Abstract
Latent nuclear antigen (LNA) is implicated in Kaposi's sarcoma-associated herpesvirus (KSHV) episome persistence. LNA colocalizes with KSHV episomes on chromosomes in metaphase, and it maintains the stability and replication of KSHV terminal repeat-containing plasmids. In this study, we examined the function of LNA in episome persistence in the context of full-length KSHV genome by mutagenesis analysis. We generated a KSHV mutant, BAC36-ΔLNA, with LNA disrupted by transposon-based mutagenesis with a KSHV BAC clone, BAC36, as a template. Immunofluorescence antibody staining revealed that the insertion of a transposon cassette into LNA disrupted its expression but had no effect on the expression of two adjacent genes, the vCyclin and vFLIP genes. Using a green fluorescent protein (GFP) cassette as a tracking marker for the KSHV episome, we found 8.7-fold-fewer GFP-positive cells in BAC36-ΔLNA cultures than in wild-type BAC36 cultures at the early stage following episome delivery into 293 cells by transfection, which could be partially rescued by cotransfection with a LNA expression plasmid but not a control plasmid. Cells harboring BAC36-ΔLNA with or without transient complementation rapidly lost episomes and became virus-free after 2 weeks of culture based on GFP expression and Gardella gel analysis and quantitative PCR assays for detecting KSHV genomes. In contrast, BAC36 episomes were stably maintained during the same period. Stable cultures with close to 100% of cells harboring KSHV episomes were readily established by hygromycin selection for BAC36 but not for BAC36-ΔLNA. These results conclusively indicate that LNA is essential for the establishment and persistence of KSHV episomes in mammalian cells.
Kaposi's sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8, is a gamma-2 herpesvirus discovered in a Kaposi's sarcoma (KS) lesion from a patient with AIDS (5). A large number of studies have shown that KSHV infection is associated with the development of all clinical forms of KS and several other lymphoproliferative diseases, including primary effusion lymphoma and multicentric Castleman's disease (28).
Similar to other herpesviruses, the life cycle of KSHV consists of latent and lytic replication phases. Following primary infection, KSHV establishes lifelong latent infection in host cells, which can be reactivated into lytic replication upon induction by intracellular or environmental factors. In KS lesions and other KSHV-related malignancies, the tumor cells are latently infected by the virus and express viral latent antigens (28). As with Epstein-Barr virus, which is another tumorigenic gammaherpesvirus, latent infection with KSHV is important in the development of KS.
In KSHV latent infection, viral genomes persist as nonintegrated circular episomes. To sustain latent infection, KSHV episomes have to be stably maintained in cells, efficiently replicated before cell division, and faithfully segregated into daughter cells during mitosis. A KSHV latent gene(s) that has an essential role in episome persistence should also be important for latent infection.
The latent nuclear antigen (LNA, also known as LANA) encoded by open reading frame 73 (ORF73) is a multifunctional protein. It consists of a doublet of two proteins of 222 and 234 kDa in the prototype BC-1 isolate but is polymorphic in different isolates because of the highly variable internal repeat domain (10, 12, 39). LNA targets tumor suppressor pathways via direct interactions with p53 and pRb tumor suppressors (8, 32) and regulates viral and cellular gene transcription via direct interaction with various transcriptional factors (1, 19, 22-26, 31, 34-37). A recent study has shown that LNA interacts with a novel transcriptional repressor, KLIP1, and relieves its transcriptional repression activity (29). LNA also dysregulates β-catenin pathways through the stabilization of β-catenin, by binding to the negative regulator glycogen synthase kinase 3β and causing a cell cycle-dependent nuclear accumulation of glycogen synthase kinase 3β (9). It has been reported that transduction with LNA promotes cell proliferation and prolongs the life span in primary human umbilical endothelial cells (38). Besides these functions, LNA is also likely to have an important role in KSHV episome persistence. LNA displays a punctate pattern in the nucleus of KSHV-infected cells in an immunofluorescence antibody assay (IFA) (11, 20, 21, 33). Such patterning is due to the colocalization of LNA with KSHV episomes on cellular chromosomes, implicating LNA's involvement in the replication and maintenance of KSHV episomes in latently infected cells (2). Indeed, LNA alone is sufficient for the maintenance, replication, and segregation of terminal repeat (TR)-containing plasmids via binding of its C-terminal domain to the TR sequence of the plasmids, which are then tethered to cellular chromosomes via its N-terminal domain (2, 3, 6, 7, 14, 15, 18, 30, 35). The results of these studies have provided evidence to support LNA's essential role in KSHV episome persistence. Nevertheless, these studies were conducted with plasmids and have not been confirmed for the full-length KSHV genome. Furthermore, no study has conclusively determined whether LNA is the only KSHV protein to carry out such functions. One of the best approaches for clarifying these issues is to functionally disrupt LNA in the KSHV genome and to assay the ability of the mutant episome to persist in cells. The recent cloning of the full-length KSHV genome as a bacterial artificial chromosome (BAC), BAC36, has made such genetic manipulation possible (40). In this study, we generated a recombinant KSHV BAC36-ΔLNA with LNA disrupted. Examination of the mutant virus in mammalian cells has shown that LNA is indeed essential for the establishment and persistence of KSHV episomes in mammalian cells.
MATERIALS AND METHODS
Generation of a recombinant KSHV mutant BAC36-ΔLNA with LNA disrupted.
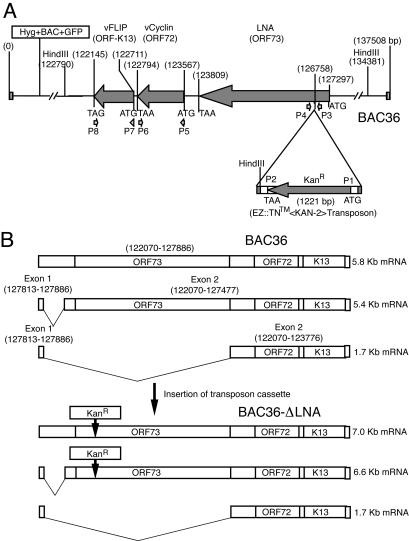
We used the approach of random transposon-based mutagenesis to generate KSHV mutants (Fig. 1). The EZ::TN <KAN-2> insertion kit (Epicentre, Madison, Wis.), originally developed from the hyperactive Tn5 transposition system, is highly efficient in randomly inserting its specific primer binding sites and a kanamycin-resistance (Kanr) cassette into the target DNA in vitro. Briefly, purified recombinant KSHV BAC36 DNA was reacted with the transposon cassette (transposon and Kanr) DNA in the presence of Tn5 transposase for 2 h. An aliquot of the reaction mixture was then used to transform Escherichia coli strain DH10B by electroporation, and bacterial clones carrying the transposon cassette were selected on Luria-Bertani agar plates in the presence of 25 μg of kanamycin/ml. To identify the insertion point, BAC DNA purified from each of the kanamycin-resistant clones was subjected to DNA sequencing with two primers (P1 and P2) that are located at both ends of the transposon cassette. One of the clones, designated BAC36-ΔLNA, with a transposon cassette inserted at genomic position 126,758 near the N-terminal domain of LNA, was selected for further characterization in this study.
FIG. 1.
(A) Schematic illustration of transposon-based mutagenesis for generating KSHV mutant BAC36-ΔLNA with LNA disrupted. (B) Expected LNA transcripts from wild-type KSHV BAC36 and mutant BAC36-ΔLNA.
Genetic analysis of BAC36-ΔLNA.
Different techniques were used to analyze the mutant BAC36-ΔLNA to ensure that the insertion site indeed occurred as revealed by DNA sequencing and that the mutant genome contains the full-length KSHV genome without any other unexpected rearrangements. PCR-based genotyping was carried out as a quick diagnostic assay to confirm the insertion position of the transposon cassette. The following set of primers was used in the PCRs: P3 (5′TACGGTTGGCGAAGTCACATC3′) was 158 bp upstream of the insertion site and P4 (5′CCTCGCAGCAGACTACACCTCCAC3′) was 22 bp downstream of the insertion site. If the insertion position revealed by DNA sequencing is correct, these primers should amplify a product of 207 bp from wild-type KSHV BAC36 and a product of 1,428 bp from mutant KSHV BAC36-ΔLNA. The amplified PCR products were analyzed by electrophoresis on a 1.2% agarose gel.
Restriction enzyme digestion was carried out to analyze BAC36 and BAC36-ΔLNA genomes. DNA was isolated from bacteria harboring BAC36 or BAC36-ΔLNA by a standard alkaline lysis method, digested with HindIII, and analyzed with a 0.7% agarose gel. To further identify bands that have altered migration because of the insertion of a transposon cassette, Southern blot hybridizations were carried out. The DNA restriction fragments were transferred onto nylon membranes, which were then probed with P32-labeled Kanr DNA and a 578-bp DNA fragment corresponding to the N-terminal domain of LNA. Specific signals were detected with a PhosphorImager.
Transfection of episome DNA and monitoring episome persistence.
293 cells were split into a 6-well plate 1 day before transfection and grown to 80% confluency. Purified episomal DNA from BAC36 and BAC36-ΔLNA was transfected into 293 cells at 2 μg/well with the SuperFect transfection kit according to the instructions of the manufacturer (QIAGEN, Valencia, Calif.). Each transfection experiment was repeated three times. Transfected cells were cultured and monitored daily for the percentage of green fluorescent protein (GFP)-positive cells with a Zeiss inverted fluorescence microscope. Transfected cells were split every 2 days, and GFP-positive cell numbers were continuously monitored for a period of 2 weeks. To isolate stable clones, the cultures were subjected to hygromycin selection 2 days posttransfection. For genetic complementation to rescue the lost function of LNA in BAC36-ΔLNA, a plasmid named pcDNA3.1His-LNA, expressing full-length LNA in mammalian cells, was cotransfected with BAC36-ΔLNA episome DNA into 293 cells. As a control, BAC36-ΔLNA was simultaneously cotransfected with plasmid pcDNA3.1His into 293 cells. Cotransfection of 293 cells with BAC36 together with pcDNA3.1His-LNA or pcDNA3.1His was also conducted as an additional control.
IFA.
IFA was carried out as previously described (13) to determine the protein expression of LNA, vCyclin, and vFLIP in 293 cells transfected with BAC36 or BAC36-ΔLNA. Briefly, 293 cells were washed three times with phosphate-buffered saline by centrifugation and evenly spread onto 10-well slides. After fixation at 56°C for 1 h, the slides were blocked with 3% bovine serum albumin. To detect LNA, the slides were incubated with a rat anti-LNA monoclonal antibody (ABI, Baltimore, Md.). Since the GFP fluorescence intensity was weakened after heat fixation, a mouse anti-GFP antibody (Sigma, St. Louis, Mo.) was simultaneously added to detect GFP-positive cells. Specific signals were revealed with a goat anti-rat immunoglobulin G (IgG)-ALEXA568 conjugate (Molecular Probes, Eugene, Oreg.) and a rabbit anti-mouse IgG-fluorescein isothiocyanate conjugate (Sigma) and captured with a digitalized Zeiss fluorescent microscope. A rat monoclonal antibody (a kind gift from Mary Collins at the Windeyer Institute of Medical Sciences, London, England) was used to detect vFLIP protein (27). A rabbit polyclonal antibody was used to detect vCyclin protein (4). Specific signals of vCyclin were revealed with a donkey anti-rabbit IgG-ALEXA568 conjugate (Molecular Probes).
Detection of KSHV episomes by Gardella gel analysis.
Detection of KSHV genomes of both wild-type and mutant KSHV in 293 cells was carried out with a Gardella gel as previously described (16). After electrophoresis, viral DNA was blotted onto a nylon membrane and then detected by Southern-blot hybridization with a P32-labeled full-length LNA probe. Specific signals were captured with a PhosphorImager.
Semiquantitative PCR (sqPCR) and quantitative real-time SYBR Green PCR (qPCR).
The diagnostic PCR described above for the genetic analysis of KSHV mutants was also used in a sqPCR assay to detect KSHV genomes in 293 cultures harboring BAC36 or BAC36-ΔLNA. A second PCR assay amplifying a 62-bp product of the ORF16 gene was also used to detect KSHV genomes. The primers used were ORF16F (5′ACCAGCTTGGGTTGAGCATG3′) and ORF16R (5′GGCTCGCCCCCAGTTC3′). Cellular DNA was calibrated by a PCR assay for glyceraldehye-3-phosphate dehydrogenase (GAPDH) with primers GAPDH-F (5′ACAGTCAGCCGCATCTTCTT3′) and GAPDH-R (5′ACGACCAAATCCGTTGACTC3′) that amplify a product of 94 bp.
qPCR was used to determine the copy number of KSHV genomes per cell. The primers for ORF16 and GAPDH were also used in qPCR. ORF65 primers used in qPCR were ORF65F (5′ATATGTCGCAGGCCGAATAC3′) and ORF65R (5′CCACCCATCCTCCTCAGATA3′), which amplify a product of 97 bp. qPCR was carried out with a total volume of 20 μl, containing 10 μl of DyNAmo SYBR Green qPCR kit (Finnzymes Oy, Espoo, Finland), 0.5 μl of each primers at 50 mM, and 1 μl of DNA at 30 ng/μl. Thermal amplification was performed with a DNA Engine Opticon 2 continuous fluorescence detector (MJ Research, Inc.) with the following linked profile: 10 min at 96°C; 45 amplification cycles, each cycle consisting of denaturation (95°C for 10 s), annealing (60°C for 20 s), and extension (72°C for 15 s); and a 50-step melting curve analysis where the annealing temperature was decreased by 0.2°C at each step. Fluorescence intensity was detected after the extension step at five temperature steps (72, 74, 76, 78, and 80°C), and calculated with Opticon MONITOR analysis software (MJ Research, Inc.). Each sample was examined in triplicate for each set of primers.
RESULTS
Generation of a KSHV mutant with LNA disrupted by transposon-based mutagenesis.
Using recombinant KSHV BAC36 as a template, we applied random transposon-based mutagenesis to generate recombinant mutants with disruptions in the genes of interest (Fig. 1). DNA sequencing of integration junctions identified one of the mutants as having an insertion of the transposon cassette into the N-terminal domain of LNA at genomic position 126,758, which was located 528 bp downstream of the translation start codon ATG of ORF73. The insertion, if confirmed, would disrupt the proper expression and function of LNA protein. We named this KSHV mutant BAC36-ΔLNA. To further confirm the insertion of transposon cassette in BAC36-ΔLNA, we performed diagnostic PCR assays with primers localized downstream and upstream of the insertion site. The PCR assay should amplify a fragment of 207 bp from the BAC36 genome. However, if the transposon cassette insertion occurred at genomic position 126,758, as revealed by DNA sequencing, the PCR would amplify a fragment of 1,428 bp from the BAC36-ΔLNA genome. Figure 2A showed that the PCRs indeed amplified DNA fragments with the expected sizes from both BAC36 and BAC36-ΔLNA genomes, confirming the revealed transposon insertion position.
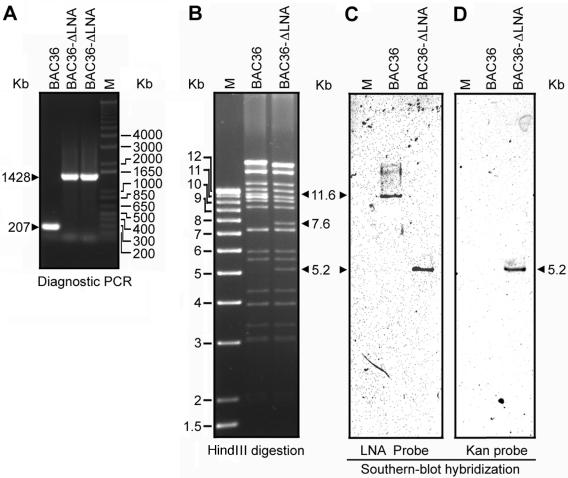
FIG. 2.
Genetic analysis of wild-type KSHV BAC36 and mutant BAC36-ΔLNA. (A) Diagnostic PCR for the differential detection of BAC36 and BAC36-ΔLNA. (B) Restriction analysis of BAC36 and BAC36-ΔLNA by HindIII digestion. The 11.6-kb band in BAC36 was replaced by two bands of 7.6 and 5.2 kb in BAC36-ΔLNA because of the presence of a HindIII site in the transposon sequence. (C) Southern blot hybridization with a 578-bp DNA fragment from the LNA N-terminal domain as a probe. (D) Southern blot hybridization with Kanr DNA as a probe. M, molecular weight marker.
To further confirm that the insertion occurred correctly and BAC36-ΔLNA contained the full-length KSHV genome without any other unexpected genomic rearrangements, we performed restriction analysis of the BAC36-ΔLNA genome. Purified BAC36-ΔLNA DNA was digested with HindIII and resolved on an agarose gel. Based on sequence analysis of both BAC36 and BAC36-ΔLNA, the insertion should occur in an 11.6-kb HindIII fragment, adding the 1.2 kb of the transposon-Kanr sequence to the fragment. However, because of the presence of a HindIII site in the transposon sequence, two HindIII fragments (a 5.2-kb fragment containing the Kanr sequence and one 7.6-kb fragment) should be generated. As expected, BAC36-ΔLNA had all the HindIII bands identified in BAC36 except the 11.6-kb fragment, which was digested into one 5.2-kb band and one 7.6-kb band (Fig. 2B). With a 578-bp DNA fragment from the LNA N-terminal domain sequence as a probe, Southern hybridization detected an 11.6-kb band in BAC36 but a 5.2-kb band in BAC36-ΔLNA. When the Kanr sequence was used a probe, only the 5.2-kb band was detected in BAC36-ΔLNA, while no band was detected in BAC36. These results indicated that BAC36-ΔLNA contained the full-length KSHV genome with only a single insertion of the transposon cassette into LNA without any other unexpected genomic rearrangements.
BAC36-ΔLNA no longer expresses LNA protein.
To determine whether the transposon-Kanr insertion disrupted LNA protein expression in mammalian cells, we transiently transfected BAC36 and BAC36-ΔLNA episomal DNA into 293 cells. Two days posttransfection, both BAC36- and BAC36-ΔLNA-transfected cells expressed GFP with BAC36-ΔLNA-transfected cells expressing GFP at a much lower rate (see below). We performed an IFA to detect LNA protein expression in GFP-positive cells. All BAC36 GFP-positive cells expressed LNA as expected, with a typical speckled pattern (Fig. 3). No LNA expression was detected in BAC36 GFP-negative cells. In contrast, we detected no LNA expression in the already small number of BAC36-ΔLNA GFP-positive cells or in the BAC36-ΔLNA GFP-negative cells. As a negative control, we stained 293 cells with the anti-LNA antibody. None of the 293 cells was positive for LNA (data not shown). These results indicated that the transposon insertion in BAC36-ΔLNA efficiently disrupted the protein expression of LNA.
FIG. 3.
IFAs for detecting LNA, vCyclin, and vFLIP proteins in BAC36- and BAC36-ΔLNA-transfected cells. GFP-positive cells were positive for LNA in BAC36-transfected cells. LNA protein expression was not detected in any BAC36-ΔLNA-transfected cells. vCyclin and vFLIP were expressed in all GFP-positive cells but not in GFP-negative cells in both BAC36- and BAC36-ΔLNA-transfected cells.
Insertion of transposon cassette into LNA did not disrupt the expression of vCyclin and vFLIP proteins.
The vCyclin and vFLIP genes are adjacent to the LNA gene, and the three genes share multiple transcripts (Fig. 1B). We determined whether the insertion of transposon cassette into LNA would also disrupt the expression of vCyclin and vFLIP genes. BAC36- and BAC36-ΔLNA-transfected 293 cells were stained for the expression of vCyclin and vFLIP proteins by IFA. All of the BAC36 and BAC36-ΔLNA GFP-positive cells were positive for vCyclin and vFLIP, while all the BAC36 and BAC36-ΔLNA GFP-negative cells were negative (Fig. 3). Untransfected 293 cells were negative for vCyclin and vFLIP (data not shown), demonstrating the specificity of the antibodies. These results indicated that the transposon cassette insertion in BAC36-ΔLNA did not disrupt the expression of vCyclin and vFLIP proteins.
BAC36-ΔLNA is inefficient in the establishment and maintenance of KSHV episome persistence in mammalian cells.
To determine whether LNA disruption affects KSHV episome persistence, we reconstituted the recombinant genome in mammalian cells. Equal amounts of BAC36 and BAC36-ΔLNA DNA were used to transfect 293 cells. Because of the presence of a GFP cassette in the original BAC36, we were able to use GFP expression to conveniently track the presence of recombinant genomes in mammalian cells (40). GFP expression was observed in cells transfected with both BAC36 and BAC36-ΔLNA DNA 1 day posttransfection and reached a peak 2 days posttransfection (Fig. 4A and B). BAC36-transfected cells had approximately 6.5% of GFP-positive cells at the peak of expression. Surprisingly, the number of GFP-positive cells in BAC36-ΔLNA-transfected cells was approximately 8.7 times smaller than in BAC36-transfected cells (0.75 versus 6.5%) (Fig. 4A and B). This difference was due neither to variations in quality of the episomal DNA nor to variations of transfection efficiencies, because these results were consecutively reproduced five times, each with three repeats using separately prepared episomal DNA. To investigate whether fewer numbers of GFP-positive cells in BAC36-ΔLNA-transfected cells were due to the disruption of LNA expression, we conducted genetic complementation for the loss of LNA protein. Cotransfection of BAC36-ΔLNA episomal DNA together with an LNA expression plasmid, pcDNA3.1His-LNA, into 293 cells rescued GFP-positive cells to 3.9%, while cotransfection of BAC36-ΔLNA with a control plasmid pcDNA3.1His did not alter the number of GFP-positive cells (Fig. 4A and B). Cotransfection of BAC36 with pcDNA3.1His-LNA or pcDNA3.1His had no effect on the number of GFP-positive cells (Fig. 4A and B). Together, these results indicated that the disruption of LNA reduced the efficiency in the initial establishment of KSHV episome persistence in mammalian cells.
FIG. 4.
GFP-positive cells in BAC36- and BAC36-ΔLNA-transfected 293 cultures with or without cotransfection with LNA expression plasmid pcDNA3.1His-LNA or a control plasmid, pcDNA3.1His. (A) Representative GFP and phase fields of the cultures. (B) GFP-positive cells over a period of 2 weeks. The results are averages from one representative experiment with standard deviations. (C) Relative GFP-positive cells from the results shown in Fig. 4B after normalization against peak values 2 days posttransfection (100%), showing the rapid loss of GFP-positive cells in all BAC36-ΔLNA cultures. BAC36-DLNA, BAC36-ΔLNA.
We continued to monitor GFP-positive cells in the cultures. Cells were split every 2 days, and the numbers of GFP-positive cells and total cells were counted. While the percentage of GFP-positive cells in BAC36-transfected cells remained relatively stable, the percentage in BAC36-ΔLNA-transfected cells rapidly decreased to less than 0.1% 14 days posttransfection (Fig. 4A and B). Cotransfection with either pcDNA3.1His-LNA or pcDNA3.1His had no effects on BAC36-transfected cells, but it also did not prevent the loss of GFP-positive cells in BAC36-ΔLNA transfected cells. To better illustrate the rapid loss of GFP-positive cells in BAC36-ΔLNA cultures, we normalized the relative percentage of GFP-positive cells using 100% for the peak value (2 days posttransfection) (Fig. 4C). The results showed a rapidly decreasing slope of GFP-positive cells for BAC36-ΔLNA-transfected cells with or without complementation, and again, a relatively stable slope for BAC36-transfected cells. In one of the experiments, we maintained the cultures for an additional 2 weeks, for a total of 4 weeks. No GFP-positive cells were observed in any BAC36-ΔLNA-transfected cultures, while the number of GFP-positive cells in BAC36-transfected cultures remained at approximately 80% of the peak level. These results indicated that BAC36-ΔLNA episomes could not be stably maintained in 293 cells.
Both BAC36 and BAC36-ΔLNA genomes contained a hygromycin-resistance cassette, allowing the establishment of stable cell lines harboring KSHV genomes by hygromycin selection. We determined the efficiencies of isolating stable mammalian cell lines harboring BAC36 and BAC36-ΔLNA genomes. Again, episomal DNA of BAC36 and BAC36-ΔLNA was transfected into 293 cells, which were then treated with hygromycin 2 days posttransfection. All the cells transfected with BAC36-ΔLNA died after 2 weeks of hygromycin treatment. While the GFP-negative cells also died in BAC36-transfected cultures, the GFP-positive cells continued to grow. The number of GFP-positive cells increased daily to close to 100% after 6 weeks of hygromycin selection in BAC36-transfected cultures. These results further confirmed that BAC36-ΔLNA could not be stably maintained in 293 cells as wild-type BAC36 could.
BAC36-ΔLNA episomes failed to persist in mammalian cells.
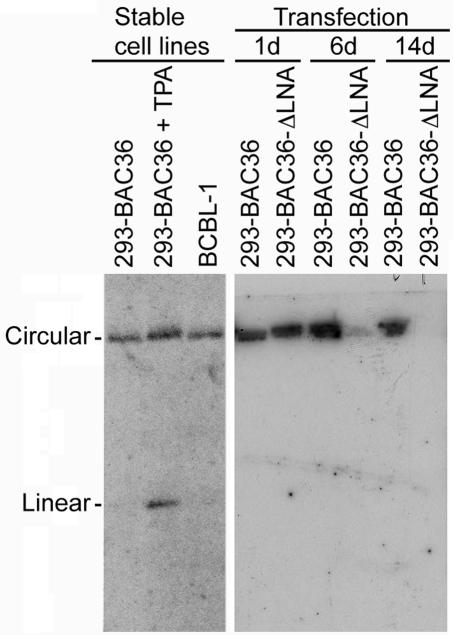
To confirm that the rapid decrease of GFP-positive cells in BAC36-ΔLNA-transfected cultures was due to the loss of KSHV episomes, we performed Gardella gel electrophoresis with BAC36- and BAC36-ΔLNA-transfected cells on different days posttransfection (Fig. 5). The DNA was transferred to a membrane and hybridized with a P32-labeled LNA probe to detect the presence of KSHV genomes. Linear KSHV genomes had a size of about 170 kb, while circular genomes representing episomes had a much slower migration band, as demonstrated with a previously established BAC36-infected stable 293 cell line. Relatively strong signals of KSHV episomes were detected in both BAC36- and BAC36-ΔLNA-transfected cells 1 day posttransfection. The episome signal in BAC36-ΔLNA-transfected cells was reduced approximately 10-fold 6 days posttransfection and became invisible 14 days posttransfection. In contrast, episome signals in BAC36-transfected cells remained relatively stable. No signal of the KSHV linear genome was detected at any time point examined for either BAC36- or BAC36-ΔLNA-transfected cells, indicating that the recombinant viruses did not have any or had only very weak viral lytic replication, which was consistent with previous observations (40). These results indicated that BAC36-ΔLNA episomes failed to be stably maintained in 293 cells, accounting for the rapid loss of GFP-positive cells in BAC36-ΔLNA-transfected cultures.
FIG. 5.
Gardella gel analysis of KSHV episomes. 293 cells transfected with BAC36 and BAC36-ΔLNA were examined by Gardella gel electrophoresis to detect KSHV episomes. The BCBL-1 cell line and a previously established stable 293 cell line harboring BAC36 were used as controls. P32-labeled full-length LNA was used as a probe.
We further performed sqPCR to detect the presence of episomes in both BAC36- and BAC36-ΔLNA-transfected cells. We used DNA isolated from equal amounts of BAC36- and BAC36-ΔLNA-transfected cells for the experiments. A GAPDH signal was used to calibrate the quantity of DNA used in each sample. We detected the 1,428-bp PCR fragment containing the transposon-Kanr insertion only in BAC36-ΔLNA-transfected cells, and the 207-bp fragment only in BAC36-transfected cells 1 day posttransfection, indicating that we introduced the correct recombinant genomes into the cells. However, the signal of the 1,428-bp band was significantly reduced 6 days posttransfection, and no signal was detectable 14 days posttransfection in BAC36-ΔLNA-transfected cultures, while a strong signal from the 207-bp band was maintained throughout the same period in BAC36-transfected cultures (Fig. 6A). Two additional PCR assays amplifying ORF16 and ORF65 in the KSHV genome were used to confirm these results. Again, BAC36-transfected cells maintained similar signal levels from 1 to 14 days posttransfection, while the signal of BAC36-ΔLNA-transfected cells started to weaken 6 days posttransfection and became almost invisible 14 days posttransfection (Fig. 6A). We further performed qPCR to monitor the copy numbers of KSHV genomes with primers designed from ORF65 and ORF16 (Fig. 6B and C). Although both BAC36- and BAC36-ΔLNA-transfected cells had similar levels of KSHV genomes 1 day posttransfection, those of BAC36-ΔLNA-transfected cells dropped significantly 6 days posttransfection. By 14 days posttransfection, the signal from BAC36-ΔLNA-transfected cells was barely detectable by the ORF65 assay and was completely undetectable by the ORF16 assay. In contrast, the KSHV genome signal from BAC36-transfected cells remained relatively stable throughout the period. These results were consistent with those of GFP tracking and Gardella gel analysis and further indicated that BAC36-ΔLNA episomes were rapidly lost while those of BAC36 were stably maintained in 293 cells following multiple passages of cell cultures.
FIG. 6.
sqPCR and qPCR analyses for the detection of KSHV genomes in BAC36- and BAC36-ΔLNA-transfected 293 cells. (A) sqPCR for detecting LNA, ORF16, and ORF65 KSHV genes. GAPDH was used to calibrate DNA loading and cell number. (B and C) qPCR analysis for detecting KSHV genomes. Results were the averages of threshold cycles (Ct values) after GAPDH calibration.
DISCUSSION
KSHV LNA has been extensively studied in vitro. One of LNA's potential functions is KSHV episome persistence. Colocalization of LNA with KSHV episomes on chromosomes strongly suggests its involvement in episome maintenance (2). Indeed, LNA alone is sufficient to maintain the replication and persistence of TR-containing plasmids (2, 3, 6, 7, 14, 15, 18, 30, 35). It has not been actually determined whether LNA is required for the maintenance and persistence of full-length KSHV episome, although it has been speculated that it is. It is unknown whether LNA alone is sufficient for carrying out these functions and whether there is a second viral protein carrying out similar functions. In this study, we generated a recombinant KSHV BAC36-ΔLNA with LNA disrupted. Upon delivery of BAC36-ΔLNA DNA into 293 cells, we unambiguously demonstrated that LNA is essential for the establishment and long-term persistence of KSHV episomes in mammalian cells.
BAC36-ΔLNA was generated by transposon-based mutagenesis with BAC36 as a template. Analysis of the mutant virus showed that it had a transposon cassette insertion in the N-terminal domain of LNA and that it maintained the full-length KSHV genome without any other genomic rearrangements. Consequently, the mutant virus no longer expressed the LNA protein (Fig. 3). Reconstitution in 293 cells showed that immediately following delivery into 293 cells, BAC36-ΔLNA-transfected cells had a substantially lower percentage of cells expressing GFP than wild-type BAC36-transfected cells did (Fig. 4). The fact that this deficiency can be partially rescued by complementing with a LNA expression plasmid strongly indicates that LNA is responsible for the lower percentage of GFP-positive cells in BAC36-ΔLNA-transfected cells and suggests that LNA is important for the initial establishment of KSHV episomes in mammalian cells. Nevertheless, it is also possible that the disruption of LNA directly interferes with the expression of GFP cassette. A number of reports have shown that LNA regulates transcription via direct interactions with transcriptional factors or via binding to nuclear heterochromatin (22-26, 34, 37). It is possible that the loss of LNA could render viral genomes in an environment unsuitable for the transcription of the GFP gene.
Our results demonstrate that LNA is essential for the maintenance and long-term persistence of KSHV genomes in mammalian cells. When monitored over a period of 2 weeks, the number of GFP-positive cells decreased rapidly in BAC36-ΔLNA-transfected cultures while those in BAC36-transfected cells remained relatively stable. Gardella gel, sqPCR, and qPCR analyses further confirmed that BAC36-ΔLNA-transfected cultures quickly lost KSHV episomes 14 days posttransfection while those of BAC36-transfected cells remained relative stable during the same period (Fig. 5 and 6).
A recent report described the rapid loss of KSHV episomes and TR-containing plasmids upon delivery into mammalian cells (17). In our study, an approximately 10 to 18% loss of wild-type KSHV BAC36 episomes was observed in the first week posttransfection; however, BAC36 episomes remained stable afterwards (Fig. 4). It would be interesting to determine whether epigenetic factors might contribute to the initial establishment and long-term maintenance of KSHV episomes in mammalian cells.
LNA, together with vCyclin (ORF72) and vFLIP (ORF-K13), is positioned as a cluster of latent genes in the KSHV genome. These three genes expressed from the same promoter constitute three latent transcripts: two tricistronic transcripts containing all three genes, and one bicistronic transcript containing vCyclin and vFLIP (Fig. 1B). Thus, vCyclin and vFLIP, if any, would be the most likely genes affected by LNA disruption. We have shown that the insertion of a transposon cassette into LNA did not disrupt the expression of vCyclin and vFLIP (Fig. 3). Hence, the reduced efficiency in the establishment of persistence and the subsequent rapid loss of KSHV episomes in the BAC36-ΔLNA-transfected cultures were solely due to the disruption of LNA. Using the same transposon-based mutagenesis approach, we recently isolated KSHV mutants with vCyclin and vFLIP disrupted, respectively. The vCyclin and vFLIP mutants behaved similarly to wild-type BAC36 when their episomes were delivered into 293 cells. Both of the mutants had relatively large numbers of GFP-positive cells (7 to 8%) after initial transfection, which could be further maintained after long-term culture. We have also isolated stable cultures of both mutants by hygromycin selection. These results suggest that vCyclin and vFLIP are not essential for the establishment and persistence of KSHV episomes in 293 cells. Therefore, the results obtained in this study are unique to the disruption of LNA but are not due to the disturbance of vCyclin and vFLIP genes.
Acknowledgments
We thank Mary Collins at the Windeyer Institute of Medical Sciences, London, England, for generously providing us a rat monoclonal antibody to vFLIP protein.
This work was supported in part by the Association for International Cancer Research, the Charlotte Geyer Foundation, and NIH grant RO1CA096512 to S.-J.G.
REFERENCES
- 1.An, J., A. K. Lichtenstein, G. Brent, and M. B. Rettig. 2002. The Kaposi's sarcoma-associated herpesvirus (KSHV) induces cellular interleukin 6 expression: role of the KSHV latency-associated nuclear antigen and the AP1 response element. Blood 99:649-654. [DOI] [PubMed] [Google Scholar]
- 2.Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. [DOI] [PubMed] [Google Scholar]
- 3.Ballestas, M. E., and K. M. Kaye. 2001. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J. Virol. 75:3250-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbone, A., A. Gloghini, D. Bontempo, P. Monini, U. Tirelli, R. Volpe, P. J. Browning, and G. Gaidano. 2000. Proliferation in HHV-8-positive primary effusion lymphomas is associated with expression of HHV-8 cyclin but independent of p27(kip1). Am. J. Pathol. 156:1209-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 6.Cotter, M. A., II, and E. S. Robertson. 1999. The latency-associated nuclear antigen tethers the Kaposi's sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology 264:254-264. [DOI] [PubMed] [Google Scholar]
- 7.Fejer, G., M. M. Medveczky, E. Horvath, B. Lane, Y. Chang, and P. G. Medveczky. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus interacts preferentially with the terminal repeats of the genome in vivo and this complex is sufficient for episomal DNA replication. J. Gen. Virol. 84:1451-1462. [DOI] [PubMed] [Google Scholar]
- 8.Friborg, J., Jr., W. Kong, M. O. Hottiger, and G. J. Nabel. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889-894. [DOI] [PubMed] [Google Scholar]
- 9.Fujimuro, M., F. Y. Wu, C. ApRhys, H. Kajumbula, D. B. Young, G. S. Hayward, and S. D. Hayward. 2003. A novel viral mechanism for dysregulation of beta-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nat. Med. 9:300-306. [DOI] [PubMed] [Google Scholar]
- 10.Gao, S.-J., L. Kingsley, D. R. Hoover, T. J. Spira, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, P. Parry, Y. Chang, and P. S. Moore. 1996. Seroconversion of antibodies against Kaposi's sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N. Engl. J. Med. 335:233-241. [DOI] [PubMed] [Google Scholar]
- 11.Gao, S.-J., L. Kingsley, M. Li, W. Zheng, C. Parravicini, J. Ziegler, R. Newton, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, Y. Chang, and P. S. Moore. 1996. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat. Med. 2:925-928. [DOI] [PubMed] [Google Scholar]
- 12.Gao, S.-J., Y.-J. Zhang, J.-H. Deng, C. S. Rabkin, O. Flore, and H. B. Jenson. 1999. Molecular polymorphism of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) latent nuclear antigen: evidence for a large repertoire of viral genotypes and dual infection with different viral genotypes. J. Infect. Dis. 180:1466-1476. [DOI] [PubMed] [Google Scholar]
- 13.Gao, S. J., J. H. Deng, and F. C. Zhou. 2003. Productive lytic replication of a recombinant Kaposi's sarcoma-associated herpesvirus in efficient primary infection of primary human endothelial cells. J. Virol. 77:9738-9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garber, A. C., J. Hu, and R. Renne. 2002. Latency-associated nuclear antigen (LANA) cooperatively binds to two sites within the terminal repeat, and both sites contribute to the ability of LANA to suppress transcription and to facilitate DNA replication. J. Biol. Chem. 277:27401-27411. [DOI] [PubMed] [Google Scholar]
- 15.Garber, A. C., M. A. Shu, J. Hu, and R. Renne. 2001. DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:7882-7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardella, T., P. Medveczky, T. Sairenji, and C. Mulder. 1984. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J. Virol. 50:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grundhoff, A., and D. Ganem. 2004. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J. Clin. Investig. 113:124-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grundhoff, A., and D. Ganem. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus permits replication of terminal repeat-containing plasmids. J. Virol. 77:2779-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyun, T. S., C. Subramanian, M. A. Cotter II, R. A. Thomas, and E. S. Robertson. 2001. Latency-associated nuclear antigen encoded by Kaposi's sarcoma-associated herpesvirus interacts with Tat and activates the long terminal repeat of human immunodeficiency virus type 1 in human cells. J. Virol. 75:8761-8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kedes, D. H., M. Lagunoff, R. Renne, and D. Ganem. 1997. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi's sarcoma-associated herpesvirus. J. Clin. Investig. 100:2606-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kellam, P., C. Boshoff, D. Whitby, S. Matthews, R. A. Weiss, and S. J. Talbot. 1997. Identification of a major latent nuclear antigen, LNA-1, in the human herpesvirus 8 genome. J. Hum. Virol. 1:19-29. [PubMed] [Google Scholar]
- 22.Knight, J. S., M. A. Cotter, and E. S. Robertson. 2001. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus transactivates the telomerase reverse transcriptase promoter. J. Biol. Chem. 276:22971-22978. [DOI] [PubMed] [Google Scholar]
- 23.Krithivas, A., D. B. Young, G. Liao, D. Greene, and S. D. Hayward. 2000. Human herpesvirus 8 LANA interacts with proteins of the mSin3 corepressor complex and negatively regulates Epstein-Barr virus gene expression in dually infected PEL cells. J. Virol. 74:9637-9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim, C., Y. Gwack, S. Hwang, S. Kim, and J. Choe. 2001. The transcriptional activity of cAMP response element-binding protein-binding protein is modulated by the latency associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Biol. Chem. 276:31016-31022. [DOI] [PubMed] [Google Scholar]
- 25.Lim, C., D. Lee, T. Seo, C. Choi, and J. Choe. 2003. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus functionally interacts with heterochromatin protein 1. J. Biol. Chem. 278:7397-7405. [DOI] [PubMed] [Google Scholar]
- 26.Lim, C., H. Sohn, Y. Gwack, and J. Choe. 2000. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) binds ATF4/CREB2 and inhibits its transcriptional activation activity. J. Gen. Virol. 81:2645-2652. [DOI] [PubMed] [Google Scholar]
- 27.Low, W., M. Harries, H. Ye, M. Q. Du, C. Boshoff, and M. Collins. 2001. Internal ribosome entry site regulates translation of Kaposi's sarcoma-associated herpesvirus FLICE inhibitory protein. J. Virol. 75:2938-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore, P. S., and Y. Chang. 2001. Molecular virology of Kaposi's sarcoma-associated herpesvirus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:499-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan, H. Y., Y. J. Zhang, X. P. Wang, J. H. Deng, F. C. Zhou, and S. J. Gao. 2003. Identification of a novel cellular transcriptional repressor interacting with the latent nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 77:9758-9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piolot, T., M. Tramier, M. Coppey, J. C. Nicolas, and V. Marechal. 2001. Close but distinct regions of human herpesvirus 8 latency-associated nuclear antigen 1 are responsible for nuclear targeting and binding to human mitotic chromosomes. J. Virol. 75:3948-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Platt, G. M., G. R. Simpson, S. Mittnacht, and T. F. Schulz. 1999. Latent nuclear antigen of Kaposi's sarcoma-associated herpesvirus interacts with RING3, a homolog of the Drosophila female sterile homeotic (fsh) gene. J. Virol. 73:9789-9795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radkov, S. A., P. Kellam, and C. Boshoff. 2000. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat. Med. 6:1121-1127. [DOI] [PubMed] [Google Scholar]
- 33.Rainbow, L., G. M. Platt, G. R. Simpson, R. Sarid, S.-J. Gao, H. Stoiber, C. S. Herrington, P. S. Moore, and T. F. Schulz. 1997. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J. Virol. 71:5915-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renne, R., C. Barry, D. Dittmer, N. Compitello, P. O. Brown, and D. Ganem. 2001. Modulation of cellular and viral gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:458-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwam, D. R., R. L. Luciano, S. S. Mahajan, L. Wong, and A. C. Wilson. 2000. Carboxy terminus of human herpesvirus 8 latency-associated nuclear antigen mediates dimerization, transcriptional repression, and targeting to nuclear bodies. J. Virol. 74:8532-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang, J., G. M. Gordon, M. G. Muller, M. Dahiya, and K. E. Foreman. 2003. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen induces expression of the helix-loop-helix protein Id-1 in human endothelial cells. J. Virol. 77:5975-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viejo-Borbolla, A., E. Kati, J. A. Sheldon, K. Nathan, K. Mattsson, L. Szekely, and T. F. Schulz. 2003. A domain in the C-terminal region of latency-associated nuclear antigen 1 of Kaposi's sarcoma-associated herpesvirus affects transcriptional activation and binding to nuclear heterochromatin. J. Virol. 77:7093-7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe, T., M. Sugaya, A. M. Atkins, E. A. Aquilino, A. Yang, D. L. Borris, J. Brady, and A. Blauvelt. 2003. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen prolongs the life span of primary human umbilical vein endothelial cells. J. Virol. 77:6188-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, Y. J., J. H. Deng, C. Rabkin, and S. J. Gao. 2000. Hot-spot variations of Kaposi's sarcoma-associated herpesvirus latent nuclear antigen and application in genotyping by PCR-RFLP. J. Gen. Virol. 81:2049-2058. [DOI] [PubMed] [Google Scholar]
- 40.Zhou, F. C., Y. J. Zhang, J. H. Deng, X. P. Wang, H. Y. Pan, E. Hettler, and S. J. Gao. 2002. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J. Virol. 76:6185-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]