Abstract
Autoimmune T and B cell responses to CNS antigen(s) are thought to drive the pathogenesis of multiple sclerosis (MS), and thus are logical targets for therapy. Indeed, several immunomodulatory agents, including IFN-β1b, IFN-β1a, glatiramer acetate, and mitoxantrone, have had beneficial clinical effects in different forms of MS. However, because the available treatments are only partially effective, MS therapy needs to be further improved. Selective (antigen-specific) immunotherapies are especially appealing because in theory they combine maximal efficacy with minimal side effects. Indeed, several innovative immunotherapies have been successfully applied in experimental autoimmune encephalomyelitis. For example, autoreactive T cells can be selectively targeted by means of antigen, T cell receptor, or activation markers. However, experimental autoimmune encephalomyelitis is far from being a perfect approximation of MS because MS is more heterogeneous and the target antigen(s) is (are) not known. Further advances in MS therapy will depend on our growing understanding of the pathogenesis of this still incurable disease.
Multiple sclerosis (MS) is one of the most common neurological diseases of young adults in Europe and North America (1). Worldwide, ≈1 million people are afflicted by this chronic inflammatory disease of the CNS. Histological hall-marks of active MS include infiltrations of T cells, macrophages, and B cells, degradation of myelin, and, to a lesser extent, axons, and reactive changes of astrocytes and microglia (2). It is usually assumed that these inflammatory changes reflect an autoimmune attack against myelin components. Other perhaps less likely possibilities are that the inflammatory reaction is primarily directed against an unknown infectious agent (3, 4), or that the inflammatory changes are secondary to a primary degenerative process.
In the following, we will broadly trace the steps by which the pipe dream of selective immunotherapy might become a reality: beginning with experimental autoimmune encephalomyelitis (EAE), confronting the obstacles in the search for a target antigen of MS, and concluding with the selective immunotherapies currently being explored.
MS and EAE
The current concepts of MS as an autoimmune disease are based on direct studies of MS lesions and animal models of inflammatory demyelination. Among these disease models, EAE is one of the most widely studied autoimmune disease paradigms. This research has provided profound insights into not only autoimmune but also basic immunological mechanisms (5). EAE can be induced in many species by active immunization with myelin antigens. The classic encephalitogenic antigen is myelin basic protein (MBP), the first to be identified. Later, other myelin and non-myelin antigens were also shown to induce EAE [e.g., proteolipid protein, myelin oligodendrocyte glycoprotein (MOG), and S-100β].
Particularly striking was the observation that the transfer of purified, activated MBP-specific CD4+ T cells into healthy syngeneic animals can induce EAE in the recipients (“transfer EAE”) (6). This study was the first formal demonstration that an autoaggressive T cell is sufficient to launch an organ-specific autoimmune disease. Subsequent studies revealed that injection of autoantibodies against a surface-exposed myelin antigen, MOG, can amplify the T cell attack by inducing large-scale demyelination (7, 8). More recent work has revealed that not only CD4+ myelin-specific T cells but also CD8+ T cells have encephalitogenic potential (9, 10).
Whereas EAE is considered useful, it is far from the ideal model for investigating novel therapies (5, 11). Many ingenious therapeutic strategies were first developed and successfully tested in EAE, but several therapies that had certain beneficial effects in EAE-exacerbated MS (e.g., inhibitors of tumor necrosis factor-α; ref. 12). So far, only a few of the therapies that were successful in EAE could be shown to be efficacious in human MS (13, 14). This finding holds particularly true for antigen- and antigen receptor-selective therapies. Likely reasons are that the inciting antigen(s) are not known in individual cases of MS, that the B and T cell receptor (TCR) repertoire of the participating inflammatory cells is more diverse in MS than it is in certain EAE models, and that MS is heterogeneous.
MS Heterogeneity and the Search for Target Antigen(s)
The search for a target antigen of MS is hindered by the fact that human MS is very heterogeneous. Not only is its clinical course and presentation extremely diverse, but there is increasing evidence of pathogenetic heterogeneity as well. Recently, criteria for the histological classification of MS subtypes have been developed. Lassmann et al. (2) have proposed four basic types of lesions: macrophage-mediated demyelination (type I), antibody-mediated demyelination (type II), distal oligodendrogliopathy (type III), and demyelination secondary to oligodendrocyte damage (type IV) (2). These patterns were originally distinguished in a cross-sectional study of biopsy specimens from MS patients who had an unusual clinical presentation that required biopsy for diagnostic clarification (15).
Two principal approaches have been taken to search for the target (auto)antigens of MS. One method is to study human B and T cell responses directed against established antigens like MBP, MOG, or proteolipid protein, i.e., antigens known to be capable of inducing EAE. The other approach is to investigate the repertoire of the antigen receptors expressed by human B and T cells. In contrast to the first approach, the second strategy does not depend on a priori knowledge of the antigen (16). Neither of these strategies has yet led to unequivocal identification of the target antigen(s) of MS.
Current Knowns and Unknowns About CNS Antigen-Specific Immune Cell Responses
Myelin-Specific T Cells Are Constitutive Components of the Healthy Immune System. Classic experiments demonstrated that MBP-specific T cells from immunized donors are capable of transferring EAE in the Lewis rat (6). Subsequently, MBP-specific T cells were isolated in vitro, even from unmanipulated, naïve rats, and these T cells could also transfer EAE to syngeneic naïve recipients (17). These findings were an early demonstration that autoreactive T cells are not generated de novo after immunization in vivo, but they already exist as preformed elements in the healthy immune system.
These studies raised the question of whether myelin-autoreactive T cells are also present in the human immune repertoire, and if so, why they seem to remain innocuous in healthy people but become encephalitogenic in MS patients. The “split-well” cloning technique has allowed the isolation of myelin autoantigen-specific T cells from MS patients and normal subjects (18, 19). Stimulation of blood T cells with MBP in vitro leads to the proliferation of MBP-specific CD4+ T cells, which, in humans, recognize different epitope clusters spread along the entire length of the MBP molecule (18, 20–24). In contrast, in several rodent models the MBP-specific T cell response is strikingly focused on only one immunodominant epitope (25). Also, in humans, some MBP epitopes are recognized more frequently than others, both at the population level and in individual subjects. These relatively immunodominant epitopes include region MBP 85–99, which is presented in the context of the HLA allele DRB1*1501, and is associated with MS. It should be noted, however, that the human T cell response to MBP region 85–99 shows remarkable microheterogeneity, both in terms of fine epitope specificity and TCR use (23).
MBP and Other Autoantigens Have Pathogenic Potential in Humans.
MBP-specific T cells are autoreactive, but can they become pathogenic? The only way to formally demonstrate the encephalitogenic potential of human myelin-specific T cells in healthy people is to adoptively transfer these cells into an autologous recipient, on analogy to the pioneering experiment in the Lewis rat (6). Although it is impossible to do such transfer experiments for ethical reasons, several indirect arguments strongly suggest that human MBP-specific CD4+ T cells are indeed potentially pathogenic.
First, the split-well technique allowed the isolation of MBP-specific T cells from outbred primates. The immune system of rhesus monkeys is very similar to the human immune system, and even shares similar molecular structures (26). MBP-specific T cells from rhesus monkeys recognize different epitopes of MBP, as do their human counterparts (27). The encephalitogenic potential of the rhesus cells was directly demonstrated by autologous transfer. Similar results were reported by the group of Genain et al. (28) who isolated MBP- and MOG (29)-specific T cells from the peripheral blood of healthy, nonimmunized marmosets, and adoptively transferred EAE to a chimeric twin. Because the in vitro conditions in these experiments were the same as in the experiments with human T cells, it is reasonable to extrapolate the results to humans.
A second line of evidence rests on experiments with “humanized” transgenic mice. Fugger and coworkers (30) were the first to express three human components involved in T cell recognition of MBP in transgenic mice: the appropriate DRA*0101/DRB1*1501 (HLA-DR2) MHC restriction molecule, a TCR specific for the HLA-DR2-bound MBP 84–102 peptide, and the human CD4 coreceptor. When the transgenic mice were backcrossed to recombination activating gene-deficient mice, the incidence of spontaneous disease drastically increased. These experiments clearly demonstrate that T cells specific for the HLA-DR2-bound MBP peptide can induce disease. They also suggest these cells are normally held in check by regulatory T cells, which are absent in the recombination activating gene-deficient mice.
A third argument comes from observations in MS patients. In a phase II clinical trial, an altered peptide ligand (APL) of MBP seemed to exacerbate the disease in a small number of MS patients. This finding was accompanied by a strong cross-reactive T cell response against MBP83–99, which, in one patient, was restricted to HLA-DRB1*0404 (31). It is therefore likely that APL treatment in these patients stimulated a cross-reactive pathogenic anti-MBP response, thus providing further indirect evidence of the pathogenic potential of MBP-specific T cells in humans.
Suggestive as these findings may be, they do not prove that MBP or any other CNS autoantigen acts as the “antigenic target” of MS. Various B and T cell responses to CNS autoantigens have been described in MS patients, but none of the many candidate antigens could be pinpointed as the target of the autoimmune response (32). For example, a number of reports have described subtle changes of various properties of myelin-specific T cells in MS patients, including IL-2 responsiveness (33), hypoxanthine-guanine phosphoribosyltransferase resistance (34), (co)stimulation requirements (35–37), IL-7 sensitivity (38), and avidity for antigen (39). So far, none has been sufficiently reproducible to become generally accepted.
The difficulties in establishing the target antigens of MS are not surprising, in view of the heterogeneity of the disease. Another obstacle is the dynamic nature of the autoimmune response. In rodents the response can spread to different antigens (40). It is difficult to prove that such “epitope spreading” actually occurs in human MS. If it did occur, it would make the identification of the initial triggering antigen very difficult, if not impossible, because even patients who present with their first clinical symptoms usually have had subclinical disease for many years.
The Triggering Process of MS Is Still Unknown. Self-tolerance develops and is maintained by several complementary mechanisms, including clonal deletion, anergy, ignorance, and active suppression. At least in theory, failure of any of these mechanisms, e.g., tolerogenic display of autoantigens in the thymus regulated by the autoimmune regulator gene (41), can result in autoimmune disease.
One way to break tolerance is by “antigenic mimicry” (42). Originally, the mimicry concept referred to contiguous sequence identities between peptides from microbes and autoantigens. Subsequently, the concept has been extended to structural resemblance between different peptide–MHC complexes. For example, one human MBP-specific TCR can recognize two completely different peptides: one derived from MBP (bound to HLA-DR2b) and one from the Epstein–Barr virus (bound to HLA-DR2a; ref. 43). In this case, molecular mimicry involves not only two distinct peptides but also two different HLA-DR molecules. Mimicry reactions are favored by the “degeneracy” of T cell recognition (44). Furthermore, autoreactive T cells could be stimulated by microbial “superantigens” or by antigen-nonspecific proinflammatory factors of the innate immune system, which are abundantly expressed in the inflammatory milieu.
Another mechanism causing loss of tolerance is the weakening of the regulatory mechanisms that normally help to keep the autoreactive T cells in check. Cellular suppressor mechanisms are still poorly understood. More recent evidence indicates that distinct populations of CD4+CD25+, CD4+CD25-, and CD8+ regulatory T cells contribute to the maintenance of self-tolerance, and that the function of CD4+CD25+ regulatory T cells might indeed be disturbed in MS (45). The precise mechanisms of regulatory cell function and dysfunction have yet to be defined (46–48).
Very recently, a different line of evidence has provided support for the possibility that the inflammatory, presumably autoimmune reaction might by secondary to some form of degenerative or infectious injury. At least in some MS cases and lesions, oligodendrocyte apoptosis seems to precede leukocyte infiltration and demyelination (49, 50). The cause of these oligodendrocyte changes, which have not been observed in EAE models, is unknown.
Techniques for Finding the Target Antigen(s) of MS
A number of approaches have emerged for the identification and functional reconstruction of pathogenic T cells directly from biopsy or autopsy tissue (16). Oksenberg et al. (51, 52) were the first to use PCR techniques to demonstrate expanded TCR V-α (51) and V-β chain (52) sequences in CNS tissue homogenates of MS patients. Intriguingly, one TCR V-β complementarity-determining region (CDR)-3 motif was identical to that of a described MBP-specific human T cell clone (52).
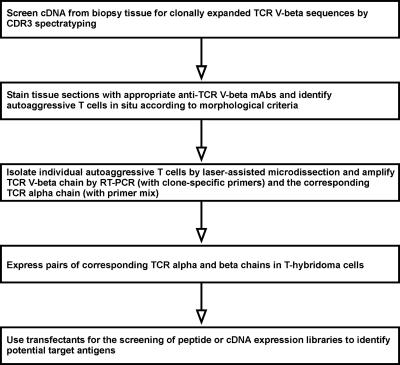
However, for the full characterization of a TCR, it is necessary to identify its paired α and β chains. This goal can only be achieved with single-cell techniques, as they were pioneered by Küppers and Rajewsky and coworkers (164) for studying B cell development and lymphomas. One approach developed by several groups, including our own, is shown in Fig. 1 (16).
Fig. 1.
Proposed strategy for the identification of the unknown targets antigens of MS, starting with the characterization of the antigen-specific TCRs expressed by autoaggressive T cell infiltrates in biopsy tissue (16).
So far, the whole series of steps, beginning with the patient's biopsy and ending with the clear-cut identification of the target antigen, has not yet been achieved. In particular, it has been very difficult to coamplify both TCR chains from single T cells. However, the application of single-cell PCR for detailed analysis of the TCR Vβ repertoire in MS lesions has not only been feasible, but has revealed intriguing insights. For example, we identified several CD8+ (but not CD4+) expanded T cell clones, which were originally present in brain biopsy tissue (53) and subsequently persisted for many years in the blood and cerebrospinal fluid of the same MS patient (54). Some of the expanded CD8+ T cells showed markers of recent activation. These observations would be consistent with a pathogenic role played by the expanded CD8+ T cells (55, 56). It would be interesting to identify the pairing α chains from these T cell clones. If the full TCR were reconstructed, it could be used in the search for the target antigen.
Similar strategies can be applied for the analysis of B cells. Increased synthesis of intrathecal IgG and its distribution as “oligoclonal bands” have long been recognized as diagnostic hallmarks of MS. However, the antigen specificity of these oligoclonal bands has remained a mystery (57). Several studies (58–61) have demonstrated clonally expanded B cells in the CNS and cerebrospinal fluid of MS patients, and this result has been confirmed by single-cell repertoire analysis (62). Similar strategies as described above for T cell repertoires can be used for the identification of the pairing light and heavy Ig chains from individual infiltrating B cells. The pairing chains can be incorporated into recombinant antibodies that can be used in the search for target antigens. This search will be facilitated by progress in “proteomics.” For example, Steinman and colleagues (63, 64) have applied miniaturized autoantigen arrays for large-scale multiplex characterization of autoantibody responses directed against diverse autoantigens. At the present time, these protein arrays are best suited for the detection of antibodies directed against conformation-independent epitopes, but future progress in this area will help to identify new antibody targets.
MS Immunotherapy Today
Our expanding knowledge of the immunopathogenesis of MS has led to several therapeutic advances, which, have over the past decade, drastically changed the therapy for MS. Several new disease-modifying agents, including IFN-β1a, IFN-β1b, glatiramer acetate (GA), and mitoxantrone, have been approved and are now being widely used (65–67). It is interesting to note that the methodology and design of MS trials has evolved in parallel with the therapeutic agents. In particular, increasingly sophisticated MRI techniques are used to great advantage for the identification and quantification of CNS lesions (68, 69).
Despite this growing number of available agents, however, it is clear that none of the existing therapies can stop the disease process. Therefore, the search for more efficient therapies continues (13, 14, 70). The list of planned, on-going, or recently completed clinical MS trials (published and regularly updated by the National Multiple Sclerosis Society, which can be accessed at: www.nationalmssociety.org) currently contains >150 entries. In addition, many potentially promising new agents are being investigated in preclinical studies. These agents can be broadly divided into nonselective (antigen-nonspecific) and selective (antigen-specific) approaches (Tables 1 and 2).
Table 1. Antigen-nonselective candidate agents for the immunomodulatory treatment of MS.
| Class | Agents |
|---|---|
| Cytotoxic agents and procedures | Azathioprine; mycophenolate mofetil; cyclophosphamide; mitoxantrone; methotrexate; linomide derivative (Laquinimod); immunosuppressive fungal macrolides and cyclic peptides (e.g., Cyclosporin-A, Tacrolimus, Sirolimus, and CCI-779); immunoablation, followed by hematopoetic stem cell transplantation. |
| Immunosuppressive mAbs | Anti-CD3; anti-CD4; anti-CD52 (Campath-1H and Alemtuzumab); anti-IL-2 receptor α-subunit (Daclizumab and Basiliximab); anti-CD20 (Rituximab). |
| Cytokines and cytokine inhibitors | IFNs (IFN β-1a, IFN β-1b, IFN-α, and IFN-τ); [tumor necrosis factor (TNF) inhibitors (TNF-α receptor-IgG soluble dimeric p-55 (LenerceptR)]; anti-TNF-α human/murine chimeric mAb cA2; metalloprotease inhibitors; down-modulatory cytokines [IL-1 inhibitors: IL-4, IL-10, and IL-13; TGF-β2 (BetaKine)]; antibodies against proinflammatory cytokines (e.g., anti-IL-12p40). |
| Chemokine antagonists and receptor blockers | MCP-1 receptor antagonist; CXCR3 antagonist; CCR1 antagonist; CCR5 antagonist. |
| Therapies directed at cell adhesion and costimulatory molecules | Humanized anti-CD11/CD18 mAb (Hu23F2G); small-molecule inhibitors of integrins; anti-α4 integrin mAb (Natalizumab); anti-ICAM-1 (CD54) mAb; anti-CD2 mAb; anti-LFA-3 (CD58) mAb; anti-CD154 mAb; CTLA4-Ig; anti-CD45 mAb. |
| Others | Plasmapheresis; intravenous immunoglobulins; statins; sphingosine-1-phosphate receptor agonist (FTY720). |
Table 2. Immunomodulatory therapies targeting the trimolecular complex.
| Therapies targeting antigen/MHC
| ||||||
|---|---|---|---|---|---|---|
| Agent | Study design | Type of MS | No. of subjects | Duration | Status of trial (as of 2004) | Ref(s). |
| Oral tolerance (bovine myelin) | Randomized, double-blinded | RR | 30 | 1 year | Completed | 110 |
| Intravenous infusion of synthetic MBP peptide (regions 82-98 and 85-96) | Double-blinded, placebo-controlled | SP, PP | 32 | 2 years | Completed | 152, 153 |
| Solubilized DR2:MBP84-102 complex (AG284) | Randomized, double-blinded, placebo-controlled | SP | 33 | 84 days | Completed | 154 |
| NBI-5788 (altered peptide ligand) | Randomized, double-blinded, placebo-controlled | RR, SP | 150 | 46 weeks | Ongoing | 31, 75–77, 155 |
| GA* plus natalizumab | Randomized, double-blinded, placebo-controlled | RR | 110 | 28 weeks | Ongoing | † |
| GA* vs. IFNβ-1b | Randomized, rater-blinded | RR | 110 | 1 year | Ongoing | † |
| GA* | Randomized, double-blinded, placebo-controlled | PP | 900 | 3 years | Terminated | † |
| GA* (oral) | Randomized, double-blinded, placebo-controlled | RR | 1300 | 56 weeks | Terminated | † |
| GA* plus albuterol | Randomized, double-blinded, placebo-controlled | RR | 40 | 2 years | Ongoing | † |
| GA* plus mitoxantrone | Open label | RR, SP | 50 | 12 months | Completed | † |
| GA* vs. different inerteron-β peparations | Prospective, controlled, open label | RR | 250 | Ongoing | † | |
| Therapies targeting TCR | ||||||
| Humanized mAb (ATM-027) against TCR V-β5.2/5.3 | Double-blinded, placebo-controlled | RR | 50 | 16 months | Completed | 137 |
| Vaccination with TCR Vβ6 CDR2 peptide | Open label (phase I) | RR, CP | 10 (pre-screened for BV6 over-expression) | 24 weeks | Completed | 156, 157 |
| Vaccination with TCR peptide (CDR2 and BV6S2/6S5) | Open label (phase I) | RR, CP | 10 (not pre-screened for BV6 over-expression) | 48 weeks | Completed | 136 |
| Vaccination with three TCR peptides (BV5S2, BV6S5, and BV13S1) NeuroVax | Partially blinded | RR, SP | 20 (interim analysis) | 24 weeks | Interim analysis completed | 150 |
| T cell vaccination (autologous MBP-specific T cells) | Open label | RR, CP | 8 | 2–5 years | Completed | 158, 159, 160 |
| T cell vaccination (autologous MBP-specific T cells) | Open label (extended phase I) | RR, CP | 49 | variable | Completed | 161 |
| T cell vaccination (autologous irradiated MBP-specific T cells) | Open label | RR, SP | 54 | 24 months | Completed | 122 |
| T cell vaccination | Double-blinded | RR | 30 | 1 year | Ongoing | † |
| T cell vaccination (T cells specific for MBP, PLP, or MOG synthetic peptides) | Randomized, double-blinded, placebo-controlled | Probable MS, after first attack | 76 | Ongoing | 151 | |
| T cell vaccination (IL-2-responsive CD4+ T cells from CSF) | Double-blinded, placebo-controlled | RR | 60 | 18 months | Ongoing | 123 |
| T cell vaccination (whole-myelin-reactive T cells) | Double-blinded, placebo-controlled (phase II) | SP | 80 | 3 years | Ongoing | 162 |
| T cell vaccination (MBP-specific autologous T cells) | Open label | RR | 18 | 24 months | Ongoing | † |
List of recently completed and ongoing therapeutic trials of selective immunotherapies in multiple sclerosis. RR, relapsing-remitting; SP, secondary progressive; PP, primary progressive; CP, chronic progressive.
For GA, only unpublished trials are shown
Based on the National Multiple Sclerosis Society list of clinical trials, which can be accessed at www.nationalmssociety.org; refs. 150 and 151
One principle emerging from our improved understanding of autoimmune mechanisms is that it is more promising to actively strengthen physiological counterregulatory mechanisms than to attempt to passively “delete” putative autoreactive cells from the immune repertoire. Many of the new therapeutic approaches can be viewed as “vaccination” strategies. Their most obvious target is the “immunological synapse” formed between a T cell and an antigen-presenting cell (APC) (71). Located in the center of this synapse, the trimolecular complex of antigen/MHC (on the APC) and the TCR (on the T cell), which confers antigen specificity, are therefore quite literally the “bull's-eye” of the target at which antigen-selective therapies must aim (72).
Putatively Selective Immunotherapies Already Tested or Currently Under Development
APLs. APLs are variant peptides that have been modified from an autoantigenic peptide in such a way that the original MHC-binding moieties are retained, but one or several of the TCR-binding amino acids have been changed (73). APLs can bind to TCRs without triggering the full program of T cell activation. For example, an APL may partially activate a T helper (TH) cell to produce IL-4 and help B cells, but fail to induce proliferation. Often, APL stimulation leads to a TH1-to-TH2 cytokine shift. Further, some APLs can induce a form of “anergy” in T cells (anergic T cells are unable to respond to stimulatory ligands). If APL therapy affected only T cells capable of reacting with the APL, then this strategy would most likely fail in human disease. However, APL treatment can have widespread effects by means of bystander suppression (74, 75).
Two phase II trials of an APL derived from an immunodominant peptide of MBP (MBP 83–99) were halted prematurely (31, 75, 76). In one of the trials, a high incidence of immediate-type hypersensitivity reactions occurred, mostly at higher dosage (76). Furthermore, a tendency to trigger exacerbations was observed in one of the two studies (31). These inadvertent results indicate that the APL can cross-stimulate encephalitogenic, MBP (83–99)-specific T cells in certain patients. These trials, however, also showed that APL treatment induces a shift of the T cell response from TH1 to TH2 (76, 77), which is reminiscent of the immunological effect of GA.
GA (Copolymer 1, Copaxone). GA is a synthetic random copolymer of the four amino acids l-glutamic acid, l-lysine, l-alanine, and l-tyrosine (78–80). After clinical trials had shown benefit (81–86), GA was approved for the treatment of relapsing remitting MS. The beneficial effects of GA were subsequently confirmed by MRI studies (87). Compared with the MRI-confirmed effects of IFN-β, those of GA are delayed (87). This delay appears to be consistent with the time course of the GA-induced immunological changes (88).
GA treatment induces a population of GA-reactive, TH2-type regulatory T cells (“GA-induced TH2 shift”; Fig. 2 and refs. 89–92). Because they are activated, the GA-reactive TH2-like T cells are able to cross the blood–brain barrier. Inside the CNS, they secrete their beneficial antiinflammatory cytokines, including IL-4, IL-6, and IL-10, and thereby create an “antiinflammatory milieu.” Production of the beneficial factors could occur spontaneously, or after cross-stimulation with products of myelin turnover (e.g., MBP and MOG) presented by local APCs. Subsequently, the local milieu is changed in such a way that the production of proinflammatory cytokines (e.g., IL-2 and IFN-γ) by other inflammatory cells is reduced by means of a suppressive bystander effect (93, 94). In addition, the GA-specific regulatory T cells might exert a neurotrophic effect, because GA-specific T cells are capable of producing brain-derived nerve growth factor (95, 96), and the corresponding tyrosine kinase receptor B is up-regulated in neurons in MS lesions (97, 98),
Fig. 2.
Proposed mechanism of action of GA, an approved agent for the immunomodulatory treatment of relapsing-remitting MS. See text for details.
The GA-induced TH2 shift might be explained at least partly by a direct action on APCs, especially dendritic cells and monocytes (99–102). After in vitro treatment with GA, dendritic cells have an impaired capacity to secrete TH1 polarizing factors, and therefore preferentially induce TH2 cells (100). Furthermore, GA treatment inhibits monocyte reactivity not only in vitro but also in vivo (101). In addition, there could be a positive feedback loop between the GA-reactive T cells and the APCs, because the TH2-polarized T cells can modulate the properties of the APC (103).
The T and B cell responses to GA treatment can be monitored with enzyme-linked immunospot (104) and ELISA (105, 106), respectively. In a pilot study involving a small number of MS patients, the immunological response to GA correlated well with the treatment response (107). The clinical value of these assays for monitoring the therapeutic response needs to be further investigated.
Tolerogenic Application of Autoantigen. The aim of several therapies is to induce antigen-specific tolerance by variously modifying the method of applying the antigen. For example, administration of autoantigens via the mucosal surfaces of the gut (“oral tolerance”) or respiratory tract (“nasal tolerance”) can efficiently suppress experimental autoimmune diseases (108, 109). Unfortunately, however, in MS the oral administration of myelin was ineffective, despite encouraging results of a small pilot trial (110).
An interesting emerging approach is the active induction of tolerance by “DNA vaccination.” For example, vaccination with DNA encoding an autoantigenic peptide of MBP or proteolipid protein can suppress EAE (111–113). It should be noted, however, that vaccination with a MOG-encoding DNA made the disease worse in another EAE model (114). An important advantage of DNA vaccination is that it permits easy modification of the therapeutic vaccine. For example, codelivery of the DNA vaccine and the IL-4 gene provided protective immunity against EAE (115). This approach combines the antigen-specific effects of DNA vaccination and the beneficial effects of local gene delivery.
T Cell and TCR Vaccination. The term T cell vaccination was coined in 1981 by Cohen and coworkers (116, 117). He reasoned that, as in microbiology, autoaggressive T cells can be attenuated to eliminate their pathogenic potential, while conserving their capacity to stimulate counterregulatory mechanisms. The original concept of T cell vaccination relies on the injection of autoantigen-specific T cell clones, which must be isolated from the prospective recipient, cultured, inactivated, and then reinjected as a vaccine to stimulate endogenous regulatory circuits (116–119). The regulatory mechanisms that are induced after T cell vaccination include TCR-specific CD8+ suppressor cells (120).
In pilot trials of vaccination with autologous MBP-specific T cells, the immunological and clinical response was promising, but rigorous proof of efficacy is still lacking (121, 122). Similarly, the results of a pilot vaccination trial with cerebrospinal fluid-derived activated T cells seem promising but need to be confirmed in a larger trial (123).
Peptides of the antigen-specific TCR of autoreactive T cells can also be used for TCR vaccination instead of whole T cells (reviewed in ref. 124). TCR peptide vaccination was pioneered by Brostoff and coworkers and Vandenbark et al. (125, 126), who used short synthetic peptides of TCR hypervariable (CDR) regions of the autoaggressive T cells. It is thought that vaccination with such TCR CDR region peptides stimulates TCR-specific counterregulatory T cells, which can also be demonstrated in the healthy human immune system (127, 128). The rationale for this approach is that in several rodent models of autoimmunity, the pathogenic, autoantigen-specific T lymphocytes use a strikingly limited number of available variable-region elements for their antigen receptor (129–131). Immunization of rats against synthetic peptides representing either the CDR3 (125) or CDR2 region (126) of the TCR of autoaggressive MBP-specific T cells prevented actively induced EAE and shortened ongoing disease (124).
In pilot clinical trials of TCR peptide vaccination, MS patients were immunized with synthetic peptides derived from the CDR regions of different Vβ5 and Vβ6 elements (132–136). These particular Vβ elements were chosen because earlier results had indicated that they are preferentially expressed by MBP-specific T cells from MS patients, in contrast to normal controls. The rationale for targeting Vβ5.2+ and Vβ5.3+ T cells in MS is further supported by the results of an MRI-monitored phase II trial of a humanized anti-Vβ5.2/Vβ5.3 monoclonal antibody (137). These pilot studies of TCR vaccination have not yet demonstrated significant clinical benefits, but further results are needed. In future studies, TCR vaccination is likely to be improved by DNA-based vaccination techniques (138, 139).
The success of TCR vaccination depends on the extent of diversity of the human T cell response to MBP and other encephalitogenic antigens. It is clear that the human T cell response to the most widely studied potential encephalitogen, MBP, is extremely complex, and there is no convincing evidence for an overall preference of certain TCR Vβ elements (23, 140). Therefore, it must be anticipated that TCR vaccination will work only if patients are treated with individualized, “tailor-designed” vaccines.
Genetically Engineered T Cells for Treatment of CNS Diseases. Genetic manipulation of CNS antigen-specific T cells has opened perspectives for selective immunotherapy. Transduction of antigen-specific T cells with GFP allowed the in vivo tracking of encephalitogenic T cells after transfer (141). This procedure helped to identify a special “migratory phenotype” that the T cells acquire in the periphery before they invade the CNS (142). At this migratory stage, it might be possible to target the pathogenic T cells very selectively. Furthermore, in a different approach, antigen-specific T cells could be engineered to express beneficial factors like antiinflammatory cytokines and neurotrophic factors (143–147). The genetically manipulated T cells of known receptor specificity could be used to deliver the transgene of interest specifically to the tissues that express the corresponding antigen.
Outlook: From Passive Deletion of “Forbidden Clones” to Active Induction of Self-Tolerance
The development of therapeutic strategies for MS has followed the evolution of the concepts of autoimmunity. Burnet (148) originally considered autoreactive immune cells to be “forbidden clones.” According to his view, therapy should aim to delete these putatively dangerous cells. This is the desired effect of classical immunosuppressive therapy, but this outcome would be at the cost of deleting beneficial regulatory cells. Subsequently, however, it has become more and more apparent that “autore-activity” per se is by no means a pathological phenomenon. On the contrary, autoreactive immune cells are essential components of the healthy immune system, and they might even serve (neuro) protective functions (97, 149). Accordingly, newer therapies aim at actively inducing tolerance rather than passively deleting “forbidden clones.” For example, APL, GA, DNA, and T cell vaccination all depend on active immunoregulatory mechanisms. One dilemma of the selective, antigen-specific immunotherapies is that the target antigen(s) of the pathogenic immune response in MS has (have) not been identified with certainty. New techniques may eventually help to achieve this ambitious goal and thereby provide the basis for a more efficient and “gentle” therapy and perhaps even cure of MS (163).
Acknowledgments
We thank Ms. J. Benson for editing the manuscript. This work was supported by the Max Planck Society, Deutsche Forschungsgemeinschaft Grant SFB 571, and the Hermann and Lilly Schilling Foundation.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Therapeutic Vaccines: Realities of Today and Hopes for Tomorrow,” held April 1–3, 2004, at the National Academy of Sciences in Washington, DC.
Abbreviations: APC, antigen-presenting cell; APL, altered peptide ligand; CDR, complementarity-determining region; EAE, experimental autoimmune encephalomyelitis; GA, glatiramer acetate; MBP, myelin basic protein; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis; TCR, T cell receptor; TH, T helper.
References
- 1.Noseworthy, J. H., Lucchinetti, C. F., Rodriguez, M. & Weinshenker, B. G. (2000) N. Engl. J. Med. 343, 938-952. [DOI] [PubMed] [Google Scholar]
- 2.Lassmann, H., Brück, W. & Lucchinetti, C. (2001) Trends Mol. Med. 7, 115-121. [DOI] [PubMed] [Google Scholar]
- 3.Meinl, E. (1999) Curr. Opin. Neurol. 12, 303-307. [DOI] [PubMed] [Google Scholar]
- 4.Wekerle, H. (1998) Nat. Med. 4, 770-771. [DOI] [PubMed] [Google Scholar]
- 5.Steinman, L. (2003) J. Exp. Med. 197, 1065-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Nun, A., Wekerle, H. & Cohen, I. R. (1981) Eur. J. Immunol. 11, 195-199. [DOI] [PubMed] [Google Scholar]
- 7.Schluesener, H. J., Sobel, R. A., Linington, C. & Weiner, H. L. (1987) J. Immunol. 139, 4016-4021. [PubMed] [Google Scholar]
- 8.Linington, C., Bradl, M., Lassmann, H., Brunner, C. & Vass, K. (1988) Am. J. Pathol. 130, 443-454. [PMC free article] [PubMed] [Google Scholar]
- 9.Huseby, E. S., Liggitt, D., Brabb, T., Schnabel, B., Öhlén, C. & Goverman, J. (2001) J. Exp. Med. 194, 669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun, D. M., Whitaker, J. N., Huang, Z. G., Liu, D., Coleclough, C., Wekerle, H. & Raine, C. S. (2001) J. Immunol. 166, 7579-7587. [DOI] [PubMed] [Google Scholar]
- 11.Wekerle, H., Kojima, K., Lannes-Vieira, J., Lassmann, H. & Linington, C. (1994) Ann. Neurol. 36, S47-S53. [DOI] [PubMed] [Google Scholar]
- 12.The Lenercept MS Study Group and The UBC MS/MRI Analysis Group. (1999) Neurology 53, 457-465. [PubMed] [Google Scholar]
- 13.Hohlfeld, R. (1997) Brain 120, 865-916. [DOI] [PubMed] [Google Scholar]
- 14.Wiendl, H. & Hohlfeld, R. (2003) BioDrugs 16, 183-200. [DOI] [PubMed] [Google Scholar]
- 15.Lucchinetti, C. F., Brück, W., Parisi, J., Scheithauer, B., Rodriguez, M. & Lassmann, H. (2000) Ann. Neurol. 47, 707-717. [DOI] [PubMed] [Google Scholar]
- 16.Dornmair, K., Goebels, N., Weltzien, H. U., Wekerle, H. & Hohlfeld, R. (2003) Am. J. Pathol. 163, 1215-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schluesener, H. J. & Wekerle, H. (1985) J. Immunol. 135, 3128-3133. [PubMed] [Google Scholar]
- 18.Pette, M., Fujita, K., Wilkinson, D., Altmann, D. M., Trowsdale, J., Giegerich, G., Hinkkanen, A., Epplen, J. T., Kappos, L. & Wekerle, H. (1990) Proc. Natl. Acad. Sci. USA 87, 7968-7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pette, M., Fujita, K., Kitze, B., Whitaker, J. N., Albert, E., Kappos, L. & Wekerle, H. (1990) Neurology 40, 1770-1776. [DOI] [PubMed] [Google Scholar]
- 20.Burns, J., Rosenzweig, A., Zweiman, B. & Lisak, R. P. (1983) Cell. Immunol. 81, 435-440. [DOI] [PubMed] [Google Scholar]
- 21.Ota, K., Matsui, M., Milford, E. L., Mackin, G. A., Weiner, H. L. & Hafler, D. A. (1990) Nature 346, 183-187. [DOI] [PubMed] [Google Scholar]
- 22.Martin, R., Howell, M. D., Jaraquemada, D., Flerlage, M., Richert, J., Brostoff, S., Long, E. O., McFarlin, D. E. & McFarland, H. F. (1991) J. Exp. Med. 173, 19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meinl, E., Weber, F., Drexler, K., Morelle, C., Ott, M., Saruhan-Direskeneli, G., Goebels, N., Ertl, B., Jechart, G., Giegerich, G., et al. (1993) J. Clin. Invest. 92, 2633-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goebels, N., Hofstetter, H., Schmidt, S., Brunner, C., Wekerle, H. & Hohlfeld, R. (2000) Brain 123, 508-518. [DOI] [PubMed] [Google Scholar]
- 25.Zamvil, S. S. & Steinman, L. (1990) Annu. Rev. Immunol. 8, 579-622. [DOI] [PubMed] [Google Scholar]
- 26.Meinl, E.,'t Hart, B. A., Bontrop, R. E., Hoch, R. M., Iglesias, A., De Waal Malefijt, R., Fickenscher, H., Müller-Fleckenstein, I., Fleckenstein, B., Wekerle, H., et al. (1995) Int. Immunol. 7, 1489-1495. [DOI] [PubMed] [Google Scholar]
- 27.Meinl, E., Hoch, R. M., Dornmair, K., De Waal Malefijt, R., Bontrop, R. E., Jonker, M., Lassmann, H., Hohlfeld, R., Wekerle, H. & 't Hart, B. A. (1997) Am. J. Pathol. 150, 445-453. [PMC free article] [PubMed] [Google Scholar]
- 28.Genain, C. P., Lee-Parritz, D., Nguyen, M.-H., Massacesi, L., Joshi, N., Ferrante, R., Hoffman, K., Moseley, M., Letvin, N. L. & Hauser, S. L. (1994) J. Clin. Invest. 94, 1339-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villoslada, P., Abel, K., Heald, N., Goertsches, R., Hauser, S. L. & Genain, C. P. (2001) Eur. J. Immunol. 31, 2942-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madsen, L. S., Andersson, E. C., Jansson, L., Krogsgaard, M., Andersen, C. B., Engsberg, J., Strominger, J. L., Svejgaard, A., Holmdahl, R., Wucherpfennig, K. W., et al. (1999) Nat. Genet. 23, 343-347. [DOI] [PubMed] [Google Scholar]
- 31.Bielekova, B., Goodwin, B., Richert, N., Cortese, I., Kondo, T., Afshar, G., Gran, B., Eaton, J., Antel, J., Frank, J. A., et al. (2000) Nat. Med. 6, 1167-1175. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt, S. (1999) Mult. Scler. 5, 147-160. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, J., Markovic-Plese, S., Lacet, B., Raus, J., Weiner, H. L. & Hafler, D. A. (1994) J. Exp. Med. 179, 973-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allegretta, M., Nicklas, J. A., Sriram, S. & Albertini, R. J. (1990) Science 247, 718-721. [DOI] [PubMed] [Google Scholar]
- 35.Markovic-Plese, S., Cortese, I., Wandinger, K. P., McFarland, H. F. & Martin, R. (2001) J. Clin. Invest. 108, 1185-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovett-Racke, A. E., Trotter, J. L., Lauber, J., Perrin, P. J., June, C. H. & Racke, M. K. (1998) J. Clin. Invest. 101, 725-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scholz, C., Patton, K. T., Anderson, D. E., Freeman, G. J. & Hafler, D. A. (1998) J. Immunol. 160, 1532-1538. [PubMed] [Google Scholar]
- 38.Bielekova, B., Muraro, P. A., Golestaneh, L., Pascal, J., McFarland, H. F. & Martin, R. (1999) J. Neuroimmunol. 100, 115-123. [DOI] [PubMed] [Google Scholar]
- 39.Bielekova, B., Sung, M. H., Kadom, N., Simon, R., McFarland, H. & Martin, R. (2004) J. Immunol. 172, 3893-3904. [DOI] [PubMed] [Google Scholar]
- 40.Lehmann, P. V., Sercarz, E. E., Forsthuber, T., Dayan, C. M. & Gammon, G. (1993) Immunol. Today 14, 203-208. [DOI] [PubMed] [Google Scholar]
- 41.Liston, A., Lesage, S., Wilson, J., Peltonen, J. & Goodnow, C. C. (2003) Nat. Immunol. 4, 350-354. [DOI] [PubMed] [Google Scholar]
- 42.Oldstone, M. B. A. (1987) Cell 50, 819-820. [DOI] [PubMed] [Google Scholar]
- 43.Lang, H. L. E., Jacobsen, H., Ikemizu, S., Andersson, C., Harlos, K., Madsen, L., Hjorth, P., Sondergaard, L., Svejgaard, A., Wucherpfennig, K., et al. (2002) Nat. Immunol. 3, 940-943. [DOI] [PubMed] [Google Scholar]
- 44.Gran, B., Hemmer, B., Vergelli, M., McFarland, H. F. & Martin, R. (1999) Ann. Neurol. 45, 559-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viglietta, V., Baecher-Allan, C., Weiner, H. L. & Hafler, D. A. (2004) J. Exp. Med. 199, 971-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furtado, G. D., Olivares-Villagomez, D., de Lafaille, M. A. C., Wensky, A. K., Latkowski, J. A. & Lafaille, J. J. (2001) Immunol. Rev. 182, 122-134. [DOI] [PubMed] [Google Scholar]
- 47.Bach, J.-F. (2003) Nat. Rev. Immunol. 3, 189-198. [DOI] [PubMed] [Google Scholar]
- 48.von Herrath, M. G. & Harrison, L. C. (2003) Nat. Rev. Immunol. 3, 223-232. [DOI] [PubMed] [Google Scholar]
- 49.Barnett, M. H. & Prineas, J. W. (2004) Ann. Neurol. 55, 458-468. [DOI] [PubMed] [Google Scholar]
- 50.Trapp, B. D. (2004) Ann. Neurol. 55, 455-457. [DOI] [PubMed] [Google Scholar]
- 51.Oksenberg, J. R., Stuart, S., Begovich, A. B., Bell, R. B., Erlich, H. A., Steinman, L. & Bernard, C. C. A. (1990) Nature 345, 344-346. [DOI] [PubMed] [Google Scholar]
- 52.Oksenberg, J. R., Panzara, M. A., Begovich, A. B., Mitchell, D., Erlich, H. A., Murray, R. S., Shimonkevitz, R., Sherritt, M., Rothbard, J., Bernard, C. C. A., et al. (1993) Nature 362, 68-70. [DOI] [PubMed] [Google Scholar]
- 53.Babbe, H., Roers, A., Waisman, A., Lassmann, H., Goebels, N., Hohlfeld, R., Friese, M., Schröder, R., Deckert, M., Schmidt, S., et al. (2000) J. Exp. Med. 192, 393-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skulina, C., Schmidt, S., Dornmair, K., Babbe, H., Roers, A., Rajewsky, K., Wekerle, H., Hohlfeld, R. & Goebels, N. (2004) Proc. Natl. Acad. Sci. USA 101, 2428-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hemmer, B., Archelos, J. J. & Hartung, H.-P. (2002) Nat. Rev. Neurosci. 3, 291-301. [DOI] [PubMed] [Google Scholar]
- 56.Neumann, H., Medana, I. M., Bauer, J. & Lassmann, H. (2002) Trends Neurosci. 25, 313-319. [DOI] [PubMed] [Google Scholar]
- 57.Archelos, J. J., Storch, M. K. & Hartung, H.-P. (2000) Ann. Neurol. 46, 694-706. [PubMed] [Google Scholar]
- 58.Qin, Y., Duquette, P., Zhang, Y., Poole, R. & Antel, J. P. (1998) J. Clin. Invest. 102, 1045-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Owens, G. P., Kraus, H., Burgoon, M. P., Smith-Jensen, T., Devlin, M. E. & Gilden, D. H. (1998) Ann. Neurol. 43, 236-243. [DOI] [PubMed] [Google Scholar]
- 60.Baranzini, S. E., Jeong, M. C., Butunoi, C., Murray, R. S., Bernard, C. C. A. & Oksenberg, J. R. (1999) J. Immunol. 163, 5133-5144. [PubMed] [Google Scholar]
- 61.Colombo, M., Dono, M., Gazzola, P., Roncella, S., Valetto, A., Chiorazzi, N., Mancardi, G. L. & Ferrarini, M. (2000) J. Immunol. 164, 2782-2789. [DOI] [PubMed] [Google Scholar]
- 62.Owens, G. C., Ritchie, A. M., Burgoon, M. P., Williamson, R. A., Corboy, J. R. & Gilden, D. H. (2003) J. Immunol. 171, 2725-2733. [DOI] [PubMed] [Google Scholar]
- 63.Robinson, W. H., DiGennaro, C., Hueber, W., Haab, B. B., Kamachi, M., Dean, E. J., Fournel, S., Fong, D., Genovese, M. C., De Vegvar, H. E. N., et al. (2002) Nat. Med. 8, 295-301. [DOI] [PubMed] [Google Scholar]
- 64.Robinson, W. H., Fontoura, P., Lee, B. J., De Vegvar, H. E. N., Tom, J., Pedotti, R., DiGennaro, C. D., Mitchell, D. J., Fong, D., Ho, P. P. K., et al. (2003) Nat. Biotechnol. 21, 1033-1039. [DOI] [PubMed] [Google Scholar]
- 65.Goodin, D. S., Frohman, E. M., Garmany, G. P., Halper, J., Likosky, W. H., Lublin, F. D., Silberberg, D. H., Stuart, W. H. & Van den Noort, S. (2002) Neurology 58, 169-178. [DOI] [PubMed] [Google Scholar]
- 66.Polman, C. H. & Uitdehaag, B. M. J. (2003) Lancet Neurol. 2, 563-566. [DOI] [PubMed] [Google Scholar]
- 67.Neuhaus, O., Archelos, J. J. & Hartung, H.-P. (2003) Trends Pharmacol. Sci. 24, 131-138. [DOI] [PubMed] [Google Scholar]
- 68.Miller, D. H., Grossman, R. I., Reingold, S. C. & McFarland, H. F. (1998) Brain 121, 3-24. [DOI] [PubMed] [Google Scholar]
- 69.Filippi, M. & Grossman, R. I. (2004) Neurology 58, 1147-1153. [DOI] [PubMed] [Google Scholar]
- 70.Martin, R., Sturzebecher, C. S. & McFarland, H. F. (2001) Nat. Immunol. 2, 785-788. [DOI] [PubMed] [Google Scholar]
- 71.Huppa, J. B. & Davis, M. M. (2003) Nat. Rev. Immunol. 3, 973-983. [DOI] [PubMed] [Google Scholar]
- 72.Hohlfeld, R. (1989) Ann. Neurol. 25, 531-538. [DOI] [PubMed] [Google Scholar]
- 73.Sloan-Lancaster, J. & Allen, P. M. (1996) Annu. Rev. Immunol. 14, 1-27. [DOI] [PubMed] [Google Scholar]
- 74.Steinman, L. (2000) J. Autoimmun. 14, 278-282. [DOI] [PubMed] [Google Scholar]
- 75.Genain, C. P. & Zamvil, S. S. (2000) Nat. Med. 1098-1100. [DOI] [PubMed]
- 76.Kappos, L., Comi, G., Panitch, H., Oger, J., Antel, J., Conlon, P. & Steinman, L. (2000) Nat. Med. 6, 1176-1182. [DOI] [PubMed] [Google Scholar]
- 77.Crowe, P. D., Qin, Y. F., Conlon, P. J. & Antel, J. P. (2000) Ann. Neurol. 48, 758-765. [PubMed] [Google Scholar]
- 78.Sela, M., Arnon, R. & Teitelbaum, D. (1990) Bull. Inst. Pasteur (Paris) 88, 303-314. [Google Scholar]
- 79.Teitelbaum, D., Arnon, R. & Sela, M. (1997) J. Neural Transm. Suppl. 49, 85-91. [DOI] [PubMed] [Google Scholar]
- 80.Sela, M. (2003) J. Cell Biol. 278, 48507-48519. [DOI] [PubMed] [Google Scholar]
- 81.Bornstein, M. B., Miller, A., Slagle, S., Weitzman, M., Crytsal, H., Drexler, E., Keilson, M., Merriam, A., Wassertheil-Smoller, S., Spada, V., et al. (1987) N. Engl. J. Med. 317, 408-414. [DOI] [PubMed] [Google Scholar]
- 82.Bornstein, M. B., Miller, A., Slagle, S., Weitzman, M., Drexler, E., Keilson, M., Spada, V., Weiss, W., Appel, S., Rolak, L., et al. (1991) Neurology 41, 533-539. [DOI] [PubMed] [Google Scholar]
- 83.Wolinsky, J. S. (1995) Neurology 45, 1245-1247. [DOI] [PubMed] [Google Scholar]
- 84.Johnson, K. P., Brooks, B. R., Cohen, J. A., Ford, C. C., Goldstein, J., Lisak, R. P., Myers, L. W., Panitch, H. S., Rose, J. W., Schiffer, R. B., et al. (1995) Neurology 45, 1268-1276. [DOI] [PubMed] [Google Scholar]
- 85.Johnson, K. P., Brooks, B. R., Cohen, J. A., Ford, C. C., Goldstein, J., Lisak, R. P., Myers, L. W., Panitch, H. S., Rose, J. W., Schiffer, R. B., et al. (1998) Neurology 50, 701-708. [DOI] [PubMed] [Google Scholar]
- 86.Johnson, K. P., Brooks, B. R., Ford, C. C., Goodman, A., Guarnaccia, J., Lisak, R. P., Myers, L. W., Panitch, H. S., Pruitt, A., Rose, J. W., et al. (2000) Mult. Scler. 6, 255-266. [DOI] [PubMed] [Google Scholar]
- 87.Comi, G., Filippi, M. & Wolinsky, J. S. (2001) Ann. Neurol. 49, 290-297. [PubMed] [Google Scholar]
- 88.Neuhaus, O., Farina, C., Wekerle, H. & Hohlfeld, R. (2001) Neurology 56, 702-706. [DOI] [PubMed] [Google Scholar]
- 89.Dhib-Jalbut, S., Chen, M., Said, A., Zhan, M., Johnson, K. P. & Martin, R. (2003) J. Neuroimmunol. 140, 163-171. [DOI] [PubMed] [Google Scholar]
- 90.Duda, P. W., Krieger, J. I., Cook, S. L. & Hafler, D. A. (2000) J. Clin. Invest. 105, 967-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gran, B., Tranquill, L. R., Chen, M., Bielekova, B., Zhou, W., Dhib-Jalbut, S. & Martin, R. (2000) Neurology 55, 1704-1714. [DOI] [PubMed] [Google Scholar]
- 92.Neuhaus, O., Farina, C., Yassouridis, A., Wiendl, H., Then Bergh, F., Dose, T., Wekerle, H. & Hohlfeld, R. (2000) Proc. Natl. Acad. Sci. USA 97, 7452-7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aharoni, R., Teitelbaum, D., Sela, M. & Arnon, R. (1998) J. Neuroimmunol. 91, 135-146. [DOI] [PubMed] [Google Scholar]
- 94.Weiner, H. L. (1999) Proc. Natl. Acad. Sci. USA 96, 3333-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ziemssen, T., Kumpfel, T., Klinkert, W. E. F., Neuhaus, O. & Hohlfeld, R. (2002) Brain 125, 2381-2391. [DOI] [PubMed] [Google Scholar]
- 96.Aharoni, R., Kayhan, B., Eilam, R., Sela, M. & Arnon, R. (2003) Proc. Natl. Acad. Sci. USA 100, 14157-14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kerschensteiner, M., Stadelmann, C., Dechant, G., Wekerle, H. & Hohlfeld, R. (2003) Ann. Neurol. 53, 292-304. [DOI] [PubMed] [Google Scholar]
- 98.Stadelmann, C., Kerschensteiner, M., Misgeld, T., Brück, W., Hohlfeld, R. & Lassmann, H. (2002) Brain 125, 75-85. [DOI] [PubMed] [Google Scholar]
- 99.Hussien, Y., Sanna, A., Soderstrom, M., Link, H. & Huang, Y. M. (2001) J. Neuroimmunol. 121, 102-110. [DOI] [PubMed] [Google Scholar]
- 100.Vieira, P. L., Heystek, H. C., Wormmeester, J., Wierenga, E. A. & Kapsenberg, M. L. (2003) J. Immunol. 170, 4483-4488. [DOI] [PubMed] [Google Scholar]
- 101.Weber, M. S., Starck, M., Wagenpfeil, S., Meinl, E., Hohlfeld, R. & Farina, C. (2004) Brain 127, 1370-1378. [DOI] [PubMed] [Google Scholar]
- 102.Jung, S., Siglienti, I., Grauer, O., Magnus, T., Scarlato, G. & Toyka, K. (2004) J. Neuroimmunol. 148, 63-73. [DOI] [PubMed] [Google Scholar]
- 103.Kim, H. J., Ifergan, I., Seguin, R., Duddy, M., Lapierre, Y., Jalili, F. & Bar-Or, A. (2004) J. Immunol. 172, 7144-7153. [DOI] [PubMed] [Google Scholar]
- 104.Farina, C., Then Bergh, F., Albrecht, H., Meinl, E., Yassouridis, A., Neuhaus, O. & Hohlfeld, R. (2001) Brain 124, 705-719. [DOI] [PubMed] [Google Scholar]
- 105.Brenner, T., Arnon, R., Sela, M., Abramsky, O., Meiner, Z., Riven-Kreitman, R., Tarcic, N. & Teitelbaum, D. (2001) J. Neuroimmunol. 115, 152-160. [DOI] [PubMed] [Google Scholar]
- 106.Farina, C., Vargas, V., Heydari, N., Kümpfel, T., Meinl, E. & Hohlfeld, R. (2002) J. Neuroimmunol. 123, 188-192. [DOI] [PubMed] [Google Scholar]
- 107.Farina, C., Wagenpfeil, S. & Hohlfeld, R. (2002) J. Neurol. 249, 1587-1592. [DOI] [PubMed] [Google Scholar]
- 108.Weiner, H. L. (2000) J. Clin. Invest. 106, 935-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wraith, D. C. (1996) Int. Arch. Allergy Appl. Immunol. 108, 355-359. [DOI] [PubMed] [Google Scholar]
- 110.Weiner, H. L., Mackin, G. A., Matsui, M., Orav, E. J., Khoury, S. J., Dawson, D. M. & Hafler, D. A. (1993) Science 259, 1321-1324. [DOI] [PubMed] [Google Scholar]
- 111.Lobell, A., Weissert, R., Storch, M. K., Svanholm, C., De Graaf, K. L., Lassmann, H., Andersson, R., Olsson, T. & Wigzell, H. (1998) J. Exp. Med. 187, 1543-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weissert, R., Lobell, A., De Graaf, K. L., Eltayeb, S. Y., Andersson, R., Olsson, T. & Wigzell, H. (2000) Proc. Natl. Acad. Sci. USA 97, 1689-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ruiz, P. J., Garren, H., Ruiz, I. U., Hirschberg, D. L., Nguyen, L. V. T., Karpuj, M. V., Cooper, M. T., Mitchell, D. J., Fathman, C. G. & Steinman, L. (1999) J. Immunol. 162, 3336-3341. [PubMed] [Google Scholar]
- 114.Bourquin, C., Iglesias, A., Berger, T., Wekerle, H. & Linington, C. (2000) Eur. J. Immunol. 30, 3663-3671. [DOI] [PubMed] [Google Scholar]
- 115.Garren, H., Ruiz, P. J., Watkins, T. A., Fontoura, P., Nguyen, L.-V., Estline, E. R., Hirschberg, D. L. & Steinman, L. (2001) Immunity 15, 15-22. [DOI] [PubMed] [Google Scholar]
- 116.Ben-Nun, A., Wekerle, H. & Cohen, I. R. (1981) Nature 292, 60-61. [DOI] [PubMed] [Google Scholar]
- 117.Ben-Nun, A. & Cohen, I. R. (1981) Eur. J. Immunol. 11, 949-952. [DOI] [PubMed] [Google Scholar]
- 118.Sun, D., Ben-Nun, A. & Wekerle, H. (1988) Eur. J. Immunol. 18, 1993-2000. [DOI] [PubMed] [Google Scholar]
- 119.Sun, D., Qin, Y., Chluba, J., Epplen, J. T. & Wekerle, H. (1988) Nature 332, 843-845. [DOI] [PubMed] [Google Scholar]
- 120.Jiang, H., Kashleva, H., Xu, L.-X., Forman, J., Flaherty, L., Pernis, B., Braunstein, N. S. & Chess, L. (1998) Proc. Natl. Acad. Sci. USA 95, 4533-4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zang, Y. C. Q., Hong, J., Rivera, V. M., Killian, J. M. & Zhang, J. Z. (2000) J. Immunol. 164, 4011-4017. [DOI] [PubMed] [Google Scholar]
- 122.Zhang, J. Z., Rivera, V. M., Tejada-Simon, M. V., Yang, D. Y., Hong, J., Li, S. F., Haykal, H., Killian, J. & Zang, Y. C. Q. (2002) J. Neurol. 249, 212-218. [DOI] [PubMed] [Google Scholar]
- 123.Van der Aa, A., Hellings, N., Medaer, R., Gelin, G., Palmers, Y., Raus, J. & Stinissen, P. (2003) Clin. Exp. Immunol. 131, 155-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vandenbark, A. A., Hashim, G. A. & Offner, H. (1996) J. Neurosci. Res. 43, 391-402. [DOI] [PubMed] [Google Scholar]
- 125.Howell, M. D., Winters, S. T., Olee, T., Powell, H. C., Carlo, D. J. & Brostoff, S. W. (1989) Science 246, 668-670. [DOI] [PubMed] [Google Scholar]
- 126.Vandenbark, A. A., Hashim, G. & Offner, H. (1989) Nature 341, 541-544. [DOI] [PubMed] [Google Scholar]
- 127.Saruhan-Direskeneli, G., Weber, F., Meinl, E., Pette, M., Giegerich, G., Hinkkanen, A., Epplen, J. T., Hohlfeld, R. & Wekerle, H. (1993) Eur. J. Immunol. 23, 530-536. [DOI] [PubMed] [Google Scholar]
- 128.Zipp, F., Kerschensteiner, M., Dornmair, K., Malotka, J., Schmidt, S., Bender, A., Giegerich, G., De Waal Malefijt, R., Wekerle, H. & Hohlfeld, R. (1998) Brain 121, 1395-1407. [DOI] [PubMed] [Google Scholar]
- 129.Acha-Orbea, H., Mitchell, D. J., Timmermann, L., Wraith, D. C., Tausch, G. S., Waldor, M. K., Zamvil, S. S., McDevitt, H. O. & Steinman, L. (1988) Cell 54, 263-273. [DOI] [PubMed] [Google Scholar]
- 130.Chluba, J., Steeg, C., Becker, A., Wekerle, H. & Epplen, J. T. (1989) Eur. J. Immunol. 19, 279-284. [DOI] [PubMed] [Google Scholar]
- 131.Urban, J. L., Kumar, V., Kono, D. H., Gomez, C., Horvath, S. J., Clayton, J., Ando, D. G., Sercarz, E. E. & Hood, L. (1988) Cell 54, 577-592. [DOI] [PubMed] [Google Scholar]
- 132.Bourdette, D. N., Whitham, R. H., Chou, Y. K., Morrison, W. J., Atherton, J., Kenny, C., Liefeld, D., Hashim, G. A., Offner, H. & Vandenbark, A. A. (1994) J. Immunol. 152, 2510-2519. [PubMed] [Google Scholar]
- 133.Chou, Y. K., Morrison, W. J., Weinberg, A. D., Dedrick, R., Whitham, R., Bourdette, D. N., Hashim, G. A., Offner, H. & Vandenbark, A. A. (1994) J. Immunol. 152, 2520-2529. [PubMed] [Google Scholar]
- 134.Vandenbark, A. A., Chou, Y. K., Whitham, R., Mass, M., Buenafe, A., Liefeld, D., Kavanagh, D., Cooper, S., Hashim, G. A., Offner, H., et al. (1996) Nat. Med. 2, 1109-1115. [DOI] [PubMed] [Google Scholar]
- 135.Antel, J. P., Becher, B. & Owens, T. (1996) Nat. Med. 2, 1074-1075. [DOI] [PubMed] [Google Scholar]
- 136.Morgan, E. E., Nardo, C. J., Dively, J. P., Bartholomew, R. M., Moss, R. B. & Carlo, D. J. (2001) J. Neurosci. Res. 64, 298-301. [DOI] [PubMed] [Google Scholar]
- 137.Killestein, J., Olsson, T., Wallström, E., Svenningsson, A., Khademi, M., Blumhardt, L. D., Fagius, J., Hillert, J., Landtblom, A.-M., Edenius, C., et al. (2002) Ann. Neurol. 51, 467-474. [DOI] [PubMed] [Google Scholar]
- 138.Chunduru, S. K., Sutherland, R. M., Stewart, G. A., Doms, R. W. & Paterson, Y. (1996) J. Immunol. 156, 4940-4945. [PubMed] [Google Scholar]
- 139.Waisman, A., Ruiz, P. J., Hirschberg, D. L., Gelman, A., Oksenberg, J. R., Brocke, S., Mor, F., Cohen, I. R. & Steinman, L. (1996) Nat. Med. 2, 899-905. [DOI] [PubMed] [Google Scholar]
- 140.Hafler, D. A., Saadeh, M. G., Kuchroo, V. K., Milford, E. & Steinman, L. (1996) Immunol. Today 17, 152-159. [DOI] [PubMed] [Google Scholar]
- 141.Flügel, A., Willem, M., Berkowicz, T. & Wekerle, H. (1999) Nat. Med. 5, 843-847. [DOI] [PubMed] [Google Scholar]
- 142.Flügel, A., Berkowicz, T., Ritter, T., Labeur, M., Jenne, D., Li, Z., Ellwart, J., Willem, M., Lassmann, H. & Wekerle, H. (2001) Immunity 14, 547-560. [DOI] [PubMed] [Google Scholar]
- 143.Kramer, R., Zhang, Y., Gehrmann, J., Gold, R., Thoenen, H. & Wekerle, H. (1995) Nat. Med. 1, 1162-1166. [DOI] [PubMed] [Google Scholar]
- 144.Flügel, A., Matsumuro, K., Neumann, H., Klinkert, W. E. F., Birnbacher, R., Lassmann, H., Otten, U. & Wekerle, H. (2001) Eur. J. Immunol. 31, 11-22. [DOI] [PubMed] [Google Scholar]
- 145.Mathisen, P. M., Yu, M., Johnson, J. M., Drazba, J. A. & Tuohy, V. K. (1997) J. Exp. Med. 186, 159-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shaw, M. K., Lorens, J. B., Dhawan, A., DalCanto, R., Tse, H. Y., Tran, A. B., Bonpane, C., Eswaran, S. L., Brocke, S., Sarvetnick, N., et al. (1997) J. Exp. Med. 185, 1711-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Furlan, R., Pluchino, S. & Martino, G. (2003) Curr. Opin. Neurol. 16, 385-392. [DOI] [PubMed] [Google Scholar]
- 148.Burnet, F. M. (1959) The Clonal Selection Theory of Acquired Immunity (Cambridge Univ. Press, Cambridge, U.K.).
- 149.Moalem, G., Leibowitz-Amit, R., Yoles, E., Mor, F., Cohen, I. R. & Schwartz, M. (1999) Nat. Med. 5, 49-55. [DOI] [PubMed] [Google Scholar]
- 150.Vandenbark, A. A., Morgan, E., Bartholomew, R., Bourdette, D., Whitham, R., Carlo, D., Gold, D., Hashim, G. & Offner, H. (2001) Neurochem. Res. 26, 713-730. [DOI] [PubMed] [Google Scholar]
- 151.Achiron, A. & Mandel, M. (2004) Autoimmun. Rev. 3, 25-32. [DOI] [PubMed] [Google Scholar]
- 152.Warren, K. G. & Catz, I. (1997) J. Neurol. Sci. 148, 67-78. [DOI] [PubMed] [Google Scholar]
- 153.Warren, K. G. & Catz, I. (2000) Mult. Scler. 6, 300-311. [DOI] [PubMed] [Google Scholar]
- 154.Goodkin, D. E., Shulman, M., Winkelhake, M. J., Waubant, E., Andersson, P.-B., Stewart, T., Nelson, S., Fischbein, N., Coyle, P. K., Jacobs, L., et al. (2000) Neurology 65, 1414-1420. [DOI] [PubMed] [Google Scholar]
- 155.Kim, H. J., Antel, J. P., Duquette, P., Alleva, D. G., Conlon, P. J. & Bar-Or, A. (2002) Clin. Immunol. 104, 105-114. [DOI] [PubMed] [Google Scholar]
- 156.Gold, D. P., Smith, R. A., Golding, A. B., Morgan, E. E., Dafashy, T., Nelson, J., Smith, L., Dively, J., Laxer, J. A., Richieri, S. P., et al. (1997) J. Neuroimmunol. 76, 29-38. [DOI] [PubMed] [Google Scholar]
- 157.Wilson, D. B., Golding, A. B., Smith, R. A., Dafashy, T., Nelson, J., Carlo, D. J., Brostoff, S. W. & Gold, D. P. (1997) J. Neuroimmunol. 76, 15-28. [DOI] [PubMed] [Google Scholar]
- 158.Zhang, J., Medaer, R., Stinissen, P., Hafler, D. A. & Raus, J. (1993) Science 261, 1451-1454. [DOI] [PubMed] [Google Scholar]
- 159.Medaer, R., Stinissen, P., Truyen, L., Raus, J. & Zhang, J. (1995) Lancet 346, 807-808. [DOI] [PubMed] [Google Scholar]
- 160.Hermans, G., Medaer, R., Raus, J. & Stinissen, P. (2000) J. Neuroimmunol. 102, 79-84. [DOI] [PubMed] [Google Scholar]
- 161.Hermans, G., Denzer, U., Lohse, A., Raus, J. & Stinissen, P. (1999) J. Autoimmun. 13, 233-246. [DOI] [PubMed] [Google Scholar]
- 162.Correale, J., Lund, B., McMillan, M., Ko, D. Y., McCarthy, K. & Weiner, L. P. (2000) J. Neuroimmunol. 107, 130-139. [DOI] [PubMed] [Google Scholar]
- 163.Weiner, H. L. (2004. in Curing MS: How Science is Solving the Mysteries of Multiple Sclerosis (Crown Publishers, New York), pp. 1-309.
- 164.Küppers, R., Zhao, M., Hansmann, M. L. & Rajewski, K. (1993) EMBO J. 12, 4955-4967. [DOI] [PMC free article] [PubMed] [Google Scholar]