Abstract
Seventeen multiple-antibiotic-resistant nonpathogenic Escherichia coli strains of human, animal, and food origins showed a wide variety of antibiotic resistance genes, many of them carried by class 1 and class 2 integrons. Amino acid changes in MarR and mutations in marO were identified for 15 and 14 E. coli strains, respectively.
The emergence of Escherichia coli isolates with multiple-antibiotic-resistant phenotypes, involving coresistance to four or more unrelated families of antibiotics, has been previously reported and is considered a serious health concern (2, 5, 22). Transference of resistance determinants by mobile genetic elements including plasmids, transposons, and gene cassettes in integrons (4, 13) and the alteration in mar locus regulation (1, 2, 27) are important factors that can contribute to the increase in multiresistant bacteria.
In previous studies (7, 34), the antibiotic resistance profiles of 515 nonpathogenic E. coli isolates obtained from food products of animal origin (n = 47) and from fecal samples of healthy animals (n = 177) and humans (n = 291) were studied. Seventeen E. coli isolates from those groups (four from food, eight from animals, and five from humans) showed a multiple-antibiotic-resistant phenotype (resistance to nalidixic acid, ampicillin, rifampin, chloramphenicol, sulfamethoxazole, streptomycin [STR] and tetracycline). All 17 of these isolates were used in the present work to detect different mechanisms of antibiotic resistance and to study the antibiotic resistance genes inside integrons and the relevance of the mar locus in the multiple-antibiotic-resistant phenotype.
Additional susceptibilities to ciprofloxacin, amoxicillin-clavulanic acid, cefazolin, cefoxitin, ceftazidime, cefotaxime, ceftriaxone, imipenem, aztreonam, gentamicin (GEN), apramycin, tobramycin, kanamycin, and trimethoprim were determined by an agar dilution method (24).
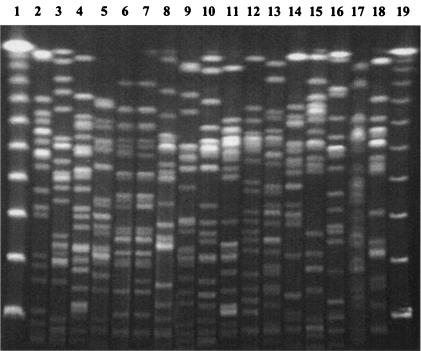
The 17 E. coli isolates showed 16 unrelated pulsed-field gel electrophoresis (PFGE) patterns with the XbaI enzyme in experiments following the method of Gautom (9) (Fig. 1). Only strains Co71 and Co82 showed closely related patterns.
FIG. 1.
PFGE patterns of XbaI-digested genomic DNA of the 17 multiresistant E. coli strains. Lanes: 1, λ ladder molecular size marker; 2, E. coli Co1; 3, E. coli Co19; 4, E. coli Co45; 5, E. coli Co53; 6, E. coli Co71; 7, E. coli Co82; 8, E. coli Co80; 9, E. coli Co110; 10, E. coli Co125; 11, E. coli Co177; 12, E. coli Co201; 13, E. coli Co227; 14, E. coli Co228; 15, E. coli Co232; 16, E. coli Co279; 17, E. coli Co354; 18, E. coli Co356; 19, λ ladder molecular size marker.
Analysis of antibiotic resistance mechanisms.
The presence of antibiotic resistance genes in the 17 E. coli strains was analyzed by PCR, PCR-restriction fragment length polymorphism analysis, and sequencing (Table 1). Table 2 shows the resistance phenotypes and genes identified.
TABLE 1.
Primers and annealing temperatures used in the PCR reactions carried out in this study
| Primer name | Sequence (5′→3′) | Target gene(s) or region | PCR product size (bp) | Annealing temp (°C) | Reference |
|---|---|---|---|---|---|
| TEM-F | ATTCTTGAAGACGAAAGGGC | blaTEM | 1,150 | 60 | 3 |
| TEM-R | ACGCTCAGTGGAACGAAAAC | ||||
| SHV-F | CACTCAAGGATGTATTGTG | blaSHV | 885 | 52 | 30 |
| SHV-R | TTAGCGTTGCCAGTGCTCG | ||||
| OXA-F | ACACAATACATATCAACTTCGC | blaOXA | 813 | 61 | 36 |
| OXA-R | AGTGTGTTTAGAATGGTGATC | ||||
| AacC1-F | ACCTACTCCCAACATCAGCC | aac(3)-I | 169 | 60 | 37 |
| AacC1-R | ATATAGATCTCACTACGCGC | ||||
| AacC2-F | ACTGTGATGGGATACGCGTC | aac(3)-II | 237 | 60 | 37 |
| AacC2-R | CTCCGTCAGCGTTTCAGCTA | ||||
| AacC3-F | CACAAGAACGTGGTCCGCTA | aac(3)-III | 185 | 60 | 37 |
| AacC3-R | AACAGGTAAGCATCCGCATC | ||||
| AacC4-F | CTTCAGGATGGCAAGTTGGT | aac(3)-IV | 286 | 60 | 37 |
| AacC4-R | TCATCTCGTTCTCCGCTCAT | ||||
| Ant(2′′)-F | ATGTTACGCAGCAGGGCAGTCG | ant(2′′) | 187 | 55 | 38 |
| Ant(2′′)-R | CGTCAGATCAATATCATCGTGC | ||||
| AphA1-F | ATGGGCTCGCGATAATGTC | aphA1 | 600 | 50 | 22 |
| AphA1-R | CTCACCGAGGCAGTTCCAT | ||||
| AphA2-F | GAACAAGATGGATTGCACGC | aphA2 | 680 | 50 | 22 |
| AphA2-R | GCTCTTCAGCAATATCACGG | ||||
| AadA-F | GCAGCGCAATGACATTCTTG | aadA1 or aadA2 | 282 | 60 | 16 |
| AadA-R | ATCCTTCGGCGCGATTTTG | 20 | |||
| TetA-F | GTAATTCTGAGCACTGTCGC | tetA | 937 | 62 | 11 |
| TetA-R | CTGCCTGGACAACATTGCTT | ||||
| TetB-F | CTCAGTATTCCAAGCCTTTG | tetB | 416 | 57 | 11 |
| TetB-R | CTAAGCACTTGTCTCCTGTT | ||||
| TetC-F | TCTAACAATGCGCTCATCGT | tetC | 570 | 62 | 11 |
| TetC-R | GGTTGAAGGCTCTCAAGGGC | ||||
| TetD-F | ATTACACTGCTGGACGCGAT | tetD | 1,104 | 57 | 11 |
| TetD-R | CTGATCAGCAGACAGATTGC | ||||
| TetE-F | GTGATGATGGCACTGGTCAT | tetE | 1,179 | 62 | 11 |
| TetE-R | CTCTGCTGTACATCGCTCTT | ||||
| CmlA-F | TGTCATTTACGGCATACTCG | cmlA | 455 | 55 | This study |
| CmlA-R | ATCAGGCATCCCATTCCCAT | ||||
| FloR1 | CACGTTGAGCCTCTATAT | floR | 868 | 55 | 26 |
| FloR2 | ATGCAGAAGTAGAACGCG | 6 | |||
| DfrIa-F | GTGAAACTATCACTAATGG | dfrA1, dfrA5, dfrA15, dfrA15b, dfrA16, dfrA16b | 474 | 55 | 25 |
| DfrIa-R | TTAACCCTTTTGCCAGATTT | ||||
| DfrIb-F | GAGCAGCTICTITTIAAAGC | dfrA14, dfrA6 | 393 | 60 | 25 |
| DfrIb-R | TTAGCCCTTTIICCAATTTT | ||||
| DfrVII-F | TTGAAAATTTCATTGATT | dfrA7, dfrA17 | 474 | 55 | 25 |
| DfrVII-R | TTAGCCTTTTTTCCAAATCT | ||||
| DfrII-F | GATCACGTGCGCAAGAAATC | dfrB1, dfrB2, dfrB3 | 141 | 60 | 25 |
| DfrII-R | AAGCGCAGCCACAGGATAAAT | ||||
| DfrXII-F | GGTGSGCAGAAGATTTTTCGC | dfrA12, dfrA13 | 319 | 60 | 25 |
| DfrXII-R | TGGGAAGAAGGCGTCACCCTC | ||||
| Sul-F | TGGTGACGGTGTTCGGCATTC | sul1 | 789 | 63 | 23 |
| Sul-R | GCGAGGGTTTCCGAGAAGGTG | This study | |||
| Sul2-F | CGGCATCGTCAACATAACC | sul2 | 722 | 50 | 22 |
| Sul2-R | GTGTGCGGATGAAGTCAG | ||||
| Sul3-F | CATTCTAGAAAACAGTCGTAGTTCG | sul3 | 990 | 51 | 29 |
| Sul3-R | CATCTGCAGCTAACCTAGGGCTTTGGA | ||||
| IntI1-F | GGGTCAAGGATCTGGATTTCG | intI1 | 483 | 62 | 23 |
| IntI1-R | ACATGGGTGTAAATCATCGTC | ||||
| IntI2-F | CACGGATATGCGACAAAAAGGT | intI2 | 788 | 62 | 23 |
| IntI2-R | GTAGCAAACGAGTGACGAAATG | ||||
| Int-F | GGCATCCAAGCAGCAAG | Class 1 integron variable region | Variable | 55 | 19 |
| Int-R | AAGCAGACTTGACCTGA | ||||
| Hep-F | CGGGATCCCGGACGGCATGCACGATTTGTA | Class 2 integron variable region | Variable | 60 | 39 |
| Hep-R | GATGCCATCGCAAGTACGAG | ||||
| Qac-F | GGCTGGCTTTTTCTTGTTATCG | qacEΔ1 | 287 | 60 | 23 |
| Qac-R | TGAGCCCCATACCTACAAAGC | ||||
| MarR-F | AGCTAGCCTTGCATCGCA | marR and marO | 568 | 55 | 28 |
| MarR-R | TACGGCAGGACTTTCTTAAGCA |
TABLE 2.
Phenotypes and mechanisms of antibiotic resistance detected in the 17 multiresistant E. coli strains of this study
| E. coli strain (origin)a | Phenotype of resistanceb | Mechanisms of resistance
|
|||
|---|---|---|---|---|---|
| Resistance genes detected | Position(s) of amino acid change(s) in:
|
CATc | |||
| GyrA | ParC | ||||
| Co1 (F) | Nal Cip Amp Kan Str Rif Tet Chl Tmp Smx | blaTEM1b, aphA1, aphA2, aadA1, tetB, dfrA1, sul2 | 83 + 87 | 80 | |
| Co19 (F) | Nal Amp Kan Str Rif Tet Chl Tmp Smx | blaTEM1b, aphA2, aadA1, tetB, dfrA1, sul2 | 83 | ||
| Co45 (F) | Nal Cip Amp Amcd Kan Str Rif Tet Chl Tmp Smx | blaTEM1b, aphA2, aadA1, tetA, dfrA1, sul2 | 83 + 87 | 80 | |
| Co53 (F) | Nal Amp Amcd Kan Str Rif Tet Chl Smx | blaTEM1b, aphA2, tetB, sul2 | 83 | ||
| Co71 (B) | Nal Cip Amp Kan Str Rif Tet Chl Tmp Smx | blaTEM1b, aphA1, aadA5, tetB, dfrA17, sul1, sul2 | 83 + 87 | 80 | + |
| Co80 (B) | Nal Amp Gen Tobd Kan Str Rif Tet Chl Smx | blaTEM1b, aac(3)-II, aphA2, aadA1, tetB, sul1, sul2 | 83 | + | |
| Co82 (B) | Nal Cip Amp Kan Str Rif Tet Chl Tmp Smx | blaTEM1b, aphA1, aadA5, tetB, dfrA17, sul1, sul2 | 83 + 87 | 80 | + |
| Co110 (B) | Nal Amp Amcd Str Rif Tet Chl Tmp Smx | blaTEM1a, aadA1, tetA, cmlA, dfrA1, sul1, sul3 | 83 | ||
| Co125 (P) | Nal Cip Amp Str Rif Tet Chl Tmp Smx | blaTEM1b, aadA1, aadA2, tetA, cmlA, dfrA12, dfrA1-like, sul3 | 83 + 87 | 80 | |
| Co279 (P) | Nal Amp Amcd Str Rif Tet Chl Tmp Smx | blaTEM1b, aadA1, aadA2, tetB, cmlA, dfrA12, sul3 | 83 | ||
| Co177 (D) | Nal Amp Amcd Str Rif Tet Chl Tmp Smx | blaTEM1b, aadA1, tetA, dfrA1, sul1, sul2 | 83 | + | |
| Co201 (D) | Nal Amp Amcd Str Rif Tet Chl Tmp Smx | blaTEM1b, aadA1, tetA, dfrA1, sul1, sul2 | 83 | + | |
| Co227 (H) | Nal Amp Amcd Gen Apr Tob Str Rif Tet Chl Tmp Smx | blaTEM1b, aac(3)-IV, aadA1, aadA2, tetA, cmlA, dfrA1, dfrA12, sul1, sul2, sul3 | 83 | ||
| Co228 (H) | Nal Amp Amcd Gen Apr Tob Kan Str Rif Tet Chl Tmp Smx | blaTEM1a, aac(3)-IV, aphA2, aadA2, tetA, cmlA, dfrA12, sul1, sul3 | 83 | ||
| Co232 (H) | Nal Cip Amp Str Rif Tet Chl Tmp Smx | blaTEM1b, aadA1, tetA, dfrA1, sul1, sul2 | 83 + 87 | 80 + 84 | + |
| Co354 (H) | Nal Cip Amp Gen Apr Tob Str Rif Tet Chld Tmp Smx | blaTEM1b, aac(3)-IV, aadA1, aadA2, tetA, dfrA1, dfrA12, sul1, sul3 | 83 + 87 | 80 | |
| Co356 (H) | Nal Cip Amp Str Rif Tet Chl Tmp Smx | blaTEM1b, aadA5, tetB, dfrA17, sul1, sul2 | 83 + 84 | 80 + 108 | + |
F, food; B, broiler; P, pig; D, dog; H, human.
Nal, nalidixic acid; Cip, ciprofloxacin; Amp, ampicillin; Amc, amoxicillin-clavulanic acid; Gen, gentamicin; Apr, apramycin; Tob, tobramycin; Kan, kanamycin; Str, streptomycin; Rif, rifampin; Tet, tetracycline; Chl, chloramphenicol; Tmp, trimethoprim; Smx, sulfamethoxazole.
CAT, chloramphenicol-acetyl-transferase enzymatic activity. +, CAT was detected for the strain indicated.
Resistance to the drug indicated is in the intermediate category according to NCCLS standards for the corresponding strain.
All strains were ampicillin resistant, and for eight of them, the minimal inhibitory concentration (MIC) of amoxicillin-clavulanic acid indicated intermediate resistance; no strain was resistant to the remaining β-lactams studied. The blaTEM-1a and blaTEM-1b genes were identified in 2 and 15 strains, respectively, whereas none of the blaSHV and blaOXA genes were found.
Four strains in which the aac3-II (found in one strain from a broiler) or aac3-IV gene (found in three strains from humans) was found were GEN resistant. The AAC(3)-IV enzyme modifies apramycin in addition to GEN. Apramycin is used exclusively in veterinary medicine, but the GEN-related chemical structure and the mobility of the aac3-IV gene inside plasmids may have contributed to the selection and dissemination of these strains in a human environment (17).
Eight of the seventeen E. coli strains were kanamycin resistant, and the aphA1 and aphA2 genes were detected in three and six strains, respectively. Both genes were found in one strain of food origin.
The following aadA genes were detected in 16 of the 17 STR-resistant strains: aadA1 was found in 12 strains, aadA2 was found in 5, and aadA5 was found in 3 strains. The aadA1 and aadA2 genes were found together in four strains (two from pigs and two from humans). No STR resistance mechanism was detected in the Co53 strain, in which case other mechanisms of STR resistance, such as the production of APH(3′′)-I or APH(6)-I phosphoryltransferases (15, 35), cannot be excluded.
The tetA or tetB gene was found in all the strains (tetA was found in nine, and tetB was found in eight strains). No tetC, tetD, or tetE genes were detected. Chloramphenicol-acetyltransferase activity was demonstrated as previously described (8) in the seven strains for which the MICs of chloramphenicol were highest (≥128 μg/ml) (Table 2). The cmlA gene was detected in five additional strains (MICs of chloramphenicol, 32 to 64 μg/ml), while the floR gene was not found.
Fifteen E. coli strains were trimethoprim resistant, and the following dfr genes were identified by PCR-restriction fragment length polymorphism analysis (25): dfrA1 was found in seven strains, dfrA1 plus dfrA12 was found in two, dfrA1-like plus dfrA12 was found in one strain, dfrA17 was found in three, and dfrA12 was found in two strains. A new type of dfrA gene, called dfrA1-like, was found in the Co125 strain. Sequencing of the Co125 amplicon indicated a deduced amino acid substitution, Asn134Asp, in contrast to DHFRIa (Swiss-Prot accession number P00382).
The sul1 and sul2 genes were detected in 11 and 12 strains, respectively, and 8 of those strains showed both genes. These findings are in agreement with the high prevalence of these genes found in Enterobacteriaceae (18, 22). The sul3 gene has recently been found in pathogenic E. coli isolates (10, 12, 29), being detected in six of our strains (one from a broiler, two from pigs, and three from humans). The mechanisms of quinolone resistance had been previously analyzed in these 17 strains (7, 33).
Integron analysis.
Class 1 and class 2 integrons, the most frequently found in resistant bacteria (14, 23, 31, 32), were analyzed in all our strains. PCR amplification was used to detect class 1 and class 2 integrase genes, intI1 and intI2, respectively, and the qacEΔ1 gene. The variable regions (VR) of these integrons were studied by PCR and sequencing (Table 1). Twelve strains presented the intI1 gene and four presented the intI2 gene; one of these was E. coli Co125, which was positive for both genes.
The VR of the class 1 integron was analyzed in the 12 intI1-positive strains, and the following gene cassette arrangements were detected (Table 3): aadA1 (one strain), dfrA1 plus aadA1 (four strains), dfrA1 plus aadA1 and dfrA12 plus orfF plus aadA2 (two strains), dfrA12 plus orfF plus aadA2 (two strains), and dfrA17 plus aadA5 (three strains). Our E. coli strain Co125 gave unexpected results: the intI1 PCR was positive, whereas no qacEΔ1 or sul1 genes and no PCR amplicon of the class 1 integron VR were detected. Thus, Co125 was studied in detail by PCR mapping. A 1,650-bp amplicon was obtained using the primers Int-F and AadA-R. Sequencing revealed the presence of the dfrA12 plus orfF plus aadA2 gene cassettes (Table 3). As in the case of our results, most class 1 integrons published are composed of two or more gene cassettes (12, 22, 40, 41).
TABLE 3.
Analysis of class 1 and class 2 integrons and their resistance gene cassettes in the 17 multiresistant E. coli strains
| E. coli strain (origin)a | Class 1 integron
|
Class 2 integron
|
|||||
|---|---|---|---|---|---|---|---|
| Detection ofb:
|
Variable region amplified by PCR
|
Detection of intI2 geneb | Variable region amplified by PCR
|
||||
| intI1 | qacEΔ1 sul1 | Size of amplicons obtained (bp) | Genes included in cassettes | Size of amplicons obtained (bp) | Genes included in cassettes | ||
| Co1 (F) | − | − | NADc | + | 2,000 | dfrA1 + sat + aadA1 | |
| Co19 (F) | − | − | NAD | + | 2,000 | dfrA1 + sat + aadA1 | |
| Co45 (F) | − | − | NAD | + | 2,000 | dfrA1 + sat + aadA1 | |
| Co53 (F) | − | − | NAD | − | NAD | ||
| Co71 (B) | + | + | 1,700 | dfrA17 + aadA5 | − | NAD | |
| Co80 (B) | + | + | 1,000 | aadA1 | − | NAD | |
| Co82 (B) | + | + | 1,700 | dfrA17 + aadA5 | − | NAD | |
| Co110 (B) | + | + | 1,500 | dfrA1 + aadA1 | − | NAD | |
| Co125 (P) | + | − | NAD | dfrA12 + orfF + aadA2d | + | 2,000 | dfrA1-like + sat + aadA1 |
| Co279 (P) | − | − | NAD | − | NAD | ||
| Co177 (D) | + | + | 1,500 | dfrA1 + aadA1 | − | NAD | |
| Co201 (D) | + | + | 1,500 | dfrA1 + aadA1 | − | NAD | |
| Co227 (H) | + | + | 1,500, 2,000 | dfrA1 + aadA1, dfrA12 + orfF + aadA2 | − | NAD | |
| Co228 (H) | + | + | 2,000 | dfrA12 + orfF + aadA2 | − | NAD | |
| Co232 (H) | + | + | 1,500 | dfrA1 + aadA1 | − | NAD | |
| Co354 (H) | + | + | 1,500, 2,000 | dfrA1 + aadA1, dfrA12 + orfF + aadA2 | − | NAD | |
| Co356 (H) | + | + | 1,700 | dfrA17 + aadA5 | − | NAD | |
F, food; B, broiler; P, pig; D, dog; H, human.
+, detected; −, not detected.
NAD, No amplicon was detected.
These gene cassettes were detected by sequencing of amplicons obtained with the primers Int-F and AadA-R.
A class 2 integron carrying the dfrA1 plus sat plus aadA1 gene cassettes was detected in four strains (three from foods and one from a pig). The dfrA1 gene cassette detected in Co125 presented the Asn134Asp amino acid change, corresponding to the sequence of the dfrA1-like gene found in this strain (Table 3).
Analysis of the mar locus.
Another mechanism contributing to a multiple-antibiotic-resistant phenotype is associated with mar locus regulation (1, 2, 5). Amino acid changes in MarR and the nucleotide mutations in the operator-promoter region marO were studied for all strains by PCR, sequencing, and comparison with the GenBank sequence found under accession number M96235 and corresponding to the mar regulon (Table 4). Fifteen strains showed Gly103Ser and Tyr137His substitutions in MarR, which had been found also in resistant clinical strains (27). Note that position 103 is inside the conserved region (between amino acids 92 and 104 in MarR) and may be important for binding with the region corresponding to marO (1). However, other authors have considered that these substitutions could correspond to genotypic variations in marR without loss of repressor activity (27). Another amino acid change in MarR, Leu36Gln, was found in only one strain in our study; this is the first time that this substitution has been reported. Further studies are necessary to relate this substitution to antibiotic resistance.
TABLE 4.
Analysis of amino acid changes in MarR protein and nucleotide mutations in marO region of 17 multiresistant E. coli strainsa
| E. coli strain(s) | Amino acid changes in MarR | Nucleotide mutation(s) in marO |
|---|---|---|
| Co53, Co356 | None | None |
| Co177 | Gly103Ser, Tyr137His | None |
| Co279 | Gly103Ser, Tyr137His | A1332C |
| Co1 | Gly103Ser, Tyr137His | A1332C, A1331G |
| Co110, Co232, Co354 | Gly103Ser, Tyr137His | A1332C, C1370T |
| Co201, Co227, Co228 | Gly103Ser, Tyr137His | A1332C, C1375T |
| Co71, Co82, Co125 | Gly103Ser, Tyr137His | A1332C, C1379T |
| Co19, Co80 | Gly103Ser, Tyr137His | A1332C, C1414T |
| Co45 | Leu36Gln, Gly103Ser, Tyr137His | A1332C |
The sequence found under GenBank accession number M96235 was used as a reference.
Regarding nucleotide mutations in marO, 14 strains showed the previously reported A1332C transversion (27) together with amino acid substitutions in MarR at positions 103 and 137. We identified other nucleotide mutations (at positions 1331, 1370, 1375, 1379, and 1414) not previously found in the literature. MarA is known to activate the marRAB operon binding the “marbox” region in marO, but the adjacent region (nucleotides 1329 to 1346) also plays a role in binding and increases this transcriptional activation (21). Indeed, the mutations found at positions 1331 and 1332 are located inside this adjacent region, but additional studies should determine their effect on MarA activity. We found no differences in resistance phenotype between the strains with and without these mutations.
Our results show a wide variety of resistance genes in multiresistant nonpathogenic E. coli strains from humans, animals, and food products. Therefore, this normal flora may play a key role as an acceptor and donor of transmissible antimicrobial resistance mechanisms. The inclusion of some resistance genes inside integrons constitutes an effective means to spread antibiotic resistance among bacteria from different ecosystems. Moreover, different amino acid changes in MarR and mutations in marO were found, possibly contributing to the multiple antibiotic resistance phenotype.
Acknowledgments
This work has been partially supported by grants from the Fondo de Investigaciones Sanitarias (FIS 01/973) and the Consejería de Educación del Gobierno de La Rioja (ACPI2001/04) of Spain. Y. Sáenz has a fellowship from the University of La Rioja (FPI-UR-00/72785864).
We are grateful to R. Del Campo for critical review of the manuscript.
REFERENCES
- 1.Alekshun, M. N., and S. B. Levy. 1997. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob. Agents Chemother. 41:2067-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ariza, R. R., S. P. Cohen, N. Bachhawat, S. B. Levy, and B. Demple. 1994. Repressor mutations in the marRAB operon that activate oxidative stress genes and multiple antibiotic resistance in Escherichia coli. J. Bacteriol. 176:143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belaaouaj, A., C. Lapoumeroulie, M. M. Caniça, G. Vedel, P. Névot, R. Krishnamoorthy, and G. Paul. 1994. Nucleotide sequences of the genes coding for the TEM-like β-lactamases IRT-1 and IRT-2 (formerly called TRI-1 and TRI-2). FEMS Microbiol. Lett. 120:75-80. [DOI] [PubMed] [Google Scholar]
- 4.Carattoli, A. 2001. Importance of integrons in the diffusion of resistance. Vet. Res. 32:243-259. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, S. P., L. M. McMurry, D. C. Hooper, J. S. Wolfson, and S. B. Levy. 1989. Cross-resistance to fluoroquinolones in multiple-antibiotic-resistant (Mar) Escherichia coli selected by tetracycline or chloramphenicol: decreased drug accumulation associated with membrane changes in addition to OmpF reduction. Antimicrob. Agents Chemother. 33:1318-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.del Cerro, A., S. M. Soto, and M. C. Mendoza. 2003. Virulence and antimicrobial-resistance gene profiles determined by PCR-based procedures for Salmonella isolated from samples of animal origin. Food Microbiol. 20:431-438. [Google Scholar]
- 7.Domínguez, E., M. Zarazaga, Y. Sáenz, L. Briñas, and C. Torres. 2002. Mechanisms of antibiotic resistance in Escherichia coli isolates obtained from healthy children in Spain. Microb. Drug Resist. 8:321-327. [DOI] [PubMed] [Google Scholar]
- 8.Gallardo, F., J. Ruiz, F. Marco, K. J. Towner, and J. Vila. 1999. Increase in incidence of resistance to ampicillin, chloramphenicol and trimethoprim in clinical isolates of Salmonella serotype Typhimurium with investigation of molecular epidemiology and mechanisms of resistance. J. Med. Microbiol. 48:367-374. [DOI] [PubMed] [Google Scholar]
- 9.Gautom, R. K. 1997. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J. Clin. Microbiol. 35:2977-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grape, M., L. Sundstrom, and G. Kronvall. 2003. Sulphonamide resistance gene sul3 found in Escherichia coli isolates from human sources. J. Antimicrob. Chemother. 52:1022-1024. [DOI] [PubMed] [Google Scholar]
- 11.Guardabassi, L., L. Dijkshoorn, J.-M. Collard, J. E. Olsen, and A. Dalsgaard. 2000. Distribution and in-vitro transfer of tetracycline resistance determinants in clinical and aquatic Acinetobacter strains. J. Med. Microbiol. 49:929-936. [DOI] [PubMed] [Google Scholar]
- 12.Guerra, B., E. Junker, A. Schroeter, B. Malorny, S. Lehmann, and R. Helmuth. 2003. Phenotypic and genotypic characterization of antimicrobial resistance in German Escherichia coli isolates from cattle, swine and poultry. J. Antimicrob. Chemother. 52:489-492. [DOI] [PubMed] [Google Scholar]
- 13.Hall, R. M., and C. M. Collis. 1995. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol. Microbiol. 15:593-600. [DOI] [PubMed] [Google Scholar]
- 14.Hall, R. M., and C. M. Collis. 1998. Antibiotic resistance in gram-negative bacteria: the role of gene cassettes and integrons. Drug Resist. Updates 1:109-119. [DOI] [PubMed] [Google Scholar]
- 15.Heinzel, P., O. Werbitzky, J. Distler, and W. Peipersberg. 1988. A second streptomycin resistance gene from Streptomyces griseus codes for streptomycin-3′′-phosphotransferase. Arch. Microbiol. 150:184-192. [DOI] [PubMed] [Google Scholar]
- 16.Hollingshead, S., and D. Vapnek. 1985. Nucleotide sequence analysis of the gene encoding a streptomycin/spectinomycin adenylyltransferase. Plasmid 13:17-30. [DOI] [PubMed] [Google Scholar]
- 17.Hunter, J. E. B., J. C. Shelley, J. R. Walton, C. A. Hart, and M. Bennett. 1992. Apramycin resistance plasmids in Escherichia coli: possible transfer to Salmonella typhimurium in calves. Epidemiol. Infect. 108:271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huovinen, P. 2001. Resistance to trimethoprim-sulfamethoxazole. Clin. Infect. Dis. 32:1608-1614. [DOI] [PubMed] [Google Scholar]
- 19.Lévesque, C., and P. H. Roy. 1993. PCR analysis of integrons, p. 590-594. In D. H. Persing, T. F. Smith, F. C. Tenover, and T. J. White (ed.), Diagnostic molecular microbiology. American Society for Microbiology, Washington, D.C.
- 20.Madsen, L., F. M. Aarestrup, and J. E. Olsen. 2000. Characterisation of streptomycin resistance determinants in Danish isolates of Salmonella Typhimurium. Vet. Microbiol. 75:73-82. [DOI] [PubMed] [Google Scholar]
- 21.Martin, R. G., P. S. Nyantakyi, and J. L. Rosner. 1995. Regulation of the multiple antibiotic resistance (mar) regulon by marORA sequences in Escherichia coli. J. Bacteriol. 177:4176-4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maynard, C., J. M. Fairbrother, S. Bekal, F. Sanschagrin, R. C. Levesque, R. Brousseau, L. Masson, S. Larivière, and J. Harel. 2003. Antimicrobial resistance genes in enterotoxigenic Escherichia coli O149:K91 isolates obtained over a 23-year period from pigs. Antimicrob. Agents Chemother. 47:3214-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazel, D., B. Dychinco, V. A. Webb, and J. Davies. 2000. Antibiotic resistance in the ECOR collection: integrons and identification of a novel aad gene. Antimicrob. Agents Chemother. 44:1568-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. 2002. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 25.Navia, M. M., J. Ruiz, J. Sánchez-Céspedes, and J. Vila. 2003. Detection of dihydrofolate reductase genes by PCR and RFLP. Diagn. Microb. Infect. Dis. 46:295-298. [DOI] [PubMed] [Google Scholar]
- 26.Ng, L.-K., M. R. Mulvey, I. Martin, G. A. Peters, and W. Johnson. 1999. Genetic characterization of antimicrobial resistance in Canadian isolates of Salmonella serovar Typhimurium DT104. Antimicrob. Agents Chemother. 43:3018-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oethinger, M., I. Podglajen, W. V. Kern, and S. B. Levy. 1998. Overexpression of the marA or soxS regulatory gene in clinical topoisomerase mutants of Escherichia coli. Antimicrob. Agents Chemother. 42:2089-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park, Y.-H., J.-H. Yoo, D.-H. Huh, Y.-K. Cho, J.-H. Choi, and W.-S. Shin. 1998. Molecular analysis of fluoroquinolone-resistance in Escherichia coli on the aspect of gyrase and multiple antibiotic resistance (mar) genes. Yonsei Med. J. 39:534-540. [DOI] [PubMed] [Google Scholar]
- 29.Perreten, V., and P. Boerlin. 2003. A new sulfonamide resistance gene (sul3) in Escherichia coli is widespread in the pig population of Switzerland. Antimicrob. Agents Chemother. 47:1169-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitout, J. D. D., K. S. Thomson, N. D. Hanson, A. F. Ehrhardt, E. S. Moland, and C. C. Sanders. 1998. β-Lactamases responsible for resistance to expanded-spectrum cephalosporins in Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis isolates recovered in South Africa. Antimicrob. Agents Chemother. 42:1350-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Recchia, G. D., and R. M. Hall. 1995. Gene cassettes: a new class of mobile element. Microbiology 141:3015-3027. [DOI] [PubMed] [Google Scholar]
- 32.Sabaté, M., and G. Prats. 2002. Estructura y función de los integrones. Enferm. Infecc. Microbiol. Clin. 20:341-345. [DOI] [PubMed] [Google Scholar]
- 33.Sáenz, Y., M. Zarazaga, L. Briñas, F. Ruiz-Larrea, and C. Torres. 2003. Mutations in gyrA and parC genes in nalidixic acid-resistant Escherichia coli strains obtained from food products, humans and animals. J. Antimicrob. Chemother. 51:1001-1005. [DOI] [PubMed] [Google Scholar]
- 34.Sáenz, Y., M. Zarazaga, L. Briñas, M. Lantero, F. Ruiz-Larrea, and C. Torres. 2001. Antibiotic resistance in Escherichia coli isolates obtained from animals, foods and humans in Spain. Int. J. Antimicrob. Agents. 18:353-358. [DOI] [PubMed] [Google Scholar]
- 35.Scholz, P., V. Haring, B. Wittmann-Liebold, K. Ashman, M. Bagdasarian, and E. Scherzinger. 1989. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene 75:271-288. [DOI] [PubMed] [Google Scholar]
- 36.Steward, C. D., J. K. Rasheed, S. K. Hubert, J. W. Biddle, P. M. Raney, G. J. Anderson, P. P. Williams, K. L. Brittain, A. Oliver, J. E. McGowan, Jr., and F. C. Tenover. 2001. Characterization of clinical isolates of Klebsiella pneumoniae from 19 laboratories using the National Committee for Clinical Laboratory Standards extended-spectrum β-lactamase detection methods. J. Clin. Microbiol. 39:2864-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van de Klundert, J. A. M., and J. S. Vliegenthart. 1993. PCR detection of genes coding for aminoglycoside-modifying enzymes, p. 547-552. In D. H. Persing, T. F. Smith, F. C. Tenover, and T. J. White (ed.), Diagnostic molecular microbiology. American Society for Microbiology, Washington, D.C.
- 38.Vanhoof, R., J. Content, E. Van Bossuyt, L. Dewit, and E. Hannecart-Pokorni. 1992. Identification of the aadB gene coding for the aminoglycoside-2′′-O-nucleotidyltransferase, ANT (2′′), by means of the polymerase chain reaction. J. Antimicrob. Chemother. 29:365-374. [DOI] [PubMed] [Google Scholar]
- 39.White, P. A., C. J. McIver, and W. D. Rawlinson. 2001. Integrons and gene cassettes in the Enterobacteriaceae. Antimicrob. Agents Chemother. 45:2658-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White, P. A., C. J. McIver, Y. Deng, and W. D. Rawlinson. 2000. Characterization of two new gene cassettes, aadA5 and dfrA17. FEMS Microbiol. Lett. 182:265-269. [DOI] [PubMed] [Google Scholar]
- 41.Yu, H. S., J. C. Lee, H. Y. Kang, D. W. Ro, J. Y. Chung, Y. S. Jeong, S. H. Tae, C. H. Choi, E. Y. Lee, S. Y. Seol, Y. C. Lee, and D. T. Cho. 2003. Changes in gene cassettes of class 1 integrons among Escherichia coli isolates from urine specimens collected in Korea during the last two decades. J. Clin. Microbiol. 41:5429-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]