary inflammatory and immune responses contributing to disease. Apoptosis has increasingly been viewed as an important mechanism of myocardial injury in noninfectious models of cardiac disease, including ischemia and failure. Using a reovirus murine model of viral myocarditis, we characterized and targeted apoptosis as a key mechanism of virus-associated myocardial injury in vitro and in vivo. We demonstrated caspase-3 activation, in conjunction with terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling and annexin binding, in cardiac myocytes after myocarditic viral infection in vitro. We also demonstrated a tight temporal and geographical correlation between caspase-3 activation, histologic injury, and viral load in cardiac tissue after myocarditic viral infection in vivo. Two pharmacologic agents that broadly inhibit caspase activity, Q-VD-OPH and Z-VAD(OMe)-FMK, effectively inhibited virus-induced cellular death in vitro. The inhibition of caspase activity in vivo by the use of pharmacologic agents as well as genetic manipulation reduced virus-induced myocardial injury by 40 to 60% and dramatically improved survival in infected caspase-3-deficient animals. This study indicates that apoptosis plays a critical role in mediating cardiac injury in the setting of viral myocarditis and is the first demonstration that caspase inhibition may serve as a novel therapeutic strategy for this devastating disease.
Viral myocarditis remains a disease without a reliable or effective treatment, resulting in chronic dilated cardiomyopathy or death in up to 20% of affected children and 50% of affected adults (18). Unfortunately, the events following viral attachment and replication that produce the cytopathic effect and lead to myocarditis are not clearly understood. Several mechanisms have been suggested (32), including direct viral injury and persistence (10, 40, 58), autoimmune phenomena (6, 17, 25, 38, 52), cytokine fluxes (20, 28, 39), and T-cell-mediated inflammatory responses (27, 33, 53). Direct damage to cardiac myocytes plays a key role in the pathogenesis of nearly all models of viral myocarditis. A clearer understanding of the pathogenic mechanisms of virus-induced cardiac myocyte death is crucial for the development of effective therapeutic strategies since currently employed antiviral agents do not result in a significantly improved clinical outcome.
Murine models of viral infection utilizing coxsackievirus, murine encephalomyocarditis virus, and reovirus have been utilized to define key events in the pathogenesis of viral myocarditis. Reovirus strain 8B produces lethal myocarditis in infected neonatal mice (55, 56, 58). SCID mice still develop myocarditis, and the passive transfer of reovirus-specific immune cells is protective, rather than harmful, to 8B-infected mice (57). These findings indicate that myocardial damage is a direct effect of viral infection of cardiac myocytes and that immune mechanisms contribute to amelioration rather than an induction of reovirus-induced viral injury. Reoviral myocarditis therefore allows dissection of the events leading to direct myocardial injury.
Apoptosis is an important mechanism of active cellular death that is distinct from necrosis and has been implicated in the pathogenesis of a variety of degenerative, ischemic, toxin-induced, and infectious human diseases (34, 41, 64). Increasing evidence indicates that apoptosis plays a central role in microbial pathogenesis, particularly in viral infections (54), and it is now recognized as an important mechanism of virus-induced tissue injury (14, 15, 47). Apoptosis has been increasingly implicated in multiple forms of cardiac pathology (reviewed in references 4, 30, and 46), including in vivo models of hypertrophic and dilated failure (5, 16, 49, 59), myocardial infarction (3), ischemia-reperfusion (23, 43), and beta-adrenergic stimulation (59). Apoptosis of cardiac myocytes in vitro has also been demonstrated in response to serum and glucose deprivation (components of ischemia in vivo) (3) as well as during cardiac myocyte differentiation (37). Although there have been sporadic reports suggesting the importance of apoptosis in the pathogenesis of viral myocarditis (1, 11, 13, 14, 61, 63), this mechanism of cellular death has not previously been extensively characterized for models of viral myocarditis.
It was shown previously that apoptosis occurs in cardiac tissue after the infection of animals with reovirus 8B (14). In that study, the in vivo inhibition of calpain, a cysteine protease implicated in apoptotic signaling, was protective against virus-induced myocardial injury. Taken together, these results indicated that apoptosis is potentially an important mechanism of virus-induced tissue damage, as has been suggested for at least one other animal model of viral myocarditis (61). In the present study, we demonstrate the importance of apoptosis, and specifically caspase activation, as a critical determinant in the pathogenesis of virus-induced myocarditis and show that caspase inhibition is protective in an animal model of viral myocarditis. These results shed light on the basic pathogenesis of viral myocarditis and may have direct therapeutic implications for the treatment of this and other diseases involving apoptotic tissue damage.
MATERIALS AND METHODS
Virus and cells.
The reovirus 8B strain was passaged twice in L929 cells prior to use. 8B infectious subvirion particles (ISVPs) were generated by treating purified 8B virions with alpha-chymotrypsin at a final concentration of 150 μg/ml (from a 2-mg/ml stock solution in 150 mM NaCl and 15 mM sodium citrate diluted to 300 μg/ml in dialysis buffer) for 35 min at 37°C. Digestion was stopped by the addition of 2 μM (final concentration) phenylmethylsulfonyl fluoride on ice. ISVP preparations were verified by Western blot analysis of viral proteins. Neonatal murine cardiac myocytes were cultured in Dulbecco's modified Eagle's medium with Hanks salts supplemented with 5% fetal bovine serum, 1 mg of bovine serum albumin/ml, 50 U of penicillin G (Sigma)/ml, 2 μg of vitamin B12 (Sigma)/ml, 10 μg of transferrin (Sigma)/ml, 10 μg of insulin (Sigma)/ml, and 0.1 mM bromodeoxyuridine (Sigma) and were maintained at 37°C with 1% CO2 (50, 51).
Mice and inoculations.
For inhibitor experiments, Swiss-Webster mice were purchased from Transduction Laboratories. Caspase-3 knockout mice and C57B6 wild-type mice were a generous gift from Richard Flavell, Yale University. Two-day-old mice were infected intramuscularly (in the left hind limb) with 104 PFU of virus administered in 20 μl of gel saline (137 mM NaCl, 0.2 mM CaCl2, 0.8 mM MgCl2, 19 mM H3BO3, 0.1 mM Na2B4O7, 0.3% gelatin).
Histologic analysis.
Hearts were immersed in 10% buffered formalin for 12 h, embedded in paraffin, and sectioned transversely into 4-μm-thick sections. Hematoxylin and eosin (H&E)-stained mid-cardiac sections were examined by light microscopy, photographed at a magnification of ×2.5, and scored in a blinded fashion by two independent scorers, using a previously validated myocardial injury scoring system (58). Injury was also quantified by determining the area of injured myocardial tissue and expressing it as a percentage of the total myocardial tissue area (Adobe Photoshop Magnetic Lasso tool).
Immunohistochemistry. (i) Viral antigen detection.
After antigen retrieval and a blockade of nonspecific binding, tissues were incubated with a rabbit polyclonal anti-T3D reovirus antiserum (1:100 dilution overnight at 4°C). After washes in Tris-buffered saline-0.1% Tween 20, the tissues were incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G (Vector) (1:100 dilution for 1 h at room temperature [RT]) and were subsequently counterstained for nuclear detection with Hoechst 33342 (Molecular Probes) (5 min at RT). Sections were mounted with Vectashield (Vector) and then analyzed by fluorescence microscopy.
(ii) Activation of caspase-3.
Antigen unmasking was performed as described above, and the endogenous peroxidase activity of the tissues was blocked. Sections were incubated with a rabbit polyclonal antibody detecting cleaved (active) caspase-3 (Cell Signaling) (1:100 dilution overnight at 4°C) and then with goat anti-rabbit immunoglobulin-biotin (Vector) (1:100 dilution for 1 h at RT) and were subsequently probed with avidin-horseradish peroxidase. Cleaved caspase-3 was detected with a diaminobenzadine peroxidase substrate (Trevigen). A blue counterstain (Trevigen) was used to visualize the tissue architecture.
Immunocytochemistry.
Cells were fixed with 3.7% paraformaldehyde (20 min at RT) and then stored in a mild cell permeabilizer, Cytonin (Trevigen), at 4°C for up to 1 week. The cells were probed for reovirus antigens and cleaved caspase-3 (Cell Signaling), as described above. Fluorescence microscopy was used to visualize staining at a magnification of ×20.
Apoptosis assays and reagents.
Cardiac myocytes were harvested by using a 50-50 mixture of trypsin-EDTA (BioSource) and Accutase (Innovative Cell Technologies).
Acridine orange and ethidium bromide stains.
Cells were stained with acridine orange for determinations of nuclear morphology and with ethidium bromide to distinguish cell viability, at a final concentration of 1 μg/ml for each, as previously described (14)
Flow cytometry.
Cells were analyzed by flow cytometry for the binding of annexin V-fluorescein isothiocyanate to phosphatidylserine and were simultaneously analyzed for propidium iodide incorporation. Data were collected for populations of 10,000 cells.
TUNEL.
Tissues and cells were analyzed for evidence of DNA fragmentation by in situ terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) (TACS XL-Blue label; Trevigen). Terminal deoxynucleotidyltransferase was utilized to incorporate biotinylated nucleotides at the sites of DNA breaks. TUNEL in the tissues was detected with the TACS blue label, and the cells were counterstained with nuclear fast red for visualization of the background cellular architecture.
Caspase inhibition.
The pan-caspase inhibitors Q-VD-OPH [quinoline-Val-Asp(OMe)-CH2-PH; Enzyme Systems] and Z-VAD(OMe)-FMK [Z-Val-Ala-Asp(Ome)-FMK; Enzyme Systems] were utilized to determine the effect of caspase inhibition on cardiac myocyte death in vitro and on myocardial injury in vivo following viral infection. Both drugs are potent irreversible broad-spectrum caspase inhibitors (7, 42). Neonatal mice received daily intraperitoneal injections of Q-VD-OPH or Z-VAD(OMe)-FMK (50 mg/kg of body weight in a 10-μl volume) or of the corresponding diluent (70 or 15% dimethyl sulfoxide, respectively, in phosphate-buffered saline) on days 3 to 6 postinfection and were sacrificed on day 7. For in vitro experiments, inhibitors (50 μM) were added to primary cardiac myocytes 1 h prior to infection with virus.
Viral titer determination.
Viral titers of the tissue homogenates were determined as previously described (14) and are reported as mean titers of two or three independently infected tissues or cell lysates ± standard errors of the means.
RESULTS
Apoptosis is an important mechanism in the pathogenesis of myocardial injury after viral infection. (i) Apoptosis and caspase-3 activation in myocardial tissue in vivo.
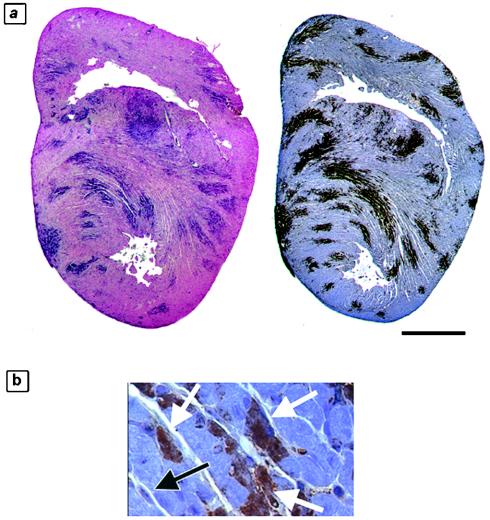
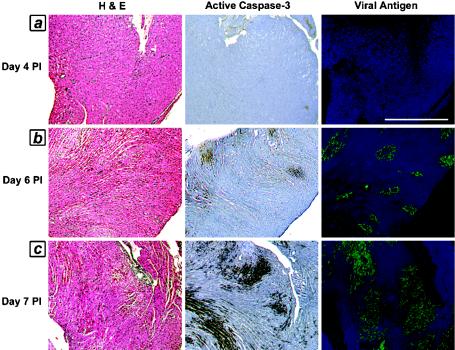
We wished to define the temporal relationship of apoptosis to tissue injury in order to confirm that apoptotic injury is an integral component of this damage. We analyzed 4-μm-thick consecutive sections from reovirus 8B-infected hearts on serial days postinfection (days 1 to 7 postinfection) for evidence of apoptosis (active caspase-3 immunohistochemistry and TUNEL), in conjunction with H&E staining for determinations of tissue injury and immunohistochemistry for viral antigens. TUNEL and active caspase-3 staining colocalized and were equally sensitive markers of apoptosis in the analyzed sections, as determined by the quantification of positive staining as a percentage of the total myocardial tissue from 4-μm-thick consecutive sections of infected hearts (Fig. 1a; 17.8% of the myocardium was TUNEL positive, and 23.3% was positive for active caspase-3). Caspase-3 activity was only noted within areas of injured myocardium and was completely absent from uninfected animals. Using high-magnification imaging of myocardial tissue with active caspase-3 positivity, we observed that the cell population undergoing apoptosis was composed of cardiac myocytes rather than fibroblasts (Fig. 1b). Using a previously validated scoring system, we determined average scores for injury and caspase-3 activation (by numerically scoring and averaging replicate sections from each day postinfection) and compared them. Myocardial tissue viral titers were also determined in parallel experiments on days 3 to 6 postinfection. On days 1 to 3 postinfection, the myocardial architecture appeared normal, viral antigen was undetectable, and neither the TUNEL nor active caspase-3 apoptotic marker was positive. Beginning on day 4 and continuing through day 7 postinfection, we detected a gradually increasing and highly correlated degree of histologic injury, apoptosis, and viral load on each day postinfection (R = 0.79 to 0.99) (Fig. 2 and Tables 1 and 2). Importantly, there was no observed discrepancy or time lag between detectable histologic injury and the presence of apoptosis (caspase-3 activity). Taken together, these results indicate that apoptosis is an integral part of the pathological response to viral infection.
FIG. 1.
Markers of apoptosis in virus-injured myocardium. Consecutive 4-μm-thick sections of hearts from reovirus-infected mice were analyzed at 7 days postinfection by two independent assays for evidence of apoptosis. (a) TUNEL (left, blue staining) and active caspase-3 staining (right, brown staining) of 4-μm-thick sections of a representative infected animal. It was calculated that 17.8% of the myocardial tissue was TUNEL positive and 23.3% was active caspase-3 positive. (b) Higher-magnification view of active caspase-3-positive area of myocardium. The black arrow indicates an example of a negatively stained cardiac fibroblast. White arrows indicate examples of positively stained cardiac myocytes. Bar = 2.5 mm.
FIG. 2.
Time course of injury, apoptosis, and viral load in virus-infected myocardium. Consecutive 4-μm-thick sections of hearts from reovirus-infected mice were analyzed on days 3 to 7 postinfection (PI) for evidence of apoptosis (active caspase-3, brown staining) in conjunction with histological injury (H&E stain) and viral load (green fluorescent staining). (a) Day 4; (b) day 6; (c) day 7. Sections are from single representative animals from each day (n = 26; two to six mice were studied from each day postinfection). See Tables 1 and 2 for quantitation and correlation coefficients. Bar = 625 μm.
TABLE 1.
Quantitation of histologic injury, caspase-3 activation, and viral load in virus-infected myocardium over time postinfectiona
| Day postinfection | Myocardial injury score | Caspase-3 activation score | Viral titer in Tissue (log10 PFU/ml) |
|---|---|---|---|
| 3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 4.4 ± 0.1 |
| 4 | 0.8 ± 0.5 | 0.5 ± 0.0 | 5.6 ± 0.4 |
| 5 | 1.0 ± 0.5 | 1.0 ± 0.0 | 7.5 ± 0.1 |
| 6 | 2.6 ± 0.3 | 2.2 ± 0.2 | 7.6 ± 0.5 |
| 7 | 3.3 ± 0.3 | 3.2 ± 0.2 | ND |
Consecutive 4-μm-thick sections of hearts from reovirus-infected mice on days 3 to 7 postinfection (n = 26) were scored quantitatively for histologic injury (0 to 4) and caspase-3 activation (0 to 4). Myocardial viral titers were also determined on days 3 to 6 postinfection. Mean scores (± standard errors of the means) for histologic injury, caspase-3 activation, and viral titers are reported for each day (derived from 2 to 6 mice per day). ND, not done.
TABLE 2.
Correlation coefficients for histologic injury, caspase-3 activation, and viral load in virus-infected myocardium over time postinfection
| Variable |
R (P value)
|
||
|---|---|---|---|
| Myocardial injury score | Caspase-3 activation score | Viral titer in tissue (log10 PFU/ml) | |
| Myocardial injury score | 0.99 (<0.001) | 0.79 (<0.02) | |
| Caspase-3 activation score | 0.99 (<0.001) | 0.85 (<0.01) | |
(ii) Apoptosis and caspase-3 activation in infected cardiac myocytes in vitro.
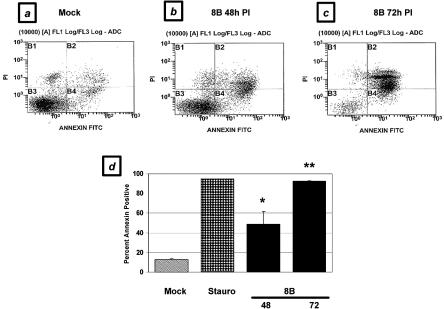
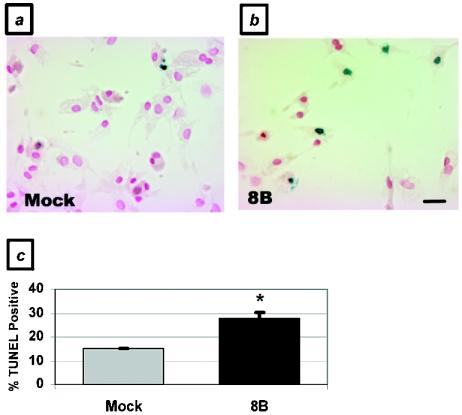
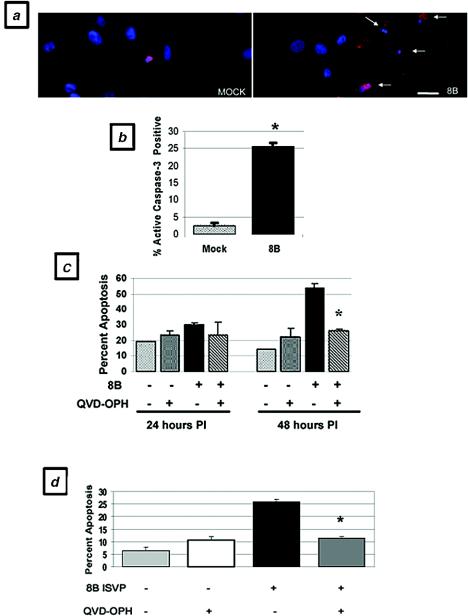
To confirm that the representative cell population undergoing apoptosis in response to viral infection in vivo was cardiac myocytes, we infected primary cardiac myocytes with the 8B virus and analyzed them for evidence of apoptosis at several time points postinfection. In a flow cytometric analysis of annexin V positivity, cardiac myocytes showed a 3.8-fold increase over basal levels of apoptosis at 48 h postinfection (48.8% ± 12.6% [8B infection] versus 13.0% ± 1.0% [mock infection]; P < 0.05), which increased to 7.1-fold at 72 h postinfection (92.3% ± 0.3%; P < 0.001) (Fig. 3). This level of apoptosis was similar to that induced by exposure to a well-characterized apoptotic stimulus, 5 μM staurosporine (94.6%). Using a TUNEL assay, we demonstrated a nearly twofold increase in apoptotic myocytes 48 h after 8B infection (28% ± 2.1% versus 15% ± 0%; P < 0.05) (Fig. 4). Similar results were obtained by analyses of nuclear morphology and cell viability by ethidium bromide and acridine orange staining (data not shown). Virus-infected cells also demonstrated a 10.3-fold increase in active caspase-3 activity compared to mock-infected cells at 48 h postinfection (25.7% ± 0.3% versus 2.5% ± 0.3%; P < 0.001) (Fig. 5a and b).
FIG. 3.
Apoptosis in virus-infected primary cardiac myocytes in vitro, as measured by annexin binding. Primary cardiac myocytes were analyzed by flow cytometry for evidence of annexin binding (representing phosphatidylserine inversion) and propidium iodide incorporation (representing cellular viability) at 48 and 72 h postinfection (PI) with reovirus and were compared to mock-infected cells. (a) Mock-infected cells; (b) 48 h after virus infection; (c) 72 h after virus infection. (d) Quantitation of replicate flow cytometry experiments, including 5 μM staurosporine treatment as a positive control. *, P < 0.05; **, P < 0.001.
FIG. 4.
Apoptosis in virus-infected primary cardiac myocytes, as determined by TUNEL. Primary cardiac myocytes were analyzed by TUNEL at 48 h postinfection. Blue-stained nuclei indicate TUNEL positivity. (a) Mock-infected myocytes; (b) 8B-infected myocytes. (c) Quantitation of staining. *, P < 0.05. Bar = 160 μm.
FIG. 5.
Caspase-3 activation in virus-infected primary cardiac myocytes in vitro and effect of caspase inhibition on virus- and ISVP-induced apoptosis. Infected and mock-infected primary cardiac myocytes were doubly labeled to allow the simultaneous detection of caspase-3 activity (red) and nuclear morphology (Hoechst staining; blue) by immunocytochemistry at 48 h postinfection. (a) Mock-infected and 8B-infected myocytes. (b) Quantitation of replicate experiments. *, P < 0.001. (c) Effect of Q-VD-OPH treatment (50 μM) on apoptosis (assessed by acridine orange and ethidium bromide staining) of mock-infected and 8B virus-infected cardiac myocytes at 24 and 48 h postinfection. *, P < 0.01. (d) Effect of Q-VD-OPH treatment (50 μM) on apoptosis of mock- and 8B ISVP-infected cardiac myocytes at 48 h postinfection. *, P < 0.01. Bar = 160 μm.
Taken together, both the in vivo and in vitro data suggest that cardiac myocytes undergo significant levels of apoptosis associated with caspase-3 activation in response to infection with myocarditic virus. As such, the apoptotic response in this cell population is likely to be an integral component of the myocardial damage observed after viral infection.
Caspase inhibition protects cardiac myocytes from virus-induced apoptosis in vitro.
Having demonstrated significant caspase-3 activation in virus-infected cardiac myocytes, we next determined the effect of caspase inhibition on virus-induced cardiac myocyte death. Using ethidium bromide and acridine orange staining, we quantified apoptosis after viral infection in the presence and absence of two broadly acting caspase inhibitors, Q-VD-OPH and Z-VAD(OMe)-FMK. Q-VD-OPH significantly protected cardiac myocytes from virus-induced apoptosis at 48 h postinfection, with a 50% (P < 0.01) reduction in the levels of apoptosis compared to untreated infected cells (Fig. 5c). Z-VAD(OMe)-FMK also provided protection, with a 33% reduction in the levels of apoptosis. To address the possibility that caspase inhibitors confer protection by interfering with viral uncoating (by nonspecifically inhibiting lysosomal cathepsins), we also performed identical experiments with 8B ISVP infections of cardiac myocytes in the presence and absence of Q-VD-OPH. Q-VD-OPH significantly protected cardiac myocytes from ISVP-induced apoptosis at 48 h postinfection, with a 50% (P < 0.01) reduction in the levels of apoptosis compared to untreated, ISVP-infected cells (Fig. 5d). These results suggest that caspase inhibition protects infected cardiac myocytes against virus-induced apoptotic death by a mechanism other than the inhibition of viral uncoating.
Caspase inhibition is protective against virus-induced myocardial injury in vivo. (i) Pharmacologic inhibitors.
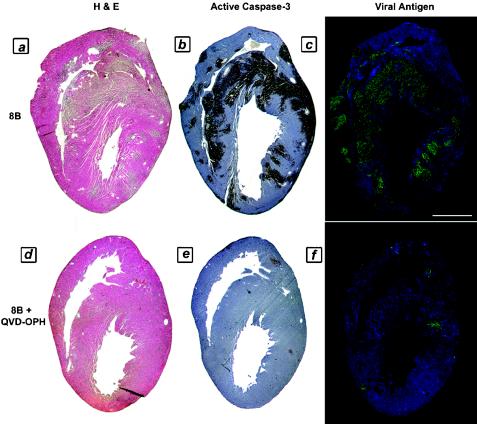
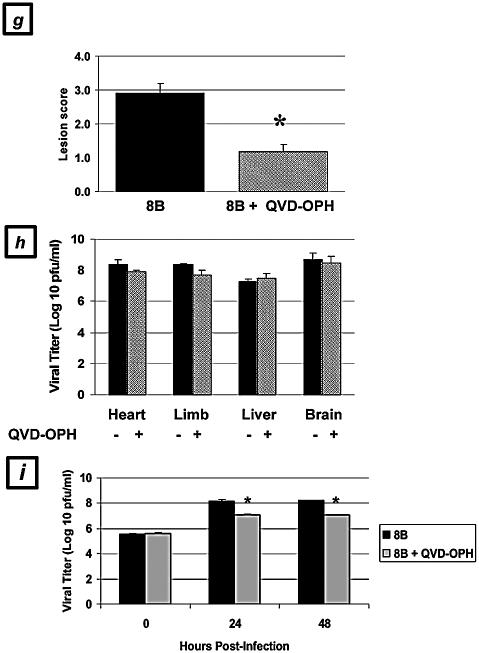
Since pharmacologic caspase inhibition was protective against virus-induced apoptosis in cardiac myocytes in vitro, we next investigated whether administering these same inhibitors to infected mice in vivo would be effective at preventing myocardial tissue injury. Two-day-old Swiss-Webster mice were infected with 104 PFU of myocarditic virus, followed by the administration of Q-VD-OPH, Z-VAD(OMe)-FMK, or their diluent controls on days 3 to 6 postinfection. Mice receiving caspase inhibitors showed statistically significant reductions in myocardial injury scores compared to controls, as measured by two independent myocardial injury scoring systems. Treatment with Q-VD-OPH resulted in a 60% reduction in the myocardial lesion score (from 2.9 ± 0.3 to 1.2 ± 0.2; P < 0.0001; n = 22) compared to untreated infected mice (Fig. 6a, d, and g), corresponding to a reduction in the injured myocardial area from 12.8% ± 2.0% to 1.4% ± 0.9% (P < 0.0001). Z-VAD(OMe)-FMK treatment also offered protection from virus-induced myocardial injury, resulting in a 39% reduction in the myocardial lesion score, from 3.1 ± 0.3 to 1.9 ± 0.6, compared to that for untreated mice, which corresponded to a reduction in the injured myocardial area from 16.4% ± 1.2% to 7.4% ± 3.3% (P < 0.05; n = 16).
FIG. 6.
Myocardial injury, apoptosis, and viral load in 8B-infected mice treated with pharmacologic caspase inhibitor (Q-VD-OPH) in vivo. Two-day-old Swiss-Webster mice were infected with myocarditic reovirus, followed by the intraperitoneal administration of Q-VD-OPH (50 mg/kg/day) or its diluent control on days 3 to 6 postinfection. The animals were sacrificed on day 7 postinfection, and consecutive 4-μm-thick sections were analyzed for histologic injury (H&E staining), active caspase-3 (brown stain), and the presence of virus (fluorescent green staining). (a to c) Consecutive sections from representative untreated infected control animal. (d to f) Consecutive sections from representative Q-VD-OPH-treated animal. (g) Quantitation of myocardial injury (n = 22). *, P < 0.001. (h) Tissue viral titers in infected animals in the presence and absence of Q-VD-OPH treatment. (i) Viral growth in primary cardiac myocytes in vitro in the presence and absence of Q-VD-OPH treatment. *, P = 0.01. Bar = 2.5 mm.
An analysis of 4-μm-thick consecutive sections from Q-VD-OPH-treated mice revealed a significant decrease in apoptosis, as measured by active caspase-3 staining (Fig. 6b and e) and TUNEL (data not shown), as well as a reduction in tissue viral antigen staining (Fig. 6c and f) compared to that in untreated animals. Since the viral load appeared to be reduced, as observed by immunohistochemical techniques, a further analysis of tissue viral titers was undertaken in order to determine if the protection conferred by Q-VD-OPH caspase inhibition was explained by a significant inhibition of viral replication within the primary site of inoculation (limb) or the target tissue (myocardium). Viral titers in tissue homogenates of limbs and hearts, as well as of brains and livers, were compared for treated and untreated animals. Small but insignificant reductions in the viral titer were observed at the primary site of inoculation (0.7-log reduction, from 8.4 ± 0.1 to 7.7 ± 0.3 log10 PFU/ml; P > 0.05) and in the heart (0.5-log reduction, from 8.4 ± 0.3 to 7.9 ± 0.1 log10 PFU/ml; P > 0.05) after treatment with Q-VD-OPH (Fig. 6h). Notably, these reduced titers still represented a 3- to 4-log increase over the input viral titer at the time of infection. We also assessed the effect of both Q-VD-OPH and Z-VAD(OMe)-FMK on the growth of the virus in infected cardiac myocytes in vitro. No reduction in viral titer was observed in Z-VAD(OMe)-FMK-treated infected myocytes compared to untreated myocytes (from 8.2 ± 0.1 to 7.9 ± 0.1 log10 PFU/ml at 48 h postinfection; P > 0.05). A modest reduction was observed following Q-VD-OPH treatment (1.2-log reduction, to 7.0 ± 0.1 log10 PFU/ml; P = 0.01) (Fig. 6i).
Taken together, these results suggest that pharmacologic caspase inhibition provides protection from virus-induced myocardial injury, with a significant reduction in caspase-3 activity within the infected myocardium in conjunction with minimal to modest reductions in the myocardial viral burden.
(ii) Transgenic (caspase-3-deficient) mice.
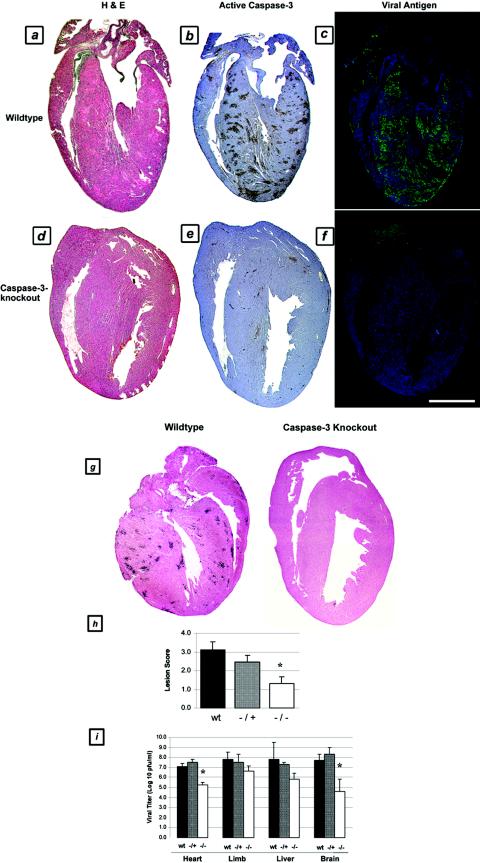
Since pharmacologic caspase inhibition was protective against virus-induced myocardial injury, we performed identical experiments with transgenic mice who were deficient in caspase-3 as well as with matched wild-type controls (littermates). Infected transgenic mice exhibited statistically significant reductions in myocardial injury after viral infection compared to wild-type and heterozygous controls, as measured by both scoring systems. Hearts from caspase-3-deficient mice showed a 58% reduction in the cardiac lesion score (from 3.1 ± 0.4 to 1.3 ± 0.4; P < 0.05; n = 32) compared to untreated infected mice (Fig. 7a, d, and h), corresponding to a reduction in the injured myocardial area from 13.3% ± 4.6% to 5.8% ± 2.5% (P < 0.05). Heterozygous mice demonstrated an intermediate injury phenotype, with a trend toward less injury than wild-type mice (lesion score, 2.5 ± 0.4; not significant) but more injury than homozygous mice (P < 0.05). Caspase-3 activation (Fig. 7b and e) and TUNEL staining (Fig. 7g) were both absent and the degree of viral antigen staining was reduced (Fig. 7c and f) in infected homozygous caspase-3-deficient mice compared to wild-type control animals. In contrast to the case in pharmacologic inhibition experiments, tissue viral titers were significantly reduced in caspase-3-deficient mice compared to wild-type and heterozygous controls, particularly in cardiac tissues. A nearly 2-log reduction was noted in the hearts of infected caspase-3-deficient mice compared to those of wild-type and heterozygous controls (from 7.1 ± 0.3 to 5.2 ± 0.3 log10 PFU/ml; P < 0.01) (Fig. 7i). A reduction in titer at the primary site of inoculation (limb) was also noted, although it was of a lesser magnitude (1.2-log reduction, from 7.8 ± 0.7 to 6.6 ± 0.5 log10 PFU/ml). Notably, these reduced titers still represented 1- to 2-log increases over the input viral titer at the time of inoculation.
FIG.7.
Myocardial injury, apoptosis, and viral load in 8B-infected caspase-3-deficient transgenic animals. Two-day-old homozygous and heterozygous caspase-3-deficient transgenic mice and wild-type controls were infected with myocarditic reovirus. The animals were sacrificed on day 7 postinfection, and consecutive 4-μm-thick sections were analyzed for histologic injury (H&E staining), activated caspase-3 (brown staining), and the presence of virus (fluorescent green staining). (a to c) Consecutive sections from a representative wild-type control animal. (d to f) Consecutive sections from a representative homozygous caspase-3-deficient transgenic animal. (g) TUNEL staining (brown) in representative wild-type and caspase-3-deficient infected animals. (h) Quantitation of myocardial injury in infected wild-type, homozygous, and heterozygous transgenic animals (n = 32). *, P < 0.05. (i) Tissue viral titers in infected wild-type, homozygous, and heterozygous transgenic animals at 7 days postinfection (n = 11). P < 0.01. Bar = 2.5 mm.
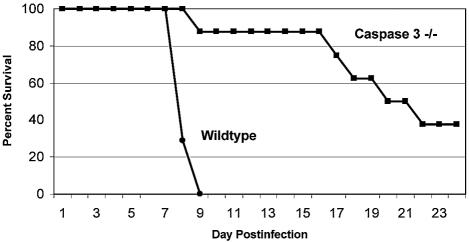
In addition to exhibiting reduced levels of injury at 7 days postinfection, caspase-3-deficient animals were also noted to have substantially prolonged survival times after viral infection (Fig. 8). Although wild-type animals exhibited 100% mortality by 9 days postinfection, 100% of the caspase-3-deficient mice were alive and well at that time. Fifty percent of the caspase-3-deficient mice were still alive at 21 days postinfection, and 37.5% of them survived indefinitely. Cardiac tissues from long-term survivors appeared normal (at 54 days postinfection).
FIG. 8.
Survival in infected caspase-3-deficient transgenic animals. The survival of wild-type and caspase-3-deficient transgenic animals was assessed daily after 8B infection (n = 8).
DISCUSSION
Apoptosis has been increasingly appreciated as an important mechanism of cardiac injury in noninfectious models of cardiac disease. We now have demonstrated the importance of apoptosis, and specifically caspase activation, as a critical determinant in the pathogenesis of virus-induced myocarditis by manipulating this process in vitro and in vivo. Our study is the first to demonstrate that caspase inhibition by pharmacologic inhibitors or genetic alteration protects cardiac myocytes from virus-induced apoptotic death and infected mice from virus-induced myocardial injury.
In contrast to the paucity of data available regarding the role of apoptosis in infectious myocarditis, there is substantial evidence suggesting that apoptosis plays an important role in the settings of myocardial ischemia-infarction and heart failure. In the case of ischemia, myocardial apoptosis has been documented by many groups, utilizing a variety of animal models (murine, porcine, canine, and lagomorph) and experimental paradigms in vitro and in vivo (reviewed extensively in reference 46). In vivo models utilizing coronary ligation and reperfusion have suggested that both the initial ischemic insult and the reperfusion phase may independently contribute to the initiation and execution of apoptosis (3, 19, 21, 31, 35). The relative contributions of apoptosis and necrosis, the role of reactive oxygen species, specific depleted energy substrates, and molecular chaperones, and the delineation of specific apoptotic signaling pathways are areas of active research in this setting. The manipulation of caspases (the effector proteases of apoptosis) has been investigated previously with ischemic models of cardiac disease in vitro (2) and in vivo (12, 26, 44, 48, 62). Numerous groups have investigated the contribution of apoptosis to the pathogenesis of heart failure in various animal models (including transgenic constructs) as well as in human tissues from patients undergoing transplantation (9, 22, 36, 45; reviewed extensively in reference 31). Although the results vary depending on the model employed, the bulk of the evidence suggests an association of cardiac myocyte apoptosis with heart failure. Recent studies provided evidence that myocyte apoptosis may be a causal mechanism of heart failure and that the inhibition of cardiac apoptosis by the manipulation of caspases largely prevents the development of cardiac dilation and contractile dysfunction (24, 60). Evidence has accumulated to indicate an important role for the activation of the adrenergic system in the regulation of cardiomyocyte apoptosis (8, 29, 65). The extent to which apoptosis occurs and its contribution as a determinant of the severity and progression of heart failure, such as in the transition from compensated hypertrophy, warrant further study.
It was previously demonstrated that apoptosis is present within cardiac lesions after reovirus infection (14). We have now shown that caspase-3 activation is detected in tight temporal and spatial association with the development of tissue injury, indicating that apoptosis is an integral mechanism of the pathological injury itself rather than a delayed effect after virus-induced tissue injury. We did not detect caspase activation preceding the time points at which histological injury was evident, suggesting that caspase activation coincided with or occurred less than 24 h preceding injury. The demonstration that cardiac myocytes underwent significant increases in baseline levels of apoptosis and caspase-3 activity after viral infection in vitro was consistent with the degree of apoptotic damage observed in the myocardium after viral infection in vivo.
Caspase inhibition may be achieved by means of pharmacologic inhibitors or genetic manipulation. Both interventional approaches resulted in significant reductions in virus-induced myocardial injury, with more efficacy observed in genetically altered animals. Caspase inhibition could potentially interfere with virus-induced tissue damage on several fronts. One possibility is that protection is conferred by inhibition of the apoptotic program itself, independent of the effects on viral replication. Alternatively, apoptosis might be required for optimal viral replication and spread (as a means of evading the host immune response's viral clearance mechanisms), and the inhibition of apoptosis might result in a reduction in the ability of the virus to replicate and spread to target tissues. Data from our experiments suggest that both mechanisms may be operative. In the case of pharmacologic inhibition, substantial reductions in myocardial injury occurred in treated animals and were accompanied by only small reductions in organ viral titers (0.5 to 0.7 log10 PFU/ml). Although they were modestly reduced, the titers achieved in all organs, including the heart, were still 3 to 4 log higher than the input viral titer at the primary site of inoculation in these animals, which is indicative of active viral replication and spread despite caspase inhibition. In caspase-3-deficient mice, more dramatic reductions in viral titers were observed in the limb and heart (1.2 and 1.9 log10 PFU/ml, respectively), suggesting the possibility that apoptosis may be a requirement for optimal viral replication and spread. The specific mechanism by which caspase activity contributes to optimal viral replication and spread is not known, but it does not appear to be at the level of viral uncoating, since the efficacy of caspase inhibitors was not diminished in the setting of infection with ISVPs.
Increased survival was observed for infected caspase-3-deficient mice, but not for those who were treated with pharmacologic inhibitors. This may be due, at least in part, to the fact that the continued dosing of pharmacologic inhibitors was not feasible beyond day 6 postinfection because of the toxicities of the drugs and/or vehicles. Although a significant proportion of caspase-3-deficient mice lived indefinitely, the majority exhibited substantially prolonged survival after infection with myocarditic virus but subsequently died. It is not clear if the mice with delayed death succumbed to delayed cardiac injury or to an alternate cause such as virus-induced hepatic injury, which may be unaffected by caspase-3 manipulation. Further study is required to identify the organ-specific signaling processes by which these diseases occur. In the case of caspase-3-deficient mice, it is possible that virus-induced apoptotic myocardial injury still occurred but was significantly delayed or blunted pending compensation by potential substitute effector caspases. From a therapeutic standpoint, such a delay in the tempo of tissue destruction would be advantageous to the host, allowing a broader window of opportunity for the development of immune responses and viral clearance.
In summary, these experiments shed light on the basic pathogenesis of viral myocarditis and have direct therapeutic implications for the treatment of this and other diseases involving apoptotic tissue damage. Studies are in progress to identify the intermediary signaling pathways leading to apoptosis after viral infection in cardiac cells and tissues. The inhibition of apoptotic signaling may provide a novel therapeutic strategy to prevent or limit cell death and to minimize tissue damage in the infected host. The development of new treatment approaches is urgently needed for serious viral infections, such as myocarditis, which result in irreversible cell damage and loss, since effective specific antiviral therapies are currently unavailable.
Acknowledgments
Research support was provided by the National Institutes of Health (5K08AI052261-02 to R.D.B.; HL66399 to C.S.L.), the U.S. Army (Medical Research Acquisition Activity grant DAMD 17-98-1-8614 to K.L.T.), Merit and REAP grants from the Department of Veterans Affairs (K.L.T.), and the Reuler-Lewin Family Professorship of Neurology (K.L.T.).
REFERENCES
- 1.Alter, P., M. Jobmann, E. Meyer, S. Pankuweit, and B. Maisch. 2001. Apoptosis in myocarditis and dilated cardiomyopathy: does enterovirus genome persistence protect from apoptosis? An endomyocardial biopsy study. Cardiovasc. Pathol. 10:229-234. [DOI] [PubMed] [Google Scholar]
- 2.Bialik, S., V. L. Cryns, A. Drincic, S. Miyata, A. L. Wollowick, A. Srinivasan, and R. N. Kitsis. 1999. The mitochondrial apoptotic pathway is activated by serum and glucose deprivation in cardiac myocytes. Circ. Res. 85:403-414. [DOI] [PubMed] [Google Scholar]
- 3.Bialik, S., D. L. Geenen, I. E. Sasson, R. Cheng, J. W. Horner, S. M. Evans, E. M. Lord, C. J. Koch, and R. N. Kitsis. 1997. Myocyte apoptosis during acute myocardial infarction in the mouse localizes to hypoxic regions but occurs independently of p53. J. Clin. Investig. 100:1363-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishopric, N. H., P. Andreka, T. Slepak, and K. A. Webster. 2001. Molecular mechanisms of apoptosis in the cardiac myocyte. Curr. Opin. Pharmacol. 1:141-150. [DOI] [PubMed] [Google Scholar]
- 5.Bueno, O. F., L. J. De Windt, K. M. Tymitz, S. A. Witt, T. R. Kimball, R. Klevitsky, T. E. Hewett, S. P. Jones, D. J. Lefer, C. F. Peng, R. N. Kitsis, and J. D. Molkentin. 2000. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 19:6341-6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caforio, A. L., N. J. Mahon, F. Tona, and W. J. McKenna. 2002. Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur. J. Heart Fail. 4:411-417. [DOI] [PubMed] [Google Scholar]
- 7.Caserta, T. M., A. N. Smith, A. D. Gultice, M. A. Reedy, and T. L. Brown. 2003. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis 8:345-352. [DOI] [PubMed] [Google Scholar]
- 8.Chesley, A., M. S. Lundberg, T. Asai, R. P. Xiao, S. Ohtani, E. G. Lakatta, and M. T. Crow. 2000. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3′-kinase. Circ. Res. 87:1172-1179. [DOI] [PubMed] [Google Scholar]
- 9.Chiu, H. C., A. Kovacs, D. A. Ford, F. F. Hsu, R. Garcia, P. Herrero, J. E. Saffitz, and J. E. Schaffer. 2001. A novel mouse model of lipotoxic cardiomyopathy. J. Clin. Investig. 107:813-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow, L. H., K. W. Beisel, and B. M. McManus. 1992. Enteroviral infection of mice with severe combined immunodeficiency. Evidence for direct viral pathogenesis of myocardial injury. Lab Investig. 66:24-31. [PubMed] [Google Scholar]
- 11.Colston, J. T., B. Chandrasekar, and G. L. Freeman. 1998. Expression of apoptosis-related proteins in experimental coxsackievirus myocarditis. Cardiovasc. Res. 38:158-168. [DOI] [PubMed] [Google Scholar]
- 12.Condorelli, G., R. Roncarati, J. Ross, Jr., A. Pisani, G. Stassi, M. Todaro, S. Trocha, A. Drusco, Y. Gu, M. A. Russo, G. Frati, S. P. Jones, D. J. Lefer, C. Napoli, and C. M. Croce. 2001. Heart-targeted overexpression of caspase3 in mice increases infarct size and depresses cardiac function. Proc. Natl. Acad. Sci. USA 98:9977-9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeBiasi, R. L., P. Clarke, S. Meintzer, R. Jotte, B. K. Kleinschmidt-Demasters, G. L. Johnson, and K. L. Tyler. 2003. Reovirus-induced alteration in expression of apoptosis and DNA repair genes with potential roles in viral pathogenesis. J. Virol. 77:8934-8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeBiasi, R. L., C. L. Edelstein, B. Sherry, and K. L. Tyler. 2001. Calpain inhibition protects against virus-induced apoptotic myocardial injury. J. Virol. 75:351-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeBiasi, R. L., B. K. Kleinschmidt-Demasters, S. Richardson-Burns, and K. L. Tyler. 2002. Central nervous system apoptosis in human herpes simplex virus and cytomegalovirus encephalitis. J. Infect. Dis. 186:1547-1557. [DOI] [PubMed] [Google Scholar]
- 16.De Windt, L. J., H. W. Lim, T. Taigen, D. Wencker, G. Condorelli, G. W. Dorn, R. N. Kitsis, and J. D. Molkentin. 2000. Calcineurin-mediated hypertrophy protects cardiomyocytes from apoptosis in vitro and in vivo: an apoptosis-independent model of dilated heart failure. Circ. Res. 86:255-263. [DOI] [PubMed] [Google Scholar]
- 17.Fairweather, D., Z. Kaya, G. R. Shellam, C. M. Lawson, and N. R. Rose. 2001. From infection to autoimmunity. J. Autoimmun. 16:175-186. [DOI] [PubMed] [Google Scholar]
- 18.Feldman, A. M., and D. McNamara. 2000. Myocarditis. N. Engl. J. Med. 343:1388-1398. [DOI] [PubMed] [Google Scholar]
- 19.Fliss, H., and D. Gattinger. 1996. Apoptosis in ischemic and reperfused rat myocardium. Circ. Res. 79:949-956. [DOI] [PubMed] [Google Scholar]
- 20.Freeman, G. L., J. T. Colston, M. Zabalgoitia, and B. Chandrasekar. 1998. Contractile depression and expression of proinflammatory cytokines and iNOS in viral myocarditis. Am. J. Physiol. 274:H249-H258. [DOI] [PubMed] [Google Scholar]
- 21.Freude, B., T. N. Masters, F. Robicsek, A. Fokin, S. Kostin, R. Zimmermann, C. Ullmann, S. Lorenz-Meyer, and J. Schaper. 2000. Apoptosis is initiated by myocardial ischemia and executed during reperfusion. J. Mol. Cell Cardiol. 32:197-208. [DOI] [PubMed] [Google Scholar]
- 22.Frustaci, A., J. Kajstura, C. Chimenti, I. Jakoniuk, A. Leri, A. Maseri, B. Nadal-Ginard, and P. Anversa. 2000. Myocardial cell death in human diabetes. Circ. Res. 87:1123-1132. [DOI] [PubMed] [Google Scholar]
- 23.Fujio, Y., T. Nguyen, D. Wencker, R. N. Kitsis, and K. Walsh. 2000. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101:660-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayakawa, Y., M. Chandra, W. Miao, J. Shirani, J. H. Brown, G. W. Dorn, R. C. Armstrong, and R. N. Kitsis. 2003. Inhibition of cardiac myocyte apoptosis improves cardiac function and abolishes mortality in the peripartum cardiomyopathy of Galpha(q) transgenic mice. Circulation 108:3036-3041. [DOI] [PubMed] [Google Scholar]
- 25.Hill, S. L., and N. R. Rose. 2001. The transition from viral to autoimmune myocarditis. Autoimmunity 34:169-176. [DOI] [PubMed] [Google Scholar]
- 26.Holly, T. A., A. Drincic, Y. Byun, S. Nakamura, K. Harris, F. J. Klocke, and V. L. Cryns. 1999. Caspase inhibition reduces myocyte cell death induced by myocardial ischemia and reperfusion in vivo. J. Mol. Cell Cardiol. 31:1709-1715. [DOI] [PubMed] [Google Scholar]
- 27.Huber, S. A., R. C. Budd, K. Rossner, and M. K. Newell. 1999. Apoptosis in coxsackievirus B3-induced myocarditis and dilated cardiomyopathy. Ann. N. Y. Acad. Sci. 887:181-190. [DOI] [PubMed] [Google Scholar]
- 28.Huber, S. A., C. J. Gauntt, and P. Sakkinen. 1998. Enteroviruses and myocarditis: viral pathogenesis through replication, cytokine induction, and immunopathogenicity. Adv. Virus Res. 51:35-80. [DOI] [PubMed] [Google Scholar]
- 29.Iwai-Kanai, E., K. Hasegawa, M. Araki, T. Kakita, T. Morimoto, and S. Sasayama. 1999. Alpha- and beta-adrenergic pathways differentially regulate cell type-specific apoptosis in rat cardiac myocytes. Circulation 100:305-311. [DOI] [PubMed] [Google Scholar]
- 30.James, T. N. 1999. Apoptosis in cardiac disease. Am. J. Med. 107:606-620. [DOI] [PubMed] [Google Scholar]
- 31.Kajstura, J., W. Cheng, K. Reiss, W. A. Clark, E. H. Sonnenblick, S. Krajewski, J. C. Reed, G. Olivetti, and P. Anversa. 1996. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab. Investig. 74:86-107. [PubMed] [Google Scholar]
- 32.Kawai, C. 1999. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation 99:1091-1100. [DOI] [PubMed] [Google Scholar]
- 33.Knowlton, K. U., and C. Badorff. 1999. The immune system in viral myocarditis: maintaining the balance. Circ. Res. 85:559-561. [DOI] [PubMed] [Google Scholar]
- 34.Krammer, P. H. 2000. CD95's deadly mission in the immune system. Nature 407:789-795. [DOI] [PubMed] [Google Scholar]
- 35.Kurrelmeyer, K. M., L. H. Michael, G. Baumgarten, G. E. Taffet, J. J. Peschon, N. Sivasubramanian, M. L. Entman, and D. L. Mann. 2000. Endogenous tumor necrosis factor protects the adult cardiac myocyte against ischemic-induced apoptosis in a murine model of acute myocardial infarction. Proc. Natl. Acad. Sci. USA 97:5456-5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latif, N., M. A. Khan, E. Birks, A. O'Farrell, J. Westbrook, M. J. Dunn, and M. H. Yacoub. 2000. Upregulation of the Bcl-2 family of proteins in end stage heart failure. J. Am. Coll. Cardiol. 35:1769-1777. [DOI] [PubMed] [Google Scholar]
- 37.Liu, Y., and R. N. Kitsis. 1996. Induction of DNA synthesis and apoptosis in cardiac myocytes by E1A oncoprotein. J. Cell Biol. 133:325-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maisch, B., A. D. Ristic, G. Hufnagel, and S. Pankuweit. 2002. Pathophysiology of viral myocarditis: the role of humoral immune response. Cardiovasc. Pathol. 11:112-122. [DOI] [PubMed] [Google Scholar]
- 39.Matsumori, A., and S. Sasayama. 2001. The role of inflammatory mediators in the failing heart: immunomodulation of cytokines in experimental models of heart failure. Heart Fail. Rev. 6:129-136. [DOI] [PubMed] [Google Scholar]
- 40.McManus, B. M., L. H. Chow, J. E. Wilson, D. R. Anderson, J. M. Gulizia, C. J. Gauntt, K. E. Klingel, K. W. Beisel, and R. Kandolf. 1993. Direct myocardial injury by enterovirus: a central role in the evolution of murine myocarditis. Clin. Immunol. Immunopathol. 68:159-169. [DOI] [PubMed] [Google Scholar]
- 41.Meier, P., A. Finch, and G. Evan. 2000. Apoptosis in development. Nature 407:796-801. [DOI] [PubMed] [Google Scholar]
- 42.Melnikov, V. Y., S. Faubel, B. Siegmund, M. S. Lucia, D. Ljubanovic, and C. L. Edelstein. 2002. Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J. Clin. Investig. 110:1083-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miao, W., Z. Luo, R. N. Kitsis, and K. Walsh. 2000. Intracoronary, adenovirus-mediated Akt gene transfer in heart limits infarct size following ischemia-reperfusion injury in vivo. J. Mol. Cell Cardiol. 32:2397-2402. [DOI] [PubMed] [Google Scholar]
- 44.Mocanu, M. M., G. F. Baxter, and D. M. Yellon. 2000. Caspase inhibition and limitation of myocardial infarct size: protection against lethal reperfusion injury. Br. J. Pharmacol. 130:197-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narula, J., P. Pandey, E. Arbustini, N. Haider, N. Narula, F. D. Kolodgie, B. Dal Bello, M. J. Semigran, A. Bielsa-Masdeu, G. W. Dec, S. Israels, M. Ballester, R. Virmani, S. Saxena, and S. Kharbanda. 1999. Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc. Natl. Acad. Sci. USA 96:8144-8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neuss, M., M. T. Crow, A. Chesley, and E. G. Lakatta. 2001. Apoptosis in cardiac disease—what is it—how does it occur. Cardiovasc. Drugs Ther. 15:507-523. [DOI] [PubMed] [Google Scholar]
- 47.Oberhaus, S. M., R. L. Smith, G. H. Clayton, T. S. Dermody, and K. L. Tyler. 1997. Reovirus infection and tissue injury in the mouse central nervous system are associated with apoptosis. J. Virol. 71:2100-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okamura, T., T. Miura, G. Takemura, H. Fujiwara, H. Iwamoto, S. Kawamura, M. Kimura, Y. Ikeda, M. Iwatate, and M. Matsuzaki. 2000. Effect of caspase inhibitors on myocardial infarct size and myocyte DNA fragmentation in the ischemia-reperfused rat heart. Cardiovasc. Res. 45:642-650. [DOI] [PubMed] [Google Scholar]
- 49.Olivetti, G., R. Abbi, F. Quaini, J. Kajstura, W. Cheng, J. A. Nitahara, E. Quaini, C. Di Loreto, C. A. Beltrami, S. Krajewski, J. C. Reed, and P. Anversa. 1997. Apoptosis in the failing human heart. N. Engl. J. Med. 336:1131-1141. [DOI] [PubMed] [Google Scholar]
- 50.Palmer, J. N., W. E. Hartogensis, M. Patten, F. D. Fortuin, and C. S. Long. 1995. Interleukin-1 beta induces cardiac myocyte growth but inhibits cardiac fibroblast proliferation in culture. J. Clin. Investig. 95:2555-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patten, M., W. Wang, S. Aminololama-Shakeri, M. Burson, and C. S. Long. 2000. IL-1 beta increases abundance and activity of the negative transcriptional regulator yin yang-1 (YY1) in neonatal rat cardiac myocytes. J. Mol. Cell Cardiol. 32:1341-1352. [DOI] [PubMed] [Google Scholar]
- 52.Rose, N. R. 2000. Viral damage or “molecular mimicry”—placing the blame in myocarditis. Nat. Med. 6:631-632. [DOI] [PubMed] [Google Scholar]
- 53.Seko, Y., N. Takahashi, M. Azuma, H. Yagita, K. Okumura, and Y. Yazaki. 1998. Expression of costimulatory molecule CD40 in murine heart with acute myocarditis and reduction of inflammation by treatment with anti-CD40L/B7-1 monoclonal antibodies. Circ. Res. 83:463-469. [DOI] [PubMed] [Google Scholar]
- 54.Shen, Y., and T. E. Shenk. 1995. Viruses and apoptosis. Curr. Opin. Genet. Dev. 5:105-111. [DOI] [PubMed] [Google Scholar]
- 55.Sherry, B. 1998. Pathogenesis of reovirus myocarditis. Curr. Top. Microbiol. Immunol. 233:51-66. [DOI] [PubMed] [Google Scholar]
- 56.Sherry, B., C. J. Baty, and M. A. Blum. 1996. Reovirus-induced acute myocarditis in mice correlates with viral RNA synthesis rather than generation of infectious virus in cardiac myocytes. J. Virol. 70:6709-6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sherry, B., X. Y. Li, K. L. Tyler, J. M. Cullen, and H. W. Virgin. 1993. Lymphocytes protect against and are not required for reovirus-induced myocarditis. J. Virol. 67:6119-6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sherry, B., F. J. Schoen, E. Wenske, and B. N. Fields. 1989. Derivation and characterization of an efficiently myocarditic reovirus variant. J. Virol. 63:4840-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shizukuda, Y., P. M. Buttrick, D. L. Geenen, A. C. Borczuk, R. N. Kitsis, and E. H. Sonnenblick. 1998. Beta-adrenergic stimulation causes cardiocyte apoptosis: influence of tachycardia and hypertrophy. Am. J. Physiol. 275:H961-H968. [DOI] [PubMed] [Google Scholar]
- 60.Wencker, D., M. Chandra, K. Nguyen, W. Miao, S. Garantziotis, S. M. Factor, J. Shirani, R. C. Armstrong, and R. N. Kitsis. 2003. A mechanistic role for cardiac myocyte apoptosis in heart failure. J. Clin. Investig. 111:1497-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang, D., J. Yu, Z. Luo, C. M. Carthy, J. E. Wilson, Z. Liu, and B. M. McManus. 1999. Viral myocarditis: identification of five differentially expressed genes in coxsackievirus B3-infected mouse heart. Circ. Res. 84:704-712. [DOI] [PubMed] [Google Scholar]
- 62.Yaoita, H., K. Ogawa, K. Maehara, and Y. Maruyama. 1998. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation 97:276-281. [DOI] [PubMed] [Google Scholar]
- 63.Yeh, E. T. 1997. Life and death in the cardiovascular system. Circulation 95:782-786. [DOI] [PubMed] [Google Scholar]
- 64.Yuan, J., and B. A. Yankner. 2000. Apoptosis in the nervous system. Nature 407:802-809. [DOI] [PubMed] [Google Scholar]
- 65.Zhu, W. Z., M. Zheng, W. J. Koch, R. J. Lefkowitz, B. K. Kobilka, and R. P. Xiao. 2001. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc. Natl. Acad. Sci. USA 98:1607-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]