Abstract
Background
Quantitative MR, including T1ρ mapping, has been extensively used to probe early biochemical changes in knee articular cartilage of subjects with osteoarthritis (OA) and others at risk for cartilage degeneration, such as those with anterior cruciate ligament (ACL) injury and reconstruction. However, limited studies have been performed aimed to assess the spatial location and patterns of T1ρ. In this study we used a novel voxel-based relaxometry (VBR) technique coupled with principal component analysis (PCA) to extract relevant features so as to describe regional patterns and to investigate their similarities and differences in T1ρ maps in subjects with OA and subjects six months after ACL reconstruction (ACLR).
Methods
T1ρ quantitative MRI images were collected for 180 subjects from two separate cohorts. The OA cohort included 93 osteoarthritic patients and 25 age-matched controls. The ACLR-6M cohort included 52 patients with unilateral ACL tears who were imaged 6 months after ACL reconstruction, and 10 age-matched controls. Non-rigid registration on a single template and local Z-score conversion were adopted for T1ρ spatial and intensity normalization of all the images in the dataset. PCA was used as a data dimensionality reduction to obtain a description of all subjects in a 10-dimensional feature space. Logistic linear regression was used to identify distinctive features of OA and ACL subjects
Results
Global prolongation of the Z-score was observed in both OA and ACL subjects compared to controls [higher values in 1st principal component (PC1); P=0.01]. In addition, relaxation time differences between superficial and deep cartilage layers of the lateral tibia and trochlea were observed to be significant distinctive features between OA and ACL subjects. OA subjects demonstrated similar values between the two cartilage layers [higher value in 2nd principal component (PC2); P=0.008], while ACL reconstructed subjects showed T1ρ prolongation specifically in the cartilage superficial layer (lower values in PC2; P<0.0001). T1ρ elevation located outside of the weight-bearing area, located in the posterior and anterior aspects of the lateral femoral compartment, was also observed to be a key feature in distinguishing OA subjects from controls [higher value in 6th principal component (PC6); P=0.007].
Conclusions
This study is the first example of T1ρ local/regional pattern analysis and data-driven feature extraction in knees with cartilage degeneration. Our results revealed similarities and differences between OA and ACL relaxation patterns that could be potentially useful to better understand the pathogenesis of post-traumatic cartilage degeneration and the identification of imaging biomarkers for the early stratification of subjects at risk for developing post-traumatic OA.
Keywords: T1ρ, voxel based relaxometry, principal component analysis (PCA), osteoarthritis (OA), anterior cruciate ligament (ACL)
Introduction
Osteoarthritis (OA) is a degenerative disease that is characterized by cartilage thinning and compositional alterations (1). The initial signs of cartilage degeneration are molecular and biochemical changes within the extracellular matrix (2). Quantitative imaging is increasingly becoming a central feature in efforts to characterize the structural and metabolic properties of musculoskeletal tissues, ranging from disease etiology to therapy response (3,4).
Quantitative MR, including T1ρ and T2 mapping has been extensively used to probe biochemical changes in the articular cartilage. While T2 relaxation times is primarily affected by hydration and collagen structure due to dipolar interactions (5), the spin-lock techniques used in T1ρ reduce dipolar interactions (6). Chemical exchange on proteoglycan and water protons was suggested to contribute to T1ρ in cartilage, although T1ρ changes in cartilage may be affected by hydration and collagen structure as well (7). T1ρ quantification was adopted in previous study to detect early signs of OA (8-10). Additionally, T1ρ quantification has been used in the attempt to stratify subjects at risk of developing post-traumatic osteoarthritis (PTOA) following anterior cruciate ligament (ACL) injury and reconstruction (11,12). Prolongation of T1ρ relaxation times has been reported in OA subjects, specifically in the medial compartment (13,14). In contrast, in subjects with ACL injury, elevation in T1ρ is observed in the posterior lateral tibia immediately after injury, the characteristic site of the pivot shift contusion (15). Elevations in this lateral region have also been observed as predictors of the changes in patient-reported outcomes 12 months after reconstruction (16). Longitudinal T1ρ progression in both injured and contralateral knees has been observed as soon as 6 months after ACL reconstruction, specifically in medial and lateral femur and femoral trochlea compartments (17).
Despite the large amount of research done in the past few years in studying compartmental average T1ρ relaxation times in both post ACL injury and OA subjects, there is no clear definition of the regions of the knee or the relaxometry features that maybe shared or those that are distinctive between these two groups: namely post traumatic OA and primary idiopathic OA.
While several studies are still limited to analyzing average values of T1ρ in specific compartments of the knee, there is growing interest in exploring spatial distribution and local patterns in T1ρ maps. Extraction of second order statistical information, or texture analysis (18-20), has been widely used to overcome the limitation of the average ROI-based approaches. However, texture analysis does not address the problem of studying local differences between two groups, and does not allow for the extraction of salient patterns that could characterize cartilage degeneration in OA and ACL injured subjects.
A novel, fully-automatic and unbiased algorithm for studying knee relaxation times has been previously proposed, by creating an atlas of the knees and using voxel-based relaxometry (VBR) (21). This technique allows for the investigation of local cartilage composition differences between two groups through voxel-based statistical parametric mapping (SPM). VBR and SPM were recently used in a multicenter study that aimed to explore how cartilage lesions at the time of ACL injury influence the longitudinal progression of cartilage degeneration (22), while a related study explored the usage of the composite R2-R1ρ metric as a possible associative biomarker for patient-reported outcomes (23).
Since all images are aligned to a single template in VBR, one can consider each patient as an individual data-point in a multi-dimensional feature space. Machine learning techniques, such as principal component analysis (PCA) can be adopted to extract latent patterns in the data, studying the characteristics of the multidimensional point cloud representing the analyzed subjects and identifying sub-clusters. In this study we propose to couple VBR and PCA to compare changes in cartilage biochemical composition patterns in subjects with OA, subjects 6 months after ACL injury and healthy controls. We hypothesize that specific patterns in the VBR maps will be distinctive for OA and ACL injured subjects, and enable separation of the two groups from controls. We expect to observe more complex and distinctive relaxometry patterns beyond the well-known global T1ρ prolongation that distinguishes both OA and ACL-injured subjects from controls.
Methods
Subjects
A total of 180 subjects from two different cohorts were considered in this study. OA cohort included 93 osteoarthritic patients [age =54.9±9.1 years, BMI =25.0±3.5 kg/m2, 62 females, mean radiographic Kellgren and Lawrence (KL) OA grade =1.96] and 25 matched controls (age =51.2±7.7 years, BMI =24.1±3.8 kg/m2, 18 females, KL =0). ACLR-6M cohort included 52 patients with unilateral ACL tears imaged 6 months after surgical reconstruction (age =28.1±12.1 years, BMI =24.3±2.8 kg/m2, 31 females) and 10 matched controls (age= 32.0±4.0 years, BMI =22.8±3.1 kg/m2, 5 females). All subjects gave informed consent, and the study was carried out in accordance with the regulations of the Committee for Human Research. OA cohort was collected at a single site: University of California, San Francisco (San Francisco, CA, USA). All subjects were recruited with age >35 years, exclusion criteria were: concurrent use of an investigational drug, history of fracture or surgical intervention in the study knee, and any contraindications to MR. Subjects were included in the OA group if they reported knee pain, aching, or stiffness on most days per month during the past year, or use of medication for knee pain on most days per month during the past year, or any possible radiographic sign of knee OA (KL >0). Subjects with KL =1 [doubtful joint space narrowing (JSN) and possible osteophytic lipping] were included in the OA group in case they met the symptomatic criteria. Subjects were included in the control group if no knee pain or stiffness in either knee or use of medications for knee pain in the last year were reported, and if no radiographic evidence of OA on either knee was noted KL =0.
Both patients and controls in the ACLR-6M cohort were collected at three sites: University of California, San Francisco (San Francisco, CA, USA), Mayo Clinic (Rochester, MN, USA), and Hospital for Special Surgery (New York City, NY, USA). ACL patients underwent anatomic single-bundle ACLR by board-certified, fellowship-trained orthopaedic surgeons. Only soft tissue grafts were used: hamstrings, either allograft or autograft, or posterior tibialis allograft. All patients underwent a standard postoperative rehabilitation protocol.
MRI protocol
All imaging was done using a 3T MRI scanner (GE Healthcare, Milwaukee, WI, USA) with an 8-channel phased array knee coil (Invivo Inc., Orlando, FL, USA). Sagittal 3D T1ρ imaging sequences were obtained using a magnetization-prepared angle-modulated partitioned 3D-MAPPS sequence (24) with the following parameters: TR/TE =8 ms/min full, FOV =14 cm, matrix =256×128, slice thickness =4 mm, in-plane pixel spacing =0.56 mm, Views Per Segment =64, time of recovery =1.2 s, spin-lock frequency =500 Hz, ARC phase AF =2, time of spin lock (TSL) =0/10/40/80 ms for the ACLR-6M cohort and 0/2/4/8/12/20/40/80 ms for the OA cohort. Strict quality control and initial calibration were performed to ensure the consistency of the protocols (25).
Image processing
The image post-processing was performed with software developed in-house using Matlab (Mathworks Inc., El Segundo, CA, USA) integrated with Elastix registration library (26,27). All images were morphed to the space of a reference to obtain anatomically matched T1ρ-weighed images using a previously developed technique (21). Briefly, we applied an intensity-based multi-resolution pyramidal approach. B-spline transformation was used for the morphing and Advance Mattes Mutual Information image similarity metric as a figure of merit of the transformation that was iteratively optimized. This process was performed on the T1ρ-weighted image (TSL =0) and the transformation obtained was applied on all the other T1ρ-weighted images.
T1ρ maps were then computed on a pixel-by-pixel basis using a two-parameter, Levenberg-Marquardt mono-exponential fit: [S(TSL) α exp(−TSL/T1ρ)]. The reference image was selected through an iterative process aimed to minimize the dataset global deformation. Having all the images in the same coordinate space allowed for local normalization of the relaxation time maps, which was an essential step for mixing images from different cohorts and/or acquired with different protocols. T1ρ values at each voxel were converted in Z-score, considering the average and standard deviation of the matched control group.
The z-score calculation normalized T1ρ in patients for both groups using their respective control average and standard deviation, which removes the spatial heterogeneity and T1ρ differences related to factors such as age, MRI system or protocol, returning a dimensionless description of the T1ρ abnormalities comparable between the two cohorts. An example is shown in Figure 1.
Figure 1.
Example of local Z-score normalization computed for one of the subjects in the OA cohort (56-year-old male, KL 2). Arrow (A) indicates a location characterized by high values of T1ρ in the patient map, but high heterogeneity in the control group, leading to a negligible increase of this area in the corresponding Z-score map. Arrow (B) shows a relatively homogeneous posterior lateral femur in the patient T1ρ, corresponding with an area of low values for the controls, thus leading to higher Z-score values. OA, osteoarthritis.
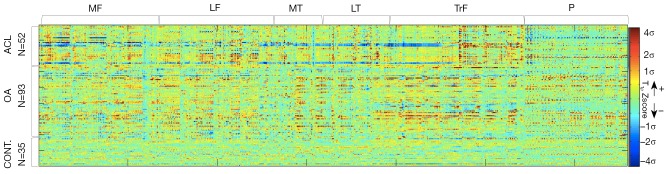
Six cartilage compartments, [medial femoral condyle (MF), lateral femoral condyle (LF), femoral trochlea (TrF), medial tibia (MT), lateral tibia (LT) and patella (P)] were segmented semi-automatically on the reference knee, and the mask obtained from the reference segmentation was applied on all the other images in the dataset. The 3D segmented cartilage regions were then reshaped in a 1D vector for each case (Figure 2).
Figure 2.
Z-score of the 180 subjects (52 ACL, 93 OA and 35 controls) across the 6,858 voxels of the reference cartilage segmentation. ACL, anterior cruciate ligament; OA, osteoarthritis.
Statistical analysis
Each relaxation time map was described as a point in L-dimension space where L is the total number of voxels in the cartilage region [6,858]. The mean Z-score can be computed as:
| [1] |
where N is the number of subjects in the dataset [180] and xi is the vector (1×6,858) of the Z-score values of each subject. The covariance matrix S was used in order to calculate the variation from the mean Z-score map:
| [2] |
Eigenvalue and eigenvector decomposition of this matrix was then used to extract principal components (PCs) in order to calculate the most important modes of variation of all the Z-score maps from the average map. Each PC describes a specific relaxometry pattern, and each Z-score map can be described by a linear combination of these patterns. The coefficient assigned to each PC defined how a specific relaxometry pattern characterizes the analyzed Z-score map. The first ten PCs, that described 87% of the overall variation, were considered in our analysis. PCA guarantees the independency of the features extracted; thus, the 180 Z-score maps are now points in a 10-D orthogonal feature space. The effect of each PC on the average Z-score map can be modeled individually, synthetizing new instances. By changing the value of each PC from the mean to mean ± standard deviations (SD) and observing the relaxometry pattern changes, the interpretation of each component was investigated.
This process was performed for both normalized Z-scores and original T1ρ values. Independent t-tests were used to study differences in the first ten PCs in the two controls groups, control group 1 from OA cohort and control group 2 from ACLR-6M cohort. The aim of this analysis was to establish the ability of the local Z-score normalization in adjusting for the differences, which were not disease (OA) related, in these two groups (age, BMI, MRI protocol settings, MRI machine).
Stepwise logistic regression was then used to identify, within the first ten PCs, possible predictors that were able to distinguish between OA and controls, and between OA and ACL subjects. Receiver operating characteristic (ROC) analysis was used to evaluate the accuracy of the classification expressed as area under the curve (AUC).
Results
PCA performed on the original T1ρ maps showed significant differences in five of the first ten PCs when the two control groups were compared (Table 1). Conversely, no significant differences were observed between the two control groups when the maps were converted in Z-score. Due to this result, we performed the analysis of relaxometry patterns using just local normalized Z-score maps.
Table 1. Comparison of the principal components (PCs) of the two controls group extracted from T1ρ maps and normalized Z-score maps. Averages and standard deviation are presented normalized between 0 and 1 to allow direct comparison between the 2 models.
| Principal components | T1ρ PCA (mean, SD) | Z-score PCA (mean, SD) | |||||
|---|---|---|---|---|---|---|---|
| Control group 1 (N=25) | Control group 2 (N=10) | P value | Control group 1 (N=25) | Control group 2 (N=10) | P value | ||
| PC1 | (1.00, 0.06) | (0.97, 0.09) | 0.2561 | (1.00, 0.18) | (1.00, 0.28) | 0.9927 | |
| PC2 | (0.35, 0.03) | (0.39, 0.12) | 0.0826# | (0.49, 0.11) | (0.49, 0.19) | 0.9865 | |
| PC3 | (0.33, 0.03) | (0.32, 0.07) | 0.0643# | (0.38, 0.11) | (0.44, 0.08) | 0.9582 | |
| PC4 | (0.43, 0.04) | (0.38, 0.04) | 0.2275 | (0.70, 0.13) | (0.68, 0.10) | 0.9839 | |
| PC5 | (0.32, 0.03) | (0.29, 0.04) | 0.0002* | (0.39, 0.12) | (0.44, 0.10) | 0.9755 | |
| PC6 | (0.22, 0.04) | (0.22, 0.02) | 0.0448* | (0.37, 0.09) | (0.49, 0.09) | 0.9987 | |
| PC7 | (0.27, 0.03) | (0.26, 0.03) | 0.0038* | (0.53, 0.10) | (0.48, 0.09) | 0.9735 | |
| PC8 | (0.11, 0.02) | (0.11, 0.04) | 0.0054* | (0.47, 0.12) | (0.44, 0.07) | 0.9960 | |
| PC9 | (0.13, 0.03) | (0.06, 0.05) | 0.0008* | (0.36, 0.09) | (0.34, 0.07) | 0.9961 | |
| PC10 | (0.15, 0.03) | (0.14, 0.06) | 0.6686 | (0.40, 0.09) | (0.33, 0.05) | 0.9966 | |
*, independent t-test significant P vale<0.05; #, approaching significant P value <0.1. PCA, principal component analysis; SD, standard deviations.
In the second experiment, we studied possible predictors of the OA and ACL groups. Four PCs were observed as significant predictors of the OA vs. controls classification, and two of these four were also predictors of ACLR-6M vs. control and ACLR-6M vs. OA classifications Figure 3 shows the values of the average principal components coefficients for the four groups. Just PCs that were observed as groups predictors are shown.
Figure 3.
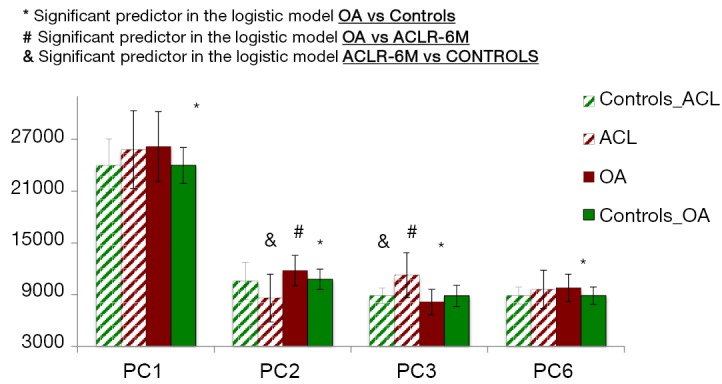
Bar graph representation of the four principal components predictors of OA (PC1, PC2, PC3 and PC6) and predictors of ACL when compared with OA and control (PC2 and PC3). OA, osteoarthritis; PC, principal component; ACL, anterior cruciate ligament.
PC1 was significantly higher in OA subjects compared to controls (P value =0.01). As expected, PC1 is the feature describing the global Z-score average; higher values of the PC1 will lead to more positive Z-score, thus correlate with T1ρ values that are globally longer. Similar values in PC1 were observed between OA and ACL subjects, even though this feature was not one of the predictors for the ACL vs. controls classification. Figure 4 shows the modeling of PC1. Lower values of this PC were observed in controls, and higher values were observed in OA and ACL subjects.
Figure 4.
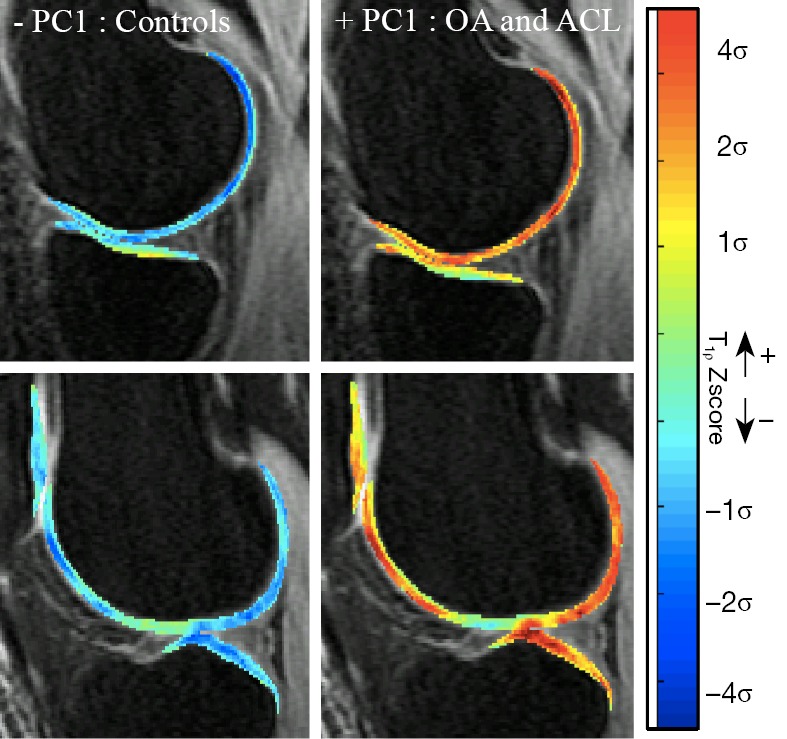
Modeling of PC1: the first column shows the Z-score maps obtained when PC1 was equal to mean − 3SD and the second column shows the Z-score maps obtained when PC1 was equal to mean + 3SD. First and second row show one representative medial and lateral slice respectively. Higher values of PC1 correspond to globally higher Z-score values. Lower values of the PC1 correspond to lower Z-score values. PC, principal component.
OA subjects showed significantly higher PC2 values (P value =0.008), while ACL subjects showed significantly lower PC2 values when compared to controls (P value <0.0001). The modeling of PC2 (Figure 5) demonstrates that higher PC2 values are related to lower Z-score values in the superficial layer, and higher Z-score values in the deep layer when compared to the average subject. However, since the deep layer shows naturally lower T1ρ values than the superficial layer, this effect in Z-score leads to a decreased difference between the two layers. This relationship was inverted for the ACLR-6M subjects, leading, contrary as what observed in OA, to a more emphasized laminar effect.
Figure 5.
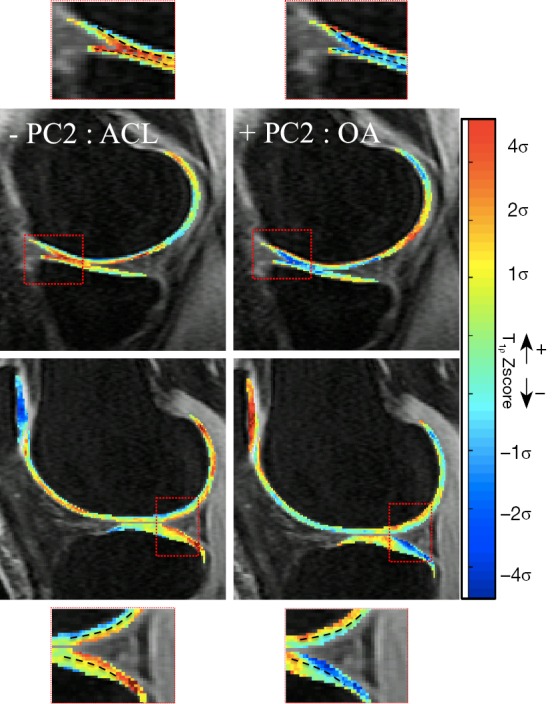
Modeling of PC2: higher values of PC2 correspond to higher deep layer Z-score and lower superficial layer Z-score leading to more similar values of the layers. Lower values of PC2 correspond to lower deep layer Z-score and higher superficial layer Z-score leading to lower difference in the values of two layers. PC, principal component.
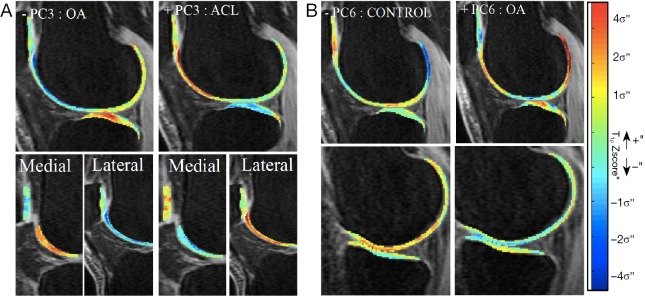
Subjects in the OA group also demonstrated significantly lower values in PC3 (P value =0.03), characterized by a concentrated higher Z-scored in weight-bearing region of lateral tibia and elevation in the trochlea that involves the medial trochlear facet more than the lateral (Figure 6A). In this case, the feature is opposite for ACLR-6M, and highly distinctive between the two groups (P value <0.0001). ACLR-6M subjects were characterized by higher Z-scores in the posterior lateral tibia, and the elevation in the trochlea compartments it is more locally located (anterior aspect of the lateral femur). Lastly, PC6 was observed to be the strongest OA predictor (P value =0.007), but no differences were observed in these features for the ACLR-6M. OA subjects showed significantly higher PC6, the pattern describing Z-score elevation in the lateral femur that is located outside of the weight-bearing area, appearing concentrated in the posterior and anterior aspects, and more centrally located in the lateral tibia (Figure 6B).
Figure 6.
Modeling of PC3 and PC6. (A) PC3: higher values of PC3 correspond to Z-score elevation in posterior lateral tibia and lateral trochlea. Lower values of PC3 correspond to Z-score elevation in central lateral tibia and medial trochlea; (B) PC6: higher values of PC6 correspond to Z-score elevation in posterior lateral femur and central lateral tibia. Lower values of PC6 correspond to Z-score reduction in posterior lateral femur. PC: principal component.
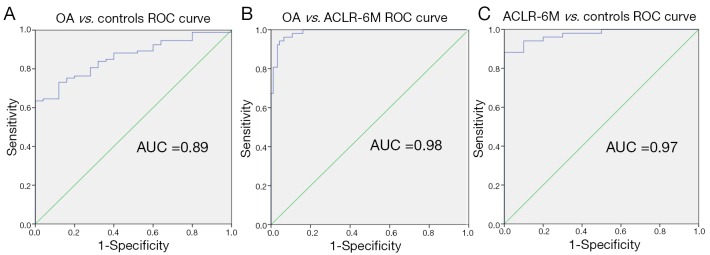
The orthogonality of the PCs makes these features good candidates for simultaneously classifying OA and ACLR-6M groups. Considering 4-dimensional description of the overall OA relaxation time map pattern, combining the significant predictors of the regression model (PC1, PC2, PC3, PC6) and a 2-dimensional description for the ACLR-6M subjects using (PC2, PC3), we were able to classify OA vs. controls, obtaining ROC AUC =0.89 (Figure 7A), to classify OA vs. ACLR-6M with ROC AUC =0.98 (Figure 7B) and to classify ACLR-6M vs. controls with ROC AUC =0.97 (Figure 7C).
Figure 7.
Receiver operating characteristic (ROC) representation of: (A) OA vs. controls binary classifications (AUC =0.89); (B) OA vs. ACLR-6A binary classifications (AUC =0.98); (C) ACLR-6A vs. controls binary classifications (AUC =0.97). OA, osteoarthritis; AUC, area under the curve; ACLR, anterior cruciate ligament reconstruction.
Discussion
In this study, two cohorts (OA and ACLR-6M) were analyzed using a PCA-VBR technique. Voxel-based Z-score maps normalized the spatial distribution of T1ρ to that seen in control subjects, enabling the direct comparison of subjects from two cohorts acquired with different scanners and with significantly different demographics. Li et al. (25) discussed the longitudinal reproducibility of T1ρ time quantification when different coils, MRI systems and sites were used. While excellent long-term reproducibility in a phantom was demonstrated, a significant effect of different models of MR systems and coils was also reported, making direct comparisons between scans done on different systems a difficult task without proper cross-calibration. It is also well-known that demographics such as age can affect T1ρ values independently from disease status. Goto et al. (28,29) studied the natural age-related changes in T1ρ, showing a significant association between T1ρ values and age. Spatial variation within the cartilage in the superficial and deep layer, and between weight-bearing and non-weight-bearing regions have also been extensively documented (30). While T1ρ may be a sensitive and early biomarker of cartilage degeneration, it is extremely important to be able to observe and quantify real relaxation time differences independently from the MRI scanner or coil used, specific demographic characteristics, and the spatial location within cartilage. By normalizing the data to that obtained in identical regions in a control cohort, we have eliminated these confounding variables. The fully automatic nature of VBR and the ability to perform local normalization is an essential step for the clinical translation of T1ρ relaxation time mapping techniques.
PCA revealed that OA subjects showed globally higher Z-score values compared with controls. This is not a surprising result as T1ρ prolongation in OA subjects is a well-known phenomenon, observed in several previous studies (31). However, our results suggested that, in addition to a global average relaxation time prolongation, anomalies observed outside of the weight-bearing area, particularly in the posterior lateral femur and femoral trochlea, could be key biomarkers for OA detection. Additionally, OA subjects showed lower differences in the two layers compared with controls and ACL subjects. While several previous studies adopted laminar analysis strategies to separately characterize the biochemical composition of the two layers to improve sensitivity (19,32), no previous report has explored the usage of the difference between the two layers to study OA. However, in OA subjects, the integrity of the collagen matrix and permeability of fluid in the layer, which is critical to maintain the cartilage mechanical properties (33), is compromised. This results in an obscure distinction between superficial and deep layers, making the difference between cartilage layer relaxation times a plausible imaging biomarker associated with OA. Souza et al. (34) observed a similar effect of decreased differences between the two layers while studying static loading in knee articular cartilage relaxation times. T1ρ values were observed to increase with loading in the deep layer and decrease in the superficial layer. In that study, changes in relaxation times due to loading were observed to be generally larger in the OA group, suggesting a reduced ability to dissipate loads and a decreased ability to retain water in OA subject cartilage.
Conversely, the cartilage laminar appearance was more emphasized in ACL patients, showing more elevated relaxation times in the superficial layer compared to the deep. This result is consistent with a previous study that demonstrated superficial layer T1ρ relaxation times increasing 1 year after ACL reconstruction; no differences in the deep layer were reported (15). Similarly, Bae et al. (35) showed prolongation in T2 relaxation times solely in the superficial layer when analyzing the differences between injured and contralateral knees 3 years after ACL reconstruction. T2 elevation in the superficial layer was also observed by Liebl et al. (36) as a significant predictor of incident OA four years before radiological signs, suggesting that changes in biochemical articular cartilage composition in the most superficial layer precede radiological manifestations of disease and could be considered as an early OA biomarker.
In both ACL and OA subjects, we observed an elevation of T1ρ in the lateral tibia compartment; however, the spatial distribution of these elevations was distinctive between the two groups. OA subjects showed elevation in the central weight-bearing area of the lateral tibia, while subjects after ACL reconstruction demonstrated a more posteriorly located elevation. Russell et al. (22) recently studied the T1ρ longitudinal progression in ACL subjects using VBR, and showed a similar effect of shifting elevation in lateral tibia between baseline and 6 months after ACL reconstruction. When the elevation was observed in the most posterior aspect of the lateral tibia at baseline, the elevation was more anteriorly located at 6 months. In the present study, we complemented those observations, demonstrating an even more anteriorly located lateral tibia elevation pattern in OA subjects. This result may suggest the potential usage of the location of T1ρ elevation in lateral tibia as a possible biomarker to early stratify subjects that may be deviating towards OA patterns after ACL reconstruction.
Another feature observed to be very distinctive between OA and ACL was the location of the T1ρ elevation in the trochlea compartment. OA subjects showed elevations in the medial trochlear facet, while ACL-injured subjects in the lateral facet. Previous studies reported that valgus knee alignment and direction or force of the quadriceps femoris muscle could influence the progression of lateral patellofemoral arthritis (37). Teng et al. (38) observed associations of T1ρ and T2 relaxation times with the presence of cartilage lesions in the patellofemoral joint, while studying the superficial and deep layers separately. However, no previous studies have considered a sub-compartmental analysis of the trochlea compartment in the medial and lateral facets for the T1ρ quantification in OA or ACL subjects.
Despite the promising results reported in the present study, some limitations need to be acknowledged. The cross-sectional nature of this study does not allow for the exploration of longitudinal changes in relaxometry patterns of ACL-injured subjects, an essential step to validate the proposed technique and establish its ability to identify subgroups of patients at risk for developing posttraumatic OA. In this analysis, we considered the compositional biochemical aspect of the cartilage degeneration process. However, it is well known that OA is a multifactorial disease, including morphological, biomechanical and inflammatory components. More comprehensive analyses, simultaneously considering those aspects, are definitely needed. PCA is one of many methods for feature extraction, and paired with supervised deep learning (39,40), could lead to an even better classification.
In conclusion, the results of this study suggest that PCA-VBR maybe a promising technique to extract patterns of change in cartilage biochemistry that may be potentially useful for OA phenotyping and early stratifying patients that are at risk for developing posttraumatic OA after ACL injury, suggesting different patient management and therapeutic approaches in the future.
Acknowledgements
This study was possible thanks to the combined efforts of the AF-ACL Consortium.
Funding: This study was funded by the Arthritis Foundation (AF) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, a subset of the National Institutes of Health, under Award Number P50 AR-060752 and R01AR046905. HSS and UCSF received funding from AF and General Electric Healthcare. Mayo received funding from AF.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Arthritis Foundation.
Ethical Statement: All subjects gave informed consent, and the study was carried out in accordance with the regulations of the Committee for Human Research.
Conflicts of Interest: The authors have no conflicts of interest to declare.
The Arthritis Foundation-ACL Consortium: The HSS (New York City, New York) team for the AF-ACL Consortium includes: Matthew F. Koff, PhD; Steven R. Goldring, MD; Mary Goldring, PhD; Jo A. Hannafin, MD, PhD; Robert G. Marx, MD, MSc, FRCSC; Danyal H. Nawabi, MD, FRCS; Miguel Otero, PhD; Hollis Potter, MD; Scott A. Rodeo, MD; Parina Shah, MSc; Russell F. Warren, MD. The Mayo Clinic (Rochester, Minnesota) team for the AF-ACL Consortium includes: Kimberly K. Amrami, MD; Joel P. Felmlee, PhD; Matthew A. Frick, MD; Aaron J. Krych, MD; Michael J. Stuart, MD; Steven L. Williams, BS. The UCSF (San Francisco, California) team for the AF-ACL Consortium includes: Keiko Amano, MD; Maxwell Cheong, MSc; Martin Kretzschmar, MD; Drew A. Lansdown, MD; Alan Li, BS; Xiaojuan Li, PhD; Thomas M. Link, MD; C. Benjamin Ma, MD; Sharmila Majumdar, PhD; Narihiro Okazaki, MD; Valentina Pedoia, PhD; Colin Russell, BA; Dragana Savic, MSc; Benedikt Schwaiger, MD; Favian Su, BS; Cory Wyatt, PhD. The Albert Einstein team (Bronx, New York) for the Arthritis Foundation Consortium includes: John A. Hardin, MD.
References
- 1.Mankin HJ, Brandt KD. Pathogenesis of arthritis. Textbook of Rheumatology. Philadelphia: W.B. Saunders, 1993. [Google Scholar]
- 2.Li X, Majumdar S. Quantitative MRI of articular cartilage and its clinical applications. J Magn Reson Imaging 2013;38:991-1008. 10.1002/jmri.24313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le J, Peng Q, Sperling K. Biochemical magnetic resonance imaging of knee articular cartilage: T1rho and T2 mapping as cartilage degeneration biomarkers. Ann N Y Acad Sci 2016;1383:34-42. 10.1111/nyas.13189 [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Regatte RR. T1ρ MRI of human musculoskeletal system. J Magn Reson Imaging 2015;41:586-600. 10.1002/jmri.24677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nieminen MT, Rieppo J, Töyräs J, Hakumäki JM, Silvennoinen J, Hyttinen MM, Helminen HJ, Jurvelin JS. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med 2001;46:487-93. 10.1002/mrm.1218 [DOI] [PubMed] [Google Scholar]
- 6.Akella SV, Regatte RR, Wheaton AJ, Borthakur A, Reddy R. Reduction of residual dipolar interaction in cartilage by spin-lock technique. Magn Reson Med 2004;52:1103-9. 10.1002/mrm.20241 [DOI] [PubMed] [Google Scholar]
- 7.Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, Ries MD, Horvai A, Link TM, Majumdar S. Quantitative MRI using T1ρ and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging 2011;29:324-34. 10.1016/j.mri.2010.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, Carballido-Gamio J, Ries M, Majumdar S. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage 2007;15:789-97. 10.1016/j.joca.2007.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Han ET, Ma CB, Link TM, Newitt DC, Majumdar S. In vivo 3T spiral imaging based multi-slice T(1rho) mapping of knee cartilage in osteoarthritis. Magn Reson Med 2005;54:929-36. 10.1002/mrm.20609 [DOI] [PubMed] [Google Scholar]
- 10.Eagle S, Potter HG, Koff MF. Morphologic and quantitative magnetic resonance imaging of knee articular cartilage for the assessment of post-traumatic osteoarthritis. J Orthop Res 2016. [Epub ahead of print]. 10.1002/jor.23345 [DOI] [PubMed] [Google Scholar]
- 11.Su F, Hilton JF, Nardo L, Wu S, Liang F, Link TM, Ma CB, Li X. Cartilage morphology and T1ρ and T2 quantification in ACL-reconstructed knees: a 2-year follow-up. Osteoarthritis Cartilage 2013;21:1058-67. 10.1016/j.joca.2013.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osaki K, Okazaki K, Takayama Y, Matsubara H, Kuwashima U, Murakami K, Doi T, Matsuo Y, Honda H, Iwamoto Y. Characterization of Biochemical Cartilage Change After Anterior Cruciate Ligament Injury Using T1ρ Mapping Magnetic Resonance Imaging. Orthop J Sports Med 2015;3:2325967115585092. 10.1177/2325967115585092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prasad AP, Nardo L, Schooler J, Joseph GB, Link TM. T1ρ and T2 relaxation times predict progression of knee osteoarthritis. Osteoarthritis Cartilage 2013;21:69-76. 10.1016/j.joca.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishioka H, Nakamura E, Hirose J, Okamoto N, Yamabe S, Mizuta H. MRI T1ρ and T2 mapping for the assessment of articular cartilage changes in patients with medial knee osteoarthritis after hemicallotasis osteotomy. Bone Joint Res 2016;5:294-300. 10.1302/2046-3758.57.BJR-2016-0057.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, Ma CB, Majumdar S. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2--initial experience with 1-year follow-up. Radiology 2011;258:505-14. 10.1148/radiol.10101006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su F, Pedoia V, Teng HL, Kretzschmar M, Lau BC, McCulloch CE, Link TM, Ma CB, Li X. The association between MR T1ρ and T2 of cartilage and patient-reported outcomes after ACL injury and reconstruction. Osteoarthritis Cartilage 2016;24:1180-9. 10.1016/j.joca.2016.01.985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedoia V, Su F, Amano K, Li Q, McCulloch CE, Souza RB, Link TM, Ma BC, Li X. Analysis of the articular cartilage T1ρ and T2 relaxation times changes after ACL reconstruction in injured and contralateral knees and relationships with bone shape. J Orthop Res 2016. [Epub ahead of print]. 10.1002/jor.23398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carballido-Gamio J, Stahl R, Blumenkrantz G, Romero A, Majumdar S, Link TM. Spatial analysis of magnetic resonance T1rho and T2 relaxation times improves classification between subjects with and without osteoarthritis. Med Phys 2009;36:4059-67. 10.1118/1.3187228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schooler J, Kumar D, Nardo L, McCulloch C, Li X, Link TM, Majumdar S. Longitudinal evaluation of T1ρ and T2 spatial distribution in osteoarthritic and healthy medial knee cartilage. Osteoarthritis Cartilage 2014;22:51-62. 10.1016/j.joca.2013.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams A, Winalski CS, Chu CR. Early articular cartilage MRI T2 changes after anterior cruciate ligament reconstruction correlate with later changes in T2 and cartilage thickness. J Orthop Res 2016. [Epub ahead of print]. 10.1002/jor.23358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedoia V, Li X, Su F, Calixto N, Majumdar S. Fully automatic analysis of the knee articular cartilage T1ρ relaxation time using voxel-based relaxometry. J Magn Reson Imaging 2016;43:970-80. 10.1002/jmri.25065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell C, Pedoia V, Amano K, Potter H, Majumdar S; AF-ACL Consortium. Baseline cartilage quality is associated with voxel-based T1ρ and T2 following ACL reconstruction: A multicenter pilot study. J Orthop Res 2016. [Epub ahead of print] 10.1002/jor.23277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell C, Pedoia V, Majumdar S; AF-ACL Consortium. Composite metric R2- R1ρ (1/T2 - 1/T1ρ) as a potential MR imaging biomarker associated with changes in pain after ACL reconstruction: A six-month follow-up. J Orthop Res 2016. [Epub ahead of print] 10.1002/jor.23400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Wyatt C, Rivoire J, Han E, Chen W, Schooler J, Liang F, Shet K, Souza R, Majumdar S. Simultaneous acquisition of T1ρ and T2 quantification in knee cartilage: repeatability and diurnal variation. J Magn Reson Imaging 2014;39:1287-93. 10.1002/jmri.24253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Pedoia V, Kumar D, Rivoire J, Wyatt C, Lansdown D, Amano K, Okazaki N, Savic D, Koff MF, Felmlee J, Williams SL, Majumdar S. Cartilage T1ρ and T2 relaxation times: longitudinal reproducibility and variations using different coils, MR systems and sites. Osteoarthritis Cartilage 2015;23:2214-23. 10.1016/j.joca.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein S, Staring M, Murphy K, Viergever MA, Pluim JP. elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 2010;29:196-205. 10.1109/TMI.2009.2035616 [DOI] [PubMed] [Google Scholar]
- 27.Shamonin DP, Bron EE, Lelieveldt BP, Smits M, Klein S, Staring M, Alzheimer's Disease Neuroimaging Initiative . Fast parallel image registration on CPU and GPU for diagnostic classification of Alzheimer's disease. Front Neuroinform 2014;7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goto H, Iwama Y, Fujii M, Aoyama N, Kubo S, Kuroda R, Ohno Y, Sugimura K. A preliminary study of the T1rho values of normal knee cartilage using 3T-MRI. Eur J Radiol 2012;81:e796-803. 10.1016/j.ejrad.2012.03.022 [DOI] [PubMed] [Google Scholar]
- 29.Goto H, Iwama Y, Fujii M, Aoyama N, Kubo S, Kuroda R, Ohno Y, Sugimura K. The natural degeneration course in the T1rho values of normal knee cartilage. Kobe J Med Sci 2012;57:E155-70. [PubMed] [Google Scholar]
- 30.Li X, Pai A, Blumenkrantz G, Carballido-Gamio J, Link T, Ma B, Ries M, Majumdar S. Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med 2009;61:1310-8. 10.1002/mrm.21877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang EY, Ma Y, Du J. MR Parametric Mapping as a Biomarker of Early Joint Degeneration. Sports Health 2016;8:405-11. 10.1177/1941738116661975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar D, Souza RB, Singh J, Calixto NE, Nardo L, Link TM, Li X, Majumdar S. Physical activity and spatial differences in medial knee T1rho and t2 relaxation times in knee osteoarthritis. J Orthop Sports Phys Ther 2014;44:964-72. 10.2519/jospt.2014.5523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health 2009;1:461-8. 10.1177/1941738109350438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Souza RB, Kumar D, Calixto N, Singh J, Schooler J, Subburaj K, Li X, Link TM, Majumdar S. Response of knee cartilage T1rho and T2 relaxation times to in vivo mechanical loading in individuals with and without knee osteoarthritis. Osteoarthritis Cartilage 2014;22:1367-76. 10.1016/j.joca.2014.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bae JH, Hosseini A, Wang Y, Torriani M, Gill TJ, Grodzinsky AJ, Li G. Articular cartilage of the knee 3 years after ACL reconstruction. A quantitative T2 relaxometry analysis of 10 knees. Acta Orthop 2015;86:605-10. 10.3109/17453674.2015.1039426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liebl H, Joseph G, Nevitt MC, Singh N, Heilmeier U, Subburaj K, Jungmann PM, McCulloch CE, Lynch JA, Lane NE, Link TM. Early T2 changes predict onset of radiographic knee osteoarthritis: data from the osteoarthritis initiative. Ann Rheum Dis 2015;74:1353-9. 10.1136/annrheumdis-2013-204157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim YM, Joo YB. Patellofemoral osteoarthritis. Knee Surg Relat Res 2012;24:193-200. 10.5792/ksrr.2012.24.4.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teng HL, Calixto NE, MacLeod TD, Nardo L, Link TM, Majumdar S, Souza RB. Associations between patellofemoral joint cartilage T1ρ and T2 and knee flexion moment and impulse during gait in individuals with and without patellofemoral joint osteoarthritis. Osteoarthritis Cartilage 2016;24:1554-64. 10.1016/j.joca.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LeCun Y, Bengio Y, Hinton G. Deep learning. Nature 2015;521:436-44. 10.1038/nature14539 [DOI] [PubMed] [Google Scholar]
- 40.Lee H, Grosse R, Ranganath R, Ng AY. Unsupervised Learning of Hierarchical Representations with Convolutional Deep Belief Networks. Communications of the ACM 2011;54:95-103. 10.1145/2001269.2001295 [DOI] [Google Scholar]