Abstract
We compared monocyte-derived dendritic cells and transforming growth factor-β1-induced Langerhans-like cells (LCs) for their capacity to cross-present exogenous NY-ESO-1 protein/antibody immune complexes to an NY-ESO-1-specific CD8+ T cell clone. In contrast to dendritic cells, LCs were not able to cross-present NY-ESO-1 to the T cell clone constitutively but did so after treatment with IFN-γ. Remarkably, this IFN-γ-inducible characteristic was due neither to enhanced antigen uptake nor to facilitated antigen processing in LCs. Rather, IFN-γ acted at least in part by potentiating the maturation of otherwise refractory LCs, enabling in turn exogenous antigen to reach the processing machinery. This model of conditional cross-presentation establishes an original level of action for IFN-γ as an effective immune modulator and supports the use of IFN-γ in protein vaccination strategies targeting LCs.
In the development of strategies to vaccinate cancer patients with tumor antigens, soluble whole proteins produced by recombinant technologies are attractive agents because they include all potential epitopes recognized by antibodies and T cells. To be naturally processed into MHC/peptide complexes, proteins have to be taken up by antigen-presenting cells, of which dendritic cells (DCs) are perhaps the most sophisticated kind due to their array of unique characteristics. In peripheral tissues, DCs internalize antigens and then migrate to lymphoid organs where they process the sequestered antigens into peptides, load them onto MHC molecules, and finally present peptide/MHC complexes on their cell surface (1, 2). To present antigens effectively, DCs must undergo maturation, a process triggered by various signals that induces phenotypic changes enabling the functional priming of T cells. When exogenous free proteins are added to DCs in vitro, most of the presentation will occur on MHC class II (2), but proteins may also be processed and presented by the MHC class I pathway if high concentrations of protein are used (3, 4). In contrast, if proteins are preincubated with specific antibodies to form immune complexes (IC), antigenic peptides derived from exogenous proteins are very efficiently presented by DCs onto MHC class I through a pathway known as cross-presentation (5, 6). The enhanced cross-presentation reflects, at least in part, the efficient uptake of IC-bound antigen by means of plasma membrane Fcγ receptors (5, 7, 8). Also, DCs seem to have a unique capacity that functionally links the lumen of endocytic compartments with the cytosol (9, 10). Internalized antigens can overcome the topology barrier normally created by the endosome-lysosome membrane and egress into cytosol, where they become susceptible to proteasome proteolysis and subsequent translocation into the endoplasmic reticulum (ER). It has been suggested that these events may in part reflect the introduction of ER components into endocytic organelles after phagocytosis (11, 12).
By using IC-loaded DCs, ovalbumin (OVA)-specific CD4+ and CD8+ T cells have been successfully induced in mice (8), suggesting that effective cross-presentation also occurs in vivo. In humans, it has been shown that IC with prostate-specific antigen (PSA) loaded on DCs induce specific CD4+ and CD8+ T cells derived from a healthy donor in vitro (13), indicating that IC-containing specific antigen proteins loaded on DCs are potentially powerful tools for cancer vaccine. To develop a vaccine strategy by using IC, it is essential to understand the mechanism of antigen transport from the membrane to the cytosol by means of the cross-presentation pathway in DCs, which remains to be elucidated.
Previously, we showed that IC formed with cancer/testis antigen NY-ESO-1 were efficiently cross-presented by monocyte-derived DCs, but not by CD34-derived DCs (6). This difference did not seem linked to uptake capacity of IC by the two DC populations. In the present follow-up work, we investigated whether maturation may be an important factor enabling cross-presentation in DCs. Because we observed that monocyte-derived DCs (mo-DCs) were extremely sensitive to maturation, we sought an alternative source of DCs that would be more refractory to maturation while still being easily accessible from peripheral blood. Geissmann et al. (14) have recently found that transforming growth factor-β1, in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4, induces differentiation of peripheral blood monocytes into DCs with Langerhans cell characteristics, and these cells have been shown to exhibit resistance to some maturation signal (15). We therefore turned our attention to these monocyte-derived Langerhans-like cells (LCs) and tested their capacity to present NY-ESO-1 protein and IC to T cells. We found that LCs were defective for cross-presentation of exogenous antigen into the MHC class I pathway. Remarkably, treatment with IFN-γ rescued the deficient phenotype and enabled LCs to acquire the capacity to cross-present antigen similarly to mo-DCs. Moreover, LCs treated with IFN-γ became susceptible to maturation signals. This system for conditional cross-presentation is a useful model to understand the mechanism of antigen transport from membrane to cytosol.
Materials and Methods
Cell Lines. HLA-A*0201+ mutant cell line T2 (CEMx721.174.T2), and NY-ESO-1+, HLA-A*0201+ melanoma cell lines SKMEL-37 and MZ-MEL-19 were cultured in RPMI medium 1640 supplemented with 10% FCS (Summit Biotechnology, Ft. Collins, CO), L-glutamine (2 mM), penicillin (100 units/ml), streptomycin (100 μg/ml), and 1% nonessential amino acids.
Peptides and Viral Vectors. HLA-A*0201-restricted NY-ESO-1 peptide 157-165 (SLLMWITQC) (16) and HLA-DR*0701-restricted peptide p115-132 (PLPVPGVLLKEFTVSGNI) (17) were synthesized by Multiple Peptide Systems (San Diego) and by Bio-Synthesis (Lewisville, TX) with a purity of >86% as determined by reverse phase HPLC. Vaccinia virus (v.v.WT) and vaccinia virus recombinant for full-length NY-ESO-1 (v.v.ESO) have been described (18).
Antibodies. Monoclonal antibodies against NY-ESO-1, ES121 IgG1, and E978 IgG1 were obtained from mice immunized with recombinant NY-ESO-1 protein (19). Monoclonal antibodies against MAGE-1 and MAGE-3 were also prepared from mice immunized with recombinant MAGE-1 and MAGE-3, respectively (19). Purified or FITC-labeled anti-human CD32, purified or phycoerythrin (PE)-labeled anti-human CD16, purified or FITC-labeled anti-human CD64, FITC-labeled anti-human CD1a, FITC-labeled anti-human CD14, FITC-labeled anti-HLA-A, -B, and -C, PE-labeled anti-HLA-DR, PE-labeled anti-human CD83, and PE-labeled anti-human CD86 were purchased from BD Pharmingen.
Recombinant Proteins. NY-ESO-1 full-length, NY-ESO-1 short (truncated form of NY-ESO-1, AA 10-121), MAGE-1, and MAGE-3 proteins were prepared as His-tagged proteins in Escherichia coli as described (19). Briefly, proteins with a six-histidine tag at the amino terminus were purified from washed and solubilized inclusion bodies by nickel chelate affinity chromatography (Chelating Sepharose FF; Amersham Pharmacia Biotech) by using a pH gradient. The purified proteins were reactive with suitable monoclonal antibodies by Western blot analysis; purity was >80% by SDS/PAGE.
Generation of DCs and Langerhans Cells. To generate mo-DCs, peripheral blood mononuclear cells were isolated from buffy-coat of healthy individuals as described (20). CD14+ monocytes were enriched by negative selection by using magnetic beads (Dynal, Oslo) and then cultured in RPMI medium 1640 with 2.5% FCS, L-glutamine (2 mM), penicillin (100 units/ml), streptomycin (100 μg/ml), supplemented with 100 ng/ml of GM-CSF (Immunex) and 20 ng/ml of IL-4 (R & D Systems). LCs were cultured in the same medium as mo-DCs supplemented with 10 ng/ml transforming growth factor-β1. Some LCs were treated with 750 units/ml IFN-γ (R & D Systems) (LCs/IFN-γ) for the last 24 h before cells were harvested. On day 6 or day 7, mo-DCs, LCs, and LCs/IFN-γ were harvested, characterized by fluorescence-activated cell sorter (FACS, Becton Dickinson), and used for antigen presentation assays or antigen uptake assays.
Immunocytochemistry. To examine cells morphologically, 4 × 105 cells were cultured in chambers on Super Cell Culture Slides (Fisher Scientific). Cells were washed by PBS, dried, fixed by 100% Met-OH, and stained by Giemsa staining. Cytospin preparations (Shandon, Pittsburgh) of 5 × 105 cells per slide were fixed in ice-chilled acetone for 15 min, then air-dried. Immunocytochemistry with a biotin-streptavidin system (Vector ABC Elite kit, Vector Laboratories) was performed as described by the manufacturer's manual. Mouse anti-human E-cadherin mAb (2.5 μg/ml) was used as primary antibody, followed by biotinylated goat anti-rabbit antibody (5 μg/ml, Vector Laboratories). Liquid DAB Concentrated Substrate Pack (BioGenex Laboratories, San Ramon, CA) was used for detection, and CoCl2 and NiCl2 were added in substrate/chromogen solution at the concentration of 0.25% and 0.20%, respectively, to increase sensitivity as described (21).
Antigen Loading. IC were prepared by mixing ES121 and E978 monoclonal antibodies with NY-ESO-1 protein for 30 min at 37°C at final concentrations indicated in the figure legends. IC were incubated with 1.5 × 105 mo-DCs, LCs, or LCs/IFN-γ in complete medium with IL-4 and GM-CSF at 37°C for 7 h. NY-ESO-1 short protein, MAGE-1 or MAGE-3 and suitable monoclonal antibodies were also used in the same conditions as control IC. For proteolysis inhibition assay, LCs/IFN-γ were treated with 40 μM lactacystin for 1 h before adding IC. Peptides (10 μM) were pulsed on cells for 7 h at 37°C. Cells were infected with recombinant vaccinia viruses at 100 plaque-forming units per cell for 7 h at 37°C.
T Cell Effectors. The HLA-A*0201-restricted CD8+ T cell clone specific for NY-ESO-1 peptide 157-165, clone49, was generated by limiting dilution from tumor-infiltrating lymphocytes of melanoma patient (22). HLA-DRB1*0701-restricted CD4+ T cell line specific for NY-ESO-1 peptide 115-132 was derived from patient NW29 as described (23).
Enzyme-Linked Immunospot (ELISPOT) Assays. For ELISPOT assays, flat-bottomed, 96-well nitrocellulose plates (MultiScreen-HA; Millipore) were coated with anti-IFN-γ mAb (2 μg/ml, 1-D1-K; MABTECH, Stockholm) and incubated overnight at 4°C. After washing with RPMI medium 1640, plates were blocked with 10% human AB type serum for 1 h at 37°C. Clone49 effector cells (1.5 × 103, 3 × 102, and none) or CD4+ T cell effectors (1 × 104, 2 × 102, and none) and 5 × 104 target cells (antigen-loaded mo-DCs, LCs, LCs/IFN-γ, or tumor cells) were added to each well and incubated for 20 h in RPMI medium 1640 without serum. Plates were then washed thoroughly with water containing 0.05% Tween 20 to remove cells, and anti-IFN-γ mAb (0.2 μg/ml, 7-B6-1-biotin; MABTECH) was added to each well. After incubation for 2 h at 37°C, plates were washed and developed with streptavidin-alkaline phosphatase (1 μg/ml; MABTECH) for 1 h at room temperature. After washing, substrate (5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium; Sigma) was added and incubated for 5 min. After final washes, plate membranes displayed dark-violet spots that were counted under the microscope.
Endocytosis Assay. For endocytosis assay by fluorescent IC, mo-DCs, LCs, or LCs/IFN-γ were incubated for 1 h at 37°C or on ice with IC made of NY-ESO-1 protein (25 μg/ml) and E978 mAb (5 μg/ml) conjugated with fluorescein by using Alexa Fluor 488 labeling kit (Molecular Probes). After washing, some cells were briefly incubated with 0.2% trypan blue (Sigma) to quench extracellular fluorescence, and analyzed by FACS.
Maturation. To induce maturation, cells were incubated with lipopolysaccharide (LPS) (100 ng/ml, Sigma), IC, or CD40L (1 μg/ml CD40L soluble set with enhancer:mouse IgG to cross-link the CD40L monomers into oligomers; Alexis, San Diego) in complete medium containing IL-4 and GM-CSF. After 2 days, cells were washed and stained with fluorescence-labeled antibodies against CD86, CD83, and HLA-DR, and analyzed by FACS.
Results
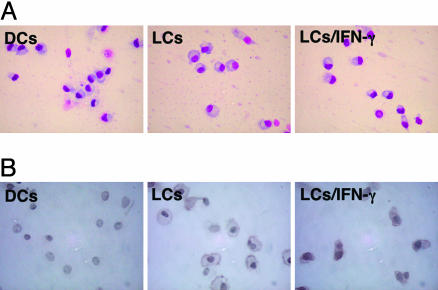
Phenotype of mo-DCs, LCs, and LCs Treated with IFN-γ. Morphologically, mo-DCs, LCs, and LCs treated for 24 h with IFN-γ (LCs/IFN-γ) were similar in appearance (Fig. 1A). However, LCs and LCs/IFN-γ displayed characteristic expression of E-cadherin in immunocytochemical staining, predominantly on their membranes, whereas mo-DCs did not (Fig. 1B). Cells were then characterized by flow cytometry. As shown in Fig. 2, mo-DCs and LCs expressed high CD1a, but low CD86 and HLA-DR, and nearly no CD83, consistent with an immature phenotype. Expression of CD32 (FcγRII) was moderate on mo-DCs, but nearly absent on LCs. Notably, LCs/IFN-γ retained an immature phenotype by flow cytometry, although HLA class I and HLA-DR were slightly elevated compared with untreated LCs. Surprisingly, expression of CD32 (FcγRII), CD64 (FcγRI), and CD16 (FcγRIII) was barely altered by IFN-γ treatment (Fig. 2).
Fig. 1.
Immunocytochemistry of mo-DCs, LCs, and LCs/IFN-γ. Cells were stained with simple Giemsa staining (A) and mouse anti-human E-cadherin mAb followed by biotinylated goat anti-rabbit antibody (B) as described in Materials and Methods.
Fig. 2.
Phenotype of mo-DCs, LCs, and LCs/IFN-γ. Monocytes isolated by magnetic bead selection from peripheral blood mononuclear cells were cultured with GM-CSF and IL-4 for 6 days to induce mo-DCs, or with additional transforming growth factor-β1 to induce LCs. IFN-γ was added for the last 24 h of culture on LCs to induce LCs/IFN-γ. Cells were analyzed by flow cytometry for the indicated markers. Results were representative of at least five independent reproducible experiments.
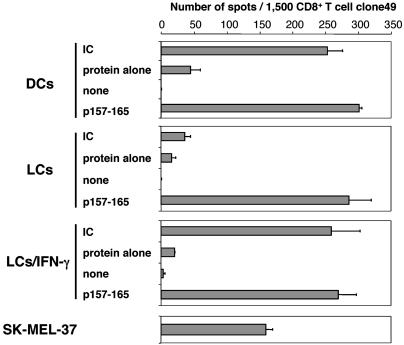
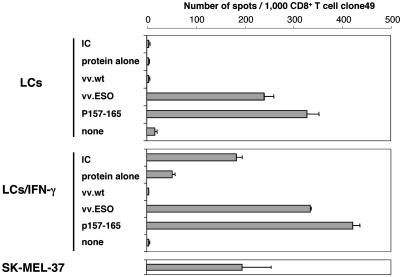
Capacity of Cross-Presentation of mo-DCs, LCs, and LCs/IFN-γ. To determine whether LCs and LCs/IFN-γ derived from healthy HLA-A*0201-positive donors were capable of generating peptide-MHC class I complexes from exogenous antigen, cultures were exposed to either free NY-ESO-1 protein or NY-ESO-1 IC and assayed by ELISPOT by using CD8+ T cell clone49 specific for NY-ESO-1 p157-165 (Fig. 3). As reported (6), mo-DCs exposed to exogenous NY-ESO-1 IC were capable of presenting the antigenic epitope onto MHC class I molecule, whereas free protein was inefficiently presented. LCs fed with either NY-ESO-1 protein or IC were, in contrast, incapable of effectively sensitizing clone49. Remarkably, LCs/IFN-γ exposed to exogenous NY-ESO-1 IC sensitized clone49 as effectively as mo-DCs. All three cell types were recognized by clone49 in the presence of exogenous peptide p157-165, and so was NY-ESO-1+ HLA-A2+ melanoma cell line SK-MEL-37.
Fig. 3.
ELISPOT assay for presentation of HLA-A*0201-restricted NY-ESO-1-derived epitope to antigen-specific CD8+ T cell clone49 by mo-DCs, LCs, and LCs/IFN-γ exposed to NY-ESO-1 IC. All three cell types were derived from the same HLA-A*0201-positive healthy donor. DCs, LCs, and LCs/IFN-γ were incubated with NY-ESO-1 IC (25 μg/ml protein and 5 μg/ml mAb preincubated for 30 min), protein alone (25 μg/ml), or synthetic peptide p157-165 (10 μM) for 7 h at 37°C. HLA-A*0201+,NY-ESO-1+ melanoma cell line SK-MEL-37 was used as a positive control for clone49. Error bars indicate standard deviation in duplicate wells. Results were representative of at least five independent reproducible experiments.
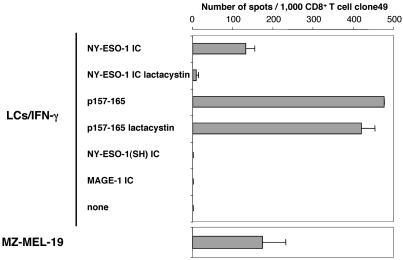
To assess whether the response of clone49 to LCs/IFN-γ exposed to NY-ESO-1 IC was due to specific recognition of MHC-peptide complexes and required proteasome proteolysis, experiments were performed by using the proteasome inhibitor lactacystin. As shown in Fig. 4, recognition by clone49 of LCs/IFN-γ fed with NY-ESO-1 IC was markedly inhibited by lactacystin treatment, whereas p157-165 presentation was not affected. Furthermore, LCs/IFN-γ fed with IC containing irrelevant proteins such as MAGE-1 or the NY-ESO-1 truncated protein (which does not include the epitope but is still recognized by anti-NY-ESO-1 mAb) were not able to sensitize clone49. Also, LCs/IFN-γ derived from HLA-mismatched donors and fed with NY-ESO-1 IC were not able to sensitize clone49 (data not shown). These results suggested that LCs/IFN-γ fed with NY-ESO-1 IC acquired the capacity to cross-present antigen in a proteasome-dependent pathway likely to require uptake and internalization of exogenous IC containing NY-ESO-1 protein.
Fig. 4.
Lactacystin inhibits cross-presentation of an NY-ESO-1 epitope in LCs/IFN-γ. Cells were preincubated in the presence or absence of lactacystin (40 μM) for 1 h before exposure to NY-ESO-1 IC (3 μg/ml protein and 2 μg/ml specific mAb) or p157-165 (10 μM) and were then tested by ELISPOT for recognition by clone49. As negative controls, IC made with truncated NY-ESO-1 SH protein (AA10-121) or with MAGE-1 protein and their respective specific mAb were used at the same concentration. HLA-A*0201+,NY-ESO-1+ melanoma cell line MZ-MEL-19 was used as positive control for clone49. Error bars indicate standard deviation in duplicate wells. Results were representative of at least three independent reproducible experiments.
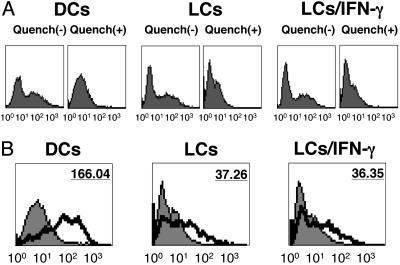
Antigen Uptake by LCs and LCs/IFN-γ. To investigate whether the distinct cross-presentation capacity of mo-DCs, LCs, and LCs/IFN-γ reflected a differential endocytic uptake of IC, we tried to directly visualize the uptake capacity of NY-ESO-1 IC in the three cell types using anti-NY-ESO-1 monoclonal antibody E978 conjugated to a fluorescent marker to form labeled IC. Quenching cells with trypan blue (25) was necessary to regulate extracellular fluorescence and thus visualize internalized antigen only, because cells incubated with labeled IC at 0°C showed surface binding in the absence of quenching (Fig. 5A). As shown in Fig. 5B, there was clear uptake of fluorescent IC in mo-DCs at 37°C. However, both LCs and LCs/IFN-γ exhibited five times lower mean fluorescence intensity compared with mo-DCs at 37°C, and they did not show a significant uptake difference with each other. As a control, matured mo-DCs induced by exposing to LPS for 48 h showed modest mean fluorescence intensity level at 37°C (data not shown).
Fig. 5.
Endocytosis of fluorescent IC by mo-DCs, LCs, and LCs/IFN-γ. Cells were incubated with IC containing NY-ESO-1 protein (25 μg/ml) and specific E978 mAb (5 μg/ml) conjugated with fluorescein for 1 h at 37°C (thick line) or on ice (gray area). After washing, half of the cells were briefly incubated with 0.2% trypan blue to quench extracellular fluorescence, and analyzed by FACS. (B) Values indicate the mean fluorescence intensity of staining at 37°C (thick line), compared with negative control on ice (gray area). Results were representative of at least three independent reproducible experiments.
Similar results were obtained in experiments measuring the uptake capacity of fluorescence-labeled dextran (24) or of peroxidase-anti-peroxidase soluble immune complexes (data not shown). Together, these data suggested that failure in cross-presentation in LCs was unlikely to be due to their lower endocytotic potential compared with LC/IFN-γ, but rather to intracellular events after the uptake of exogenous IC.
Conventional Antigen Presentation by Means of Endogenous MHC Class I Pathway by LCs and LCs/IFN-γ. To investigate whether LCs were impaired for antigen processing in general as compared with LCs/IFN-γ, we assayed antigen presentation by means of conventional (i.e., endogenous) MHC class I pathway. First, vaccinia virus recombinant for NY-ESO-1 (vv.ESO) or wild type (vv.WT) was used to infect cells and target the endogenous class I pathway. Fig. 6 shows that LCs infected with vv.ESO were able to effectively present the HLA-A*0201-restricted epitope to clone49 in ELISPOT despite the fact that they failed to cross-present the same antigen when delivered as an exogenous IC. LCs infected with Fowlpox or adenovirus recombinant for NY-ESO-1 were also capable of presenting effectively (data not shown). On the other hand, LCs/IFN-γ were able to present the epitope onto MHC class I effectively by means of both conventional and cross-presentation pathways (Fig. 6).
Fig. 6.
Conventional and cross-presentation pathways to MHC class I in LCs and LCs/IFN-γ. Cells were simultaneously prepared from the same HLA-A*0201-positive healthy donor. Cells were incubated with NY-ESO-1 IC (3 μg/ml protein, 2 μg/ml specific mAb), free NY-ESO-1 protein (3 μg/ml), vaccinia virus recombinant for NY-ESO-1 (vv.ESO), or vaccinia virus wild type (vv.WT) (100 plaque-forming units/cell), p157-165 (10 μM) for 7 h, and tested by ELISPOT with clone49. SK-MEL-37 cells were used as positive control. Error bars indicate standard deviation in duplicate wells. Results were representative of at least three independent reproducible experiments.
We also examined the HLA class II presentation pathway in LCs and LCs/IFN-γ fed with NY-ESO-1 protein or IC, by using a CD4+ T cell line specific for NY-ESO-1 peptide 115-132 presented by HLA-DR7 (23). We found that histocompatible LCs and LCs/IFN-γ were equally capable of presenting not only NY-ESO-1 IC but also free protein (data not shown). As controls, NY-ESO-1 short truncated protein and MAGE-3, or their counterpart IC, did not sensitize the NY-ESO-1 specific CD4 line (data not shown).
These results indicated that conventional antigen-processing pathways, i.e., MHC class I presentation from endogenous antigen and MHC class II presentation from exogenous antigen, were intact in both LCs and LCs/IFN-γ. Taken together, it suggested that LCs were impaired only for cross-presentation pathway of MHC class I presentation from exogenous antigen. The defect likely occurred after antigen uptake but before proteasome processing, and treatment of LCs with IFN-γ enables a mechanism to acquire cross-presentation capacity.
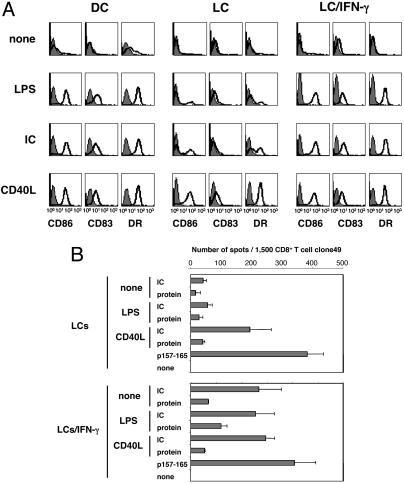
Susceptibility to Maturation Signal of LCs and LCs/IFN-γ. We next investigated responses to maturation signals such as IC, LPS, or CD40L on mo-DCs, LCs, and LCs/IFN-γ. In Fig. 7A, mo-DCs up-regulated CD83, CD86, and HLA-DR expression after 48-h treatment with either IC or LPS. In contrast, LCs were resistant to maturation under the same conditions. Only CD40L could induce maturation on all cell types. If treated with IFN-γ, however, LCs became susceptible to maturation not only by LPS but also by IC, even though IFN-γ treatment by itself did not induce maturation (Fig. 2). These results suggested that resistance of LCs to certain maturation signals could be overcome by treatment with IFN-γ. To investigate whether maturation is directly linked with the capacity to cross-present exogenous antigen, LCs were exposed to NY-ESO-1 IC in the presence of CD40L and assayed with clone49 by ELISPOT (Fig. 7B). Remarkably, CD40L treatment enabled cross-presentation of NY-ESO-1 IC by LCs in the absence of IFN-γ, linking the cross-presentation and maturation processes together.
Fig. 7.
Susceptibility of mo-DCs, LCs, and LCs/IFN-γ to various maturation signals. (A) Cells were harvested at day 6 and treated with LPS (100 ng/ml), NY-ESO-1 IC (10 μg/ml protein, 5 μg/ml specific mAb), or CD40L (1 μg/ml and enhancer) for 2 days, then stained for CD86, CD83, and HLA-DR expression and analyzed by FACS. (B) LCs were incubated with NY-ESO-1 IC (25 μg/ml protein and 5 μg/ml mAb preincubated for 30 min), protein alone (25 μg/ml) in the presence of LPS (100 ng/ml) and CD40L (1 μg/ml and enhancer) or not, or synthetic peptide p157-165 (10 μM) for 7 h at 37°C. ELISPOT was performed by using clone49. Error bars indicate standard deviation in duplicate wells. Results were representative of at least three independent reproducible experiments.
Discussion
Previously, we showed that monocyte-derived DCs efficiently presented antigenic epitopes derived from exogenous NY-ESO-1 IC by means of a proteasome-dependent pathway (6). Treating DCs with anti-CD32 (FcγRII) monoclonal antibody inhibited their antigen presentation partially. Moreover, DCs exposed to NY-ESO-1 IC formed with a Fab fragment of anti-NY-ESO-1 IgG1 did not sensitize specific cytotoxic T lymphocyte (CTL) clones, suggesting that Fc portions of antibodies are critical for IC uptake by DCs. In the present study, we analyzed LCs differentiated from monocytes by using IL-4, GM-CSF, and transforming growth factor-β1, and they exhibited a similar phenotype to DCs except for lower CD32 expression levels. We observed that LCs had a poor cross-presentation capacity, and we predicted this result could be due to lack of Fcγ receptor in addition to low capacity of fluid phase endocytosis (Fig. 5 and data not shown). Remarkably, treatment for 24 h with IFN-γ caused LCs to acquire the ability to cross-present exogenous antigen. Two questions arose from these results. One was whether IFN-γ treatment affected the capacity of LCs to internalize IC in a quantitative or qualitative manner. The other was whether LCs/IFN-γ uptake of IC still occurred by means of Fcγ receptors despite poor expression levels. We found that the quantitative endocytic capacity of LCs, as measured by uptake of fluorescent IC, was not up-regulated significantly by IFN-γ treatment. We thus favor the explanation that a qualitative difference in endocytic capacity occurs, potentially targeting Fcγ receptor-mediated uptake pathways, even though IFN-γ treatment of LCs up-regulated Fcγ receptor surface expression by only 2% (data not shown). Still, LCs/IFN-γ exposed to NY-ESO-1 IC formed with Fab fragment of anti-NY-ESO-1 IgG1 or with monoclonal anti-NY-ESO-1 IgM did not sensitize clone49 (data not shown). Even though LCs/IFN-γ do not significantly differ from LCs for uptake capacity, they seem to make better use of antigen derived from IC once internalized.
We tried to assess differences in routing of internalized antigen to the processing machinery between LCs and LCs/IFN-γ. Cross-presentation by LCs/IFN-γ incubated with NY-ESO-1 IC was inhibited by lactacystin, suggesting a proteasome-dependent mechanism as has been found in other examples of cross-presentation. In this case, a fraction of antigen proteins internalized by LCs/IFN-γ likely must exit or be translocated from endosome compartments to the cytosol by an unknown mechanism. Both LCs and LCs/IFN-γ retained the function to present antigen from cytosol to class I by means of a proteasome-dependent pathway because virus recombinant with NY-ESO-1 could be presented effectively. Virus infection by using vaccinia, fowlpox, or adenovirus recombinant for NY-ESO-1 did not lead to induction of maturation for mo-DCs, LCs, and LCs/IFN-γ (data not shown). A conventional class II presentation pathway was also preserved in both cell types. Taken together, these findings suggest that treatment with IFN-γ potentiated cross-presentation in LCs due to qualitative enhancement of IC trafficking from membrane to cytosol. One level of action could be a recently proposed model of fused endoplasmic reticulum-phagosome compartments in cross-presenting cells, provided these compartments are not constitutively expressed in LCs (11, 12).
We also indicated that LCs/IFN-γ became susceptible to maturation signals, such as LPS and IC, which did not induce maturation in LCs. We tried to assess whether induction of maturation itself was directly linked with capacity of cross-presentation, because it has been shown that presentation of exogenous antigens by MHC class I pathway is subject to developmental control by inducing maturation by CD40L or disruption of cell-cell contact but not by LPS in mouse bone marrow-derived DCs (26). We investigated the cross-presentation of antigen in LCs exposed to IC, simultaneously treated with CD40L, which is one of the only signals to induce LCs maturation effectively. We observed an enhancement in the capacity of LCs to cross-present IC when maturation was forced by CD40 ligation. Because LCs treated with IFN-γ were susceptible to maturation by IC treatment alone, the link between maturation and cross-presentation would explain that IFN-γ enabled cross-presentation by LCs at least in part by allowing cellular maturation changes. Thus, our monocyte-derived human antigen presentation system argues for facilitated cross-presentation when maturation is conditionally enabled by IFN-γ. It will be interesting to correlate these findings with protein vaccination studies targeting dermal Langerhans cells, and ask whether local coadministration of IFN-γ may enhance in vivo cross-presentation.
Additional hypotheses will have to be tested to unravel the mechanism of cross-presentation, particularly the potential involvement of protein ubiquitination after IC internalization. It has been shown that DCs exposed to maturation signals displayed transient aggregation of ubiquitinated proteins called DALIS (DC aggresome-like inducible structures), which are most likely derived from newly synthesized proteins such as DRiPs (defective ribosomal products) (27, 28). It will be interesting to test whether internalized proteins derived from exogenous IC can form DALIS-like structures in matured cells, and whether they might be involved in cross-presentation.
Previously, we showed that NY-ESO-1 IC were not efficiently cross-presented by CD34-derived DCs despite the fact that CD32 was expressed moderately in these cells (6). Preliminary data indicate that treatment of CD34-derived DCs with IFN-γ does not enhance cross-presentation, in contrast with its effect on LCs (data not shown). Importantly, CD34-derived DCs have been shown to have phenotypic characteristics of LCs, but they seem more committed and less susceptible to IFN-γ. Given the very low capacity of CD34-derived DCs to take up exogenous soluble antigen, it is also possible that the threshold of internalized antigen needed to stimulate clone49 cells is not reached, independently of IFN-γ treatment, although this finding remains to be confirmed.
NY-ESO-1, a cancer-testis antigen, was originally discovered by its capacity to induce a humoral response (29). Both CD8+ (30) and CD4+ T cell (23) responses against NY-ESO-1 spontaneously occur in cancer patients, and they strongly correlate with the presence of NY-ESO-1-specific antibodies in patient sera. As reported previously, epitopes from NY-ESO-1 recognized by specific CD4+ and CD8+ T cells have been identified (16-18, 23, 31-34). When mapping the distribution of epitopes along the NY-ESO-1 sequence, there are large overlaps between CD4+ and CD8+ T cell-recognized peptides (23), which may mirror the linked destiny of internalized protein antigens in endosome, some being processed into long peptides loaded onto class II, other being transported to cytosol, processed with proteasome, and present onto class I in DCs. The presence of concomitant antibody to NY-ESO-1 suggests that priming NY-ESO-1-specific T cells in naturally immunized patient could involve cross-presentation mechanisms in vivo.
Acknowledgments
We thank the Cancer Research Institute for its support.
Abbreviations: DC, dendritic cell; mo-DC, monocyte-derived DC; IC, immune complex; LC, Langerhans-like cell; GM-CSF, granulocyte-macrophage colony-stimulating factor; elispot, enzyme-linked immunospot; FACS, fluorescence-activated cell sorter; LPS, lipopolysaccharide.
References
- 1.Mellman, I. & Steinman, R. M. (2001) Cell 106, 255-258. [DOI] [PubMed] [Google Scholar]
- 2.Guermonprez, P., Valladeau, J., Zitvogel, L., Thery, C. & Amigorena, S. (2002) Annu. Rev. Immunol. 20, 621-667. [DOI] [PubMed] [Google Scholar]
- 3.Norbury, C. C., Chambers, B. J., Prescott, A. R., Ljunggren, H. G. & Watts, C. (1997) Eur. J. Immunol. 27, 280-288. [DOI] [PubMed] [Google Scholar]
- 4.Brossart, P. & Bevan, M. J. (1997) Blood 90, 1594-1599. [PMC free article] [PubMed] [Google Scholar]
- 5.Regnault, A., Lankar, D., Lacabanne, V., Rodriguez, A., Thery, C., Rescigno, M., Saito, T., Verbeek, S., Bonnerot, C., Ricciardi-Castagnoli, P. & Amigorena, S. (1999) J. Exp. Med. 189, 371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagata, Y., Ono, S., Matsuo, M., Gnjatic, S., Valmori, D., Ritter, G., Garrett, W., Old, L. J. & Mellman, I. (2002) Proc. Natl. Acad. Sci. USA 99, 10629-10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amigorena, S. (2002) J. Exp. Med. 195, F1-F3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rafiq, K., Bergtold, A. & Clynes, R. (2002) J. Clin. Invest. 110, 71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez, A., Regnault, A., Kleijmeer, M., Ricciardi-Castagnoli, P. & Amigorena, S. (1999) Nat. Cell Biol. 1, 362-368. [DOI] [PubMed] [Google Scholar]
- 10.Fonteneau, J. F., Kavanagh, D. G., Lirvall, M., Sanders, C., Cover, T. L., Bhardwaj, N. & Larsson, M. (2003) Blood 102, 4448-4455. [DOI] [PubMed] [Google Scholar]
- 11.Guermonprez, P., Saveanu, L., Kleijmeer, M., Davoust, J., Van Endert, P. & Amigorena, S. (2003) Nature 425, 397-402. [DOI] [PubMed] [Google Scholar]
- 12.Houde, M., Bertholet, S., Gagnon, E., Brunet, S., Goyette, G., Laplante, A., Princiotta, M. F., Thibault, P., Sacks, D. & Desjardins, M. (2003) Nature 425, 402-406. [DOI] [PubMed] [Google Scholar]
- 13.Berlyn, K. A., Schultes, B., Leveugle, B., Noujaim, A. A., Alexander, R. B. & Mann, D. L. (2001) Clin. Immunol. 101, 276-283. [DOI] [PubMed] [Google Scholar]
- 14.Geissmann, F., Prost, C., Monnet, J. P., Dy, M., Brousse, N. & Hermine, O. (1998) J. Exp. Med. 187, 961-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geissmann, F., Revy, P., Regnault, A., Lepelletier, Y., Dy, M., Brousse, N., Amigorena, S., Hermine, O. & Durandy, A. (1999) J. Immunol. 162, 4567-4575. [PubMed] [Google Scholar]
- 16.Jäger, E., Chen, Y.-T., Drijfhout, J. W., Karbach, J., Ringhoffer, M., Jäger, D., Arand, M., Wada, H., Noguchi, Y., Stockert, E., et al. (1998) J. Exp. Med. 187, 265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jäger, E., Jäger, D., Karbach, J., Chen, Y.-T., Ritter, G., Nagata, Y., Gnjatic, S., Stockert, E., Arand, M., Old, L. J. & Knuth, A. (2000) J. Exp. Med. 191, 625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gnjatic, S., Nagata, Y., Jäger, E., Stockert, E., Shankara, S., Roberts, B. L., Mazzara, G. P., Lee, S. Y., Dunbar, P. R., Dupont, B., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 10917-10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stockert, E., Jäger, E., Chen, Y.-T., Scanlan, M. J., Gout, I., Karbach, J., Arand, M., Knuth, A. & Old, L. J. (1998) J. Exp. Med. 187, 1349-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Doherty, U., Steinman, R. M., Peng, M., Cameron, P. U., Gezelter, S., Kopeloff, I., Swiggard, W. J., Pope, M. & Bhardwaj, N. (1993) J. Exp. Med. 178, 1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams, J. C. (1981) J. Histochem. Cytochem. 29, 775. [DOI] [PubMed] [Google Scholar]
- 22.Valmori, D., Dutoit, V., Liénard, D., Rimoldi, D., Pittet, M. J., Champagne, P., Ellefsen, K., Sahin, U., Speiser, D., Lejeune, F., et al. (2000) Cancer Res. 60, 4499-4506. [PubMed] [Google Scholar]
- 23.Gnjatic, S., Atanackovic, D., Jäger, E., Matsuo, M., Selvakumar, A., Altorki, N. K., Maki, R. G., Dupont, B., Ritter, G., Chen, Y. T., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 8862-8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallusto, F., Cella, M., Danieli, C. & Lanzavecchia, A. (1995) J. Exp. Med. 182, 389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjerknes, R. & Bassoe, C. F. (1984) Blut 49, 315-323. [DOI] [PubMed] [Google Scholar]
- 26.Delamarre, L., Holcombe, H. & Mellman, I. (2003) J. Exp. Med. 198, 111-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lelouard, H., Gatti, E., Cappello, F., Gresser, O., Camosseto, V. & Pierre, P. (2002) Nature 417, 177-182. [DOI] [PubMed] [Google Scholar]
- 28.Schild, H. & Rammensee, H. G. (2000) Nature 404, 709-710. [DOI] [PubMed] [Google Scholar]
- 29.Chen, Y.-T., Scanlan, M. J., Sahin, U., Türeci, Ö., Güre, A. O., Tsang, S., Williamson, B., Stockert, E., Pfreundschuh, M. & Old, L. J. (1997) Proc. Natl. Acad. Sci. USA 94, 1914-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jäger, E., Nagata, Y., Gnjatic, S., Wada, H., Stockert, E., Karbach, J., Dunbar, P. R., Lee, S. Y., Jungbluth, A., Jäger, D., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 4760-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, R.-F., Robbins, P. F., Kawakami, Y., Kang, X.-Q. & Rosenberg, S. A. (1995) J. Exp. Med. 181, 799-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarour, H. M., Storkus, W. J., Brusic, V., Williams, E. & Kirkwood, J. M. (2000) Cancer Res. 60, 4946-4952. [PubMed] [Google Scholar]
- 33.Zeng, G., Wang, X., Robbins, P. F., Rosenberg, S. A. & Wang, R. F. (2001) Proc. Natl. Acad. Sci. USA 98, 3964-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jäger, E., Karbach, J., Gnjatic, S., Jäger, D., Maeurer, M., Atmaca, A., Arand, M., Skipper, J., Stockert, E., Chen, Y.-T., et al. (2002) Cancer Immun. 2, 12. [PubMed] [Google Scholar]