Abstract
Aspergillus is one of the economically most important fungal genera. Recently, the ICN adopted the single name nomenclature which has forced mycologists to choose one name for fungi (e.g. Aspergillus, Fusarium, Penicillium, etc.). Previously two proposals for the single name nomenclature in Aspergillus were presented: one attributes the name “Aspergillus” to clades comprising seven different teleomorphic names, by supporting the monophyly of this genus; the other proposes that Aspergillus is a non-monophyletic genus, by preserving the Aspergillus name only to species belonging to subgenus Circumdati and maintaining the sexual names in the other clades. The aim of our study was to test the monophyly of Aspergilli by two independent phylogenetic analyses using a multilocus phylogenetic approach. One test was run on the publicly available coding regions of six genes (RPB1, RPB2, Tsr1, Cct8, BenA, CaM), using 96 species of Penicillium, Aspergillus and related taxa. Bayesian (MrBayes) and Ultrafast Maximum Likelihood (IQ-Tree) and Rapid Maximum Likelihood (RaxML) analyses gave the same conclusion highly supporting the monophyly of Aspergillus. The other analyses were also performed by using publicly available data of the coding sequences of nine loci (18S rRNA, 5,8S rRNA, 28S rRNA (D1-D2), RPB1, RPB2, CaM, BenA, Tsr1, Cct8) of 204 different species. Both Bayesian (MrBayes) and Maximum Likelihood (RAxML) trees obtained by this second round of independent analyses strongly supported the monophyly of the genus Aspergillus. The stability test also confirmed the robustness of the results obtained. In conclusion, statistical analyses have rejected the hypothesis that the Aspergilli are non-monophyletic, and provided robust arguments that the genus is monophyletic and clearly separated from the monophyletic genus Penicillium. There is no phylogenetic evidence to split Aspergillus into several genera and the name Aspergillus can be used for all the species belonging to Aspergillus i.e. the clade comprising the subgenera Aspergillus, Circumdati, Fumigati, Nidulantes, section Cremei and certain species which were formerly part of the genera Phialosimplex and Polypaecilum. Section Cremei and the clade containing Polypaecilum and Phialosimplex are proposed as new subgenera of Aspergillus. The phylogenetic analysis also clearly shows that Aspergillus clavatoflavus and A. zonatus do not belong to the genus Aspergillus. Aspergillus clavatoflavus is therefore transferred to a new genus Aspergillago as Aspergillago clavatoflavus and A. zonatus was transferred to Penicilliopsis as P. zonata. The subgenera of Aspergillus share similar extrolite profiles indicating that the genus is one large genus from a chemotaxonomical point of view. Morphological and ecophysiological characteristics of the species also strongly indicate that Aspergillus is a polythetic class in phenotypic characters.
Key words: Aspergillus, Multigene phylogeny, Monophyly, Nomenclature, Teleomorphs
Taxonomic novelties: Aspergillus subgenus Cremei, subgen. nov.; Aspergillus subgenus Polypaecilum, subgen. nov.; Aspergillago Samson, Houbraken & Frisvad, gen. nov.
New combinations: Aspergillago clavatoflava (Raper & Fennell) Samson, Houbraken & Frisvad, comb. nov.; Penicilliopsis zonatus (Kwon-Chung & Fennell) Samson, Houbraken & Frisvad, comb. nov.
Introduction
The genus Aspergillus contains some of the most abundant and widely distributed organisms on earth, and comprises approximately 350 accepted species (Samson et al. 2014). It is one of the fungal genera with the highest economic importance in biotechnology (enzymes, organic acids, bioactive metabolites), but members of the genus are also frequently reported as foodborne contaminants (food spoilage and mycotoxin contamination), or as causal agents of human mycoses (pulmonary aspergillosis, otomycosis, keratitis). Aspergillus is also one of the oldest names in fungal taxonomy since it was applied by Micheli (1729), who gave it this name because the spore-bearing structure characteristic of the genus resembled an aspergillum (a device used by the Catholic church to sprinkle holy water). However this morphological characteristic resulted in a broad generic concept because it is associated to twelve quite different teleomorphs demonstrating the variation in physiological and morphological features (Houbraken and Samson, 2011, Pitt and Taylor, 2014). Houbraken et al. (2014) have reduced the number of teleomorphic names to ten (Petromyces, Neopetromyces, Saitoa, Fennellia, Emericella, Hemisartorya, Neosartorya, Neocarpenteles, Cristaspora, and Eurotium) and showed that the teleomorphs Warcupiella and Sclerocleista do not belong to the Aspergillus monophyletic clade.
The most important change in recent fungal nomenclature is the abandonment of dual nomenclature for pleomorphic fungi, following the decision taken at the International Botanical Congress in Melbourne (24–30 July, 2011). In the latest International Code of Nomenclature for algae, fungi and plants (ICN, McNeill et al. 2012), the single name nomenclature was adopted. This has forced mycologists to choose one name for each fungal genus (i.e Aspergillus, Fusarium, Penicillium, etc.). The ICN recommended that either the sexual or asexual name can be chosen, in contrast to the earlier recommendation that the name of the sexual state should always be preferred. Several sexual names have priority over the asexual ones, but the final choice among the names should also be strongly supported by the (mycological) community. In general, the nomenclatural decision has been easily assigned for most fungal genera, but it sometimes became complicated for economically and socially important fungi having a well-established sexual and asexual name (Zhang et al. 2013). Even though taxonomy contains the rather independent disciplines such as classification, nomenclature and identification, decisions concerning nomenclature should take into account both the other two. In recent years cladonomy has having a more and more important impact on taxonomy, to a degree where monophyly is the overruling factor in deciding which taxa (clada) should be accepted and which names to give to them, rather than classificatory principles.
Phylogenetic approaches have helped to solve taxonomical and nomenclatural problems. A clear example is evident in the paper of Kepler et al. (2014) in which the robust monophyly of the genus Metharrizum included the majority of species recognized in Metacordyceps as well as the green-spored Nomuraea species and those in the more recently described genus Chamaeleomyces. In the same analysis Pochonia was shown to be polyphyletic and the description of Metapochonia gen. nov. was done to accommodate these species forming a separate clade. In this regard, a dispute on the asexual genus Aspergillus and its sexual generic names, started after the International Commission of Penicillium and Aspergillus (ICPA) discussed the single nomenclature and made a decision on April 12 2012 (www.aspergilluspenicillium.org).
Two proposals for the single name nomenclature in Aspergillus have been presented: one attributes the name “Aspergillus” to clades comprising ten different teleomorphic names, by supporting the monophyly of this genus (Houbraken and Samson, 2011, Samson et al., 2014). In the second proposal Aspergillus is considered to be a non-monophyletic genus, and it recommends the preservation of the name Aspergillus only to species belonging to subgenus Circumdati while maintaining the sexual names in the other clades (Pitt and Taylor, 2014, Pitt and Taylor, 2016, Taylor et al., 2016).
The first proposal considers the use of Aspergillus in a wide sense and preserves this large important genus, with the exclusion of some minor species with the anamorph of Aspergillus (i.e. A. clavatoflavus, A. zonatus and the Sclerocleista and Warcupiella teleomorphs) and the inclusion of some taxa lacking Aspergillus anamorph (Polypaecilum and Phialosimplex). As alternative to the “wide” Aspergillus, the second proposal suggests the non–monophyletic feature of Aspergillus and maintains existing teleomorph names (i.e. Eurotium, Emericella, Neosartorya, etc.) reducing Aspergillus mainly to species important for food fermentation, spoilage and mycotoxin contaminations. In this second proposal, as the type of Aspergillus belongs to the Eurotium clade, it was also proposed to move the type of Aspergillus to the subgenus Circumdati. In this respect, Taylor et al. (2016) provided data to suggest that if the genus Aspergillus should be considered monophyletic the Penicillium clade will belong within Aspergillus and the new nomenclatorial rules would lead, e.g., to Aspergillus subgenus Penicillium. Therefore, they propose to keep the sexual name Eurotium for subgenus Aspergillus, Neosartorya for subgenus Fumigati, Emericella for subgenus Nidulantes and Chaetosartorya for sect. Cremei. Additionally, they propose the retypification of Aspergillus with A. niger and to maintain Aspergillus names for some economically relevant species in the subgenus Circumdati. However, this proposal is based on phylogenetic studies using the data set of Houbraken & Samson (2011), that was set up to resolve the phylogeny of the family Trichocomaceae and not specifically for the genus Aspergillus. In fact, their analysis did not show enough phylogenetic signals to unambiguously show the monophyly or paraphyly of the wide Aspergillus genus.
To resolve the discussion of the two proposals it is important re-examining the phylogenetic analysis to assess the monophyly or paraphyly of this group of taxa with the “aspergillum” as the main spore-bearing structure. Therefore, the aim of our study was to test the monophyly of Aspergilli by a multilocus phylogenetic approach and this was achieved by two independent analyses. The phylogenetic analysis using six loci were performed by GP and DM at Bari, Italy whereas the nine loci analysis was carried out by SK, JV and GS at Szeged, Hungary.
Materials and methods
Phylogenetic analysis using six loci
Ninety six strains belonging to species of Penicillium, Aspergillus and related taxa were studied for their phylogenetic relationship by using their publicly available sequences of the following six loci: RPB1 and RPB2 genes coding for subunits of RNA polymerase II; Tsr1, coding for a putative ribosome biogenesis protein; Cct8, coding for the theta subunit of the TCP-1 chaperonin complex; BenA coding for the beta-tubulin protein, and CaM coding for the calcium binding protein calmodulin. The list of strains and the relevant sequences accession number used is reported in Supplementary Table 1.
DNA sequences of the six loci were singularly aligned with Muscle (for RPB1, RPB2, CaM, BenA, and Cct8) and ClustalW (for Tsr1) algorithms using the software MEGA7 (Kumar et al. 2016), manually optimized and trimmed to make sequences of equal length, and then concatenated. The alignment is deposited at TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S20285). Successively, the Multiple Sequence Alignment (MSA) was evaluated for quality using Transitive Consistence Score (TCS) offered by the T-Coffee web server (Chang et al. 2015). The presence of rogue taxa in the set of data was evaluated through the RogueNaRok web server analysis, because the presence of these taxa can frequently have a negative impact on the results of a bootstrap analysis (e.g., the overall support in consensus trees, Aberer et al. 2013). Then the sequences were manually controlled and substituted if necessary to settle the MSA. JModelTest2 (v2.1.6) (Darriba et al. 2012) was used to find the preferred model of evolution for the concatenated dataset, PartitionFinder (v1.1.1) (Lanfear et al. 2012) was used to investigate the best-fit partitioning schemes and models of molecular evolution to be adopted in RaxML analysis of the partitioned dataset, models were selected according to Bayesian Information Criterion (BIC) for both tools. The different tools performed to infer the phylogenetic tree were as follows: a) MrBayes v3.2.6 (Ronquist et al. 2012) for posterior probabilities (Bpp) using models of evolution on concatenated dataset from JmodelTest; b) RAxML-HPC2 (v8.2.8) (Stamatakis 2014) for rapid bootstrap support (Rbs) using models of evolution defined by JmodelTest and PartitionFinder on concatenated and partitioned dataset, respectively; c) IQ-Tree-omp (v1.4.1) (Minh et al., 2013, Nguyen et al., 2015, Chernomor et al., 2016) for UFML (Ultra Fast Maximul Likelihood) support (Ibs).
The CIPRES Science Gateway V 3.3 (Miller et al. 2010) was used to perform MrBayes analysis, setting GTR + invgamma, 107 generations, sampling every 1 000 generations with a burnin fraction of 0.25; and RaxML analyses, setting GTR + GAMMA + P-Invar, executing 1 000 rapid bootstrap inferences and thereafter a thorough ML search, for the concatenated and partitioned dataset respectively.
IQ-Tree analysis were done locally, setting GTR + I + G4 for the concatenated dataset and the calculated charpartition BIC (GTR + I + G4: RPB1, RPB2, CaM, BenA, and Cct8, TPM2 + I + G4: Tsr1) for the partitioned dataset, both analyses were run with 104 ultrafast bootstrap replicates.
Phylogenetic analysis using nine loci
Phylogenetic analyses were conducted using nine loci (18S rDNA, 5.8S rDNA, 28S rDNA (D1-D2), RPB1, RPB2, CaM, BenA, Tsr1, Cct8) with intron regions excluded from CaM and BenA sequences. The dataset primarily consisted of publicly available sequences which are listed in Supplementary Table 2. Additional Cct8, RPB1, RPB2 and Tsr1 loci of Aspergillus species were amplified and sequenced using the methods described previously by Houbraken & Samson (2011). Sequences were deposited into GenBank under the accession numbers KY006730-KY006827. All sequences were aligned by PRANK v.140603 (Löytynoja 2014) with default settings. Individual alignments were concatenated by using SequenceMatrix 1.8 (Vaidya et al. 2011) and the dataset was partitioned by the nine loci. An initial maximum likelihood (ML) tree was generated from the dataset by raxmlGUI 1.5b1 (Silvestro & Michalak 2012) using the executables of RAxML 8.2.7 (Stamatakis 2014) under the GTR model with gamma-distributed rate heterogeneity with 500 rapid bootstrap replicates. Sequences encoding Tsr1 are containing large number of indels therefore this initial tree was used to refine the alignment of the Tsr1 sequences by PRANK with the -F option. In the case of SSU, RPB2 and Tsr1 alignments FastGap 1.2 (Borchsenius 2009) was used to code the phylogenetic information of gaps as binary characters implementing the “simple indel coding” algorithm. The refined alignment of partial Tsr1 sequences and the indel matrix was incorporated in the concatenated dataset. The final ML trees and branch supports were estimated by 1 000 thorough bootstrap replicates under the GTR + Γ model with ten partitions. Bootstrap support was mapped on the ML tree using the SumTrees script of the Dendropy v4.2.0 package (Sukumaran & Holder 2010). The resulted best tree and the bootstrap replicates were submitted for rogue taxon identification by the RogueNaRok (http://rnr.h-its.org/, Aberer et al. 2013) web service. Bayesian analyses were performed on the partitioned dataset using MrBayes 3.2.6 (Ronquist et al. 2012) with GTR substitution model with gamma-distributed rate variation across sites for 107 generations with four chains and two replicates sampling every 1 000th generations. The burnin proportion was set to 0.25. Convergence and ESS values of the runs were examined by Tracer 1.6 (Rambaut et al. 2014).
To test the phylogenetic hypotheses of the monophyly of Aspergilli, a constraint tree was generated by Mesquite v3.04 (Maddison & Maddison 2016). Per-site log likelihoods were calculated for 20 unconstrained and 20 constrained ML searches by using RAxML. To measure the support of the two hypotheses Approximately Unbiased (AU) test was conducted by CONSEL 0.1j (Shimodaira & Hasegawa 2001) with 105 replicates.
Tree space visualization of ML and Bayesian analyses was carried out by using the TreeSetVis v3.01 (Hillis et al. 2005) package for Mesquite and the RWTY v1.0.1 package for R v3.3.1 (R Core Team 2016). Sorting of the bootstrap replicates was conducted by PhySortR v1.0.7 (Stephens et al. 2016) package in R.
Branch support analysis
To verify the robustness of the six and nine-genes phylogeny the branch supports of the principal nodes depicting the Aspergillus and Penicillium monophyletic topology were evaluated. Three categories of branch support (Anisimova et al., 2011, Minh et al., 2013) were considered: parametric (Bpp, aLRT-Chi2, aBayes), nonparametric (Rbs, SH-aLRT) and hybrid (Ibs).
To compute, aLRT-Chi2, SH-aLRT and aBayes branches support of the six-genes phylogeny, PhyML (v20130805) (Guindon & Gascuel 2003) and IQ-Tree-omp (v1.4.1) analyses were performed locally (Guindon et al., 2010, Anisimova et al., 2011). The single branch tests (SH-aLRT, aBayes) and ultrafast bootstrap approximation of the nine-genes phylogeny were also conducted by using IQ-Tree v1.4.2 in 50.000 replicates under the GTR + Γ model.
Analysis of extrolites
Strains of species expected to be outside Aspergillus were analysed by HPLC-DAD (high performance liquid chromatography with diode array detection as described by Frisvad & Thrane (1987), using the agar plug method of Smedsgaard (1997), as updated by Nielsen et al. (2011).
Results
Phylogenetic analysis using six loci
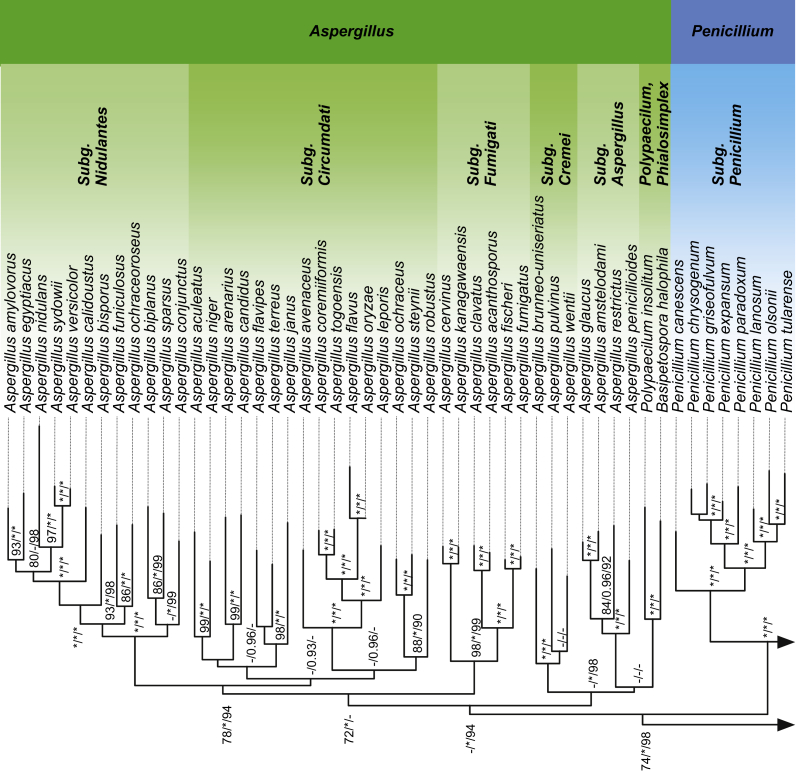
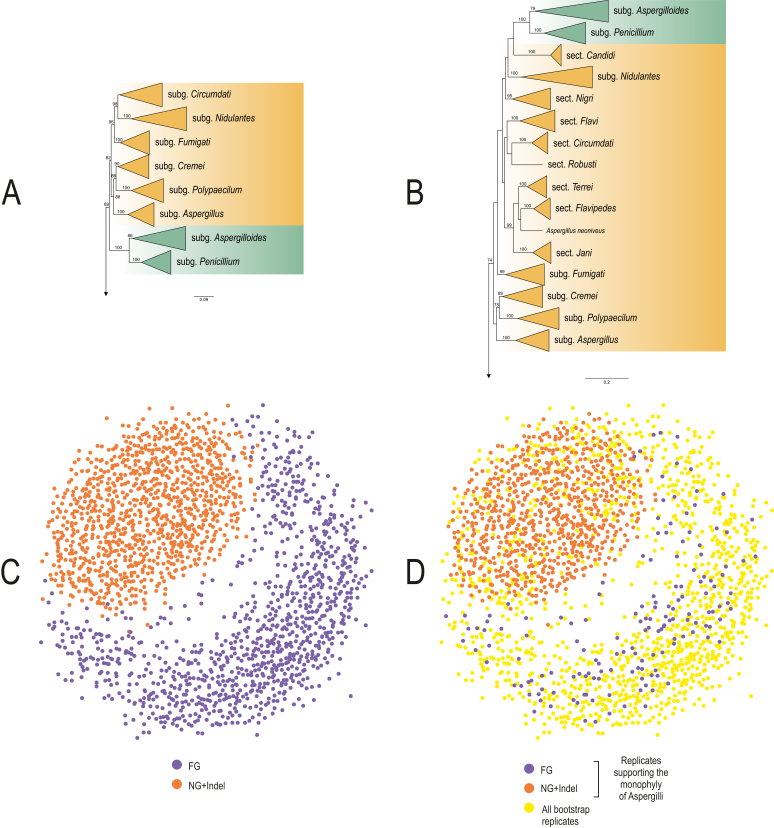
The results of the six-gene phylogenetic analysis of the 96 strains belonging to species of Penicillium, Aspergillus and related taxa highly supported the monophyly of Aspergillus and its sister genus Penicillium in terms of Bayesian, UFML (IQ-Tree) and RAxML analyses. In particular, the six genes MSA consisted of 3 395 bps containing only the exons of each gene with the respective length of RPB1 (767 bps), RPB2 (963 bps), Tsr1 (640 bps), CaM (150 bps), BenA (164 bps), and Cct8 (711 bps). The number of conserved sites was 1 368, the number of variable sites was 2 008, with 1 755 parsimony informative sites. The Transitive Consistence Score (TCS) evaluate the robustness of the six-gene MSA with the high score of 996. No rogue taxa have been identified among the sequences of the strains used, confirming the absence of taxa that could have a negative impact on the bootstrap analysis. The best model of evolution calculated with the JModelTest2 tool was the GTR + I + G (General Time Reversible + Invariant Site and Gamma Distribution) used for non-partitioned analysis in RAxML and MrBayes analysis. The best model of evolution for the RAxML partitioned analysis calculated from Partition Finder was confirmed as GTR + I + G for each partition of the six-gene MSA. The phylogenetic tree comprehensive of the ML analysis (RAxML and IQ-TREE) and the posterior probabilities Bayesian analysis with the same topology is represented in Fig. 1. All five phylogenetic trees supported the monophyly of the genus Aspergillus respectively with the higher bootstrap support of 94 % for the partitioned IQ-TREE, 1.0 for MrBayes and 63 % for RAxML not partitioned (see Fig. S1). Interestingly all the resolved trees highly supported (98 % IQ-TREE, 77 % RAxML and 1.0 MrBayes) the principal node clustering genera Penicillium and Aspergillus together. In addition, the five subgenera of Aspergillus are conserved in all the phylogenetic analysis with the same topology (Fig. 1).
Fig. 1.
Tree based on six genes. The tree shown is a rooted consensus tree inferred by maximum likelihood with partitioned dataset (IQ-TREE) and 10 000 bootstrap replicates, branch support values are given for two maximum-likelihood implementations and one Bayesian inference method (from left to right: RaxML bootstrap support; MrBayes posterior probabilities; IQ-TREE bootstrap support; respectively).
The phylogenetic analysis clearly showed that Aspergillus clavatoflavus, A. zonatus, Penicillium megasporum, and P. arenicola, do not belong to their respective sister genera, being outside of the two lineages. In addition, the teleomorphic genera Warcupiella and Sclerocleista, formerly assigned with an Aspergillus anamorph, were found to be outside the Aspergillus monophyletic clade.
Phylogenetic analysis using nine loci
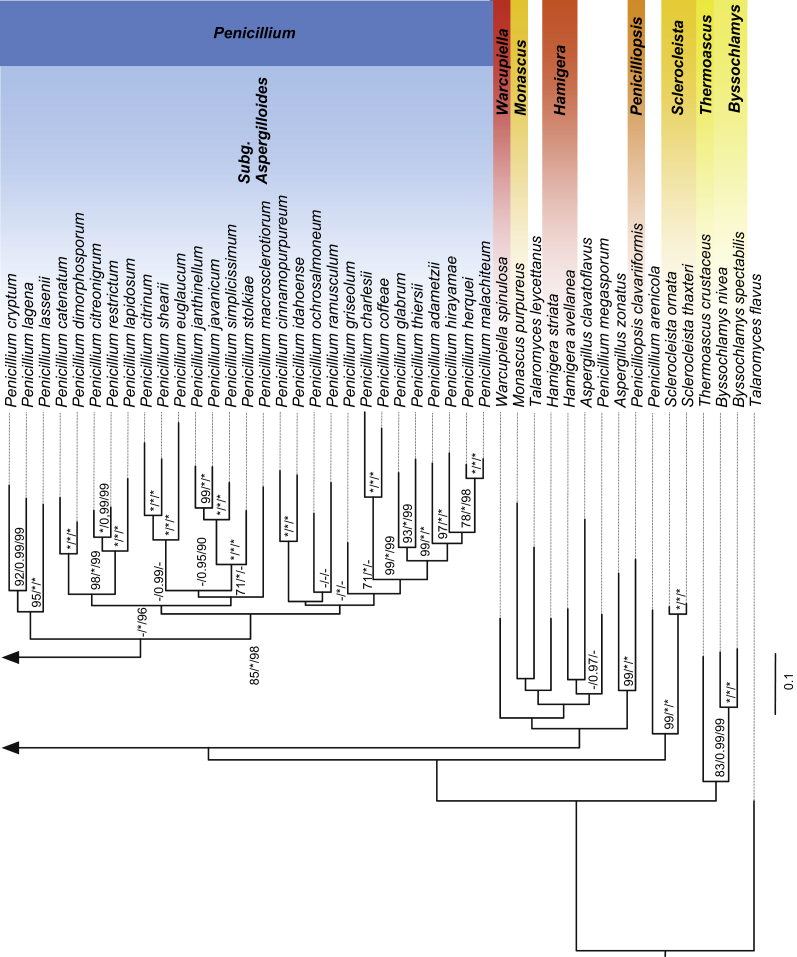
The 204 species analysed in the concatenated alignment included 86 Aspergillus, 66 Penicillium and 52 species from other genera with 6 603 nucleic sites (18S rDNA: 1 792 sites, 5,8S rDNA: 161, 28S rDNA: 647 sites, BenA: 241 sites, CaM: 402 sites, Cct8: 718 sites, RPB1: 768 sites, RPB2: 983 sites, Tsr1: 891 sites) and 201 binary sites of indels. Phylogenetic trees obtained from both ML and Bayesian analyses (Figs 2 and S2, Fig 5B) were highly congruent and both analyses have shown that the genus Aspergillus is monophyletic with high support values. The results have evidenced that the genus Aspergillus can be divided into six subgenera comprising 22 sections. Maximum likelihood and Bayesian inference strategies recovered subgenus Aspergillus (100/1), Polypaecili (100/1), Cremei (90/1), Fumigati (100/1) and Nidulantes (100/1) as strongly supported clades with the exception of subgenus Circumdati (47/1), which was strongly supported by Bayesian analysis but had low support by the ML method.
Fig. 2.
Phylograms obtained by Maximum Likelihood (ML) and Bayesian analysis inferred from nine loci (18S rDNA, 5.8S rDNA, 28S rDNA (D1-D2), RPB1, RPB2, CaM, BenA, Tsr1, Cct8). Monophyletic groups are collapsed and shown as triangles. A. Best-scoring ML tree obtained by RAxML. B. 50 % majority rule phylogram of Bayesian analysis. Numbers above or below branches are bootstrap values (A) and posterior probabilities (B). Only support values greater than 60 % and 0.95 are shown.
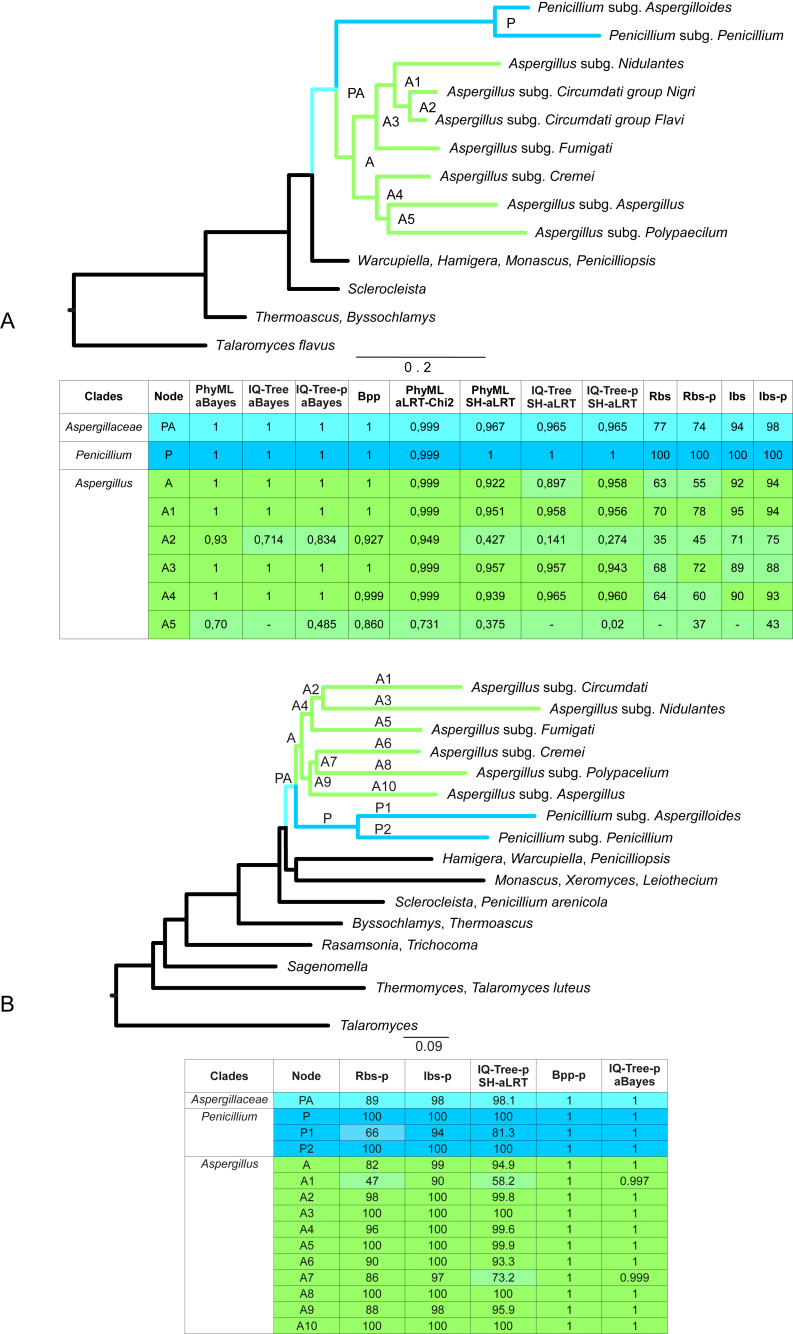
Fig. 5.
Collapsed phylograms showing the support values of the principal nodes involved in the monophyly of Aspergillus based on six (A) and nine (B) genes. The tables are summarizing the values of these nodes obtained by different methods (Bpp – Bayesian posterior probabilities, Rbs – RAxML bootstrap support, Ibs – IQ-Tree UFBoot support). Single branch tests (aBayes, SH-aLRT and aLRT-Chi2) were conducted with PhyML and IQ-Tree. The use of partitioned data set is indicated by -p in the tables.
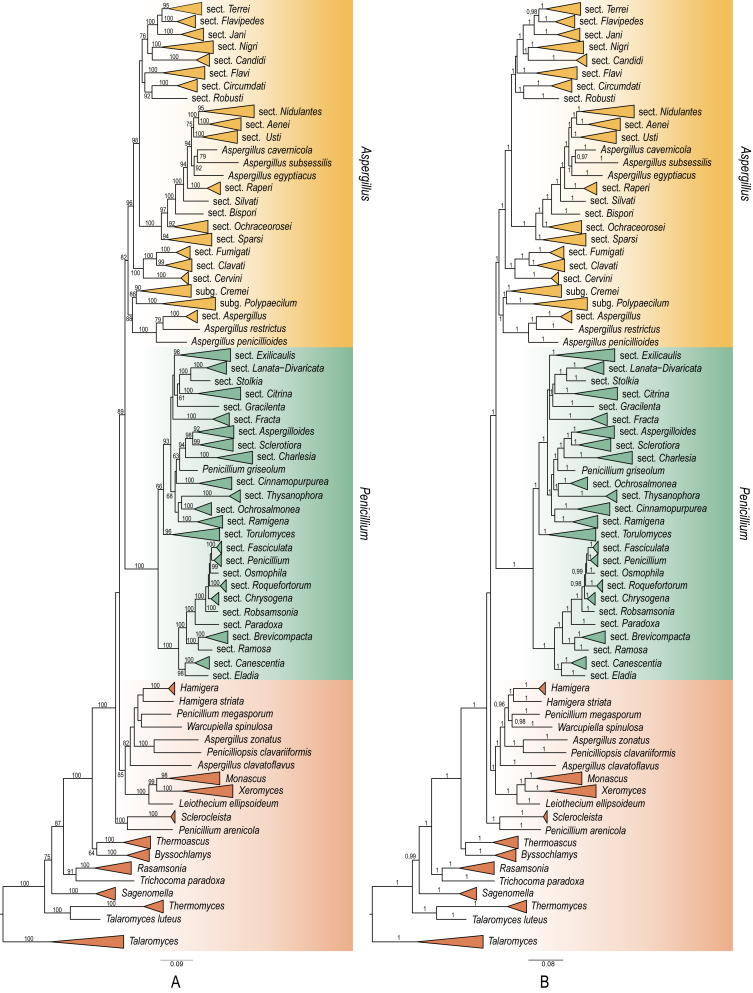
The hypothesis of monophyly was tested using the constrained tree that is likely to be multifurcating to indicate uncertainty between the two competing hypotheses and let the algorithm find the most realistic ML solution for a given constraint. Our constrained tree was drawn in Mesquite 3.04 forcing the two genera, Aspergillus and Penicillium to be paraphyletic. Branches encompassing the members of genus Penicillium were collapsed into polytomy as well as the members of sections Terrei, Flavipedes, Jani, Nigri, Candidi, Flavi and Circumdati in the Aspergillus clade. Altogether 20 constrained and 20 unconstrained topologies were compared using the approximately unbiased test with CONSEL. The test resulted in the complete rejection of the hypothesis of Taylor et al. (2016). The monophyly of the genus Aspergillus was accepted with p values ranging from 0.323 to 0.706, with mean of 0.45815. The hypothesis that genus Aspergillus is paraphyletic and Penicillium is a sister clade of subgenus Nidulantes was rejected with low p values in the range of 0.005–0.023, with mean of 0.0134.
Tree space of the bootstrap replicates
To investigate the background of the high dissimilarity between the results of Taylor et al. (2016) and our results we analysed the tree space of the bootstrap replicates and the trees obtained from Bayesian MCMC analysis by multi-dimensional scaling.
We reduced our dataset to Cct8, RPB1, RPB2 and Tsr1 genes without removing taxa to have only those genes that had been used in the analysis of Taylor et al. (2016). The dataset was un-partitioned without a binary matrix of indels. Both ML and Bayesian analysis were conducted with the same settings as applied on the nine-gene dataset. Our results with the four-gene dataset differed from those of Taylor et al. (Fig. S3). Briefly, the genus Aspergillus was a sister group and paraphyletic to the genus Penicillium and subgenus Circumdati was not recovered as a monophyletic clade. The most closely related group to Penicillia was section Candidi. Subgenus Nidulantes formed a well-defined monophyletic clade with a sister clade of the members of section Nigri. Other sections from the subgenus Circumdati were clustered together with high support except sect. Circumdati however, the deeper branching was not statistically supported. Members of subgenera Fumigati, Cremei and Aspergillus formed monophyletic clades with moderate to high support, but deeper nodes were poorly supported.
The results of the Bayesian analysis were similar to the results of the ML analysis. The relationship between Aspergilli and Penicillia was the same as in the ML analysis. Five subgenera formed well-defined clades with high statistical support, while sections in subgenus Circumdati were not monophyletic (Fig. S3). We re-analysed the dataset of Taylor et al. (2016) without any modification, and the resulting trees were highly congruent to the ones obtained with our reduced dataset. We were not able to obtain a tree with a monophyletic clade containing all sections from subgenus Circumdati regardless the use of Bayesian or ML approaches. However, this difference from the tree shown in the article of Taylor et al. (2016) can be the result of the different parsimony starting tree between the two analyses, as different seeds will generate different starting trees, which can have an impact on the final ML tree.
We used the TreeSetViz package for Mesquite to investigate the distribution of the bootstrap replicates in the tree space of our and the reduced dataset. To visualize the tree space 1 000 bootstrap replicates were used from both runs. The topological distances between all replicates were measured by the calculation of pairwise unweighted Robinson-Foulds (Robinson and Foulds, 1979, Robinson and Foulds, 1981) distances. The distribution of the replicates was visualized in two dimensions by multidimensional scaling (MDS) (Lingoes et al., 1979, Young and Hamer, 1987, Borg and Groenen, 1997). The MDS search was run until no major changes were observed in the value of the stress function to minimize the distortion between the true distance and the two-dimensional distance. The analysis showed that the bootstrap replicates of the nine-gene dataset were grouped together in a well-defined island, while the replicates of the four-gene dataset were much more widely distributed in the tree space (Fig. 3C). This indicates that the variation between the bootstrap samples in the reduced dataset is higher, suggesting that the alignment used in the analysis has substantially lower phylogenetic signal, which is not strong enough to resolve all clades with high confidence and by the addition of more genes and partitioning the dataset the signal became more balanced.
Fig. 3.
Visualization of 1 000–1 000 bootstrap replicates obtained by using nine (NG) and four (FG) loci. Best-scoring ML tree using nine (A) and four loci (B) are shown with bootstrap support above branches higher than 60 %. Monophyletic groups are collapsed and shown as triangles. (C) Orange dots represent bootstrap replicates from the analysis encompassing nine genes, while purple dots are trees obtained with four genes. (D) Visualization of those replicates which support the monophyly of Aspergilli. Orange and purple dot are replicates from the nine-gene and the four-gene analysis respectively. Yellow dots represent the tree space occupied by all replicates from both runs.
Replicates which support the monophyly of Aspergilli were sorted out from both analyses by PhySortR and mapped on the tree space of all bootstrap samples. The bootstrap samples supporting the monophyly of Aspergilli from the dataset encompassing nine genes were distributed uniformly suggesting that there is no high variability in the branching patterns between the replicates (Fig. 3D). Samples sorted out by the same criterion from the four genes analysis were more distinct to each other suggesting that the uncertainty of the dataset is not exclusive to those clades that contains Aspergilli.
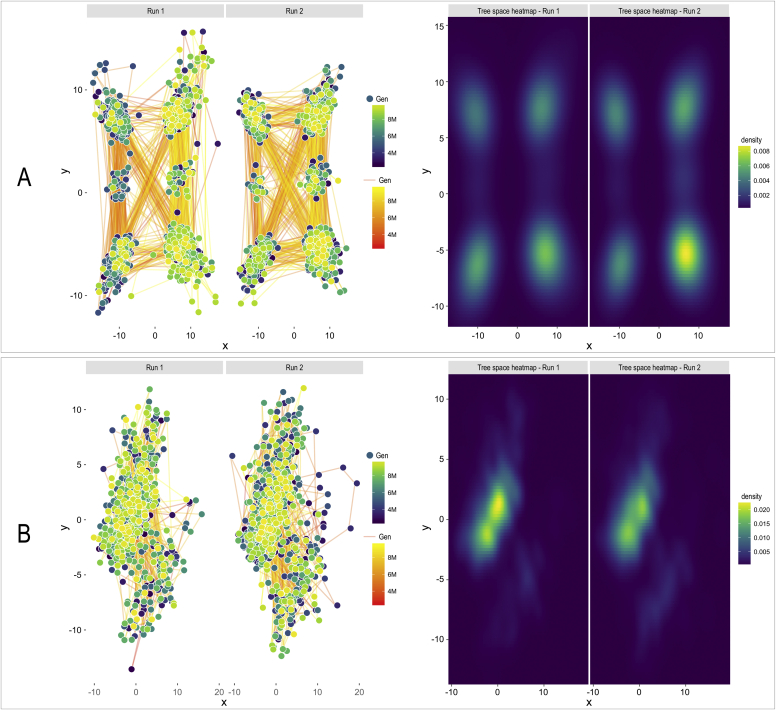
The results of Bayesian analysis were examined by using Tracer and the RWTY package. The ESS values were above 200 for all parameters in all runs. The topological convergence for each run was assessed using the cumulative split frequency plots of RWTY package (Fig. S4) examining the split frequencies of the worst 40 clades. With minor movements all split frequencies reached stationarity during the run indicating that all chains reached convergence. Tree space visualisation of the MCMC analysis showed high similarity to those obtained from the bootstrap samples. Altogether 1 000 trees were visualized after removing 25 % of the generations as burnin. In the case of the four-gene analysis the posterior distribution of tree topologies were not concentrated into one region. It is common that during the MCMC analysis the trees are moving through the tree-space from regions with low optimum to regions with high likelihood scores, but in an analysis with stable data this region should form a single, well-defined island in the tree space. Our data (Fig. 4A) show that the dataset with four genes has four almost equally optimal solutions and these are present in the later generations. These observations suggest that the phylogenetic signal in the dataset is not strong enough to have a well-defined set of trees and therefore, this dataset is not suitable to draw conclusions regarding the phylogenetic relationship of Aspergilli and Penicillia. The MCMC analysis of the dataset with nine genes resulted in a more compact set of trees occupying the tree space (Fig. 4B). The earlier generations showed relatively high movements in the space, but after the initial search the trees settled down in a more compact region with optimal solutions close to each other, suggesting that the phylogeny obtained with this dataset is more reliable than the results of the four-gene dataset.
Fig. 4.
Post-burnin tree space plots of 1 000 trees of Bayesian analysis with four (A) and nine (B) loci. Lines represent the connections between the subsequent generations while dots represent the two-dimensional place of the trees in the space. The colour of the lines and dots represents the generations. On the heat map green coloured areas represent the space occupied by larger number of trees.
Branch support analysis
The test of branch support for the six-genes phylogeny, by SH-aLRT, aLRT-Chi2 and aBayes values, give additional strength to the principal nodes depicting Penicillium and Aspergillus monophyletic topology (Fig. 5A, nodes P, A and PA). The lower bootstrap support observed in some nodes is generally balanced by high branch supports, except for the A2 node where the monophyly of subgenus Circumdati is not supported strongly. The A5 node resulted not supported due to the variable position of the Polypaecilum clade, clustering with subgenus Aspergillus or with section Cremei, as it is clearly visible when comparing partitioned to non-partitioned trees (Fig. S1). Single branch tests conducted with the nine-gene dataset support the monophyly of Aspergillus, confirming the subdivision of the genus into six subgenera with high values except subgenus Circumdati (Fig. 5B).
Phenotypic data supporting taxonomy and cladonomy
Species in Aspergillus subgenus Circumdati have most extrolites in common with the other subgenera/sections in Aspergillus, indicating that Aspergillus is one large genus. Subgenus Nidulantes is closely related to Circumdati, but even subgenus Fumigati and subgenus Aspergillus have several extrolites or heteroisoextrolites (Frisvad & Larsen 2016) in common. Data listed in Table 1 shows that at least xanthocillins, terphenyllins and emodin are in common within all the subgenera of the genus Aspergillus. Heveadrides are common also in section Aspergillus (Slack et al. 2009).
Table 1.
Isoextrolites and heteroisoextrolites in Aspergillus subgenera (see Frisvad & Samson 2004; Samson et al. 2004; Nielsen et al. 2009; Frisvad and Larsen, 2015, Frisvad and Larsen, 2016; Ma et al. 20161).
| Aspergillus and Cremei | Fumigati | Nidulantes | Circumdati | |
|---|---|---|---|---|
| Pseurotins | − | + | − | + |
| Kojic acid | − | − | + | + |
| Terrein | − | − | + | + |
| Asperphenamate | + | − | − | + |
| Sterigmatocystin | + | − | + | + |
| Cyclopiazonic acid | − | + | − | + |
| Malformins | − | + | − | + |
| Fumitremorgins | − | + | + | + |
| Emodin (as precursor) | + | + | + | + |
| 6-Methylsalicylic acid (as precursor) | − | + | − | + |
| Itaconic acid | + | − | − | + |
| Viridicatins | − | + | + | + |
| Penicillins | − | + | + | + |
| Notoamides | − | − | + | + |
| Aflavinins | − | + | + | + |
| Echinulins | + | +2 | − | +2 |
| Diketopiperazines | + | − | − | + |
| Polythiodiketopiperazines | − | + | + | + |
| Kotanins/desertorins | + | − | + | + |
| Falconensin type azaphilones | − | + | + | + |
| Xanthocillins and terphenyllins | + | + | + | + |
| Mycophenolic acid | + | + | − | − |
| Heveadrides | + | + | − | − |
| Patulin | + | + | − | − |
Even though Ma et al. (2016) identified their strain as Aspergillus tamarii, their strain was clearly an A. fumigatus.
While Aspergillus subgenus Aspergillus species produce echinulins and neoechinulins, species from Fumigati and Circumdati produce the related cycloechinulin.
An important example of chemical and morphological relationships in Aspergillus is A. cejpii (subgenus Fumigati). This species has a polypaecilum-like asexual morph, but it is phylogenetically placed “between” section Clavati and Fumigati, two sections in which all species have uniseriate aspergilla. Aspergillus cejpii is phylogenetically placed into an intermediate position between Fumigati and Clavati (Varga et al., 2007, Houbraken and Samson, 2011), and thus had to be transferred from Dichotomomyces (anamorphs had been named both Polypaecilum and Talaromyces) to Aspergillus (Samson et al. 2014). In subgenus Aspergillus, A. pisci (formerly Polypaecilum pisci) is placed in a sister-clade to Aspergillus section Aspergillus, containing species with phialosimplex-like and polypaecilum-like morphs, while in the clade based on A. wentii, a species with a penicillium-like morph is placed as A. inflatus (Samson et al. 2014). Most, if not all species in the subgenus Aspergillus are species able to grow well at very low water activities, while species in subgenus Fumigati are adapted to higher water activities. Yet species with polypaecilum-like morphs are placed in both subgenera. Aspergillus cejpii has heat resistant ascospores in common with species in section Fumigati with neosartorya-like morphs (Jesenska et al., 1992, Jesenska et al., 1993), while A. pisci has salt tolerance in common with most species in subgenus Aspergillus. Thus one can predict that if a fungus in subgenus Fumigati produces ascospores, those ascospores are heat-resistant, while if a new species is found to belong to subgenus Aspergillus, one can predict that it can grow under conditions with very low water activity, despite the differences in micro-morphology.
Regarding extrolites, A. cejpii also has an intermediate position between sections Fumigati and Clavati, while the species also show some chemical similarities with subgenus Aspergillus, and even with subgenus Circumdati. A. cejpii has been shown to produce gliotoxins and fiscalin B in common with A. fumigatus and A. fischeri (Varga et al., 2007, Frisvad and Larsen, 2015, Harms et al., 2015a, Rodrigues et al., 2015, Fan et al., 2016), xanthocillins (Kitahara and Endo, 1981, Harms et al., 2015b) in common with A. fumigatus (Zuck et al. 2011), showing several chemical similarities between A. cejpii with its phylogenetic sister group section Fumigati. Furthermore, indoloterpenes, such as JBIR-03, emeniveol, emindol SB, emindole SB mannoside, asporyzin A-C, 27-O-methylasporyzin C (Ogata et al., 2007, Qiao et al., 2010a, Qiao et al., 2010b, Harms et al., 2014) can be also found in common with species in subgenus Circumdati and Nidulantes (Nozawa et al., 1988, Kimura et al., 1992). Finally, tryptoquivalones in common with species of section Clavati and Fumigati (Varga et al., 2007, Frisvad and Larsen, 2016), while asporyergosterols and similar bioactive sterols (Qiao et al., 2010a, Harms et al., 2015b) in common with several Aspergilli, and heveadrides in common with Aspergillus section Aspergillus (Slack et al., 2009, Harms et al., 2015a) have also been found. Aspergillus arxii (formerly Cristaspora arxii) was found to produce heveadrides, in common with Aspergillus cejpii (new data provided here) and Aspergillus species in section Aspergillus (Table 1). Thus, A. cejpii has several physiological, chemical and phylogenetic similarities with other species of Aspergillus.
Discussion
In our study we compared 96 and 204 species using six and nine genes phylogenies, respectively. The involved species covered all sections from genus Aspergillus, except sections Tanneri and Petersonii (Samson et al., 2014, Hubka et al., 2014, Jurjevic et al., 2015), all accepted sections from the genus Penicillium except section Turbata (Visagie et al. 2014, Houbraken et al. 2015) and species from other genera of the family Aspergillaceae, Thermoascaceae and Trichocomaceae (Peterson et al., 2010, Houbraken and Samson, 2011, Yilmaz et al., 2014). Both phylogenetic studies supported the monophyly of the genus Aspergillus using Bayesian and ML approaches. These findings are contradictory to those of Pitt & Taylor (2014), as well as Taylor et al. (2016), while they are in agreement with the previous studies of Houbraken & Samson (2011), and Houbraken et al. (2014).
Both results are in accordance regarding the subgenus Circumdati as this clade was resolved with low support values in all analyses except the Bayesian approaches. In the ML analysis all sections formed monophyletic groups with moderate to high support except for species previously assigned to section Usti and Restricti. Both the ML and Bayesian approach divided section Usti into two separate groups in which A. amylovorus, A. subsessilis and A. egyptiacus formed a well-defined clade with high posterior probabilities and ML bootstrap values (1/92). Members of section Restricti did not form a separate clade however; this can be due to the inadequate taxon sampling as a recent phylogenetic analysis across species diversity in the subgenus Aspergillus strongly supported monophyly of both, sect. Aspergillus and sect. Restricti (unpublished data). Both analyses rendered the genus Penicillium as a monophyletic sister group to Aspergilli with high support (100/1). The genus can be divided into two subgenera: Aspergilloides and Penicillium comprising 25 sections with high statistical support obtained by Bayesian analysis. The results of the ML analysis were largely congruent with those of Bayesian approach except for the moderate support (66) for the subgenus Aspergilloides. Regarding the basal genera the topology of the tree was mainly in agreement with previous studies (Peterson 2008, Houbraken & Samson 2011).
Taylor et al. (2016) tested several hypotheses regarding the monophyly of Aspergilli, however most of these tests did not reflect the current knowledge on Aspergilli. Their tests rejected the inclusion of A. penicilliformis, A. zonatus, Sclerocleista ornata and S. thaxteri in the genus Aspergillus. Previous studies (Peterson, 2008, Houbraken and Samson, 2011, Samson et al., 2014) have proven that these species are phylogenetically distinct from the Aspergilli and therefore the rejection of these hypotheses is in agreement with recent phylogenies. The inclusion of A. clavatoflavus was not rejected but the p value of the hypothesis did not indicate strong support for the inclusion of this species to the Aspergilli. However, the taxonomic position of this species remained unclear. Several studies have demonstrated that A. clavatoflavus is not a member of the genus Aspergillus (Peterson, 2008, Peterson et al., 2010, Houbraken and Samson, 2011, Samson et al., 2014). The reason of this contradictory result can be that the dataset used in their study had low resolving power restricting the estimation of a well-established phylogeny. On the tree obtained by Taylor et al. (2016), the deeper clades were poorly supported; therefore the inclusion of A. clavatoflavus may not have altered the overall likelihood value of the constrained tree substantially.
Our main concern about the tests conducted by Taylor et al. (2016) is that it is not clear whether they had used multifurcating or fully resolved constraints for estimating ML trees before the calculation of the site-wise likelihoods. Using fully resolved trees as constraints can lead to the underestimation of the probabilities of hypotheses, which can explain the unexpectedly low p values in some of their analyses. In our experiments the hypothesis of Taylor et al. (2016) was rejected with a mean p value of 0.0134, when a constrained tree containing polytomies was used. When the ML likelihood search was conducted with the completely resolved best tree obtained by RAxML the approximately unbiased test in CONSEL also rejected the hypothesis but with values very close to zero.
The exclusion of subgenus Polypaecilum from a monophyletic Aspergillus clade was also rejected indicating that the species of this section are members of the genus Aspergillus. Moreover, when this section was included in a monophyletic Aspergillus clade, the hypothesis was accepted. This finding is in agreement with the previous results of Houbraken and Samson, 2011, Samson et al., 2014 and our recent findings.
Additional evidences of the robustness of our analysis with respect to that of Taylor et al. (2016) could be retrieved from the recently guidelines published on IMA Fungus for introducing new genera of fungi (Vellinga et al. 2015). The authors proposed six criteria; our analysis is in accordance with all the criteria but in particular two of these criteria are fully in accordance with our results and not with those of Taylor et al. (2016). They have assessed that: 1) all genera that are recognized should be monophyletic, not only the one that is the focus of the study, but also the group from which it is separated and the group to which it is added (the reciprocal monophyly criterion), 2) the branching of the phylogenetic trees should have sufficient and strong statistical support. Finally, also the extrolite data support the clustering of the wide Aspergillus genus evidencing that at least xanthocillins, terphenyllins and emodin are in common within all the subgenera of the genus (Table 1). In particular, some species that have been shown to be outside Aspergillus, despite having an Aspergillus conidiophore, appear to be unique chemically: Aspergillus clavatoflavus has been analysed chemically and produced a series of unique secondary metabolites never found in any species of Aspergillus and does not produce kojic acid, produced by all species in Aspergillus section Flavi except A. avenaceus and A. togoensis (Varga et al. 2011). Aspergillus zonatus was reported to produce aszonalenin and aszonapyrone (Kimura et al., 1982a, Kimura et al., 1982b, Katsube et al., 1985, Bhat et al., 1993), but several chemical analysis of the ex-type strain of this fungus showed that it only produces some few unique extrolites, and that aszonalenin and aszonapyrone was not among them (Frisvad, unpublished). Aszonalenin and aszonapyrone was found in several species in Aspergillus section Fumigati (Larsen et al., 2007, Frisvad et al., 2009, Frisvad and Larsen, 2016) however, indicating that the culture of A. zonatus was contaminated with an isolate from section Fumigati. Also Throckmorton et al. (2015) did not find biosynthetic gene clusters coding for aszonapyrone when examining the genome sequenced isolate of A. zonatus, but they did find a PKS Aspzol_2112764 coding for an unknown non-reduced polyketide. Aflatoxin B1 was also reported from a strain of A. zonatus (El Kady et al. 1994), but this was obviously a mistake.
Sclerocleista ornata and S. thaxteri produce viriditoxin in common with both Paecilomyces variotii and Aspergillus section Fumigati species such as A. viridinutans, and citrinin in common with Monascus spp. and Aspergillus sections Flavipedes and Terrei. Apart from this, they produce at least two types of secondary metabolites not yet found in any Aspergillus section. Given that at least S. thaxteri occupies a dung habitat; it is interesting to note that the two Sclerocleista species grow very poorly on media containing sucrose, thus making them pretty unique. It is recommended to use the genus name Sclerocleista for those two closely related species. Thus, the phenotyping data confirm the grouping of the wide Aspergillus genus with the exclusion of A. clavatoflavus and A. zonatus species, and of the Warcupiella and Sclerocleista clades, previously treated as Aspergillus subgenera.
Taxonomic discussion and conclusions
The phylogenetic analyses show that the Polypaecilum clade and section Cremei are strongly supported therefore should be treated as subgenera:
Aspergillus subgenus Cremei Samson, Houbraken & Frisvad, subgen. nov. MycoBank MB819182.
Etymology: named after the epithet of the type species.
Diagnosis: Conidia en masse grey-green to yellow brown, globose to subglobose, biseriate or uniseriate conidial heads, metulae and phialides produced synchronously, except in A. inflatus, where they are produced successively. Species are moderately osmophilic and halophilic (Wheeler & Hocking 1993).
Type species: Aspergillus cremeus Kwon-Chung & Fennell
Aspergillus subgenus Polypaecilum Samson, Houbraken & Frisvad, subgen. nov. MycoBank MB819184.
Etymology: named after the genus Polypaecilum.
Diagnosis: Conidia formed on reduced phialides (as in Phialosimplex salinarum, Greiner et al. 2014, appearing as phialide collula only), small phialides with long collula often with a thickened centre part (like in Phialosimplex caninus, Sigler et al. 2010) or on polyphialides (as in Polypaecilum insolitum, Smith 1961), with the common theme of a thin, long collulum producing chains of conidia that are large compared to the diameter of the collulum. Aspergilla are not produced. The species are halophilic or osmophilic (Wheeler et al., 1988, Wheeler and Hocking, 1993, Greiner et al., 2014, Piñar et al., 2015, Piñar et al., 2016). The subgenus Polypaecilum contains species of the previously known genera Polypaecilum and Phialosimplex.
Type species: Polypaecilum insolitum G. Sm. = Aspergillus insolitus (G. Smith) Houbraken, Visagie & Samson
Our analysis shows that A. zonatus does not belong to Aspergillus, which was already demonstrated by Peterson (2008), and Houbraken & Samson (2011). Together with Penicilliopsis clavariiformis the taxon forms a strongly supported clade. Penicilliopsis is typified by P. clavariiformis and characterized by seed-borne, stipitate stromata often occurring in tropical forests. The anamorph genera Pseudocordyceps, Sarophorum and Stilbodendron are phenotypically related (Samson and Seifert, 1985, Hsieh and Ju, 2002). The former two genera have conidiogenous structures similar to those of Penicillium and the latter has Aspergillus-like conidiogenous structures. Therefore it is possible that A. zonatus belongs to Penicilliopsis. The type culture of A. zonatus was found a sample of forest soil in Costa Rica and Penicilliopsis occurs in a similar habitat. Since A. zonatus is known only from this type culture the accommodation in a new genus might be premature until more material is collected. For the time being the species is recombined in Penicilliopsis.
Penicilliopsis zonata (Kwon-Chung & Fennell) Samson, Houbraken & Frisvad, comb. nov. Mycobank MB819185.
Basionym: Aspergillus zonatus Kwon-Chung & Fennell, The Genus Aspergillus: 377 (1965) [MB#326666]
A detailed description of the species is provided by Raper & Fennel (1965: 377).
Aspergillus clavatoflavus described from rain forest soil, collected in Australia, is also not related to Aspergillus. Our analyses confirm its position outside Aspergillus as it was already demonstrated by Peterson (2008), and Houbraken & Samson (2011) without any closely related taxon. Although the species is only known from its ex-type culture the erection of a new genus is proposed herein:
Aspergillago Samson, Houbraken & Frisvad, gen. nov. MycoBank MB819186.
Etymology: Resembling Aspergillus
Diagnosis: Morphologically resembles Aspergillus by its typical aspergillum, but phylogenetically distant.
Type species: Aspergillus clavatoflavus Raper & Fennell, Gen Aspergillus: p. 378 (1965).
Aspergillago clavatoflava (Raper & Fennell) Samson, Houbraken & Frisvad, comb. nov. MycoBank MB819187.
Basionym: Aspergillus clavatoflavus Raper & Fennell, Gen Aspergillus: p. 378 (1965)
For a full description, see Raper & Fennell (1965: 378–381).
Raper & Fennell (1965) proposed A. clavatoflavus as a new taxon because it resembled the morphology of A. clavatus and A. flavus. However, the conidiophores were produced in loose synnemata, a feature not observed in Aspergillus. In that respect the synnematous conidiophores of A. clavatoflavus resembles those of Stilbothamnium which is considered to be a synonym of Aspergillus (Varga et al., 2011, Samson et al., 2014).
Conclusion
From our extensive and independent phylogenetic multilocus analyses of 96 and 204 species respectively, it can be concluded that there is no phylogenetic evidence to split Aspergillus into several genera and the name Aspergillus can be used for all the species which have been proven taxonomically to belong to Aspergillus. The monophyly of the genus Aspergillus supports the use of Aspergillus in a wide sense.
Acknowledgement
During the research and writing of the manuscript our good friend and colleague János Varga, a member of the IUMS International Commission of Penicillium and Aspergillus, passed away. We and the Aspergillus community owe him an enormous gratitude for his expertise and inspiration. János was the first who initiated the concept of using the name Aspergillus in the One Fungus One Name discussion. The research of János Varga was supported by the Hungarian Research Fund (OTKA K115690).
Footnotes
Peer review under responsibility of CBS-KNAW Fungal Biodiversity Centre.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.simyco.2016.11.006.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Fig. S1. The trees were indicated as follows: IQ-TREE (rooted consensus maximum likelihood tree inferred by IQ-TREE), IQ-TREE partitioned (rooted consensus partitioned maximum likelihood tree inferred by IQ-TREE), RaxML (rooted consensus maximum likelihood tree inferred by RAxML), RaxML partitioned (rooted consensus partitioned maximum likelihood tree inferred by RAxML), MrBayes (rooted Bayesian consensus tree inferred by MrBayes). Numbers at the node represent SH-aLRT, aBayes and UFML bootstrap support for IQTREE; Rapid ML bootstrap support values for RaxML; Bayesian posterior probabilities for MrBayes.
Fig. S2. Phylograms obtained by Maximum Likelihood (ML) and Bayesian analysis inferred from nine loci (18S rDNA, 5.8S rDNA, 28S rDNA (D1-D2), RPB1, RPB2, CaM, BenA, Tsr1, Cct8). A. Best-scoring ML tree obtained by RAxML.B. 50 % majority rule phylogram of Bayesian analysis. Numbers above or below branches are bootstrap values (A) and posterior probabilities (B). Only support values greater than 60 % and 0.95 are shown.
Fig. S3. Phylograms obtained by Maximum Likelihood (ML) and Bayesian analysis inferred from the Cct8 + RPB1 + RPB2 + Tsr1 dataset. Monophyletic groups are collapsed and shown as triangles. A. Best-scoring ML tree obtained by RAxML. B. 50 % majority rule phylogram of Bayesian analysis. Numbers above or below branches are bootstrap values (A) and posterior probabilities (B). Only support values greater than 60 % and 0.95 are shown.
Fig. S4. Post-burnin cumulative split frequencies of the worst 40 clades of Bayesian analysis with four and nine loci. The colour of the line represents the standard deviation of the split frequencies of a given clade.
References
- Aberer A.J., Krompass D., Stamatakis A. Pruning rogue taxa improves phylogenetic accuracy: an efficient algorithm and webservice. Systematic Biology. 2013;62:162–166. doi: 10.1093/sysbio/sys078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimova M., Gil M., Dufayard J.F. Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Systematic Biology. 2011;60:685–699. doi: 10.1093/sysbio/syr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat B., Harrison S.M., Lamont H.M. The biosynthesis of the mould metabolites roquefortine and aszonalenin from L-[2,4,5,6,7-2H5]tryptophan. Tetrahedron. 1993;49:10663–10668. [Google Scholar]
- Borchsenius F. FastGap 1.2. Department of Biosciences, Aarhus University, Denmark. 2009. http://www.aubot.dk/FastGap_home.htm Published online at:
- Borg I., Groenen P. Springer-Verlag; Heidelberg: 1997. Modern multidimensional scaling. [Google Scholar]
- Chang J.M., Di Tommaso P., Lefort V. TCS: a web server for multiple sequence alignment evaluation and phylogenetic reconstruction. Nucleic Acids Reserch. 2015;43:W3–W6. doi: 10.1093/nar/gkv310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomor O.A., von Haeseler A., Minh B.Q. Terrace aware data structure for phylogenomic inference from supermatrices. Systematic Biology. 2016 doi: 10.1093/sysbio/syw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba D., Taboada G.L., Doallo R. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods. 2012;30:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kady I., El Maraghy S., Zsohri A.N. Mycotoxin producing potential of some isolates of Aspergillus flavus and Eurotium groups from meat products. Microbiological Research. 1994;149:297–307. doi: 10.1016/S0944-5013(11)80073-X. [DOI] [PubMed] [Google Scholar]
- Fan Z., Sun Z.-H., Liy Z. Dichotocejpins A-C; new diketopiperazines from a deep-sea derived fungus Dichotomomyces cejpii FS110. Marine Drugs. 2016;14 doi: 10.3390/md14090164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisvad J.C., Larsen T.O. Chemodiversity in the genus Aspergillus. Applied Microbiology and Biotechnology. 2015;99:7859–7877. doi: 10.1007/s00253-015-6839-z. [DOI] [PubMed] [Google Scholar]
- Frisvad J.C., Larsen T.O. Extrolites of Aspergillus fumigatus and other pathogenic species in Aspergillus section Fumigati. Frontiers in Microbiology. 2016;6 doi: 10.3389/fmicb.2015.01485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisvad J.C., Samson R.A. Emericella venezuelensis, a new species with stellate ascospores producing sterigmatocystin and aflatoxin B1. Systematic and Applied Microbiology. 2004;27:672–680. doi: 10.1078/0723202042369910. [DOI] [PubMed] [Google Scholar]
- Frisvad J.C., Thrane U. Standardized high-performance liquid chromatography of 182 mycotoxins and other fungal metabolites based on alkylphenone indices and UV–VIS spectra (diode-array detection) Journal of Chromatography. 1987;404:195–214. doi: 10.1016/s0021-9673(01)86850-3. [DOI] [PubMed] [Google Scholar]
- Frisvad J.C., Rank C., Nielsen K.F. Metabolomics of Aspergillus fumigatus. Medical Mycology. 2009;47:S53–S71. doi: 10.1080/13693780802307720. [DOI] [PubMed] [Google Scholar]
- Greiner K., Peršoh D., Weig A. Phialosimplex salinarum, a new species of Eurotiomycetes from a hypersaline habitat. IMA Fungus. 2014;5:161–172. doi: 10.5598/imafungus.2014.05.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S., Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Guindon S., Dufayard J.F., Lefort V. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Harms H., Rempel V., Kehraus S. Indoloterpenes from a marine-derived fungal strain of Dichotomomyces cejpii with antagonistic activity at GRR18 and cannabinoid receptors. Journal of Natural Products. 2014;77:673–677. doi: 10.1021/np400850g. [DOI] [PubMed] [Google Scholar]
- Harms H., Orlikova B., Seungwon J. Epipolythiodiketopiperazines from the marine derived fungus Dichotomomyces cejpii with NF-κB inhibitory potential. Marine Drugs. 2015;13:4949–4966. doi: 10.3390/md13084949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms H., Kehraus S., Nesaei-Mosaferan D. Ab-42 lowering agents from the marine-derived fungus Dichotomomyces cejpii. Steroids. 2015;104:182–188. doi: 10.1016/j.steroids.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Hillis D.M., Heath T.A., St John K. Analysis and visualization of tree space. Systematic Biology. 2005;54:471–482. doi: 10.1080/10635150590946961. [DOI] [PubMed] [Google Scholar]
- Houbraken J., de Vries R.P., Samson R.A. Modern taxonomy of biotechnologically important Aspergillus and Penicillium species. Advances in Applied Microbiology. 2014;86:199–249. doi: 10.1016/B978-0-12-800262-9.00004-4. [DOI] [PubMed] [Google Scholar]
- Houbraken J., Samson R.A. Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Studies in Mycology. 2011;70:1–51. doi: 10.3114/sim.2011.70.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbraken J., Wang L., Lee H.B. New sections in Penicillium containing novel species producing patulin, pyripyropens or other bioactive compounds. Persoonia: Molecular Phylogeny and Evolution of Fungi. 2015;36:299–314. doi: 10.3767/003158516X692040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H.-M., Ju Y.-M. Penicilliopsis pseudocordyceps, the holomorph of Pseudocordyceps seminicola, and notes on Penicilliopsis clavariaeformis. Mycologia. 2002;94:539–544. [PubMed] [Google Scholar]
- Hubka V., Nováková A., Kolarík A. Revision of Aspergillus section Flavipedes: seven new species and proposal of section Jani sect. nov. Mycologia. 2014;107:169–208. doi: 10.3852/14-059. [DOI] [PubMed] [Google Scholar]
- Jesenska Z., Pieckova E., Bernat D. Heat-resistance fungi in the soil. International Journal of Food Microbiology. 1992;16:209–214. doi: 10.1016/0168-1605(92)90081-d. [DOI] [PubMed] [Google Scholar]
- Jesenska Z., Pieckova E., Bernat D. Heat-resistance of fungi from soil. International Journal of Food Microbiology. 1993;19:187–192. doi: 10.1016/0168-1605(93)90076-s. [DOI] [PubMed] [Google Scholar]
- Jurjevic Ž., Kubátová A., Kolařík M. Taxonomy of Aspergillus section Petersonii sect. nov. encompassing indoor and soil-borne species with predominant tropical distribution. Plant Systematics and Evolution. 2015;301:2441–2462. [Google Scholar]
- Katsube Y., Kimura Y., Hamasaki T. Structure of aszonapyrojne A monomethyl ether-1, a derivative of aszonapyrone A, produced by Aspergillus zonatus. Agricultural and Biological Chemistry. 1985;49:551–553. [Google Scholar]
- Kepler R.M., Humber R.A., Bischoff J.F. Clarification of generic and species boundaries for Metarhizium and related fungi through multigene phylogenetics. Mycologia. 2014;106:811–829. doi: 10.3852/13-319. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Hamasaki T., Nakajima H. Structure of aszonalenin, a new metabolite of Aspergillus zonatus. Tetrahedron Letters. 1982;23:225–228. [Google Scholar]
- Kimura Y., Hamasaki T., Isogai A. Structure of aszonapyrone, a new metabolite produced by Aspergillus zonatus. Agricultural and Biological Chemistry. 1982;46:1963–1965. [Google Scholar]
- Kimura Y., Nishibe M., Nakajima H. Emeniveol; a new pollen growth inhibitor from the fungus, Emericella nivea. Tetrahedron Letters. 1992;33:6987–6990. [Google Scholar]
- Kitahara N., Endo A. Xanthocillin X monomethyl ether, a potent inhibitor of prostaglandin biosynthesis. Journal of Antibiotics. 1981;34:1556–1561. doi: 10.7164/antibiotics.34.1556. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecular Biology and Evolution. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfear R., Calcott B., Ho S.Y. Partitionfinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution. 2012;29:1695–1701. doi: 10.1093/molbev/mss020. [DOI] [PubMed] [Google Scholar]
- Larsen T.O., Smedsgaard J., Nielsen K.F. Production of mycotoxins by Aspergillus lentulus and other medically important and closely related species in section Fumigati. Medical Mycology. 2007;45:225–232. doi: 10.1080/13693780601185939. [DOI] [PubMed] [Google Scholar]
- Lingoes J.C., Roskam E.E., Borg I. 2nd edn. Mathesis Press; Ann Arbor, Michigan: 1979. Geometric representations of relational data. [Google Scholar]
- Löytynoja A. Phylogeny-aware alignment with PRANK. Methods in Molecular Biology. 2014;1079:155–170. doi: 10.1007/978-1-62703-646-7_10. [DOI] [PubMed] [Google Scholar]
- Ma Y-M., Liang X-A., Zhang H-C. Cytotoxic and antibiotic cyclic pentapeptide from an endophytic Aspergillus tamarii of Ficus carica. Journal of Agricultural and Food Chemistry. 2016;64:3789–3793. doi: 10.1021/acs.jafc.6b01051. [DOI] [PubMed] [Google Scholar]
- Maddison W.P., Maddison D.R. Mesquite: a modular system for evolutionary analysis. Version 3.10. 2016. http://mesquiteproject.org
- McNeill J., Barrie F.R., Buck W.R. International Code of Nomenclature for algae, fungi, and plants (Melbourne Code) Regnum Vegetabile. 2012;154:208. [Google Scholar]
- Micheli P.A. Nova plantarum genera; 1729. [Google Scholar]
- Miller M.A., Pfeiffer W., Schwartz T. Gateway Computing Environments Workshop (GCE), IEEE. 2010. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees; pp. 1–8. [Google Scholar]
- Minh B.Q., Nguyen M.A.T., von Haeseler A. Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution. 2013;30:1188–1195. doi: 10.1093/molbev/mst024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L.T., Schmidt H.A., von Haeseler A. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Molecular Biology and Evolution. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen K.F., Månsson M., Rank C. Dereplication of microbial natural products by LC-DAD-TOFMS. Journal of Natural Products. 2011;74:2338–2348. doi: 10.1021/np200254t. [DOI] [PubMed] [Google Scholar]
- Nielsen K.F., Mogensen J.M., Johansen M. Review of secondary metabolites and mycotoxins from the Aspergillus niger group. Analytical and Bioanalytical Chemistry. 2009;395:1225–1242. doi: 10.1007/s00216-009-3081-5. [DOI] [PubMed] [Google Scholar]
- Nozawa K., Nakajima S., Kawai K. Isolation and structures of indoloditerpenes, possible biosynthetic intermediates to the tremorgenic mycotoxin, paxillin, from Emericella striata. Journal of the Chemical Society Perkin Transactions I. 1988;1988:2607–2610. [Google Scholar]
- Ogata M., Ueda J., Hoshi M. A novel indole-diterpenoid, JBIR-03 with anti-MSRA activity from Dichotomomyces cejpii var. cejpii NBRC 103559. Journal of Antibiotics. 2007;60:645–648. doi: 10.1038/ja.2007.83. [DOI] [PubMed] [Google Scholar]
- Peterson S.W. Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia. 2008;100:205–226. doi: 10.3852/mycologia.100.2.205. [DOI] [PubMed] [Google Scholar]
- Peterson S.W., Jurjevic Z., Bills G.F. Genus Hamigera, six new species and multilocus DNA sequence based phylogeny. Mycologia. 2010;102:847–864. doi: 10.3852/09-268. [DOI] [PubMed] [Google Scholar]
- Piñar G., Dalnodar D., Voiti C. Biodeterioration risk threatens the 3100 year old staircase of Hallstatt (Austria): possible involvement of halophilic microorganisms. PLOS ONE. 2016;11:e0148279. doi: 10.1371/journal.pone.0148279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñar G., Tafer H., Sterflinger K. Amid the possible causes of a very famous foxing: molecular and microscopic insight into Leonardo da Vinci’s self-portrait. Environmental Microbiology Reports. 2015;7:849–859. doi: 10.1111/1758-2229.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt J.I., Taylor J.W. Aspergillus, its sexual states, and the new International Code of Nomenclature. Mycologia. 2014;106:1051–1052. doi: 10.3852/14-060. [DOI] [PubMed] [Google Scholar]
- Pitt J.I., Taylor J.W. (2441) Proposal to conserve the name Aspergillus (Fungi: Eurotiales: Trichocomaceae) with a conserved type to maintain also the name Eurotium. Taxon. 2016;65:631–632. [Google Scholar]
- Qiao M.-F., Ji N.-Y., Liu X.-H. Asporyergosterol, a new steroid from an algicolous isolate of Aspergillus oryzae. Natural Products Communications. 2010;5:1575–1578. [PubMed] [Google Scholar]
- Qiao M.-F., Ji N.-Y., Liu X.-H. Indoloterpenes from an algicolous isolate of Aspergillus oryzae. Bioorganic and Medicinal Chemistry Letters. 2010;20:5677–5680. doi: 10.1016/j.bmcl.2010.08.024. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2016. R: a language and environment for statistical computing.https://www.R-project.org/ [Google Scholar]
- Rambaut A., Suchard M.A., Xie D. Tracer v. 1.6. 2014. http://beast.bio.ed.ac.uk/Tracer
- Raper K.B., Fennell D.I. Williams & Wilkins; Baltimore: 1965. The genus Aspergillus. [Google Scholar]
- Robinson D.F., Foulds L.R. Comparison of weighted labeled trees. Lecture Notes in Mathematics. 1979;748:119–126. [Google Scholar]
- Robinson D.F., Foulds L.R. Comparison of phylogenetic trees. Mathematical Biosciences. 1981;53:131–147. [Google Scholar]
- Rodrigues B.S.F., Sahm B.D.B., Jimenez P.C. Bioprospection of cytotoxic compounds in fungal strains recovered from sediments of the Brazilian coast. Chemistry & Biodiversity. 2015;12:432–442. doi: 10.1002/cbdv.201400193. [DOI] [PubMed] [Google Scholar]
- Ronquist F., Teslenko M., van der Mark P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson R.A., Houbraken J.A.M.P., Kuijpers A.F.A. New ochratoxin or sclerotium producing species in Aspergillus section Nigri. Studies in Mycology. 2004;50:45–61. [Google Scholar]
- Samson R.A., Seifert K.A. The ascomycete genus Penicilliopsis and its anamorphs. In: Samson R.A., Pitt J.I., editors. Advances in Penicillium and Aspergillus systematic. Plenum Press; New York, USA: 1985. pp. 397–426. [Google Scholar]
- Samson R.A., Visagie C.M., Houbraken J. Phylogeny, identification and nomenclature of the genus Aspergillus. Studies in Mycology. 2014;78:141–173. doi: 10.1016/j.simyco.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimodaira H., Hasegawa M. CONSEL: for assessing the confidence of phylogenetic tree selection. Bioinformatics. 2001;17:1246–1247. doi: 10.1093/bioinformatics/17.12.1246. [DOI] [PubMed] [Google Scholar]
- Sigler L., Sutton D.A., Gibas C.F.C. Phialosimplex, a new anamorphic genus associated with infections in dogs and having phylogenetic affinity to the Trichocomaceae. Medical Mycology. 2010;48:335–345. doi: 10.3109/13693780903225805. [DOI] [PubMed] [Google Scholar]
- Silvestro D., Michalak I. raxmlGUI: a graphical front-end for RAxML. Organisms Diversity and Evolution. 2012;12:335–337. [Google Scholar]
- Slack G., Puniani E., Frisvad J.C. Secondary metabolites from Eurotium species, A. calidoustus and A. insuetus common in Canadian homes with a review of their chemistry and biological activities. Mycological Research. 2009;113:480–490. doi: 10.1016/j.mycres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Smedsgaard J. Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. Journal of Chromatography A. 1997;760:264–270. doi: 10.1016/s0021-9673(96)00803-5. [DOI] [PubMed] [Google Scholar]
- Smith G. Polypaecilum gen. nov. Transactions of the British Mycological Society. 1961;44:437–440. [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens T.G., Bhattacharya D., Ragan M.A. PhySortR: a fast, flexible tool for sorting phylogenetic trees in R. PeerJ. 2016;4:e2038. doi: 10.7717/peerj.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran J., Holder M.T. DendroPy: a Python library for phylogenetic computing. Bioinformatics. 2010;26:1569–1571. doi: 10.1093/bioinformatics/btq228. [DOI] [PubMed] [Google Scholar]
- Taylor J.W., Göker M., Pitt J.I. Choosing one name for pleomorphic fungi: the example of Aspergillus versus Eurotium, Neosartorya and Emericella. Taxon. 2016;65:593–601. [Google Scholar]
- Throckmorton K., Wiemann P., Keller N.P. Evolution of chemical diversity in a group of non-reduced polyketide gene clusters: using phylogenetics to inform the search for novel natural products. Toxins. 2015;7:3572–3607. doi: 10.3390/toxins7093572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya G., Lohman D.J., Meier R. SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics. 2011;27:171–180. doi: 10.1111/j.1096-0031.2010.00329.x. [DOI] [PubMed] [Google Scholar]
- Varga J., Due M., Frisvad J.C. Taxonomic revision of Aspergillus section Clavati based on molecular, morphological and physiological data. Studies in Mycology. 2007;59:89–106. doi: 10.3114/sim.2007.59.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J., Frisvad J.C., Samson R.A. Two new aflatoxin producing species, and an overview of Aspergillus section Flavi. Studies in Mycology. 2011;69:57–80. doi: 10.3114/sim.2011.69.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellinga E.C., Kuyper T.W., Ammirati J. Six simple guidelines for introducing new genera of fungi. IMA Fungus. 2015;6:65–68. [Google Scholar]
- Visagie C.M., Houbraken J., Frisvad J.C. Identification and nomenclature of the genus Penicillium. Studies in Mycology. 2014;78:343–371. doi: 10.1016/j.simyco.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler K.A., Hocking A.D. Interactions among xerophilic fungi associated with dried salted fish. Journal of Applied Bacteriology. 1993;74:164–169. doi: 10.1111/j.1365-2672.1993.tb03010.x. [DOI] [PubMed] [Google Scholar]
- Wheeler K.A., Hocking A.D., Pitt J.I. Influence of temperature on the water relations on Polypaecilum pisce and Basipetospora halophila, two halophilic fungi. Journal of General Microbiology. 1988;134:2255–2260. [Google Scholar]
- Yilmaz N., Visagie C.M., Houbraken J. Polyphasic taxonomy of the genus. Talaromyces. Studies in Mycology. 2014;78:175–341. doi: 10.1016/j.simyco.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F.W., Hamer R.M. Erlbaum; New York: 1987. Multidimensional scaling: history, theory and applications. [Google Scholar]
- Zhang N., Rossman A.Y., Seifert K. American Phytopathological Society; St. Paul: 2013. Impacts of the International Code of Nomenclature for algae, fungi and plants (Melbourne Code) on the scientific names of plant pathogenic fungi. Online. APSnet Feature. [Google Scholar]
- Zuck K.M., Shipley S., Newman D.J. Induced production of N-formyl alkaloids from Aspergillus fumigatus by co-culture with Streptomyces peuceticus. Journal of Natural Products. 2011;74:1653–1657. doi: 10.1021/np200255f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.