Abstract
Background
Obesity increases cardiovascular risk. However, the extent to which various measures of body composition are associated with abnormalities in cardiac structure and function, independent of comorbidities commonly affecting obese individuals, is not clear. This study sought to examine the relationship of body mass index (BMI), waist circumference (WC), and percent body fat (BF) with conventional and advanced measures of cardiac structure and function.
Methods and Results
We studied 4343 participants of the Atherosclerosis Risk in Communities Study who were aged 69-82 years, free of coronary heart disease and heart failure, and underwent comprehensive echocardiography. Increasing BMI, WC, and BF were associated with greater left ventricular (LV) mass and left atrial volume indexed to height2.7 in both men and women (P<0.001). In women, all three measures were associated with abnormal LV geometry, and increasing WC and BF were associated with worse global longitudinal strain, a measure of left ventricular systolic function. In both sexes, increasing BMI was associated with greater right ventricular (RV) end-diastolic area and worse RV fractional area change (P≤0.001). We observed similar associations for both waist circumference and percent body fat.
Conclusions
In a large, biracial cohort of older adults free of clinically overt coronary heart disease or heart failure, obesity was associated with subclinical abnormalities in cardiac structure in both men and women and with adverse left ventricular remodeling and impaired left ventricular systolic function in women. These data highlight the association of obesity and subclinical abnormalities of cardiac structure and function, particularly in women.
Keywords: obesity, body mass index, echocardiography, remodeling, gender differences
The prevalence of obesity has grown over the past several decades with 68% of U.S. adults currently considered overweight or obese (1). While obesity has been associated with increased all-cause mortality, after adjustment for traditional cardiovascular (CV) risk factors, body mass index (BMI) and waist circumference (WC) remain only minimally associated with coronary heart disease and stroke (1,2,3) There are conflicting data as to whether greater BMI is associated with an increased risk of heart failure (4,5).
Although many of the mechanisms remain unexplained, a growing body of evidence demonstrates there are detrimental effects of obesity on cardiac structure and function (6,7,8,9). The majority of studies examining the effects of obesity on the heart used anthropomorphic measures such as BMI, which does not distinguish between fat and lean tissue and may misclassify individuals with excess fat mass as non-obese, especially in older adults in whom sarcopenia is common. Bioimpedence offers a means of directly measuring body composition and enables body mass to be classified as fat or lean, and may help to elucidate the true relationship between excess body fat and abnormalities of cardiac structure and function. Additionally, waist circumference more directly reflects abdominal obesity, which is associated with increased metabolic abnormalities and cardiovascular risk.
We analyzed variations in body composition (percent body fat) and traditional anthropomorphic measures (BMI and waist circumference) and their association with cardiac structure and function in an elderly, biracial cohort that was free from clinically overt coronary heart disease or heart failure.
Methods
Study Population
The Atherosclerosis Risk in Communities (ARIC) Study is a prospective cohort designed to investigate the causes of atherosclerosis and its related clinical outcomes in 15,792 men and women recruited from four U.S. communities (Forsyth County, NC; Jackson, MS; suburbs of Minneapolis, MN; and, Washington County, MD) (10). Participants were aged 45 to 64 years at the time of the baseline examination (1987-1989) and were subsequently evaluated during four follow-up clinical visits (Visits 2, 3, 4 and 5) at approximately 3-year intervals, except for visit 5 (15 y interval). Annual telephone follow-up of participants has been conducted since the baseline visit. Institutional review boards from each site approved the study, and informed consent was obtained from all participants.
The present investigation is a cross-sectional analysis of 4343 ARIC participants during visit 5 (2011-2013). Of the 6538 participants at visit 5, we first restricted the sample to 5741 who had complete data for echocardiography, BMI, waist circumference, and percent body fat. We then excluded participants with prevalent coronary heart disease (n=853) or heart failure (n=480), race other than black or white (n=12), as well as those who were underweight at visit 5 (n=53), yielding a final n of 4343.
Ascertainment and Categorization of Body Composition
Standardized anthropomorphic measurements of weight, height, and waist circumference were obtained and bioelectric impedance (measured using the Tanita Body Composition Analyzer, TBF-300A) was utilized to calculate percent body fat, fat mass and lean body mass. Obesity was defined using three different criteria: 1. Body-mass index [defined as body weight (in kilograms) divided by height (in meters) squared] was categorized as normal (18.5 ≥ BMI <25 kg/m2), overweight (25 ≥ BMI <30 kg/m2), or obese (BMI ≥30 kg/m2). 2. Waist circumference (cm) with abdominal obesity categorized defined as waist circumference >102 in men or >88 in women. 3. Percent body fat was defined as obese, if >25% in men or 35% in women (11,12,13).
Echocardiographic Methods and Measurements
Echocardiograms were obtained from participants at all four sites at visit 5 on identically configured systems using a standardized protocol (14). Images were digitally transferred to the Cardiovascular Imaging Core Lab at Brigham and Women's Hospital, Boston, MA for offline analysis. The intra-observer variability (coefficient of variation and intra-class correlation) for key echocardiographic measures has been previously published (14).
Images were obtained in the parasternal long- and short-axis and apical 2- and 4-chamber views. Primary measures of left ventricular (LV) dimensions, volumes, and wall thickness; right ventricular (RV) area; left atrial (LA) dimension, volume, and area; and Doppler measures of mitral inflow, tricuspid regurgitation, and mitral annular relaxation velocities were made in triplicate from the 2D views in accordance with the recommendations of the American Society of Echocardiography (15).
Calculation of Derived Echocardiographic Variables
LV ejection fraction was calculated as 100*(LV end-diastolic volume- LV end-systolic volume)/ LV end-diastolic volume. LV mass was determined according to the ASE recommendations and indexed to height to the power of 2.7. Total LV wall thickness (TWT) was the sum of anteroseptal and posterior wall thickness (PWT), and relative wall thickness (RWT) was calculated as (2*PWT) / LV end-diastolic dimension. LV hypertrophy (LVH) was defined as LV mass indexed to BSA >95 g/m2 in women and >115 g/m2 in men. Normal geometry was classified as RWT ≤ 0.42 and no LVH; Abnormal geometry was defined as the presence of either concentric remodeling (RWT >0.42 and no LVH), concentric hypertrophy (RWT <0.42 and LVH),or eccentric hypertrophy (RWT ≥ 0.42 and LVH). RV fractional area change (%) was calculated as 100*(RV end-diastolic area-RV end-systolic area)/RV end-diastolic area. LA volume was indexed to height to the power of 2.7. Abnormalities of diastolic function were assessed using a previously published approach in a community based cohort (16). Our population was classified using the following schema: normal diastolic function (deceleration time of E wave (DT) >140ms, 0.75<E/A<2 and E/E’<10); mild diastolic dysfunction (DT>140ms, E/A<0.75 and E/E’<10); moderate to severe diastolic dysfunction (0.75<E/A<2 and E/E’≥10; or DT <140ms, E/A>2 and E/E’≥10); and unclassifiable if participants did not fall into one of these three categories. Diastolic function was collapsed into normal diastolic function and abnormal diastolic function (mild or moderate to severe diastolic dysfunction) for all analyses.
Statistical Analysis
Data are presented as mean +/− SD, median [IQR], or N (%). Due to gender specific norms for many of the outcome variables as well as significant interactions between sex and the outcomes all analyses were performed stratified by sex. Interactions with race were tested for in all outcomes. Intergroup comparisons were compared using non-parametric trend, t-test, and χ2 tests. Skewed variables were analyzed following log-transformation. Unadjusted and adjusted associations were assessed using linear and logistic regression. Restricted cubic spline models were used to assess all relationships for linearity. Chi-square tests were used to compare categorical variables. Potential confounders were identified a priori and used to correct all models. Covariates included in the regression models were age, race, heart rate, systolic blood pressure, anti-hypertensive use, diabetes, HbA1c, and current smoking. Bonferroni corrected P-values were used to determine statistical significant for all analyses. A corrected p-value of <0.005 was considered statistically significant for the primary echocardiographic measures reported. All reported p values are 2-sided. STATA v12.1 (College Station, TX) was used.
Results
Patient Characteristics
The characteristics of our study sample (n=4343) are shown in Table 1 and Supplemental Table 1. Compared to participants with a normal BMI, those who were overweight and obese were younger and more likely to be black. The prevalences of diabetes and hypertension were high and increased along with BMI and other measures of obesity. Participants with higher BMIs also had significantly larger waist circumferences as well as fat mass (p for trend <0.001). There was an inverse relationship observed between BMI and current smoking.
Table 1.
Characteristics at Visit 5 Stratified by Body Mass Index Category
| Normal | Overweight | Obese | |
|---|---|---|---|
| n=1137 | n=1732 | n=1474 | |
| Clinical Characteristics | |||
| Age (years) | 76±5 | 76 ±5 | 75±5 |
| Male | 377 (33.2%) | 732 (42.3%) | 547 (37.1%) |
| Female | 760 (66.8%) | 1000 (57.7%) | 927 (62.9%) |
| Black | 160 (14.1%) | 351 (20.3%) | 413 (28.0%) |
| White | 977 (85.9%) | 1381 (79.7%) | 1061 (72.0%) |
| Center | |||
| Forsyth Co. | 327 (28.8%) | 392 (22.6%) | 259 (17.6%) |
| Jackson | 146 (12.8%) | 314 (18.1%) | 379 (25.7%) |
| Minneapolis | 390 (34.3%) | 563 (32.5%) | 408 (27.7%) |
| Washington Co. | 274 (24.1%) | 463 (26.7%) | 428 (29.0%) |
| Diabetes mellitus | 214 (18.8%) | 559 (32.3%) | 692 (46.9%) |
| Hypertension | 796 (70.0%) | 1378 (79.6%) | 1300 (88.2%) |
| Current Smoker | 97 (8.7%) | 85 (5.0%) | 62 (4.3%) |
| Lipid lowering therapy | 474 (41.7%) | 1027 (53.9%) | 889 (60.3%) |
| Heart rate (bpm) | 62±10 | 62±10 | 64±11 |
| SBP (mmHg) | 130±18 | 131±17 | 130±17 |
| DBP (mmHg) | 65± 10 | 68±10 | 69±10 |
| Body Composition | |||
| Height (cm) | 165±9 | 166±10 | 165±9 |
| Weight (kg) | 62±9 | 76±10 | 94±14 |
| BMI (kg/m2) | 22.8 ±1.7 | 27.4±1.4 | 34.5±4.4 |
| Body fat (%) | 28.8±13.9 | 34.4±7.1 | 41.3±7.2 |
| Waist circumference (cm) | 88±8 | 99±8 | 113±11 |
| Waist hip ratio | 0.90±0.07 | 0.94±0.07 | 0.95±0.08 |
| Laboratory Values | |||
| HbA1c (%) | 5.7±0.5 | 5.9±0.7 | 6.1±1.0 |
| Total Chol (mg/dL) | 194±38 | 187±42 | 180±40 |
| LDL (mg/dL) | 112±31 | 110±35 | 104±34 |
| HDL (mg/dL) | 60±15 | 52±13 | 49±11 |
| Triglycerides (mg/dL) | 108±48 | 127±62 | 137±71 |
| hs-CRP (mg/L) | 1.27 [0.65,2.56] | 1.86 [0.92, 3.80] | 2.95 [1.47, 5.62] |
| NT-proBNP (pg/mL) | 141 [75, 243] | 105 [56, 201] | 101 [54, 196] |
| eGFR (ml/min/1.73m2) | 71.6±15.4 | 70.8±16.0 | 70.9±17.1 |
Values are shown as mean±SD, median [IQR], or N (%).
Left Ventricular Dimensions
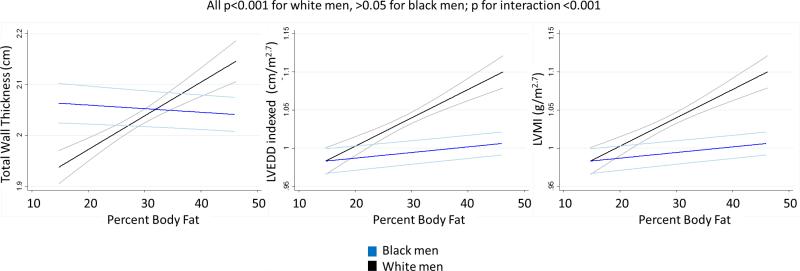
In both women and men, greater anthropomorphic measures of obesity, BMI and WC, were associated with a significantly increased thickness of the left ventricular (LV) walls, larger LV cavity size, and higher LV mass (Table 2). A similar association was seen when percent body fat (BF) was used as a measure of adiposity in women. There was a significant interaction with race in the relationship between left ventricular dimensions and BF in men that was not observed with BMI or WC; a positive correlation between BF and LV dimensions was not seen in black men, though in white men the findings were consistent with those in women (Figure 1).
Table 2.
Associations between measures of obesity and cardiac structure and function by sex. Model 1 is adjusted for age and race; model 2 is adjusted for age, race, systolic blood pressure, anti-hypertensive use, diabetes, HbA1c, and current smoking. Strain measures were also adjusted for heart rate.
| Women | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P values |
||||||||||||
| Normal BMI | Overweight | Obese | BMI | Waist Circumference | Body Fat | |||||||
| n= 760 | n= 1000 | n= 927 | unadj | model 1 | model 2 | unadj | model 1 | model 2 | unadj | model 1 | model 2 | |
| Left Ventricular Dimensions | ||||||||||||
| IVS (cm) | 0.94±0.12 | 1.00±0.14 | 1.06±0.15 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| PWT (cm) | 0.84±0.10 | 0.89±0.11 | 0.94±0.13 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| LVEDD index (cm/m2.7) | 1.15±0.14 | 1.20±0.15 | 1.24±0.16 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.19 | <0.001 | 0.001 |
| LV RWT (cm) | 0.41±0.06 | 0.43±0.07 | 0.43±0.08 | <0.001 | <0.001 | 0.10 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | 0.002 |
| LV mass index (g/m2.7) | 31.78±7.18 | 36.50±7.84 | 41.78±10.12 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Left Ventricular Systolic Function | ||||||||||||
| LVEF (%) | 66.87±5.17 | 66.92±5.10 | 66.67±5.62 | 0.04 | 0.17 | 0.19 | 0.38 | 0.36 | 0.35 | 0.05 | 0.23 | 0.14 |
| Longitudinal strain (%) | −18.63±2.20 | −18.37±2.28 | −18.28±2.49 | <0.001 | 0.001 | 0.36 | <0.001 | <0.001 | 0.01 | <0.001 | <0.001 | 0.04 |
| Circumferential strain (%) | −28.74±3.50 | −28.51±3.55 | −27.93±3.80 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.02 |
| Left Ventricular Diastolic Function | ||||||||||||
| LA volume (mL) | 38.24±12.61 | 41.97±12.30 | 48.34±14.69 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| LA vol index (mL/m2.7) | 10.76±3.53 | 11.87±3.34 | 13.64±4.21 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Peak E (cm/s) | 68.35±17.37 | 66.96±16.46 | 69.88±17.88 | <0.001 | <0.001 | <0.001 | 0.01 | 0.004 | <0.001 | 0.004 | 0.02 | 0.02 |
| E/A | 0.90±0.29 | 0.82±0.24 | 0.83±0.25 | <0.001 | <0.001 | 0.04 | <0.001 | <0.001 | 0.01 | <0.001 | <0.001 | <0.001 |
| e′ lateral (cm/s) | 7.11±2.01 | 6.79±1.92 | 6.88±2.01 | 0.45 | 0.01 | 0.43 | 0.01 | <0.001 | 0.08 | 0.007 | <0.001 | <0.001 |
| E/e′ lateral | 10.23±3.56 | 10.52±3.75 | 10.85±3.80 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.77 | 0.01 | 0.10 |
| Right Ventricular Size and Function | ||||||||||||
| RV FAC (%) | 0.55±0.08 | 0.54±0.07 | 0.53±0.08 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| RVEDA (cm2) | 16.38±3.76 | 17.18±3.62 | 19.00±4.24 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| RVEDA/Ht^2.7 (cm2/m2.7) | 4.61±1.06 | 4.87±1.02 | 5.38±1.24 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Peak TR velocity (cm/s) | 233.99±26.15 | 238.53±27.08 | 243.20±31.74 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.04 | 0.001 | 0.02 |
| Men | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P values |
||||||||||||
| Normal BMI | Overweight | Obese | BMI | Waist Circumference | Percent Body Fat | |||||||
| n=377 | n=732 | n=547 | unadj | model 1 | model 2 | unadj | model 1 | model 2 | unadj | model 1 | model 2 | |
| Left Ventricular Dimensions | ||||||||||||
| IVS (cm) | 1.03±0.14 | 1.07±0.16 | 1.11±0.15 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.02 | 0.02 | 0.09 |
| PWT (cm) | 0.92±0.14 | 0.96±0.13 | 1.00±0.14 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.04 | 0.02 | 0.22 |
| LVEDD index (cm/m2.7) | 0.99±0.13 | 1.02±0.13 | 1.06±0.13 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 |
| LV RWT (cm) | 0.42±0.08 | 0.42±0.07 | 0.43±0.08 | 0.05 | 0.01 | 0.17 | 0.10 | 0.01 | 0.20 | 0.55 | 0.76 | 0.33 |
| LV mass index (g/m2.7) | 32.33±7.89 | 36.22±9.12 | 40.62±9.61 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Left Ventricular Systolic Function | ||||||||||||
| LVEF (%) | 64.71±6.02 | 64.66±5.92 | 64.38±5.56 | 0.34 | 0.32 | 0.54 | 0.15 | 0.06 | 0.13 | 0.54 | 0.49 | 0.71 |
| Longitudinal strain (%) | −17.93±2.35 | −17.79±2.40 | −17.44±2.45 | 0.20 | <0.001 | 0.07 | <0.001 | <0.001 | 0.04 | 0.35 | 0.48 | 0.85 |
| Circumferential strain (%) | −27.20±3.97 | −27.33±3.60 | −27.07±3.77 | <0.001 | 0.28 | 0.90 | 0.30 | 0.19 | 0.89 | 0.70 | 0.93 | 0.59 |
| Left Ventricular Diastolic Function | ||||||||||||
| LA volume (mL) | 47.16±14.93 | 52.47±21.80 | 57.10±17.81 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.54 | 0.32 | 0.41 |
| LA vol index (mL/m2.7) | 10.46±3.22 | 11.70±4.96 | 12.79±3.98 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.25 | 0.07 | 0.11 |
| Peak E (cm/s) | 62.43±17.84 | 62.29±16.53 | 64.54±17.50 | <0.001 | <0.001 | 0.002 | <0.001 | 0.001 | <0.001 | 0.79 | 0.74 | 0.96 |
| E/A | 0.89±0.28 | 0.85±0.28 | 0.83±0.27 | 0.01 | <0.001 | 0.004 | 0.02 | <0.001 | 0.01 | 0.01 | 0.001 | 0.004 |
| e′ lateral (cm/s) | 7.51±2.10 | 7.33±2.17 | 7.09±2.07 | 0.05 | 0.001 | 0.01 | 0.02 | 0.001 | 0.01 | 0.36 | 0.09 | 0.14 |
| E/e′ lateral | 8.81±3.26 | 9.07±3.23 | 9.68±3.34 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.41 | 0.12 | 0.25 |
| Right Ventricular Size and Function | ||||||||||||
| RV FAC (%) | 0.52±0.08 | 0.51±0.07 | 0.50±0.08 | <0.001 | <0.001 | 0.001 | 0.001 | 0.001 | 0.003 | 0.15 | 0.17 | 0.17 |
| RVEDA (cm2) | 21.86±5.46 | 22.08±4.90 | 23.42±5.21 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.99 | 0.86 | 0.75 |
| RVEDA/Ht^2.7 (cm2/m2.7) | 4.86±1.23 | 4.92±1.06 | 5.23±1.16 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | 0.004 | 0.43 | 0.34 | 0.43 |
| Peak TR velocity (cm/s) | 231.82±24.51 | 234.70±25.32 | 239.30±28.12 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.96 | 0.84 | 0.78 |
Values shown are mean±SD.
Figure 1.
Higher percent body fat is positively associated with increased LV wall thickness, higher LV mass, and a larger LVEDD in white but not black men.
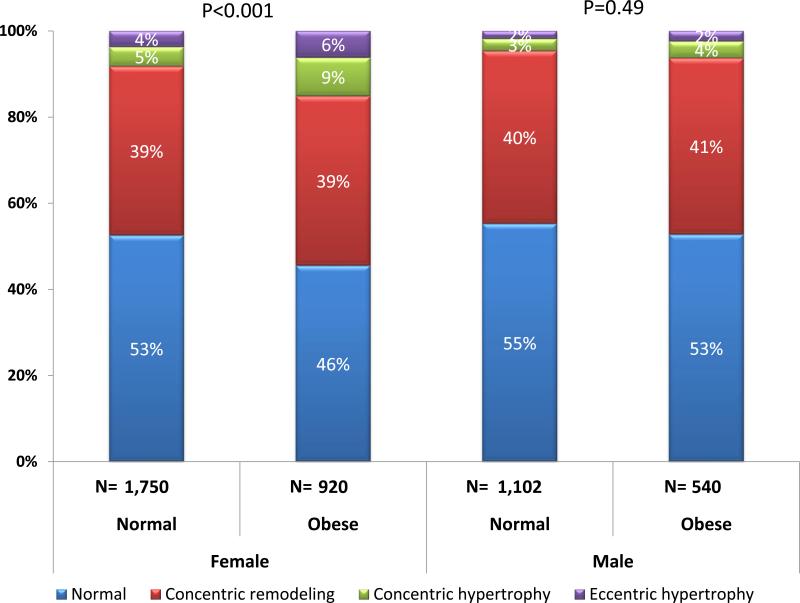
Obese women but not men were significantly more likely to exhibit abnormal LV geometry when compared to participants with a BMI < 30 kg/m2 (p<0.001 women, p=0.49 men) (Figure 2).
Figure 2.
Obesity is associated with abnormal LV geometry in women but not men.
Left Ventricular Systolic Function
The presence of obesity was not associated with LV systolic function as assessed by ejection fraction in either women or men. In women but not men, circumferential strain, a more sensitive measure of impaired systolic function, was highly associated with all three measures of obesity. Global longitudinal strain was also significantly associated with WC and BF in women but not men.
Diastolic Function and the Left Atrium
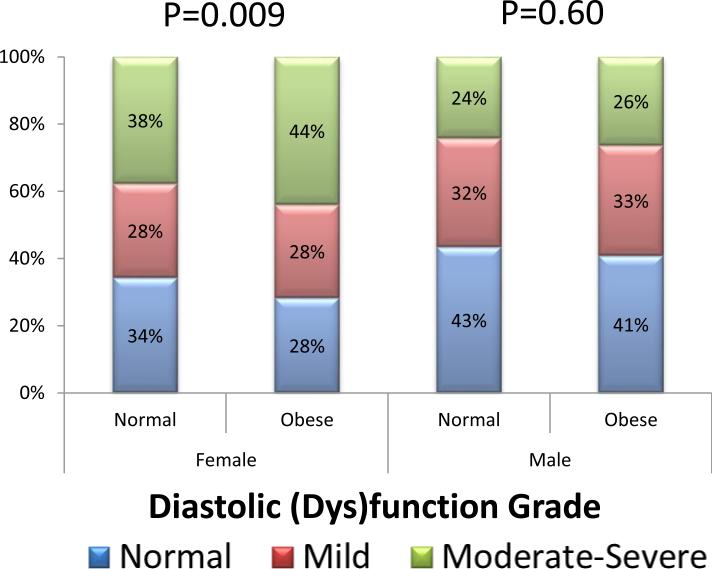
In women but not men, obesity was associated with significantly worse overall diastolic function (Figure 3). In both sexes, left atrial volumes increased significantly in the presence of increasing measures of obesity, even when scaled by height (Table 2).
Figure 3.
Obesity is associated with worse diastolic grade in women but not men.
Right Ventricular Structure and Function
The size of the right ventricle (RV) as assessed by the RV end diastolic area indexed to height2.7 was associated positively with body mass index, and RV systolic function was negatively associated with BMI in both men and women (p≤0.001). The relationship between RV size and function and measures of obesity remained significant across all measures of body composition in women (p<0.001). In men, the association between BF and RV size and function was significant in white but not black men.
Discussion
In a community-based, bi-racial population of older adults free of cardiovascular disease, greater metrics of body size and adiposity were associated with subclinical abnormalities in LV structure and function which differed somewhat by sex and race. In women, obesity was associated with a higher LV mass coupled with a disproportionately smaller increase in LV diameter, which resulted in concentric and eccentric hypertrophy with a concurrent decrement in strain and abnormalities of diastolic function. Men also exhibited greater total wall thickness and a positive association between LV diameter and mass in relation to greater BMI and WC, though these findings were not significantly accompanied by body size related impairments of LV function or a particular pattern of remodeling; the relationship with BF was modified by race and among men was found to be significant only in whites.
Similar to the changes that occur in hypertension or as a result of aortic stenosis, obesity is associated with cardiac remodeling. Previous studies using echocardiography have also demonstrated healthy obese men and women are more likely to have increased LV wall thickness and LV mass than their non-obese counterparts which may represent the earliest moment on the path to developing heart failure (8,9,17). There is emerging evidence from animal models that this change in wall thickness may be mediated by leptin, an adipocyte hormone that promotes collagen synthesis (18). Despite these changes in LV structure, greater BMI has not previously been associated with a significant decrement in systolic function as assessed by ejection fraction (8,19), or abnormalities of diastolic function after adjustment for clinical covariates (20).
Our results demonstrate that whether assessed via traditional anthropomorphic measures such as BMI and WC, or newer measures of body composition such as BF, obesity is associated with cardiac geometry and function differently in elderly men compared with elderly women. In particular, women are more likely to display primarily LV hypertrophy coupled with diastolic dysfunction and subclinical decrements in systolic function. Less negative circumferential strain has been shown to add incremental prognostic value for the development of incident heart failure (21) and may partially account for the female predominance of heart failure with preserved ejection fraction (HFpEF). By contrast, more obese men were not observed to exhibit similar patterns of remodeling or subclinical decrements in left ventricular systolic function. This preservation of biventricular systolic function could represent relative protection against the development of clinical heart failure with obesity and a type of ‘obesity paradox’ in men.
Both sexes and races demonstrated similar decrements in RV function and a positive association between RV size and greater body size. There are a paucity of data regarding the echocardiographic relationships between right ventricular size and obesity, though subclinical dysfunction has been described in association with increasing BMI. (7) To our knowledge these data are the first from a large, biracial contemporary cohort to examine RV morphology and function and its relation to obesity. We found that there is a significant decrement in RV systolic function coupled with RV enlargement in overweight and obese individuals that was not explained by comorbid hypertension or diabetes. These changes are probably multifactorial and may result from increased intravascular volume or sleep disordered breathing and warrant further study to elucidate the etiological mechanisms. In addition, as the prevalence of obesity continues to rise, the potential implications of RV dysfunction on long term survival will be increasingly important.
Several limitations of our study warrant consideration. First, this is an ambulatory elderly cohort and the findings may not be applicable to all obese individuals. Our study is cross-sectional and therefore hypothesis generating as to the causal relationship between obesity and HFpEF in women. There may also be a healthy survivor bias observed among the small black male cohort that resulted in their findings differing from the other participants. Additionally, there was a small number of participants who were excluded from the analysis due to missing data. These individuals were slightly older, more likely to be black, and slightly more hypertensive than those included. Multiple comparisons were made between three indices of obesity and echocardiographic measures. To minimize the possibility of in increased risk of a type I error, we used the highly conservative Bonferroni adjustment to account for multiple testing which may have consequentially resulted in limited power. However, this allows us to focus on the most clinically significant differences. Although echocardiography has been well validated for the assessment of cardiac structure and function, MRI is considered the gold standard for the assessment of the RV. However, our results are consistent with those from a prior study that examined the effect of obesity on the RV using cardiac MRI (22).
In conclusion, we observed in a large community-based cohort of elderly individuals that subclinical abnormalities of cardiac structure function are associated with measures of obesity. Both sexes demonstrated similar increases in LV mass and biventricular size as well as decrements in RV function with obesity, although women manifested subclinical left ventricular systolic dysfunction while men did not. These sex-specific abnormalities may contribute to different patterns of obesity-related cardiovascular disease.
Supplementary Material
Clinical Perspective.
The prevalence of obesity continues to rise in the US and around the world, and threatens to erase the gains made in the reduction of cardiovascular morbidity and mortality over the past several decades. Detrimental effects on cardiac structure and function are a potential mechanism by which obesity increases future risk of cardiovascular disease. The current study examined an elderly, biracial community-based cohort free from overt cardiovascular disease and found larger body size was associated with alterations in cardiac structure and function which varied by race and sex. Despite similar changes in left ventricular cavity size and mass in both sexes, only obese elderly women exhibited adverse remodeling, left ventricular diastolic dysfunction, and a subclinical decline in systolic function. However, abnormalities of right ventricular size and function associated with obesity were found in both sexes. Future studies are needed to determine whether this sex-specific variation in the cardiac effects of obesity contributes to different patterns of obesity-related cardiovascular disease in men and women, and whether weight loss can reverse these abnormalities.
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions.
Sources of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This work was also supported NHLBI cooperative agreement NHLBIHC-11-08 (Brigham and Women's Hospital). Support was provided for NAB by the NHLBI training grant (T32 HL007374-34). ABSS acknowledges support from CAPES grant 0281-12-3 (Brazil). SC is supported in part by R00-HL-107642 and the Ellison Foundation. AS is supported in part by K08-HL-116792.
Footnotes
Disclosures
None.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation. 2016;133:e38–60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA, Nordestgaard BG, Ridker P, Salomaa V, Stevens J, Woodward M, Sattar N, Collins R, Thompson SG, Whitlock G, Danesh J. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: A pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970–983. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 5.Voulgari C, Tentolouris N, Dilaveris P, Tousoulis D, Katsilambros N, Stefanadis C. Increased heart failure risk in normal-weight people with metabolic syndrome compared with metabolically healthy obese individuals. J Am Coll Cardiol. 2011;58:1343–1350. doi: 10.1016/j.jacc.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 6.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC. Heart failure. 2013;1:93–102. doi: 10.1016/j.jchf.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Wong CY, O'Moore-Sullivan T, Leano R, Hukins C, Jenkins C, Marwick TH. Association of subclinical right ventricular dysfunction with obesity. J Am Coll Cardiol. 2006;47:611–616. doi: 10.1016/j.jacc.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Wong CY, O'Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–3087. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 9.Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA. 1991;266:231–236. [PubMed] [Google Scholar]
- 10.ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 11.Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 12.The Evidence Report. National Institutes of Health. National Heart, Lung, and Blood Institute; Sep, 1998. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. NIH publication No. 98-4083. [Google Scholar]
- 13.World Health Organization . Physical status: The use and interpretation of anthropometry. World Health Organization; Geneva, Switzerland: 1995. WHO Technical Report Series. [PubMed] [Google Scholar]
- 14.Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, Cook N, Ni H, Coresh J, Mosley TH, Heiss G, Folsom AR, Solomon SD. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: The Atherosclerosis Risk in Communities Study. Circulation. Cardiovascular imaging. 2014;7:173–181. doi: 10.1161/CIRCIMAGING.113.000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: A report from the American Society of Echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Ech. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 17.Lauer MS, Anderson KM, Levy D. Separate and joint influences of obesity and mild hypertension on left ventricular mass and geometry: the Framingham Heart Study. Journal of the American College of Cardiology. 1992;19:130–4. doi: 10.1016/0735-1097(92)90063-s. [DOI] [PubMed] [Google Scholar]
- 18.Zibadi S, Cordova F, Slack EH, Watson RR, Larson DF. Leptin's regulation of obesity-induced cardiac extracellular matrix remodeling. Cardiovascular Toxicology. 2011;11:325–333. doi: 10.1007/s12012-011-9124-0. [DOI] [PubMed] [Google Scholar]
- 19.Lavie CJ, Milani RV, Ventura HO, Cardenas GA, Mehra MR, Messerli FH. Disparate effects of left ventricular geometry and obesity on mortality in patients with preserved left ventricular ejection fraction. American Journal of Cardiology. 2007;100:1460–4. doi: 10.1016/j.amjcard.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 20.Fischer M, Baessler A, Hense HW, Hengstenberg C, Muscholl M, Holmer S, Doring A, Broeckel U, Riegger G, Schunkert H. Prevalence of left ventricular diastolic dysfunction in the community. Results from a Doppler echocardiographic-based survey of a population sample. Eur Heart J. 2003;24:320–328. doi: 10.1016/s0195-668x(02)00428-1. [DOI] [PubMed] [Google Scholar]
- 21.Choi EY, Rosen BD, Fernandes VR, Yan RT, Yoneyama K, Donekal S, Opdahl A, Almeida AL, Wu CO, Gomes AS, Bluemke DA, Lima JA. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2013;34:2354–2361. doi: 10.1093/eurheartj/eht133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chahal H, McClelland RL, Tandri H, Jain A, Turkbey EB, Hundley WG, Barr RG, Kizer J, Lima JA, Bluemke DA, Karut SM. Chest. 2012;141:388–95. doi: 10.1378/chest.11-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.