Abstract
Irreversible Bruton tyrosine kinase (BTK) inhibitors, ibrutinib and acalabrutinib have demonstrated remarkable clinical responses in multiple B-cell malignancies. Acquired resistance has been identified in a sub-population of patients in which mutations affecting BTK predominantly substitute cysteine 481 in the kinase domain for catalytically active serine, thereby ablating covalent binding of inhibitors. Activating substitutions in the BTK substrate phospholipase Cγ2 (PLCγ2) instead confers resistance independent of BTK. Herein, we generated all six possible amino acid substitutions due to single nucleotide alterations for the cysteine 481 codon, in addition to threonine, requiring two nucleotide substitutions, and performed functional analysis. Replacement by arginine, phenylalanine, tryptophan or tyrosine completely inactivated the catalytic activity, whereas substitution with glycine caused severe impairment. BTK with threonine replacement was catalytically active, similar to substitution with serine. We identify three potential ibrutinib resistance scenarios for cysteine 481 replacement: (1) Serine, being catalytically active and therefore predominating among patients. (2) Threonine, also being catalytically active, but predicted to be scarce, because two nucleotide changes are needed. (3) As BTK variants replaced with other residues are catalytically inactive, they presumably need compensatory mutations, therefore being very scarce. Glycine and tryptophan variants were not yet reported but likely also provide resistance.
Introduction
Bruton tyrosine kinase (BTK) is a member of the tyrosine kinase expressed in hepatocellular carcinoma (TEC) family, which is the second largest family of human non-receptor tyrosine kinases.1, 2, 3 BTK is an essential component of B-cell receptor (BCR) signaling and has a crucial role in B-cell development and activation.4, 5, 6 Loss-of-function variations of BTK cause X-linked agammaglobulinemia (XLA) in humans.7, 8, 9, 10, 11, 12 BTK is a multi-domain protein of 659 amino acids, consisting of N-terminal Pleckstrin homology (PH) and Tec homology (TH) domains, followed by Src homology 3 (SH3), 2 (SH2) and C-terminal catalytic (SH1) domains.1, 2, 3
BTK is found in cells of hematopoietic origin, including both lymphoid and myeloid lineages and participates in different pathways in B-cell signaling.13, 14, 15 It is also highly expressed in many B-cell leukemias and lymphomas. BTK-dependent signaling pathways are involved in the pathogenesis of B-cell leukemia and lymphoma, as this protein is crucial for the survival and growth of the malignant cells.16, 17 BTK is important for chemotaxis and adhesion, controlling the homing and migration of tumor cells.18, 19, 20 Crucially, based on recent clinical trials, BTK is considered as an important therapeutic target for the treatment of B-cell malignancies.16, 17, 18, 21, 22, 23, 24, 25, 26
Although several inhibitors for BTK have been developed, the most studied drug, ibrutinib is the first compound in a new class of orally administered, irreversible inhibitors binding covalently to cysteine 481 in the catalytic kinase domain. Ibrutinib thereby blocks BTK activation and inhibits downstream BCR signaling.17, 21, 27, 28, 29, 30 Ibrutinib has demonstrated clinically significant activity in several B-cell malignancies, and is approved by FDA for the treatment of chronic lymphocytic leukemia (CLL), mantle cell lymphoma and Waldenström's macroglobulinemia.21, 22, 23 Recently, a second-generation BTK inhibitor, acalabrutinib, has been developed and demonstrated very good treatment effects.26
Drug resistance is a common problem during cancer treatment as it limits the effectiveness of the therapy. The resistance can arise before or during treatment.31 Recent studies report the development of acquired resistance to both ibrutinib and acalabrutinib in a sub-population of patients with CLL and mantle cell lymphoma.26, 32, 33, 34 Until now, point mutations causing single amino acid replacement in BTK as well as acquired activating variations in PLCγ2 have been reported.32 In most patients with progressive CLL after ibrutinib therapy, the resistance has been shown to result from substitution of C481 by serine at the ibrutinib-binding site in BTK, altering the irreversible covalent binding of ibrutinib to a reversible interaction and decreasing ibrutinib's affinity for BTK, leading to drug resistance.34, 35 However, rare cases with other BTK variations like C481F/R/Y, T474I/S and L528W have also been identified.36 PLCγ2 variations also appear in a subset of mutation-prone patients with CLL.32, 36, 37 The PLCγ2 variations are gain-of-function substitutions causing BTK-independent activation of BCR signaling owing to that PLCγ2 is a substrate for BTK.37, 38 As it is plausible that other BTK variations could also cause ibrutinib resistance, the aim of this study was to determine the effect of all possible amino acid substitutions resulting from the most frequent mutational event, namely single nucleotide changes at the C481 codon in BTK gene. Given threonine's structural and functional similarity to serine, we also investigated the effect of replacing C481 with threonine for which two nucleotide changes are needed.
Materials and methods
Plasmids
Plasmids encoding BTK substitutions (C481 to arginine (R), glycine (G), phenylalanine (F), serine (S), tryptophan (W), tyrosine (Y) and threonine (T)) were generated by site-directed mutagenesis, and the resulting variants were verified by sequencing.
Cell culture and transfections
COS-7 (African green monkey fibroblast-like kidney), HEK-293T (human embryonic kidney cells) and DT40 (chicken lymphoma cells) were obtained from the American Type Culture Collection. The B7.10 cell line (DT40 chicken lymphoma cells in which the BTK gene is inactivated) was generated in Dr T. Kurosaki's laboratory,5 and has been characterized in great detail previously.39 COS-7 and HEK-293T cells were cultured in Dulbecco's modified eagle medium supplemented with 10% heat-inactivated fetal bovine serum. DT40 and B7.10 cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 5% chicken serum, 5% glutamine, 50 μm 2-mercaptoethanol and penicillin/streptomycin. Cells were grown at 37 °C under 5% CO2. COS-7 and HEK-293T cells were transiently transfected in six-well plates using polyethylenimine (Polyscience, Inc., Warrington, PA, USA). B7.10 cells were transfected using the Neon transfection system (Life technologies, La Jolla, CA, USA).
Antibodies and chemical reagents
The following antibodies were used in this study: polyclonal rabbit anti-BTK and anti-actin were from Sigma-Aldrich, St. Louis, MO, USA. Anti-PLCγ2, anti-extracellular signal-regulated kinases (ERK) and anti-phospho-ERK (pY204) were from Santa Cruz Biotechnology, Dallas, TX, USA, anti-phospho-BTK (pY551) from BD Pharmingen, Franklin Lakes, NJ, USA. Anti-phospho-PLCγ2 (pY753) and anti-phospho-BTK (pY223) were from Abcam, Cambridge, UK. Anti-chicken IgM was from Southern Biotech (Birmingham, AL, USA) and ibrutinib was obtained from Selleckchem, Houston, TX, USA.
Immunoprecipitation and immunoblotting
Immunoprecipitation and immunoblotting analyses were performed as previously described.40 The following secondary antibodies were used, goat anti-mouse 800CW, goat anti-rabbit 800CW, goat anti-mouse 680LT or goat anti-rabbit 680 (all from LI-COR Biosciences GmbH, Lincoln, NE, USA). The membranes were scanned using Odyssey Imager from LI-COR Biosciences GmbH.
In vitro kinase assay
Plasmids expressing the wild-type PLCγ2, BTK or the BTK variants were transfected into HEK-293T cells. The cells were starved overnight and subsequently the proteins were purified and isolated by immunoprecipitation. Enzymatic assays were carried out using kinase buffer obtained from Cell Signaling Technology, Danvers, MA, USA. The buffer consists of 25 mm Tris–HCl (pH 7.5), 5 mm β-glycerophosphate, 2 mm dithiothreitol, 0.1 mm Na3VO4, 10 mm MgCl2. The kinase reaction mixture was incubated for 30 min at 30 °C in the presence of 100 μm adenosine triphosphate (ATP). The reactions were stopped by the addition of sample buffer, and the proteins were resolved on 4–12% SDS–PAGE and the phosphorylation was visualized by the western blot analysis.
Results
All BTK variants are stable and expressed at the protein level
The variant forms of BTK were created by replacing C481 with all six different amino acids, due to single nucleotide substitutions in this codon (Figure 1a). Owing to the structural and functional similarity between serine and threonine, we also generated this replacement, even though two nucleotide substitutions are required. In order to determine the expression of the different BTK variants, we first transiently transfected the BTK variant plasmids into a non-lymphoid cell lines, COS-7, enabling the analysis without any influence of the endogenous protein. We detected protein expression by immunoblotting. All variants demonstrated equal expression at the protein level (Figure 1b).
Figure 1.
Expression of the BTK protein variants. (a) Substitution of C481 with seven different amino acids was generated by replacing one (all variants except for threonine) or two (threonine) nucleotides. (b) Whole-cell lysates of COS-7 cells transfected with plasmids encoding the BTK variants were processed for western blotting and proteins were detected using rabbit polyclonal antibody to BTK.
BTK variants show differential catalytic activity
BTK has two critical tyrosine phosphorylation sites, Y551 and Y223. Y551 in the kinase domain is phosphorylated by SRC family kinases (SFKs), whereas Y223, located in the SH3 domain, is autophosphorylated.41, 42, 43 In order to investigate the correlation between the structure and the function of the BTK variants, we analyzed their phosphorylation status as well as their enzymatic activity to phosphorylate PLCγ2, which is a bona fide BTK substrate. We transiently co-transfected HEK-293T and COS-7 cells with plasmids expressing the wild type or the variant forms of BTK together with plasmids encoding PLCγ2. As expected, both the serine variant and wild-type BTK demonstrated substantial phosphorylation at both tyrosines (Y223, Y551), and they potently phosphorylated PLCγ2 at Y753 (Figures 2a and b; Supplementary Figure 1). The same was true for the threonine variant, for the first time demonstrating retained catalytic activity following substitution of C481 with another residue than serine.
Figure 2.
Functional characterization of the BTK C481 variants. (a and b) Whole-cell lysates of COS-7 and HEK-293T cells expressing either wild type or variant BTK along with the substrate PLCγ2. Thirty-six hours post transfection, the cells were serum starved overnight and then treated with serum, and the phosphatase inhibitor pervanadate for 5 min at room temperature. Subsequently, total and phosphorylated proteins were detected. (c) BTK-deficient B7.10 cells were transfected with either wild type or variant BTK. Forty-two hours post transfection, the cells were serum starved for 6 h, and then activated with anti-chicken IgM (10 μg/ml) and H2O2 (4 mm) for 5 min at room temperature.
The glycine variant was phosphorylated both at Y223 and Y551 and phosphorylated PLCγ2 at Y753, but to a much lower extent than BTK with serine or threonine substitution. In contrast, phosphorylation at both tyrosine sites in BTK was severely compromised when C481 was replaced with any of the other amino acids, and these variants were unable to phosphorylate PLCγ2 (Figures 2a and b; Supplementary Figure 1). As these results were obtained from cells outside the B-cell lineage, we subsequently assessed the catalytic activity of the variants in a B-cell line. We used BTK-deficient B7.10 chicken B cells and the corresponding wild-type lymphoma cell, DT40. The results from these experiments confirm that the serine and threonine substitutions, and to a much lesser extent, the glycine variant retain kinase activity (Figure 2c; Supplementary Figures 1 and 3). In order to investigate further downstream signaling, we have also studied ERK phosphorylation in B cells. Interestingly, after activation, all variants showed measurable phosphorylation (Figure 2c; Supplementary Figure 3).
In order to demonstrate whether PLCγ2 was directly phosphorylated by the BTK variants, we performed in vitro kinase assays. Only BTK with serine or threonine replacement phosphorylated PLCγ2 (Figure 3). Thus, under these conditions, the glycine variant did not detectably phosphorylate PLCγ2 (Figure 3).
Figure 3.
Kinase assay for the BTK variants. BTK variants and PLCγ2 were transfected in HEK-293T cells, the cells were serum starved overnight, whereafter the proteins were purified and isolated by immunoprecipitation. Subsequently, they were mixed and incubated in kinase buffer containing 100 μm ATP for 30 min at 30 °C, and probed for the detection of PLCγ2 and BTK phosphorylation by immunoblotting.
BTK C481S and C481T confer resistance to ibrutinib
In order to investigate the ibrutinib sensitivity of the two active BTK variants with serine or threonine replacement, we analyzed the phosphorylation of BTK at both tyrosine sites (Y223, Y551) and PLCγ2 at Y753 after treatment with different concentrations of the drug. The wild-type BTK and the two variants were expressed in COS-7 and B7.10 cells. For wild-type BTK, phosphorylation of PLCγ2 at Y753 and BTK at Y223 but not at Y551, was inhibited strongly after treatment with 0.5 μm ibrutinib. In contrast, at this concentration phosphorylation was not substantially diminished in the two active variants (Figure 4; Supplementary Figure 2). In addition, ibrutinib washout experiments demonstrated that the two BTK variants bound reversibly to ibrutinib. They were only inhibited when treated continuously with high concentrations of the drug, and the inhibition was eliminated when ibrutinib was washed out (Figure 5). Thus, in addition to the known resistant variant C481S, the C481T substitution also results in ibrutinib resistance.
Figure 4.
BTK C481S and C481T variants show resistance to ibrutinib. (a and b) COS-7 cells were transfected with wild-type BTK or the two variants. Thirty-six hours post transfection, the cells were serum starved and treated with ibrutinib overnight followed by activation with serum and pervanadate for 5 min at room temperature. The cell lysates were immunoblotted for pY223 BTK and pY753 PLCγ2. (c and d) B7.10 cells were transfected with wild-type BTK or with the two variants. Forty-two hours post transfection, the cells were serum starved and treated with ibrutinib for 6 h and subsequently activated with anti-chicken IgM (10 μg/ml) and pervanadate for 5 min at room temperature. The ratio of the signals obtained from phosphorylated protein to total protein for BTK and PLCγ2 was measured by Image Studio Lite Software (LI-COR Biosciences, Lincoln, NE, USA).
Figure 5.
Ibrutinib washout. COS-7 cells were transfected with wild-type BTK or with the two variants. Thirty-six hours post transfection, the cells were treated with 4 μm ibrutinib overnight and then washed four times with phosphate buffer solution. Subsequently cells were incubated at 37 °C for 30 min, and then stimulated with serum and pervanadate for 5 min at room temperature. The ratio of the signals obtained from phosphorylated BTK to total BTK was measured by Image Studio Lite Software (LI-COR Biosciences).
Structure and function correlation of BTK variants
The effects of the variants were evaluated based on the available BTK kinase domain structures. Several structures have been solved including co-crystals with potential inhibitors, however, not together with ibrutinib. C481 is located on the protein surface in the lower lobe of the kinase structure. As the position is on the surface, basically any substitution could be accommodated to the structure. However, because C481 is a functionally crucial residue involved in ATP binding and in the formation of the catalytic conformation of the enzyme, most substitutions at this position would likely be harmful (Figure 6a), as indicated both by PON-BTK12 and PON-P244 tolerance predictors.
Figure 6.
BTK kinase domain structure. (a) Location of C481 in BTK kinase domain structure (PDB entry 3GEN). Ibrutinib (in cyan) was built by modifying another inhibitor, B43 (4-Amino-5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl-cyclopentane), present at 3GEN. C481 (in yellow) has contacts with N485, Y486 and C527 via backbone, and hydrogen bonds with ibrutinib. The side chain has contacts with L483, R525 and ibrutinib. The structure can accommodate all the amino acid substitutions without structural clashes, however, affecting the interactions with the inhibitor, ATP and the activation loop. Y551 and R520 are in blue and the residues G409 and F413 are in green indicating the position of the missing structure for amino acids in the glycine-rich loop. (b) Close up of the variants at position 481. The original cysteine residue is in yellow. The polar –OH head groups of serine (green) and threonine (magenta) can have the same orientation as –SH in cysteine and at least partially substitute for its interaction, despite not being able to bind covalently to ibrutinib.
C481 participates in the orientation of the catalytic R525 in the active conformation. The reason for the C481S and C481T variants to be functional is that both serine and threonine can form similar interactions as the original cysteine. Instead of the –SH group they have an –OH end group, which provides similar electrostatic properties and hence interactions. Modeling predicts that the polar groups can take the same position and thus substitute for each other (Figure 6b). The crucial difference compared to the wild-type protein is that serine and threonine cannot form covalent bonds with ibrutinib. Therefore, these variants are insensitive to ibrutinib.
The aromatic side chains of F, W and Y are big and bulky, and therefore these substitutions lead to structural clashes within the kinase domain as well as intermolecularly with both substrate and inhibitor. Although these side chains are flexible, in the sense that they can adopt different angles relative to the backbone, they cannot form the polar interaction with ibrutinib. The binding site is quite crowded because several residues are involved in substrate and ATP binding, with the catalytic loop tethering to this area as well as to the lid on top of the catalytic site. Although the side chain of arginine is long, it is flexible and can be accommodated to the structure.
The reason for glycine substitution to show activity is probably because it does not have any side chain and thus allows binding. On the other hand, it lacks the polar side chain to interact with R525 and ibrunitib, and therefore has lower catalytic activity. Thus, this variant likely has the correct conformation but lacks functionally important side-chain interactions.
Discussion
Many BTK variants block B-cell development and cause XLA, and the distribution of disease-causing variants in BTK domains varies widely.7, 8, 11, 12, 45 A recent study has evaluated all possible single-nucleotide change originating amino acid substitutions in the BTK kinase domain, and altogether two-thirds of these variations are likely harmful.12
BTK has some mutational hotspots with large numbers of variations. These appear typically at arginine residues.11 The reason for the vulnerability is firstly that some, but not all, arginine codons contain CpG dinucleotides, which are well known mutational hotspots and arginine side chains are difficult to replace by any other residue. Second, as the side chains are elongated and charged, they have many functional and structural interactions.46
Here, we studied variations related to the ibrutinib-binding site in BTK, which cause drug resistance. This study clearly demonstrates that in addition to known resistant variant C481S, replacement with threonine (C481T) also causes ibrutinib resistance according to the biochemical assays (Figures 2 and 3; Table 1). Our ibrutinib sensitivity assay is based on the in vitro testing of the activation status of BTK and the downstream pathway by measuring the phosphorylation of BTK as well as of the substrate PLCγ2 after drug treatment. We believe that the C481T variant is also likely to induce resistance to ibrutinib in a clinical setting, although this has not been formally proven. The C481T variant requires two nucleotide substitutions and the doublet mutation rate has been estimated to only 0.3% of the singleton alteration rate.47 Sequential mutations could also take place, but would first yield serine substitution, which would likely prevail.
Table 1. The characteristics of the BTK variants.
| BTK and variants | Protein | BTK Y551 phosphorylationa | Intracellular BTK Y223 and PLCγ2 Y753 phosphorylationa | IVK BTK Y223 and PLCγ2 Y753 phosphorylationa | Ibrutinib sensitivity |
|---|---|---|---|---|---|
| Cysteine (WT) | Detectable | ++++ | ++++ | ++++ | Yes |
| Serine | Detectable | ++++ | ++++ | ++++ | No |
| Threonine | Detectable | +++ | +++ | +++ | No |
| Glycine | Detectable | ++ | + | — | NR |
| Tyrosine | Detectable | — | — | — | NR |
| Tryptophan | Detectable | — | — | — | NR |
| Phenylalanine | Detectable | — | — | — | NR |
| Arginine | Detectable | — | — | — | NR |
Abbreviations: IVK, in vitro kinase assay; NR, not relevant; WT, wild-type BTK.
Intensity scoring based on visual inspection.
Under activation conditions, both C481S and C481T variants showed substantial phosphorylation of tyrosines Y223 and Y551, and they potently phosphorylated PLCγ2. The two active variants bound reversibly to the drug, thereby conferring treatment resistance. Thus, the ibrutinib washout study shows that the two variants are inhibited under continuous treatment with high concentration of ibrutinib and that this inhibition is abolished when the drug was washed out. In serum of treated patients an ibrutinib concentration of 0.4 μm has been observed,21 which is in the same range as the 0.5 μm that profoundly impaired the activity of wild-type BTK in our assays. Consequently, if these less sensitive variants appear in patient tumor cells, the BTK signaling pathway will remain active during treatment with inhibitors binding irreversibly to C481, favoring the growth and survival of this subset of cancer cells. Even if higher ibrutinib concentrations in cell cultures inhibit the activity of BTK with serine or threonine replacement, in vitro the concentrations are maintained, whereas in patients the drug is continuously turned over. This suggests that increased dosing of ibrutinib will not be sufficient to overcome the effects of such substitutions in a clinical setting.
The C481G variant is phosphorylated at both Y223 and Y551, but to a lower extent than C481S or C481T, and it only weakly phosphorylates PLCγ2. Taken together, this suggests that the glycine variant is incapable of adequately activating downstream signaling, and hence, is not normally expected to provide any survival advantage during treatment with BTK inhibitors.
When C481 was substituted by F, R, W or Y, the BTK protein was expressed, but was catalytically inactive in our assays, as demonstrated by the fact that the phosphorylation of both Y223 and Y551 was severely compromised in COS-7 and HEK-293T cells. Furthermore, we showed that for these four replacements, neither Y223 and Y551 nor the downstream substrate PLCγ2 were phosphorylated in B cells, further strengthening the likelihood of a severe BCR signaling impairment. As active BTK is crucial for the survival of the B-cell-derived cancer cells, we can assume that such acquired variations therefore in most cases would hamper tumor cell survival. A possible exception to the reduced, or absent, signal transduction of the mutants was the finding that phosphorylation of Y204 in ERK upon activation was increased, albeit to a lower extent. Whether this has any functional consequence in terms of cell survival is not known.
On the basis of the sequence alignments comparing human BTK, human TEC and marine sponge BTK, the C481 residue is invariant,11 and replacements at this position are therefore expected to cause XLA when carried in the germline. However, the reason for the invariance is unclear, and given the retained catalytic activity of both C481S and C481T, it is possible that these two substitutions would be tolerated. This is currently under investigation by using genetically altered mice. However, we predict that the other four replacements investigated in this report would cause XLA when carried in the germline. To this end, it should also be pointed out that phylogenetic relationships, as defined by bootstrap analysis identified the insect type of BTK, with the prototype Btk29A in D. melanogaster as forming a related group of kinases.48 We have now analyzed the amino acid corresponding to cysteine 481, and found that this residue is a serine in all insects. This can be exemplified by the D. melanogaster sequence being YMKHGSLLNYL (H. sapiens sequence: YMANGCLLNYL). We have previously shown that human BTK can substitute for Btk29A and restore the activity in deficient flies by expressing human BTK as a transgene.49 Thus, this indicates that these residues may be functionally interchangeable in an insect context.
Although the C481S substitution has been observed in 16 patients treated with ibrutinib or acalabrutinib26, 32, 33, 34, 35, 36, 50 as shown in (Supplementary Table 1), Maddocks et al.36 recently reported C481F/R/Y substitutions in single patients. One CLL patient had C481F variant with an allele frequency of 84%, whereas another had two clones, C481Y with an allele frequency of 29% and C481R with an allele frequency of 24%. The high incidence of serine substitution is highly statistically significant (P<0.0001; χ2 with Yates' correction). Hence, the rarity of C481F/R/Y substitutions, and the fact that we are not aware of any underlying mutational mechanism favoring serine substitution calls for an alternative explanation. This assumption is also supported by the fact that the likelihood of finding two very rare alleles in the same patient is extremely low.
Although certain catalytically inactive cytoplasmic tyrosine kinases can substitute for the natural form, the restoration is only partial.51, 52, 53 Moreover, there are no overt phenotypic differences between patients with XLA with mutations causing complete absence of expression and those expressing a kinase-deficient protein.7 This argues against the possibility that BTK under normal conditions has important functions in the absence of catalytic activity. Moreover, in vitro, BTK-dependent phosphorylation of PLCγ2 has been investigated and an intact BTK ATP-binding site, activation loop tyrosine (Y551) and SH2 domain were required for this activity.54
However, also for BTK there is evidence from transgenic animals of a phenotype, independent of intact kinase activity. Thus, Middendorp et al.55 demonstrated that transgenic mice expressing the kinase-inactive K430R BTK mutant in a BTK/SLP-65 double-deficient background showed reversal of the severe developmental arrest at the pre-B-cell stage. Moreover, K430R BTK could functionally replace wild-type BTK as a tumor suppressor in SLP-65-deficient mice and the authors concluded that BTK exerts its tumor suppressor function in pre-B cells as an adaptor protein, independent of its catalytic activity.55
There is insufficient information about the C481F/R/Y clones appearing in the treated patients to allow for an explanation regarding their existence. It is possible that certain mutations in the BTK gene in the presence of genetic alterations affecting certain other genes could result in a compensatory activity. As in a single patient clones with two very rare substitutions, C481R and C481Y, were found,36 this suggests the existence of a common denominator. We favor the idea that an initial mutational event outside the BTK locus, perhaps appearing even before ibrutinib treatment, sensitized the tumor cell for C481 substitutions to other residues than serine. Genetic analysis of cells from this patient could lead to the discovery of the underlying alteration. We predict that in this patient substitution for glycine, phenylalanine and tryptophan would also confer resistance.
Mutation rates in lymphocytes, including cells from leukemia patients, are considerably higher than in the germline.56 Wang et al.57 detected mutations in protein-coding sequences, corresponding to a mean (±s.d.) somatic mutation rate of 0.72±0.36 per megabase, and an average of 20 non-synonymous mutations per CLL patient. Recently, 990 patients with CLL studied by whole-exome and whole-genome sequencing have been reported.58, 59 From these studies it appears that alterations in the BTK gene were not observed, suggesting that mutations substituting C481 are exclusively detectable following treatment with BTK inhibitors.
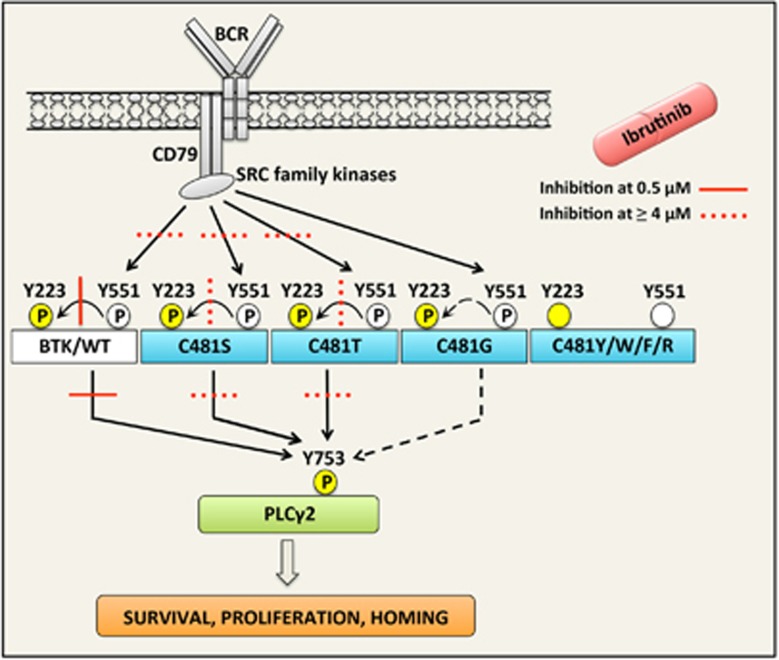
We consider phosphorylation of Y753 in PLCγ2 as the most physiologically relevant readout for BTK catalytic activity, as PLCγ2 is a direct downstream target for BTK.5 Autophosphorylation of Y223 is often used as a relevant indicator of BTK enzymatic activity. This conserved residue in the SH3 domain has a role in non-lymphoid transformation assays,60 but its function for normal B-lineage differentiation remains mainly elusive. Phosphorylation of Y551 in the kinase domain correlates rather well with the inhibitory effect of ibrutinib, although this event is only indirectly related to BTK activity. SFKs are responsible for this phosphorylation.60 In B-lymphocytes, LYN is the most abundant SFK, whereas BLK, which is highly ibrutinib-sensitive, but acalabrutinib-insensitive,26 is present in lower concentrations.61, 62 Whether the reduced pY551 noticed upon ibrutinib addition is secondary to an inhibitory effect on SFKs, or whether the activation loop of BTK is less accessible when ibrutinib binds to cysteine 481, is to the best of our knowledge not known. In contrast to BTK Y223 that shows high sensitivity to ibrutinib, the inhibitor much less affects phosphorylation of Y551. Thus, this outcome is in agreement with what is known about the enzymes involved in inducing these post-transcriptional modifications. In Figure 7, we have tried to provide a summary of how the different BTK variants affect the BCR signaling. We also depict the effect of ibrutinib treatment.
Figure 7.
Schematic representation of the BCR signaling pathway demonstrating the ibrutinib sensitivity of BTK C481 variants and their impact on downstream signaling. Stimulation of the BCR induces phosphorylation of BTK tyrosine 551 through SFKs, and of tyrosine 223 via autophosphorylation. Activated BTK also phosphorylates its downstream substrate, PLCγ2, which in turn promotes the production of the signaling mediators that ultimately promote survival, proliferation and homing. Phosphorylations are denoted with an encircled P; those with white background depict phosphorylations independent of BTK catalysis, whereas those with yellow background mark those induced by BTK. BTK C481 variants show differential catalytic activity. When C481 is substituted by R, F, W or Y the resulting proteins become catalytically inactive, as demonstrated by severely compromised phosphorylation of both Y551 and Y223. C481S and T variants are substantially phosphorylated at both tyrosines, and they potently phosphorylate PLCγ2. The effect of ibrutinib is depicted according to the inhibitory concentration required (0.5 μM and ⩾4 μm) for an effect on BTK pY223 and with the activity on PLCγ2 pY753 being considered similar. Overall, 0.4 μm is the concentration detected in patients,21 whereas the catalytic activity of C481S and T is only inhibited by ⩾4 μm of ibrutinib. The G variant is weakly phosphorylated at Y223 and Y551, and barely phosphorylates PLCγ2. The effect of ibrutinib on C481G was not analyzed, as its catalytic activity was considered insufficient.
Protein kinases undergo several major and minor structural alterations during activation. The upper lobe is turned relative to the lower lobe about 30° mainly around the hinge between the domains, but several structural changes happen when ATP is bound. The glycine-rich loop on the edge of the binding site becomes structurally altered and major changes appear at the activation loop upon phosphorylation. We have predicted pY551 to bind to R520,63 which seems plausible based on the structure in Figure 6. It is possible that binding of ibrutinib to the ATP-binding site induces structural changes similar to those in the catalytically active form. This would mean that the activation loop conformation is altered and most likely this affects the accessibility, and consequently, the phosphorylation status of Y551. Thus, a catalytic-like conformation locked by ibrutinib binding would prevent phosphorylation by SFKs.
In this study we have predicted the occurrence of a new variant of BTK, C481T, which confers resistance to ibrutinib, while being catalytically active. However, except for C481S, all other single-point mutations causing amino acid replacement were found to be catalytically inactive, and even if the C481G variant showed some residual activity, we believe that this is insufficient for survival of tumor cells, unless additional genetic alterations take place. Collectively, we therefore identify three potential ibrutinib resistance scenarios for cysteine 481 replacement: (1) Serine, being catalytically active and predominating among patients, because a single nucleotide alteration is sufficient. (2) Threonine, also being catalytically active, but predicted to be scarce, because two nucleotide changes are needed. This variant was not reported until now. Finally, (3) as BTK variants replaced with arginine, glycine, phenylalanine, tryptophan or tyrosine are all catalytically inactive, they presumably need an additional, compensatory mutation and therefore are very scarce among patients. However, they have been reported, for 3 of the 5 of the variants (F/R/Y) already found, albeit, as yet, only in single patients. Glycine and tryptophan variants were not reported as yet, but our prediction is that they should also confer resistance.
Although ibrutinib is the first-in-class BTK inhibitor, other irreversible binders are under development,28, 64 including the recently studied acalabrutinib.26 Even if they will show differential activity, including side effects, depending on their specificity,62 we predict that the resistance pattern will be similar for all compounds, which bind covalently to C481 in BTK. However, if novel irreversible C481-binders would show enhanced selectivity for BTK, yielding tolerable side effects even when given at higher doses, it may be possible to achieve levels of the inhibitor that would significantly block even the C481 escape variants. Moreover, in the event that the inhibition of the T-cell kinase ITK65, 66 could be avoided by the use of such more selective inhibitors, this might have a positive influence on the treatment outcome, as the recent development of checkpoint inhibitors67 clearly demonstrates that cellular immunity could represent a clinically high relevant form of anti-tumor activity. To this end, recent reports also demonstrate the enhanced effect of combinatorial treatment of checkpoint blockade and BTK inhibitors.68, 69
Acknowledgments
This work was supported by the Swedish Cancer Society (CAN2013/389), the Swedish Medical Research Council (K2015-68X-11247-21-3) and the Swedish County Council (ALF-project 2012006). Abdulrahman Hamasy holds a PhD fellowship from the Ministry of Higher Education and Scientific Research in the Kurdistan Regional Government (Erbil-Iraq). We would like to thank Yue Chen for the generous help with experiments.
Author contributions
AH and QW performed most of the experiments, analyzed data and wrote the manuscript; EB designed and performed some experiments; DKM performed some experiments and together with LY provided advice and edited the manuscript; MV carried out modeling of the BTK kinase domain structure, structural analysis, and contributed to writing and to the manuscript editing; AB was involved in planning the research, data interpretation, and contributed to writing and editing the manuscript; CIES conceived the project, designed the experiments, interpreted data, revised the manuscript, and obtained research funding.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- Mohamed AJ, Yu L, Backesjo CM, Vargas L, Faryal R, Aints A et al. Bruton's tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol Rev 2009; 228: 58–73. [DOI] [PubMed] [Google Scholar]
- Smith CI, Islam TC, Mattsson PT, Mohamed AJ, Nore BF, Vihinen M. The Tec family of cytoplasmic tyrosine kinases: mammalian Btk, Bmx, Itk, Tec, Txk and homologs in other species. BioEssays 2001; 23: 436–446. [DOI] [PubMed] [Google Scholar]
- Yang WC, Collette Y, Nunes JA, Olive D. Tec kinases: a family with multiple roles in immunity. Immunity 2000 Apr; 12: 373–382. [DOI] [PubMed] [Google Scholar]
- Berglof A, Turunen JJ, Gissberg O, Bestas B, Blomberg KE, Smith CI. Agammaglobulinemia: causative mutations and their implications for novel therapies. Expert Rev Clin Immunol 2013; 9: 1205–1221. [DOI] [PubMed] [Google Scholar]
- Takata M, Kurosaki T. A role for Bruton's tyrosine kinase in B cell antigen receptor-mediated activation of phospholipase C-gamma 2. J Exp Med 1996; 184: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings DJ. Bruton's tyrosine kinase controls a sustained calcium signal essential for B lineage development and function. Clin Immunol 1999; 91: 243–253. [DOI] [PubMed] [Google Scholar]
- Valiaho J, Smith CI, Vihinen M. BTKbase: the mutation database for X-linked agammaglobulinemia. Hum Mutat 2006; 27: 1209–1217. [DOI] [PubMed] [Google Scholar]
- Conley ME, Dobbs AK, Farmer DM, Kilic S, Paris K, Grigoriadou S et al. Primary B cell immunodeficiencies: comparisons and contrasts. Ann Rev Immunol 2009; 27: 199–227. [DOI] [PubMed] [Google Scholar]
- Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature 1993; 361: 226–233. [DOI] [PubMed] [Google Scholar]
- Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell 1993; 72: 279–290. [DOI] [PubMed] [Google Scholar]
- Lindvall JM, Blomberg KE, Valiaho J, Vargas L, Heinonen JE, Berglof A et al. Bruton's tyrosine kinase: cell biology, sequence conservation, mutation spectrum, siRNA modifications, and expression profiling. Immunol Rev 2005; 203: 200–215. [DOI] [PubMed] [Google Scholar]
- Valiaho J, Faisal I, Ortutay C, Smith CI, Vihinen M. Characterization of all possible single-nucleotide change caused amino acid substitutions in the kinase domain of Bruton tyrosine kinase. Hum Mutat 2015; 36: 638–647. [DOI] [PubMed] [Google Scholar]
- Smith CI, Baskin B, Humire-Greiff P, Zhou JN, Olsson PG, Maniar HS et al. Expression of Bruton's agammaglobulinemia tyrosine kinase gene, BTK, is selectively down-regulated in T lymphocytes and plasma cells. J Immunol 1994; 152: 557–565. [PubMed] [Google Scholar]
- de Gorter DJ, Beuling EA, Kersseboom R, Middendorp S, van Gils JM, Hendriks RW et al. Bruton's tyrosine kinase and phospholipase Cgamma2 mediate chemokine-controlled B cell migration and homing. Immunity 2007; 26: 93–104. [DOI] [PubMed] [Google Scholar]
- Mohammad DK, Nore BF, Hussain A, Gustafsson MO, Mohamed AJ, Smith CI. Dual phosphorylation of Btk by Akt/protein kinase b provides docking for 14-3-3zeta, regulates shuttling, and attenuates both tonic and induced signaling in B cells. Mol Cell Biol 2013; 33: 3214–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buggy JJ, Elias L. Bruton tyrosine kinase (BTK) and its role in B-cell malignancy. Int Rev Immunol 2012; 31: 119–132. [DOI] [PubMed] [Google Scholar]
- Ponader S, Burger JA. Bruton's tyrosine kinase: from X-linked agammaglobulinemia toward targeted therapy for B-cell malignancies. J Clin Oncol 2014; 32: 1830–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BY, Francesco M, De Rooij MF, Magadala P, Steggerda SM, Huang MM et al. Egress of CD19(+)CD5(+) cells into peripheral blood following treatment with the Bruton tyrosine kinase inhibitor ibrutinib in mantle cell lymphoma patients. Blood 2013; 122: 2412–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood 2012; 119: 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nore BF, Vargas L, Mohamed AJ, Branden LJ, Backesjo CM, Islam TC et al. Redistribution of Bruton's tyrosine kinase by activation of phosphatidylinositol 3-kinase and Rho-family GTPases. Eur J Immunol 2000; 30: 145–154. [DOI] [PubMed] [Google Scholar]
- Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol 2013; 31: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013; 369: 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treon SP, Tripsas CK, Meid K, Warren D, Varma G, Green R et al. Ibrutinib in previously treated Waldenstrom's macroglobulinemia. N Engl J Med 2015; 372: 1430–1440. [DOI] [PubMed] [Google Scholar]
- Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton's tyrosine kinase in B cell malignancies. Nat Rev Cancer 2014; 14: 219–232. [DOI] [PubMed] [Google Scholar]
- Burger JA. Bruton's tyrosine kinase (BTK) inhibitors in clinical trials. Curr Hematol Malig Rep 2014; 9: 44–49. [DOI] [PubMed] [Google Scholar]
- Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med 2016; 374: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman SE, Mustafa RZ, Gyamfi JA, Pittaluga S, Chang S, Chang B et al. Ibrutinib inhibits BCR and NF-kappaB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood 2014; 123: 3286–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas L, Hamasy A, Nore BF, Smith CI. Inhibitors of BTK and ITK: state of the new drugs for cancer, autoimmunity and inflammatory diseases. Scand J Immunol 2013; 78: 130–139. [DOI] [PubMed] [Google Scholar]
- Pan Z, Scheerens H, Li SJ, Schultz BE, Sprengeler PA, Burrill LC et al. Discovery of selective irreversible inhibitors for Bruton's tyrosine kinase. ChemMedChem 2007; 2: 58–61. [DOI] [PubMed] [Google Scholar]
- Burger JA, Buggy JJ. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765). Leuk Lymphoma 2013; 54: 2385–2391. [DOI] [PubMed] [Google Scholar]
- Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 2013; 13: 714–726. [DOI] [PubMed] [Google Scholar]
- Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N Engl J Med 2014; 370: 2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiron D, Di Liberto M, Martin P, Huang X, Sharman J, Blecua P et al. Cell-cycle reprogramming for PI3K inhibition overrides a relapse-specific C481S BTK mutation revealed by longitudinal functional genomics in mantle cell lymphoma. Cancer Discov 2014; 4: 1022–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman RR, Cheng S, Lu P, Setty M, Perez AR, Guo A et al. Ibrutinib resistance in chronic lymphocytic leukemia. N Engl J Med 2014; 370: 2352–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Guo A, Lu P, Ma J, Coleman M, Wang YL. Functional characterization of BTK(C481S) mutation that confers ibrutinib resistance: exploration of alternative kinase inhibitors. Leukemia 2015; 29: 895–900. [DOI] [PubMed] [Google Scholar]
- Maddocks KJ, Ruppert AS, Lozanski G, Heerema NA, Zhao W, Abruzzo L et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol 2015; 1: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SQ, Smith SM, Zhang SY, Lynn Wang Y. Mechanisms of ibrutinib resistance in chronic lymphocytic leukaemia and non-Hodgkin lymphoma. Br J Haematol 2015; 170: 445–456. [DOI] [PubMed] [Google Scholar]
- Liu TM, Woyach JA, Zhong Y, Lozanski A, Lozanski G, Dong S et al. Hypermorphic mutation of phospholipase C, gamma2 acquired in ibrutinib-resistant CLL confers BTK independency upon B-cell receptor activation. Blood 2015; 126: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz HM, Blomberg KE, Lindvall JM, Kurosaki T, Smith CI. Expression profiling of chicken DT40 lymphoma cells indicates clonal selection of knockout and gene reconstituted cells. Biochem Biophys Res Commun 2008; 377: 584–588. [DOI] [PubMed] [Google Scholar]
- Gustafsson MO, Hussain A, Mohammad DK, Mohamed AJ, Nguyen V, Metalnikov P et al. Regulation of nucleocytoplasmic shuttling of Bruton's tyrosine kinase (Btk) through a novel SH3-dependent interaction with ankyrin repeat domain 54 (ANKRD54). Mol Cell Biol 2012; 32: 2440–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl MI, Fluckiger AC, Kato RM, Park H, Witte ON, Rawlings DJ. Phosphorylation of two regulatory tyrosine residues in the activation of Bruton's tyrosine kinase via alternative receptors. Proc Natl Acad Sci USA 1997; 94: 11526–11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Wahl MI, Afar DE, Turck CW, Rawlings DJ, Tam C et al. Regulation of Btk function by a major autophosphorylation site within the SH3 domain. Immunity 1996; 4: 515–525. [DOI] [PubMed] [Google Scholar]
- Li Z, Wahl MI, Eguinoa A, Stephens LR, Hawkins PT, Witte ON. Phosphatidylinositol 3-kinase-gamma activates Bruton's tyrosine kinase in concert with Src family kinases. Proc Natl Acad Sci USA 1997; 94: 13820–13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niroula A, Urolagin S, Vihinen M. PON-P2: prediction method for fast and reliable identification of harmful variants. PLoS ONE 2015; 10: e0117380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorechovsky I, Luo L, Hertz JM, Froland SS, Klemola T, Fiorini M et al. Mutation pattern in the Bruton's tyrosine kinase gene in 26 unrelated patients with X-linked agammaglobulinemia. Hum Mutat 1997; 9: 418–425. [DOI] [PubMed] [Google Scholar]
- Ollila J, Lappalainen I, Vihinen M. Sequence specificity in CpG mutation hotspots. FEBS Lett 1996; 396: 119–122. [DOI] [PubMed] [Google Scholar]
- Smith NG, Webster MT, Ellegren H. A low rate of simultaneous double-nucleotide mutations in primates. Mol Biol Evol 2003; 20: 47–53. [DOI] [PubMed] [Google Scholar]
- Ortutay C, Nore BF, Vihinen M, Smith CI. Phylogeny of Tec family kinases identification of a premetazoan origin of Btk, Bmx, Itk, Tec, Txk, and the Btk regulator SH3BP5. Adv Genet 2008; 64: 51–80. [DOI] [PubMed] [Google Scholar]
- Hamada N, Backesjo CM, Smith CI, Yamamoto D. Functional replacement of Drosophila Btk29A with human Btk in male genital development and survival. FEBS Lett 2005; 579: 4131–4137. [DOI] [PubMed] [Google Scholar]
- Martin P, Maddocks K, Leonard JP, Ruan J, Goy A, Wagner-Johnston N et al. Post-ibrutinib outcomes in patients with mantle cell lymphoma. Blood 2016; 127: 1559–1563. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M, West SR, Gertler FB, Hoffmann FM. A novel tyrosine kinase-independent function of Drosophila abl correlates with proper subcellular localization. Cell 1990; 63: 949–960. [DOI] [PubMed] [Google Scholar]
- Schwartzberg PL, Xing L, Hoffmann O, Lowell CA, Garrett L, Boyce BF et al. Rescue of osteoclast function by transgenic expression of kinase-deficient Src in src−/− mutant mice. Genes Dev 1997; 11: 2835–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin JD, Boast S, Mendelsohn M, de los Santos K, Goff SP. Transgenes encoding both type I and type IV c-abl proteins rescue the lethality of c-abl mutant mice. Oncogene 1996; 12: 2669–2677. [PubMed] [Google Scholar]
- Fluckiger AC, Li Z, Kato RM, Wahl MI, Ochs HD, Longnecker R et al. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J 1998; 17: 1973–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middendorp S, Zijlstra AJ, Kersseboom R, Dingjan GM, Jumaa H, Hendriks RW. Tumor suppressor function of Bruton tyrosine kinase is independent of its catalytic activity. Blood 2005; 105: 259–265. [DOI] [PubMed] [Google Scholar]
- Lynch M. Rate, molecular spectrum, and consequences of human mutation. Proc Natl Acad Sci USA 2010; 107: 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Lawrence MS, Wan Y, Stojanov P, Sougnez C, Stevenson K et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N Engl J Med 2011; 365: 2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente XS, Bea S, Valdes-Mas R, Villamor N, Gutierrez-Abril J, Martin-Subero JI et al. Non-coding recurrent mutations in chronic lymphocytic leukaemia. Nature 2015; 526: 519–524. [DOI] [PubMed] [Google Scholar]
- Landau DA, Tausch E, Taylor-Weiner AN, Stewart C, Reiter JG, Bahlo J et al. Mutations driving CLL and their evolution in progression and relapse. Nature 2015; 526: 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afar DE, Park H, Howell BW, Rawlings DJ, Cooper J, Witte ON. Regulation of Btk by Src family tyrosine kinases. Mol Cell Biol 1996; 16: 3465–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam KB, Rabbani H, Larsson C, Sanders R, Smith CI. Molecular cloning, characterization, and chromosomal localization of a human lymphoid tyrosine kinase related to murine Blk. J Immunol 1995; 154: 1265–1272. [PubMed] [Google Scholar]
- Berglof A, Hamasy A, Meinke S, Palma M, Krstic A, Mansson R et al. Targets for ibrutinib beyond B cell malignancies. Scand J Immunol 2015; 82: 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihinen M, Vetrie D, Maniar HS, Ochs HD, Zhu Q, Vorechovsky I et al. Structural basis for chromosome X-linked agammaglobulinemia: a tyrosine kinase disease. Proc Natl Acad Sci USA 1994; 91: 12803–12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinleye A, Chen Y, Mukhi N, Song Y, Liu D. Ibrutinib and novel BTK inhibitors in clinical development. J Hematol Oncol 2013; 6: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013; 122: 2539–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovsky JA, Flynn R, Du J, Harrington BK, Zhong Y, Kaffenberger B et al. Ibrutinib treatment ameliorates murine chronic graft-versus-host disease. J Clin Invest 2014; 124: 4867–4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015; 161: 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng PP, Chang BY, Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci USA 2015; 112: E966–E972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiff A, Trikha P, Wesolowski R, Kendra K, Hsu V, Uppati S et al. Myeloid-derived suppressor cells express Bruton's tyrosine kinase and can be depleted in tumor bearing hosts by ibrutinib treatment. Cancer Res 2016; 76: 2125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.