Abstract
Age-related cataracts are one of the leading causes of visual impairment and blindness among the elderly worldwide. Among age-related cataracts, cortical opacities rank as the second most common type; however, little is known about their molecular pathogenesis or genetics. To identify susceptibility loci for cortical cataracts, we genotyped a subset of families (102 families; n = 224 sib pairs) from the Beaver Dam Eye Study and performed a model-free genome-wide linkage analysis for markers linked to a quantitative measure of cortical opacity. We obtained evidence for linkage at marker D1S1622 on chromosome 1p35 (P < 0.0002) and at marker D6S1053 on 6q12 (P < 0.00008) in the initial scan. Five additional regions on 1q31, 2p24, 2q11, 4q28, and 15q13 that are suggestive of linkage (P ≤ 0.01 or logarithm of the likelihood ratio ≥ 1.18) were observed. The region on chromosomes 6p12-q12 was selected for fine mapping, and the intermarker distance was reduced to 3 cM by adding 11 markers in the interval between D6S1017 and D6S1021. After fine mapping, significant evidence of linkage remained on chromosome 6p12-q12 at D6S1053 (P < 0.00005). The current genome scan for age-related cortical cataracts may lead to identification of novel genes, because few regions identified in the current scan have previously been implicated in congenital or age-related cataracts.
Keywords: age-related cataract, genome-wide scan, linkage, quantitative trait
Age-related eye diseases are the leading cause of vision impairment and blindness worldwide (1, 2). The most prevalent among age-related eye diseases is cataract formation (3), affecting nearly 20.5 million Americans age 65 and older. Cataract accounts for an estimated 16 million cases of blindness worldwide, with approximately half of all cases originating from Africa and Asia (4). In the United States, cataract is considered a treatable disease due to the wide availability of surgery with intraocular lens implantation (5). Despite the availability of treatment, cataracts still comprise a significant risk for visual impairment in the United States, particularly in older individuals who experience difficulty accessing appropriate eye care due to cost and other barriers (6).
Cataract results from impairment in lens transparency, which affects how light is scattered through the lens and images are focused onto the retina. The lens is a biconvex, avascular structure made up of elongated cells or fibers that are preserved, from fetal life to death. A thick capsular basement membrane encloses the lens, under which lies a layer of cuboidal epithelial cells that differentiate into new lens fibers, progressively overlaying the existing lens fibers. The younger, outer lens fibers are metabolically active, whereas the older fibers mature by degrading their nuclei and organelles, enabling the lens to maintain clarity. Disruption in the cellular structure of the lens due to aging can affect lens transparency and lead to considerable changes in the morphology of the nucleus, cortex, and capsule (7, 8). Thus, age-related cataracts are morphologically classified into nuclear, cortical, posterior subcapsular, and mixed cataracts, each of which has associated risk factors. In most populations, the most commonly observed age-related cataract is nuclear, followed by cortical and posterior subcapsular cataracts, respectively (9-14). The current investigation is focused on cortical cataracts, the second most prevalent age-related cataract.
Previous epidemiologic studies have established that factors affecting cortical cataract development include female gender (15), sunlight or UV light exposure (16-18), vitamin usage (19-22), myopia (23), and type 2 diabetes (24). Less well established is the role of specific genetic risk factors, although familial aggregation has been reported by the Italian American Cataract Study Group (25), Heiba et al. (26), the Framingham study (27), and Hammond et al. (28). By using a quantitative scoring system based on the percentage of cortical surface area affected, a segregation analysis was performed in families ascertained through the Beaver Dam Eye Study (BDES) (25). Fifty-eight percent of the variance in age- and sex-adjusted cortical opalescence measures could be explained by a single major gene (25). Supporting these results, broad sense heritability for cortical cataracts in twins ranges from 53-58% (28).
To comprehend the molecular basis of lens opacification, others have examined families with congenital or early-onset cataract. Linkage and mutation analyses of these families led to identification of over 20 candidate loci, the majority of which are rare single-gene disorders that follow an autosomal dominant mode of inheritance (reviewed recently in refs. 29 and 30). Mutations have been identified in a variety of proteins, such as, crystallins, gap junction proteins, and lens fiber-cell intrinsic membrane proteins. Despite the progress in identifying genes for early-onset cataracts, the significance of these genes has not been evaluated in cortical or other age-related cataracts, nor have loci specific to age-related cataracts been characterized. To identify loci for cortical cataracts, we conducted a genome-wide scan in families from the BDES. The current study represents a large-scale investigation to identify genetic risk factors for age-related cataracts. We anticipate that genes identified through this endeavor will further elucidate the biology of age-related cataract, possibly leading to preventative measures and therapeutic interventions that will reduce the global burden of this disease.
Subjects, Materials, and Methods
Study Population. The BDES is a cohort study of age-related ocular diseases. This population, the methodology, and baseline parameters are described in detail elsewhere (31). Briefly, a private census of the population of Beaver Dam, Wisconsin, was performed from autumn 1987 to the end of spring 1988. All 5,924 people who were 43-86 years of age identified as living in the township were eligible and were invited to participate in the study from spring 1988 to the end of autumn 1990. Of eligible people, 4,926 participated. Family relationships were ascertained and recorded (31). Participants were reevaluated at 5 and 10 years after the initial visit. Informed consent was obtained from the participants following a protocol approved by the Institutional Review Board of the University of Wisconsin; the tenets of the Declaration of Helsinki were observed.
Phenotypic Evaluation. Photographs of the lenses were taken with two different cameras, a slit-lamp camera and a retro-illumination camera (32). The pupil diameter at the time of the baseline photographs was recorded on the examination form for each of the two subsequent examinations. The photographer was instructed to take the lens photographs when the pupil diameter was equal to the diameter at baseline. The grading procedures for the lens were based on detailed codified decision rules (32). Graders were masked to subject identity and personal characteristics. Scores for cortical cataract were based on weighted estimates of degree of opacity of lens area, as defined by a circular grid, divided into eight “pie-wedged” peripheral areas and a central circular area overlaid on the photograph. Cases of cortical cataract were those with opacity of 5% or more of the lens “surface.” This definition is the standard in the field and has been used in other large population-based epidemiological studies (33). It was chosen because this amount of lens opacity is comparable to the amount often used to diagnose this condition, and because it can be reliably measured when grading retro-illumination images of the lens.
Cortical cataract severity scores were determined for each eye at three time points spaced at 5-year intervals. We averaged the right and left eye scores and substituted the available score, if a score was missing for either eye (which occurred for 7.2% of individuals). The final score used in the analysis was an average of either the first and last time point, or the average of scores at any two available time points. This approach ensured that the same or similar amount of information was used for each participant. We then used multiple regression analysis to investigate the effects of age, age2, sex, together with their interactions, plus the effects of alcohol consumption, smoking, multiple vitamin usage, and sunlight exposure, measured in Wisconsin sun years, on this severity score. The significant covariates were age (P < 0.0221), age2 (P < 0.0032), sex (P < 0.0293), age × sex interaction (P < 0.0055), and vitamin usage (P < 0.0150). The covariates identified were similar to those reported by Heiba et al. (26) in the original analysis of the BDES data, but the parameterization was not identical.
Genome Scan and Fine Mapping. Genotyping and linkage analyses were conducted on a subset of 244 sib pairs, obtained from 102 families in BDES. Details of the rationale for selection of families for genotyping is described elsewhere (34). DNA was extracted from the blood samples by established methods (35). We genotyped 345 markers on 22 autosomes by using the Weber Set 8 markers, with an average marker spacing of 11 cM, using previously described methods (34). After reviewing the initial linkage results, 11 additional markers were typed on chromosome 6, between markers D6S1017 and D6S1021, reducing the average intermarker spacing there to 3 cM.
Mendelian inconsistencies were identified by using the mark-erinfo program in S.A.G.E. (36). and by testing for deviation from Hardy-Weinberg proportions at each marker. No significant departure from Hardy-Weinberg proportions was observed. Inconsistencies were verified by regenotyping, and allelic inconsistencies that could not be reconciled (0.68%) were changed to missing values. Allele frequencies were estimated for each genetic marker by gene counting (disregarding relationships).
Before performing the linkage analysis, errors in relationship specification were identified with reltest (36) by using the entire genome scan. We reclassified 6 individuals in three full sibships as unrelated and 21 individuals in 12 full sibships as half-sibs.
Linkage Analysis. The power of a model-free quantitative trait linkage analysis depends on the scale of measurement (37). Because adjusting for the covariates may result in negative values and power transformation of data requires positive values, we adjusted the quantitative scores to 80 years old as was done by Heiba et al. (26). Segregation analysis of the data was used to estimate a Box-Cox transformation power parameter λ1 = 0.393 (38), under a codominant model. The age-adjusted values were raised to this power before linkage analysis. The final trait used for linkage analyses was the average cortical cataract severity score, adjusted for the covariates described above, which was subject to power transformation to maximize the trait information for linkage analyses.
The multipoint identity by descent (IBD) sharing was estimated for each chromosome by using genibd (36) followed by multipoint model-free linkage analysis with sibpal (35). Linkage analysis used a Haseman-Elston regression (39, 40), where the weighted average of the squared trait difference and the squared mean-corrected trait sum, allowing for the non-independence of the sib-pairs (41), was the dependent variable (option W4 in sibpal). To eliminate false positives, null permutation distributions were generated by using a sample of up to 1,000,000 replicates of the allele-sharing data to evaluate the nominally significant multipoint Haseman-Elston P values (HE-P). Both the Haseman-Elston and the permutation multipoint P values are reported. P values are not adjusted for multiple testing but can be interpreted as genomewide significance levels following the locus-counting method of Wiltshire et al. (42)
Results
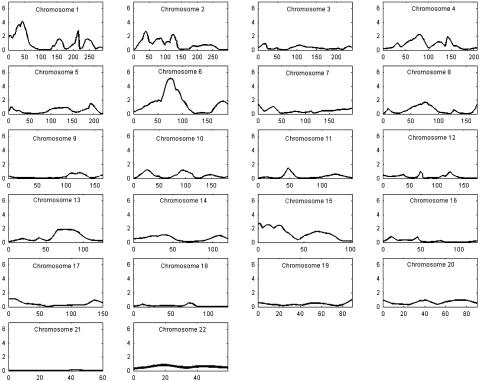
We used 102 families with 224 informative sib pairs (n = 325) to conduct a genome scan for cortical cataracts using a quantitative score for percent of cortical area affected. The sibships range in size from two to six, with an average of 2.46 persons per sibship. Similar to the original data set (26), the subset contains slightly more females (n = 176) than males (n = 149). The results of the genome scan are presented in Fig. 1; detailed information for the most significant marker at each locus with a nominal significance of P < 0.01, and distance along the chromosome are shown in Table 1.
Fig. 1.
Multipoint results of the genomewide linkage scan for cortical cataracts using the Weber panel 8 map spacing on 22 autosomes. For each chromosome, genetic distance (cM) is plotted on the x axis against pP = -log10 (P value) on the y axis.
Table 1. Genetic locations and multipoint P values (Haseman-Elston and empirical) for markers demonstrating evidence of linkage (P ≤ 0.01 or a LOD score ≥ 1.18) for cortical cataracts.
| Marker | Chromosomal location (cM)* | HE-P | Permutation P value | No. of replicates |
|---|---|---|---|---|
| D1S1622 | 1p35 (49) | 0.0002‡ | 0.0009 | 105,114 |
| D1S2625 | 1q31 (216) | 0.0079 | 0.0135 | 7,023 |
| D2S1360 | 2p24 (38) | 0.0021 | 0.0057 | 16,758 |
| GATA176C01 | 2q11 (123) | 0.0068 | 0.0102 | 9,376 |
| D4S3243 | 4q28 (83) | 0.0063 | 0.0133 | 7,146 |
| D6S1053 | 6q12 (71.4) | 7.9 × 10-6† | 0.000027 | 1,000,000 |
| D15S165 | 15q13 (5) | 0.0083 | 0.0116 | 8,189 |
The † and ‡ indicate loci meeting Lander and Kruglyak criteria (42) for significant and suggestive linkage, respectively. LOD, logarithm of odds.
Distances, in Kosambi centimorgans (cM), are plotted from the first marker genotyped on every chromosome.
Two loci on chromosomes 1p14-1p35 and 6p12-q12 met Lander and Kryuglak criteria for linkage (43). The location of the linkage signal on 1p spanned from marker D1S468 (HE-P < 0.018; 0 cM) to D1S1622 (HE-P < 0.0003; 49 cM), whereas the linkage signal on 6p12-q12 extended from D6S1959 (HE-P < 0.058; 29 cM) to D6S1056 (HE-P < 0.009; 100 cM). The maximum signals on chromosomes 1p35 and 6p12-q12 were at markers D1S1622 (HE-P < 0.0002; permutation P < 0.0009; 49 cM) and D6S1053 (HE-P < 7.9 × 10-6; permutation P < 0.000027; 71.4 cM; see supporting information, which is published on the PNAS web site), respectively (Table 1). The locus on chromosome 1p35 overlaps with previous linkage signals for congenital cataracts, the Volkman type (44), and posterior polar cataract (45, 46). In contrast, the most significant linkage peak in the entire genome scan, at 6p12-q12, is novel.
In addition to the loci on chromosomes 1p and 6p12-q12, several other loci suggestive of linkage [P ≤ 0.01 or a logarithm of the likelihood ratio (LOD) score ≥ 1.18] were located on chromosomes 1q31, 2p24, 2q11, 4q28, and 15q13 (Table 1). Markers D1S2625, D2S1360, GATA176C01, D4S2365, and D15S185 were the most significant markers at these sites. The range of the second signal on chromosome 1 (Table 1) was smaller and extended over 7 cM from marker D1S518 (HE-P < 0.016; 209 cM) to D1S2625 (HE-P < 0.0079; 216 cM). Two other weak linkage signals were observed on chromosome 1 (Fig. 1). The first occurred between markers D1S534 (HE-P < 0.029; 156 cM) and D1S1653 (HE-P < 0.096; 168 cM) and the second between GATA124F08 (HE-P < 0.038; 237 cM) and D1S549 (HE-P < 0.042; 251 cM). The gap junction protein, Connexin50 (GJA8), is located between markers D1S534 and D1S1653. Mutations in GJA8 cause zonular pulverulant cataracts that show autosomal dominant inheritance (47). Multiple linkage signals were also observed on chromosome 2 (Fig. 1 and Table 1). The first occurred between markers D2S1400 (HE-P < 0.025; 28 cM) and D2S405 (HE-P < 0.080; 50 cM), the second between D2S2739 (HE-P < 0.031; 81 cM) and D2S441 (HE-P < 0.098; 93 cM), the third between D2S1777 (HE-P < 0.058; 108 cM) and D2S410 (HE-P < 0.047; 135 cM), and the last between D2S1391 (HE-P < 0.074; 213 cM) and GATA30E06 (HE-P < 0.090; 238 cM). The third and fourth signals overlap a region on 2q33-q35 that contains the gamma crystallin family (48, 49) and a linkage signal in a consanguineous Iraqi family at 2p12 (50), respectively. The second and third linkage signals on chromosome 2 are in such close proximity (within 15 cM of each other) that it is difficult to determine whether they are independent. Further mapping will be necessary to dissect the confounding. The range of linkage on chromosomes 4 and 15 is defined by markers D4S2367 (HE-P < 0.021; 70 cM)-D4S2361 (HE-P < 0.021; 88 cM), and D15S822 (HE-P < 0.0025; 0 cM)-D15S659 (HE-P < 0.027; 30 cM), respectively. The locus on chromosome 4 is novel, but the interval on chromosome 15, and a previous report for central “pouch-like” cataracts (51), can be superimposed.
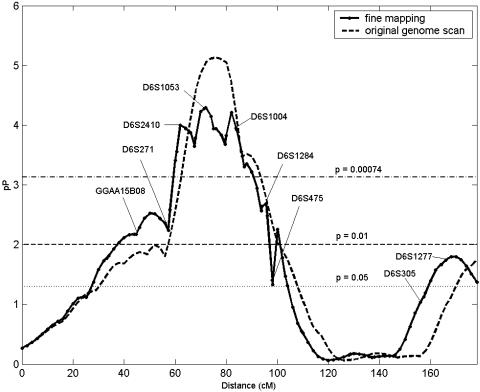
In compliance with the two-stage genome scan design (52), signals with P ≤ 0.01 or logarithm of the likelihood ratio (LOD) ≥ 1.18 were prioritized for fine mapping. The first locus to be thus characterized in the current scan is located on chromosome 6p12-q12. We genotyped 11 additional markers between D6S1017 and D6S1021, shortening the inter-marker distance to 3 cM (Fig. 2, and Table 2). Addition of the markers decreased the overall significance at 6p12-q12 (Fig. 2); for example, the HE-P value at D6S1053 decreased by an order of magnitude from 7.9 × 10-6 to 5.1 × 10-5. Despite the deflation in the P value, the linkage evidence remains consistent with a major locus for cortical cataracts on chromosome 6p12-q12 (43), with D6S1053 demonstrating the greatest significance. Although a consensus physical map for the human genome is available, marker order for closely linked markers remains uncertain due to gaps and segmental repeats in the human genome. We investigated whether small rearrangements in marker order would improve the fine mapping linkage signal. We see improvement in linkage evidence with an altered marker order during fine mapping (see supporting information). Fine mapping of the other regions with suggestive evidence of linkage is an ongoing process.
Fig. 2.
Fine-mapping multipoint results on chromosome 6p12-q12 for cortical cataracts. Genetic distance (cM) is plotted on the x axis against pP = -log10 (P value) on the y axis. Horizontal lines are drawn at P = 0.05, P = 0.01, and P = 0.00074.
Table 2. Genetic locations and multipoint P values (Haseman-Elston and empirical) for fine mapping markers on chromosome 6p12-q12.
| Marker | Genetic distance,* cM | Stage of mapping† | HE-P | Permutation P value | No. of replicates |
|---|---|---|---|---|---|
| GGAA15B08 | 45 | GS | 0.0067 | 0.0103 | 9236 |
| D6S1017 | 55 | GS | 0.0037 | 0.0048 | 19815 |
| D6S271 | 57 | FM | 0.0057 | 0.0084 | 11338 |
| GATA161E01 | 61 | FM | 0.00027 | 0.00069 | 138283 |
| D6S2410 | 65 | FM | 0.00011 | 0.00034 | 283767 |
| D6S1960 | 67 | FM | 0.00022 | 0.00072 | 132358 |
| D6S1053 | 71 | GS | 0.000053 | 0.00025 | 387643 |
| D6S421 | 75 | FM | 0.00011 | 0.00061 | 157662 |
| D6S1031 | 79 | GS | 0.00021 | 0.00072 | 133249 |
| D6S1004 | 83 | FM | 0.00011 | 0.00036 | 273164 |
| D6S1644 | 87 | FM | 0.00050 | 0.0014 | 67479 |
| D6S1570 | 89 | FM | 0.00060 | 0.0019 | 51030 |
| D6S1056 | 93 | GS | 0.0027 | 0.0080 | 11999 |
| D6S1284 | 95 | FM | 0.0020 | 0.0041 | 23526 |
| D6S475 | 99 | FM | 0.0470‡ | — | — |
| GATA164H01 | 101 | FM | 0.0056 | 0.0143 | 6641 |
| D6S1021 | 103 | GS | 0.0338‡ | — | — |
Genetic distance, in Kosambi centimorgans (cM), was calculated from the first p-arm marker (F13A1) genotyped.
To achieve an average intermarker density of 2-3 cM as per the two-stage design (51), fine mapping (FM) markers, interspersed between genome-scan (GS) markers that demonstrated evidence of linkage, were identified and genotyped.
Permutation tests were not conducted for markers with a nominal Haseman-Elston regression significance of P ≥ 0.01.
Discussion
We conducted a genome scan for cortical cataracts in a subsample from a large population-based study, possibly representing the first of such analyses for any age-related cataract. Using model-free linkage methods, we identified two major loci for cortical cataracts on chromosome 6p12-q12 and 1p14-1p35, and several possible minor loci on other chromosomal regions. The region on chromosome 6p12-q12 is novel, and neither linkage to this region, nor mutations in any positional candidates on 6p12-q12, have previously been cited for cataracts of any etiology. There is potential correspondence in the map location of loci on 1p14-1p35 identified in this study and previously identified cataract loci (44-46). The Volkman cataract is central and zonular with a sutural component, is progressive, and shows an autosomal dominant mode of inheritance (44). Two types of posterior polar cataract (stationary and progressive) also map to this region (45, 46). Interestingly, the progressive cataract initiates at the posterior pole but later develops cortical involvement. One appealing candidate gene on chromosome 1p is the DNAse2-like acid DNase (DLAD) gene. Knockout mice for this gene develop nuclear cataracts (53), but it is feasible that this gene has broader implications than originally described.
Several other loci on 1q31, 2p24, 2q11, 2q33, 4q28, 5q35, 7p22, 8q12, 13q31, and 15q13 were identified through this genome scan. Of the weaker signals, the loci on 1q near GJA8, and on 2q33 near the γ crystallins, are noteworthy. Whether mutations in these weaker loci are critical events in age-related cortical cataract, or whether these loci act as modifiers, is a topic for future investigation. Because the majority of the loci identified in this genome scan do not coincide with map coordinates for known genes, such as β A3 (17q11-q12), α A (21q22.3) and β 2 (22q) crystallins, it may be that pathways and genes that are less crucial to the development of the lens fibers may play a role in age-related cataracts.
We conducted fine mapping on 6p12-q12 to refine the position of this susceptibility locus. During our investigation, we observed that the region on chromosome 6 remained broad (≈60 cM) despite addition of 11 markers. This property is characteristic of real linkage findings (54, 55), as opposed to false positives. At this time, there are no genes known to cause cataracts at this location. We surveyed the human genome sequence (www.ensembl.org) in the interval spanning the maximal evidence of linkage (Table 2) for potential candidate genes. Counting known genes, predicted genes and expressed transcripts, ≈267 positional candidates can be enumerated in this linkage interval. Because of the length of the linkage interval, the biologically plausible candidates included gap junction proteins, heat shock proteins, and ion channels, all of which can be hypothesized to play a role in the etiology of cataract formation, but there is no a priori evidence which, if any, is the susceptibility gene.
There are some limitations to this study. Our sample size for this study is modest, and it is feasible that other loci may be identified through genome scans in independent samples. For example, in this study, loci on chromosomes 1 and 6 were the most significant. In another sample, these loci may have secondary roles or may have no discernible impact. Issues such as these are particularly important if the population under investigation is not Caucasian, because analysis of other ethnic groups may lead to quite different conclusions. Although there is considerable heterogeneity in the phenotypic expression of known cataract loci within families, it is unlikely (but not impossible) that the newly identified loci will also cause other types of age-related cataracts. Finally, even if novel susceptibility genes are identified through these investigations, the attributable risk for each gene will have to be ascertained through population surveys that are controlled for cataract type or morphology, age at onset, progression of cataract (especially in relation to morphology), and other susceptibility factors.
In summary, we have discovered multiple loci for age-related cortical cataracts that add to the molecular and locus heterogeneity observed among congenital/early-onset cataracts. We expect that cloning of susceptibility genes will lead to significant breakthroughs, particularly in deciphering which genes impact the aging process in the lens, and how these effects are mediated.
Supplementary Material
Acknowledgments
This study was supported in part by U.S. Public Health Service Research Grant GM28356 from the National Institute of General Medical Sciences, Grants U10-EY06594 and EY10605 from the National Eye Institute, Training Grant HL07567 from the National Heart, Lung, and Blood Institute, and Resource Grant RR03655 from the National Center for Research Resources.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BDES, Beaver Dam Eye Study; HE-P, Haseman-Elston P value.
References
- 1.Thylefors, B., Negrel, A. D., Pararajasegaram, R. & Dadzie, K. Y. (1995) Bull. W. H. O. 73, 115-121. [PMC free article] [PubMed] [Google Scholar]
- 2.Thylefors, B. (1999) Bull. W. H. O. 77, 453. [PMC free article] [PubMed] [Google Scholar]
- 3.Brian, G. & Taylor, H. (2001) Bull. W. H. O. 79, 249-256. [PMC free article] [PubMed] [Google Scholar]
- 4.Anonymous (1998) Afr. Health 20, 38. [Google Scholar]
- 5.Stark, W. J., Worthen, D. M., Holladay, J. T., Bath, P. E., Jacobs, M. E., Murray, G. C., McGhee, E. T., Talbott, M. W., Shipp, M. D., Thomas, N. E., et al. (1984) Aust. J. Ophthalmol. 12, 61-69. [PubMed] [Google Scholar]
- 6.Glied, S. & Little, S. E. (2003) Health Aff. (Millwood) 22, 210-219. [DOI] [PubMed] [Google Scholar]
- 7.Philipson, B. (1973) Exp. Eye Res. 16, 29-39. [DOI] [PubMed] [Google Scholar]
- 8.Tripathi, R. C. & Tripathi, B. J. (1983) J. Gerontol. 38, 258-270. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, C. Y., Liu, J. H., Chen, S. J. & Lee, F. L. (2000) Zhonghua Yi Xue Za Zhi (Taipei) 63, 641-648. [PubMed] [Google Scholar]
- 10.Klein, B. E., Klein, R. & Linton, K. L. (1992) Ophthalmology 99, 546-552. [DOI] [PubMed] [Google Scholar]
- 11.Leske, M. C., Wu, S. Y., Nemesure, B., Li, X., Hennis, A. & Connell, A. M. (2000) Ophthalmology 107, 1267-1273. [DOI] [PubMed] [Google Scholar]
- 12.Maraini, G., Pasquini, P., Sperduto, R. D., Rosmini, F., Bonacini, M., Tomba, M. C. & Corona, R. (1990) Ophthalmology 97, 752-756. [DOI] [PubMed] [Google Scholar]
- 13.Sperduto, R. D. & Hiller, R. (1984) Ophthalmology 91, 815-818. [DOI] [PubMed] [Google Scholar]
- 14.Tsai, S. Y., Hsu, W. M., Cheng, C. Y., Liu, J. H. & Chou, P. (2003) Ophthalmology 110, 1089-1095. [DOI] [PubMed] [Google Scholar]
- 15.McCarty, C. A., Mukesh, B. N., Fu, C. L. & Taylor, H. R. (1999) Am. J. Ophthalmol. 128, 446-465. [DOI] [PubMed] [Google Scholar]
- 16.Klein, B. E., Cruickshanks, K. J. & Klein, R. (1994) Doc. Ophthalmol. 88, 295-305. [DOI] [PubMed] [Google Scholar]
- 17.Sharma, Y. R., Vajpayee, R. B. & Honavar, S. G. (1994) Arch. Environ. Health 49, 414-417. [DOI] [PubMed] [Google Scholar]
- 18.Taylor, H. R., West, S. K., Rosenthal, F. S., Munoz, B., Newland, H. S., Abbey, H. & Emmett, E. A. (1988) N. Engl. J. Med. 319, 1429-1433. [DOI] [PubMed] [Google Scholar]
- 19.Creighton, M. O., Sanwal, M., Stewart-DeHaan, P. J. & Trevithick, J. R. (1983) Exp. Eye Res. 37, 65-76. [DOI] [PubMed] [Google Scholar]
- 20.Jacques, P. F., Hartz, S. C., Chylack, L. T., Jr., McGandy, R. B. & Sadowski, J. A. (1988) Am. J. Clin. Nutr. 48, 152-158. [DOI] [PubMed] [Google Scholar]
- 21.Kuzniarz, M., Mitchell, P., Cumming, R. G. & Flood, V. M. (2001) Am. J. Ophthalmol. 132, 19-26. [DOI] [PubMed] [Google Scholar]
- 22.Taylor, A., Jacques, P. F., Chylack, L. T., Jr., Hankinson, S. E., Khu, P. M., Rogers, G., Friend, J., Tung, W., Wolfe, J. K., Padhye, N., et al. (2002) Am. J. Clin. Nutr. 75, 540-549. [DOI] [PubMed] [Google Scholar]
- 23.Brown, N. A. & Hill, A. R. (1987) Br. J. Ophthalmol. 71, 405-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein, B. E., Klein, R., Wang, Q. & Moss, S. E. (1995) Ophthalmic Epidemiol. 2, 49-55. [DOI] [PubMed] [Google Scholar]
- 25.Italian-American Cataract Study Group (1991) Am. J. Epidemiol. 133, 541-553. [PubMed] [Google Scholar]
- 26.Heiba, I. M., Elston, R. C., Klein, B. E. & Klein, R. (1995) Invest. Ophthalmol. Vis. Sci. 36, 227-235. [PubMed] [Google Scholar]
- 27.Anonymous (1994) Am. J. Epidemiol. 140, 555-564. [PubMed] [Google Scholar]
- 28.Hammond, C. J., Duncan, D. D., Snieder, H., de Lange, M., West, S. K., Spector, T. D. & Gilbert, C. E. (2001) Invest. Ophthalmol. Vis. Sci. 42, 601-605. [PubMed] [Google Scholar]
- 29.Graw, J. & Loster, J. (2003) Ophthalmic Genet. 24, 1-33. [DOI] [PubMed] [Google Scholar]
- 30.Hejtmancik, J. F. & Smaoui, N. (2003) Dev. Ophthalmol. 37, 67-82. [DOI] [PubMed] [Google Scholar]
- 31.Klein, R., Klein, B. E., Linton, K. L. & De Mets, D. L. (1991) Ophthalmology 98, 1310-1315. [DOI] [PubMed] [Google Scholar]
- 32.Klein, B. E., Klein, R., Linton, K. L., Magli, Y. L. & Neider, M. W. (1990) Ophthalmology 97, 1428-1433. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell, P., Cumming, R. G., Attebo, K. & Panchapakesan, J. (1997) Ophthalmology 104, 581-588. [DOI] [PubMed] [Google Scholar]
- 34.Schick, J. H., Iyengar, S. K., Klein, B. E., Klein, R., Reading, K., Liptak, R., Millard, C., Lee, K. E., Tomany, S. C., Moore, E. L., et al. (2003) Am. J. Hum. Genet. 72, 1412-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller, S. A., Dykes, D. D. & Polesky, H. F. (1988) Nucleic Acids Res. 16, 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anonymous (2003) s.a.g.e., Statistical Analysis for Genetic Epidemiology (Dept. of Epidemiology and Biostatistics, Case Western Reserve University, Cleveland), Version 4.4.
- 37.Wilson, A. F., Elston, R. C., Tran, L. D. & Siervogel, R. M. (1991) Am. J. Hum. Genet. 48, 862-872. [PMC free article] [PubMed] [Google Scholar]
- 38.Box, G. & Cox, D. (1964) J. R. Stat. Soc. B 26, 211-254. [Google Scholar]
- 39.Elston, R. C., Buxbaum, S., Jacobs, K. B. & Olson, J. M. (2000) Genet. Epidemiol. 19, 1-17. [DOI] [PubMed] [Google Scholar]
- 40.Haseman, J. K. & Elston, R. C. (1972) Behav. Genet. 2, 3-19. [DOI] [PubMed] [Google Scholar]
- 41.Shete, S., Jacobs, K. B. & Elston, R. C. (2003) Hum. Hered. 55, 79-85. [DOI] [PubMed] [Google Scholar]
- 42.Wiltshire, S., Cardon, L. R. & McCarthy, M. I. (2002) Am. J. Hum. Genet. 71, 1175-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lander, E. & Kruglyak, L. (1995) Nat. Genet. 11, 241-247. [DOI] [PubMed] [Google Scholar]
- 44.Eiberg, H., Lund, A. M., Warburg, M. & Rosenberg, T. (1995) Hum. Genet. 96, 33-38. [DOI] [PubMed] [Google Scholar]
- 45.Ionides, A., Francis, P., Berry, V., Mackay, D., Bhattacharya, S., Shiels, A. & Moore, A. (1999) Br. J. Ophthalmol. 83, 802-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ionides, A. C., Berry, V., Mackay, D. S., Moore, A. T., Bhattacharya, S. S. & Shiels, A. (1997) Hum. Mol. Genet. 6, 47-51. [DOI] [PubMed] [Google Scholar]
- 47.Shiels, A., Mackay, D., Ionides, A., Berry, V., Moore, A. & Bhattacharya, S. (1998) Am. J. Hum. Genet. 62, 526-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nandrot, E., Slingsby, C., Basak, A., Cherif-Chefchaouni, M., Benazzouz, B., Hajaji, Y., Boutayeb, S., Gribouval, O., Arbogast, L., Berraho, A., et al. (2003) J. Med. Genet. 40, 262-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brakenhoff, R. H., Henskens, H. A., van Rossum, M. W., Lubsen, N. H. & Schoenmakers, J. G. (1994) Hum. Mol. Genet. 3, 279-283. [DOI] [PubMed] [Google Scholar]
- 50.Khaliq, S., Hameed, A., Ismail, M., Anwar, K. & Mehdi, S. Q. (2002) Invest. Ophthalmol. Vis. Sci. 43, 2083-2087. [PubMed] [Google Scholar]
- 51.Vanita, Singh, J. R., Sarhadi, V. K., Singh, D., Reis, A., Rueschendorf, F., Becker-Follmann, J., Jung, M. & Sperling, K. (2001) Am. J. Hum. Genet. 68, 509-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elston, R. C., Guo, X. & Williams, L. V. (1996) Genet. Epidemiol. 13, 535-558. [DOI] [PubMed] [Google Scholar]
- 53.Nishimoto, S., Kawane, K., Watanabe-Fukunaga, R., Fukuyama, H., Ohsawa, Y., Uchiyama, Y., Hashida, N., Ohguro, N., Tano, Y., Morimoto, T., et al. (2003) Nature 424, 1071-1074. [DOI] [PubMed] [Google Scholar]
- 54.Visscher, P. & Haley, C. (2001) Genet. Epidemiol. 20, 409-414. [DOI] [PubMed] [Google Scholar]
- 55.Terwilliger, J. D., Shannon, W. D., Lathrop, G. M., Nolan, J. P., Goldin, L. R., Chase, G. A. & Weeks, D. E. (1997) Am. J. Hum. Genet. 61, 430-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.