Abstract
Objective(s):
Maternal high-fat diet has been shown to have deleterious effects on the offspring bones. However, there is no study to assess the effects of type and amount of maternal dietary oil in an isocaloric diet, with focus on extra virgin olive oil (EVOO). The objective of the current study was to test the hypothesis that type of maternal dietary oil has more effects than its amount in an isocaloric diet during gestation and lactation on bone genes expression in offspring in adolescence.
Materials and Methods:
Virgin female C57BL/6 mice were impregnated and fed either the AIN 93G diet (received 16% of calories as soybean oil, as a control diet, or EVOO) or a high fat AIN 93G diet (received 45% of calories as soybean oil or EVOO) from the time of vaginal plug confirmation until offspring’s weaning.
Results:
After adjusting for the amount of oils, osteoprotegerin/receptor activator of nuclear factor NF-κB ligand (OPG/RANK-L) and OPG expressions were 6.1- and 2.8-folds higher in offspring born to EVOO compared with soybean oil-fed mothers. OPG, beta-catenin, and OPG/RANK-L expression were 88%, 94%, and 70% lower in offspring born to the 45% oil-fed mothers compared with the 16% group. In contrast, peroxisome proliferator-activated receptor gamma-2 (PPARγ2) gene expression was higher in the 45% oil group, adjusted for the types of oil.
Conclusion:
Maternal EVOO consumption, but not soybean oil increased osteoblastic gene expression, and high amounts of both oils decreased osteoblastic and increased adipogenic genes expression in adolescent offspring.
Keywords: Dietary oil, Fetal programming, Gestation, Lactation, Mouse, Olive oil, Osteoblastogenesis
Introduction
Osteoporosis is a serious worldwide health problem with economic implications, and it is characterized by enhanced bone fragility and increased fracture risk. These problems arise as a result of low bone mass and micro-architectural deterioration of bone tissue (1). Therefore, it is important to develop new preventive approaches for reducing the incidence of osteoporosis. Preventative strategies against osteoporosis can be aimed at either optimizing the peak bone mass obtained or reducing the rate of bone loss (2).
Evidence has shown that environmental factors may act early in development (in utero and early postnatal life), interacting with the genome to produce a persistent influence on postnatal skeletal development (3, 4). Increasing evidence suggests that maternal fatty acid status, both quantity and quality, during gestation and lactation can induce permanent changes in gene expression and metabolism (5, 6). Previous studies have shown that maternal high-fat/high-calorie diets during gestation increase bone marrow adiposity and alter bone structure in their offspring (4). However, no study has yet examined the effects of different types and amounts of maternal dietary oil in an isocaloric diet with extra virgin olive oil (EVOO) focus on bone remodeling and adipogenic markers in adolescent offspring.
A fine balance between bone resorption and formation is required for its integrity. The expression of osteoprotegerin (OPG) and receptor activator of nuclear factor NF-κB ligand (RANK-L) genes is an important marker in osteogenic cells, indicating their capacity to regulate bone remodeling. It is considered that high OPG/RANK-L ratio favors bone formation, while low ratios induce bone resorption (7). On the other hand, studies have shown that beta-catenin signaling is essential for proper bone formation and remodeling. Specifically, mice lacking beta-catenin in the bone showed increased resorption despite normal OPG production (8). Peroxisome proliferator-activated receptor gamma-2 (PPARγ2) controls bone turnover and regulates cell differentiation of both mesenchymal and hematopoietic lineages (9). The rapidly growing literature indicates an increase in dietary omega-6 attributed to high levels of consumption of vegetable oils, such as soybean, sunflower, and corn oils (10, 11). Higher levels of these oils, as the main source of omega-6 fatty acids in the diet, activate PPAR-γ expression in bone cells, inhibit osteogenesis, and increase the differentiation of mesenchymal stem cell (MSCs) to adipocytes rather than osteoblasts (11). Dietary consumption of olive oil is associated with lowered incidence of cardiovascular disease (12). Additionally, one cell culture study showed that olive leaf polyphenols have beneficial effects on osteoblastic gene expression (13).
However, the effect of differing amounts of maternal EVOO, which is composed mainly of omega-9 fatty acids and polyphenolic compounds, during gestation and lactation on bone remodeling and adipogenic markers in offspring has not been studied. Therefore, using soybean oil as a control, we hypothesized that type of maternal dietary oil has more effects than its amount in an isocaloric diet during gestation and lactation on bone genes expression in offspring at adolescence. To achieve this, virgin female C57BL/6 mice were used as a model to study the effects of two doses of EVOO, or soybean oil control, during gestation and lactation on bone osteoblastic, osteoclastic and adipogenic genes expression levels in adolescent male or female offspring.
Materials and Methods
Animals, diets, and experimental design
The experimental protocol was approved by the Animal Research Committee of Iran University of Medical Sciences (protocol number: 24208). This research conforms to the Institutional and National Guide for the Care and Use of Laboratory Animals. As shown in Figure 1, eight-week-old inbred female C57BL/6 mice (21±1.5 g) were obtained from the Razi Vaccine and Serum Research Institute, Tehran, Iran. Each mouse was individually housed at 21– 23 °C with controlled humidity (50±5%) under a 12 hr artificial light cycle (7 am to 7 pm). All animals received the AIN 93M diet two weeks before the beginning of the study for adaptation. The AIN 93M diet composition (per 1 kg) was 140 g of protein as Casein lactate (Iranian Caseinate Industry, Iran) and 1.8 g as L-cysteine (W326305, Sigma-Aldrich, Germany), 40 g of lipid as soybean oil (Kamzit Company, Iran), 630 g of carbohydrate as corn starch (corn dextrin from corn refinery, Iran), 100 g of sugar (local confectionery, Iran), 35 g of AIN 93M mineral mix (296040002, MP Biomedicals, USA), 10 g of AIN 93 vitamin mix (296040201, MP Biomedicals, USA), 2.5 g of choline bitartrate (C1629, Sigma-Aldrich, Germany), 0.008 g of tert-butylhydroquinone (112941, Sigma-Aldrich, Germany) and 50 g of fiber (wheat bran, Iran).
Figure 1.
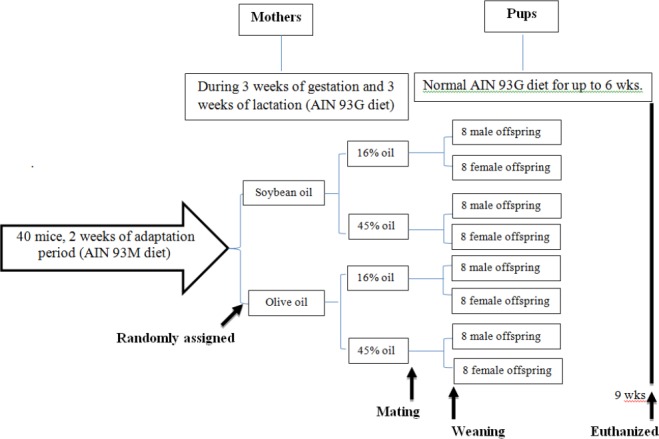
Schematic overview of the experimental design. Female C57BL/6 mice were exposed during gestation and lactation to a normal-fat (16%) regular mouse AIN 93G diet containing soybean oil or EVOO, or to a high-fat AIN 93G (45%) diet containing soybean oil or EVOO. All pups received 16% soybean oil diet after weaning and were sacrificed at 6 weeks of age
Each female mouse was mated with one male per cage overnight. After vaginal plug confirmation, mothers were randomly assigned to different dietary groups (N=10 in each group), as shown in Table 1. The diet containing 16% soybean oil was considered the control group diet.
Table 1.
Ingredient composition of the diets administered to mice (AIN 93G diet)
| Diets | Soybean | Olive | ||
|---|---|---|---|---|
| Nutrients | 16% | 45% | 16% | 45% |
| Casein (g/kg) | 200 | 200 | 200 | 200 |
| Cornstarch (g/kg) | 530 | 247 | 530 | 247 |
| Sucrose (g/kg) | 100 | 100 | 100 | 100 |
| Soybean oil (g/kg) | 70 | 198 | - | - |
| EVOO (g/kg) | - | - | 70 | 198 |
| Fiber (g/kg) | 50 | 204.5 | 50 | 204.5 |
| Mineral mix (g/kg) | 35 | 35 | 35 | 35 |
| Vitamin mix (g/kg) | 10 | 10 | 10 | 10 |
| L-cys (g/kg) | 3 | 3 | 3 | 3 |
| Choline bitartrate (g/kg) | 2.5 | 2.5 | 2.5 | 2.5 |
| tert-butylhydroquinone (g/kg) | 0.008 | 0.008 | 0.008 | 0.008 |
| Energy (Kcal/g) | 3.97 | 3.97 | 3.97 | 3.97 |
EVOO: extra virgin olive oil, L-cys: L- cysteine
During the study, all animals were isocalorically fed with the same kcal/[g body weight] of the group that ate less (pair-fed model) (14). Offspring were reduced to 4 pups in each cage, nursed by birth mothers and weaned on day 21, when they were separated according to sex with a maximum of four mice per cage. Pups received ad libitum control diet (16%-soybean oil) post-weaning. Pups were weighed on a calibrated balance scale [Marte Scale (EK-3000i, USA)] to the nearest 0.1 g weekly for determination of weight at birth and at adolescence. One male and one female offspring were randomly selected from each cage for final gene expression analysis at 6 weeks of age.
Collection and preparation of bone specimens
All pups were killed at 6 weeks of age by administration of 40 mg/kg ketamine and 8 mg/kg xylazine, following the overnight fasting conditions. The right femur was dissected out, stripped of soft tissue using a scalpel, immediately frozen, and then was powdered.
RNA isolation and real-time polymerase chain reaction (RT-PCR)
Total RNA from the powdered samples were extracted using QIAzol Lysis Reagent (QIAGEN Inc, Valencia, CA 91355, USA) according to the manufacturer’s instructions, followed by DNase digestion and column cleanup using RNeasy mini columns (Qiagen, Valencia, CA, USA) (15).
RNA quantification was assessed at 260 nm with an ND-1000 spectrophotometer (NanoDrop, Wilmington, DE, USA), and its quality (integrity) was checked by agarose gel electrophoresis.
One microgram of total RNA was retrotranscribed into cDNA using Prime-Script cDNA synthesis reagents from Takara (Takara Bio, Inc, Japan) in 20 μl volume.
The reverse transcription polymerase chain reaction)RT-PCR(was performed in an ABI StepOne sequence detection system (Applied Biosystems, California, USA) with 1 μl of cDNA and 10 pmol of each primer corresponding to the tested genes, using the SYBR Green I Master Mix (Roche). The expression assay was run in duplicate. All primers for real-time PCR analysis were designed using Primer Express software 2.0.0 (Applied Biosystems; Table 2).
Table 2.
Real-time RT-PCR primer sequences
| Genes | Primers | Length |
|---|---|---|
| OPG | F: GGGCGTTACCTGGAGATCG R:CGTTGTCATGTGTTGCATTTCC |
19 22 |
| RANK-L | F: CAGCATCGCTCTGTTCCTGTA R: CTGCGTTTTCATGGAGTCTCA |
21 21 |
| Ctnnb1* | F: CCTCCCAAGTCCTTTATGAATGG R: CCGTCAATATCAGCTACTTGCTCTT |
23 25 |
| PPARγ2 | F: GCCCTTTGGTGACTTTATGGA R: GCAGCAGGTTGTCTTGGATG |
21 20 |
| GAPDH | F: GACTTCAACAGCAACTCCCAC R: TCCACCACCCTGTTGCTGTA |
21 20 |
F, forward; R, reverse.
official name of beta-catenin
OPG: osteoprotegerin; RANK-L: nuclear factor (NF-kB) ligand; PPRRɤ2: Peroxisome proliferator-activated receptor gamma 2; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase
The relative quantity of mRNA was calculated for each sample using the copy threshold (Ct) value and normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH, housekeeping gene) mRNA. The stability of the housekeeping gene was considered a Ct standard deviation <1 and was therefore considered an appropriate control (16). The amplification profile included one cycle at 95 °C for 10 min and 40 two-step cycles: 9 5°C for 15 sec and 60 °C for 60 s. The results were generated and analyzed using the 2^-∆∆Ct method as described by Livak and Schmittgen (17).
Statistical analyses
In the designing of the study, to detect a difference in total body fat mass between two groups, we used a 90% confidence interval with a two-sided test with α=0.05 (type I error). On the basis of SDs reported in a similar study (18), the number of subjects needed to detect this difference was 16 per group. Data were tested for normal distribution using the Kolmogorov-Smirnov test and did not have normal distributions in gene expression even after all of the transformation methods. Three-way ANOVA was used for assessing the effects of sex of offspring, type and amount of maternal dietary oil on offspring food intake, birth, and adolescent weights. The differences between the two variables were measured by the Mann-Whitney-Wilcoxon test, controlling for the sex of offspring and the amounts of maternal dietary oil and/or the sex of the offspring and the types of maternal dietary oil. To assess the interactions between sex and type and amount of dietary oil on the target gene expression, a logistic regression model was used in which any gene was coded as a nominal variable (gene expression above the 50th percentile or below the 50th percentile) (19). All data are expressed as the means ± SE. The level of significance was set at P<0.05. Statistical analyses were performed using the IBM SPSS statistics software (version 18; IBM Corp).
Results
The average food intake was not significantly different between groups at the end of weeks 1, 2, or 3, post-weaning. The results showed no interaction between the sex of the offspring and the type and amount of maternal dietary oil on the offspring’s birth and adolescent weight. There was also no interaction between the sex of offspring and the type of maternal dietary oil, the sex of offspring and the amounts of maternal dietary oil, or the types and amounts of maternal dietary oil on the offspring’s birth and adolescent weight. The mean birth and adolescent weights were significantly higher in male compared with female offspring (P=0.002 and P<0.001, respectively), after being adjusted for the type and amount of maternal dietary oil. The birth and adolescent weights were significantly higher in the offspring born to soybean compared with the EVOO fed group (P<0.001 and P<0.001, respectively), after being adjusted for the amount of maternal dietary oil and the sex of offspring. Adjusting for the type of maternal dietary oil and the sex of offspring, the birth and adolescence weights were not significantly different between offspring born to 16% or 45% oil-fed mothers (Table 3).
Table 3.
Weight and food intake of pups
| Groups | Type of oils | Amounts of oils | Sex of pups | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Soybean | EVOO | P-value | 16% | 45% | P-value | Male | Female | P-value |
| Birth weight (g) | 1.39±0.3 | 1.04±0.1 | *<0.001 | 1.25±0.3 | 1.27±0.2 | 0.16 | 1.3±0.2 | 1.15±0.2 | *0.002 |
| Adolescence weight (g) | 20.1±2.1 | 15.2±1.7 | *<0.001 | 17.8±3.1 | 17.5±3.2 | 0.34 | 19.1±2.7 | 16.2±2.8 | *<0.001 |
| Food intake† (kcal/day) | 9.5±0.7 | 9.7±0.8 | 0.65 | 9.5±0.8 | 9.6±0.6 | 0.8 | 9.7±0.7 | 9.5±0.7 | 0.61 |
| Food intake‡ (kcal/day) | 11.9±0.7 | 11.6±0.5 | 0.54 | 11.7±0.7 | 11.9±0.6 | 0.58 | 11.8±0.5 | 11.6±0.5 | 0.75 |
| Food intakeǂ (kcal/day) | 23.6±1.1 | 23.5±0.8 | 0.58 | 23.5±1 | 23.7±0.8 | 0.79 | 23.6±0.9 | 23.5±0.8 | 0.81 |
Values are reported as the means±SE (N= 32 in each group)
Means of food intake at the end of week 1 after weaning
Means of food intake at the end of week 2 after weaning
Means of food intake at the end of week 3 after weaning
To adjust for confounding variables and assess the interactions among different variables (types and amounts of maternal dietary oil, as well as sex of offspring), ANCOVA was used (*statistically significant)
The regulation of target gene expression by the amounts of maternal dietary oil is controlled by the type of oil (Figure 2). In addition, the effects of the types of maternal dietary oil are controlled by the amounts of oil (Figure 3).
Figure 2.
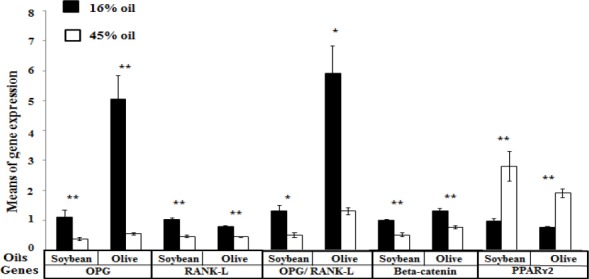
Means of target gene expression in offspring according to the amounts of maternal dietary oil. The effects of the amount of maternal dietary oil, controlled by the type of oil on gene expression in bone; statistically evaluated by the Mann-Whitney-Wilcoxon test. In offspring born to soybean oil-fed mothers, significant differences were observed in OPG, RANK-L, the OPG/RANK-L ratio, beta-catenin, and PPARɤ2 gene expression between 16% and 45% oil-fed groups. The same results were observed in the offspring born to olive oil-fed mothers. (*P-value <0.05; **P-value<0.001)
OPG: osteoprotegerin; RANK-L: nuclear factor (NF-kB) ligand; PPRRɤ2: Peroxisome proliferator-activated receptor gamma 2
Figure 3.
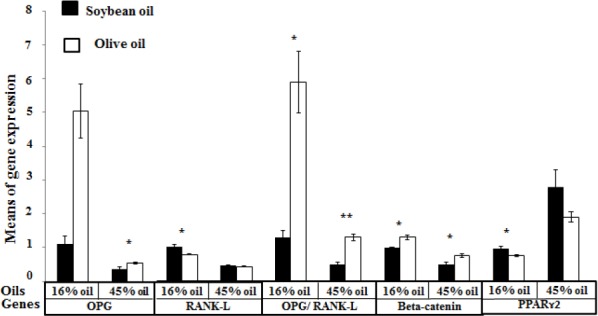
Means of target gene expression in offspring according to the type of maternal dietary oil. The effects of the type of maternal dietary oil, controlled by the amounts of maternal dietary oil on gene expression in bone; statistically evaluated by the Mann-Whitney-Wilcoxon test. In offspring born to 16% oil-fed mothers, significant differences were observed in RANK-L, the OPG/RANK-L ratio, beta-catenin and PPARɤ2 gene expression between soybean oil and olive oil-fed groups. In the 45% oil-fed mothers, significant differences were observed in OPG, the OPG/RANK-L ratio and beta-catenin gene expression between soybean oil and olive oil-fed groups. (*P-value <0.05; **P-value<0.001)
OPG: osteoprotegerin; RANK-L: nuclear factor (NF-kB) ligand; PPRRɤ2: Peroxisome proliferator-activated receptor gamma 2
Values are reported as the means±SE (N= 32 in each group)
According to the results of the logistic regression model, the types of maternal dietary oil showed significant effects on OPG and OPG/RANK-L genes expression (P=0.035 and P=0.003, respectively), after being adjusted for the amount of maternal dietary oil and the sex of offspring. Adjusting for the Values are reported as the means±SE. (N= 32 in each group amounts of maternal dietary oil and the sex of offspring, both of which had significant effects on OPG gene expression; the offspring born to olive oil-fed mothers had 2.8-fold higher OPG gene expression compared with soybean oil-fed mothers. The offspring of olive oil-fed mothers had a 6.1-fold higher OPG/RANK-L ratio compared to soybean oil-fed mothers, adjusted for the amount of maternal dietary oil and the sex of offspring. Beta-catenin gene expression in the offspring of olive oil-fed mothers was 2.4-folds higher than in soybean oil-fed mothers, but this difference was not significant after being adjusted for the sex of the offspring and the amount of maternal dietary oil. The amount of maternal dietary oil had significant effects on OPG, beta-catenin and PPARγ2 gene expression, as well as on the OPG/RANK-L ratio (P<0.001, P<0.001, P<0.001, and P=0.037, respectively), after being adjusted for the types of maternal dietary oil and sex of offspring. The offspring born to 45% oil-fed mothers had 88% lower OPG gene expression and 94% lower beta-catenin gene expression compared to those fed 16% oil diets. The offspring of the 45% oil-fed mothers had a 70% lower OPG/RANK-L ratio than the 16% oil group. PPARγ2 gene expression in the offspring of 45% oil-fed mothers increased 24-fold compared with 16% oil. OPG gene expression was significantly different between male and female offspring, after being adjusted for the type and amount of maternal dietary oil. OPG gene expression was significantly reduced by 90% in males compared to females (P<0.001). Additionally, OPG/RANK-L ratio was significantly reduced by 97% in males compared to females (P<0.001). The types of maternal dietary oil had no effect on RANK-L, beta-catenin or PPARγ2 gene expression, after being adjusted for the amounts of maternal dietary oil and sex of offspring. Additionally, the amount of maternal dietary oil did not effect RANK-L gene expression, after being adjusted for the type of maternal dietary oil and sex of offspring. The interaction effects between the type and amount of maternal dietary oil were significant and synergistic on OPG and PPARγ2 gene expression (P<0.001 and P=0.016, respectively) (Table 4).
Table 4.
Status of the target gene expression, type (soybean and olive oil), or amount (16% and 45%) of maternal dietary oil and the sex of the offspring
| OR (95% CI) | SE | B† | Variable’s level | Variable’s name | Genes |
|---|---|---|---|---|---|
| 1 | - | Soybean oil | Types of oil | OPG | |
| *2.8 (1.07, 7.48) | 0.49 | 1.04 | olive oil | ||
| 1 | - | 16% | Amounts of oil | ||
| ***0.12 (0.04, 0.35) | 0.53 | -2.08 | 45% | ||
| 1 | - | Female | Sex | ||
| ***0.09 (0.03, 0.27) | 0.5 | -2.4 | Male | ||
| 1 | - | Soybean | Types of oil | RANK-L | |
| <0.001 (0.0, 0.0) | 55 | -19.1 | olive oil | ||
| 1 | - | 16% | Amounts of oil | ||
| <0.001 (0.0, 0.0) | 5536 | -23.4 | 45% | ||
| 1 | - | Female | Sex | ||
| 0.55 (0.09, 3.3) | 0.9 | -0.59 | Male | ||
| 1 | - | Soybean | Types of oil | OPG/RANK-L | |
| **6.1 (1.85, 20.2) | 0.61 | 1.8 | olive oil | ||
| 1 | - | 16% | Amounts of oil | ||
| *0.3 (0.12, 0.94) | 0.53 | -1.1 | 45% | ||
| 1 | - | Female | Sex | ||
| ***0.03 (0.01, 0.12) | 0.63 | -3.4 | Male | ||
| 1 | - | Soybean | Types of oil | Beta-catenin | |
| 2.4 (0.92, 6.37) | 0.49 | 0.88 | olive oil | ||
| 1 | - | 16% | Amounts of oil | ||
| ***0.06 (0.025, 0.17) | 0.49 | -2.71 | 45% | ||
| 1 | - | Female | Sex | ||
| 1.31 (0.51, 3.37) | 0.48 | 0.27 | Male | ||
| 1 | - | Soybean | Types of oil | PPARɤ2 | |
| 0.37 (0.13, 1.07) | 0.53 | -0.97 | olive oil | ||
| 1 | - | 16% | Amounts of oil | ||
| ***24 (8.3, 69.9) | 0.54 | 3.18 | 45% | ||
| 1 | - | Female | Sex | ||
| 2.4 (0.85, 6.8) | 0.53 | 0.88 | Male |
Discussion
To our knowledge, these are the first results that demonstrate the effects of type and amount of maternal dietary oil (with focus on EVOO) during gestation and lactation, coupled with post-weaning control diet of the offspring on gene expression of formation and resorption markers in bones of offspring at adolescence. The results of the present study showed that EVOO consumption during gestation and lactation leads to up-regulation of OPG and OPG/RANK-L mRNA expression in the bones of offspring, which increases osteoblastogenesis. Although the mRNA expression of OPG, beta-catenin, and OPG/RANK-L were lower, PPARγ2 gene expression increased in the offspring born to 45% compared with the 16% oil-fed mothers, which increases osteoclastogenesis and adipogenesis. The present study showed that both type and amount of maternal dietary oil during gestation and lactation effect bone remodeling markers in offspring at adolescence. Previous animal and human studies have shown that diets with high saturated fat content have harmful effects on bone mineralization and hip fracture risk (20, 21). Although some studies have reported that the ratio of n-6/n-3 PUFA plays a critical role in bone formation (22), no study has yet examined the effects of differing amounts of maternal dietary oil with focus on EVOO in an isocaloric diet on the expression of bone osteoblastic and/or osteoclastic and adipogenic genes in adolescent offspring.
Interpretation of our data is challenging, given that there are only a few previous animal studies that have tested how maternal diet affects bone mass in their offspring. Previous studies have shown the deleterious effects of a maternal high-fat diet on the bone structure of offspring, but the mechanisms underlying this observation remain unclear. This could be because studies in this field have different protocols and outcome measures (4, 23, 24).
Osteoclasts and osteoblasts originate from the different cell lines (25). Osteoblasts are derived from MSCs in which cell-derived cytokines and transcription factors can switch the fate of MSCs to osteoblasts or adipocytes (26). OPG is a marker of osteoblasts that can inhibit osteoclast differentiation and activity (27), but RANK-L mRNA plays a major role in the stimulation of osteoclast differentiation, activity, and inhibition of osteoclast apoptosis (28). OPG can inhibit the RANK system by blocking the effects of RANK-L (29). Beta-catenin signaling is necessary for osteoblast and osteocyte expression of the anti-osteoclastic factor OPG, which is the decoy receptor for RANKL (8). PPARγ2 is the dominant regulator of adipogenesis. Ligand activation of PPARγ2 favors the differentiation of MSCs into adipocytes rather than osteoblasts. PPARγ2 and beta-catenin are well-known as an adipogenic and osteoblastogenic signaling molecules, respectively. We examined the expression of PPARγ2 and beta-catenin genes in bone to determine the molecular signaling events that lead to the increased bone marrow adiposity and decreased bone formation. In one study, western blot results of total proteins isolated from the femur, with bone marrow cells removed, confirmed that beta-catenin and PPARγ protein expression were significantly down-regulated and up-regulated in high fat diet-induced obese animals, respectively (30). In the present study, offspring born to 45% oil-fed mothers had lower beta-catenin and higher PPARγ2 genes expression than the 16% oil group. This means that a high fat diet increases adiposity in the bone of offspring and shifts the lineage of MSCs to adipocytes rather than osteoblasts, regardless of the type of oil used.
In an in vitro study, omega-6 fatty acid decreased the expression of the osteogenic markers and the OPG/RANK-L ratio, and also inhibited the mineralization of the bone (17). Our results are in agreement with this study. We conclude that maternal soybean oil consumption, as the main source of omega-6 fatty acids, during gestation and lactation has permanent deleterious effects on the osteoblastic markers in adolescent offspring. In our study, the type and amount of maternal dietary oil had synergistic effects on PPAR-γ2 gene expression in the bone of offspring.
In the present study, a maternal diet comprised of normal amounts of fat that contains olive oil led to an increase in OPG mRNA and OPG/RANK-L ratio in the bone of female offspring compared with males, but sex had no significant effect on the expression of other target genes. Previous studies mentioned that 17β-estradiol enhances OPG gene expression and protein secretion through a transcriptional mechanism in MSCs and mature osteoblastic cells that had been stably transfected with the ER-α (31, 32). In addition, 17β-estradiol can repress the activity of c-jun N-terminal Kinase (JNK), a critical downstream signal in the RANK pathway (33, 34). Additionally, a previous study mentioned that estrogen induces apoptosis of osteoclasts (35). The most convincing results appeared in female compared with male offspring, perhaps due to the synergistic effects of estrogen and the polyphenolic compounds of EVOO. Oleuropein as the major polyphenol in the EVOO leads to these effects (13).
In summary, a maternal diet containing olive oil increases osteogenesis through the up-regulation of OPG and OPG/RANK-L ratio. The amount of maternal dietary oil is another important factor in this regulation, and high amounts have deleterious effects on the bone of offspring through increases in PPARγ2, a marker of bone adiposity, and decreases in beta-catenin, OPG, and the ratio of OPG/RANK-L gene expression, as the markers of osteogenesis. Therefore, our hypothesis mentioned at the beginning of the essay will be rejected. One should note the importance of both the type of maternal dietary oil taken during gestation and lactation and the amount of oil consumption because both are important.
There are some limitations to our data, most importantly, the fact that we rely on measurements of mRNA to suggest the regulatory effects of the analyzed diets, but proteins or other phenotypes of bone metabolism were not assessed. Skeletal gene expression was assessed only at adolescence, even though periodic measures from birth until aging are recommended. This study does not directly measure whether different types and amounts of maternal dietary oil induce epigenetic changes in bone of offspring. In future studies, direct assessment of epigenetic changes such as histone modification or DNA methylation of the mentioned target genes will allow us to test the hypothesis that perinatal programming of the skeleton occurs through epigenetic mechanisms. Additionally, genes expression was normalized against GAPDH as the housekeeping gene. Other housekeeping genes other than GAPDH are suggested for future studies. A free-fed group along with the pair-fed groups is also suggested for the future. Another group that compares EVOO with refined olive oil is suggested because we cannot conclude that these effects are due to the type of fatty acids (omega-9) or polyphenols in EVOO. This is an animal model; therefore, the effects of the diets cannot be directly transposed to humans. These studies provide indications of the possible effects of such dietary manipulations on skeletal physiology that may suggest future insights for research of underlying mechanisms.
Longer studies that will include evaluation of the developing bone by using in vivo micro-CT as well as bone strength testing, bone mineral density, histology, and histomorphometry and measurement of bone serum parameters such as OCN, OPG, RANK-L, IGF-1, alkaline phosphatase, and calcium will lead to further insights about post-translational modifications.
Conclusion
A maternal diet containing EVOO, but not soybean oil leads to increased bone formation gene expression that modulates early-life events to potentiate protection from the risk of offspring bone disorders in adult life. But, high amounts of both oils decrease osteoblastic and increase adipogenic gene expression in adolescent offspring. Normal amount of EVOO in the maternal diet is the best advice during gestation and lactation.
Acknowledgment
Authors are very grateful to the colleagues in the Breeding Centre of Laboratory Animals at Iran University of Medical Sciences, Tehran, Iran.
References
- 1.Kanis JA, Burlet N, Cooper C, Rizzoli R, Reginster Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2008;19:399–428. doi: 10.1007/s00198-008-0560-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodfellow L, Earl S, Cooper C, Harvey NC. Maternal diet, behaviour and offspring keletal health. Int J Environ Res Public Health. 2010;7:1760–1772. doi: 10.3390/ijerph7041760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecol Evol. 2005;20:527–533. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Lanham SA, Roberts C, Hollingworth T, Sreekumar R, Elahi MM, Cagampang FR, et al. Maternal high-fat diet: effects on offspring bone structure. Osteoporosis Int. 2010;21:1703–1714. doi: 10.1007/s00198-009-1118-4. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Bernal CL, Rebagliato M, Iniguez C. Diet quality in early pregnancy and its effects on fetal growth outcomes: the tnfanciay medioambiente (childhood and environment) mother and child cohort study in Spain. Am J Clin Nutr. 2010;91:1659–1666. doi: 10.3945/ajcn.2009.28866. [DOI] [PubMed] [Google Scholar]
- 6.Niculescu MD, Lupu DS, Craciunescu CN. Perinatal manipulation of alpha-linolenic acid intake induces epigenetic changes in maternal and offspring livers. FASEB J. 2013;27:350–358. doi: 10.1096/fj.12-210724. [DOI] [PubMed] [Google Scholar]
- 7.Casado-Díaz A, Santiago-Mora R, Dorado G, Gomez J. The omega-6 arachidonic fatty acid, but not the omega-3 fatty acids, inhibit osteoblastogenesis and induces adipogenesis of human mesenchymal stem cells: potential implication in osteoporosis. Osteoporos Int. 2013;24:1647–1661. doi: 10.1007/s00198-012-2138-z. [DOI] [PubMed] [Google Scholar]
- 8.Albers J, Keller J, Baranowsky A, Timo Beil F, Catala-Lehnen Ph, Schulze J, Amling M, Schinke Th. Canonical Wnt signaling inhibits osteoclastogenesis independent of osteoprotegerin. J Cell Biol. 2013;4:537–549. doi: 10.1083/jcb.201207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lecka-Czernik B. PPARγ, an essential regulator of bone mass: metabolic and molecular cues. IBMS Bonekey. 2010;7:171–181. [Google Scholar]
- 10.Simopoulos A. Essential fatty acids in health and chronic disease. Am J Clin Nutr. 1999;70:560S–9S. doi: 10.1093/ajcn/70.3.560s. [DOI] [PubMed] [Google Scholar]
- 11.Halade GV, Roohman MM, Williams PJ, Fernandes G. High fat diet-induced animal model of age-associated obesity and osteoporosis. J Nutr Biochem. 2010;21:1162–1169. doi: 10.1016/j.jnutbio.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao K, Xu J, Zou X, Li Y, Chen C, Zheng A, et al. Hydroxytyrosol prevents diet-induced metabolic syndromeand attenuates mitochondrial abnormalities in obese mice. Free Radic Biol Med. 2014;67:396–407. doi: 10.1016/j.freeradbiomed.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Santiago-Mora R, Casado-Díaz A, De Castro MD, Quesada-Gómez JM. Oleuropein enhances osteoblastogenesis and inhibits adipogenesis: the effect on differentiation in stem cells derived from bone marrow. Osteoporos Int. 2011;22:675–684. doi: 10.1007/s00198-010-1270-x. [DOI] [PubMed] [Google Scholar]
- 14.Gamba CA, Friedman SM, Rodriguez PN, Macri EV, Vacas MI, Lifshitz F. Metabolic status in growing rats fed isocaloric diets with increased carbohydrate-to-fat ratio. Nutrition. 2005;21:249–254. doi: 10.1016/j.nut.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Chen JR, Lazarenko OP, Wu X, Kang J, Blackburn M, Shankar K, et al. Dietary induced serum phenolic acids promote bone growth via p38 MAPK/beta-catenin canonical Wnt signaling. J Bone Miner Res. 2010;25:2399–2411. doi: 10.1002/jbmr.137. [DOI] [PubMed] [Google Scholar]
- 16.Lima Reboucas E, Nascimento Costa J, Passos M, Sousa Passos J, Van den Hurk R, Viana Silva J. Real time PCR and importance of housekeepings genes for normalization and quantification of mRNA expression in different tissues. Braz Arch Biol Technol. 2013;56:143–154. [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Priego T, Sánchez J, García AP, Palou A, Picó C. Maternal dietary fat affects milk fatty acid profile and impacts on weight gain and thermogenic capacity of suckling rats. Lipids. 2013;48:481–495. doi: 10.1007/s11745-013-3764-8. [DOI] [PubMed] [Google Scholar]
- 19.Rosa BV, Blair H, Vickers MH, Dittmer KE, Morel P, Knight CG, et al. Moderate exercise during pregnancy in wistar rats alters bone and body composition of the adult offspring in a sex-dependent manner. PLoS One. 2013;8:e82378. doi: 10.1371/journal.pone.0082378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wohl GR, Loehrke L, Watkins BA, Zernicke RF. Effects of high fat diet on mature bone mineral content, structure, and mechanical properties. Calcif Tissue Int. 1998;63:74–79. doi: 10.1007/s002239900492. [DOI] [PubMed] [Google Scholar]
- 21.Orchard TS, Cauley JA, Frank GCL, Neuhouser M, Robinson JG, Snetselaar L, Tylavsky F, et al. Fatty acid consumption and risk of fracture in the Women’s Health Initiative. Am J Clin Nutr. 2010;92:1452–1460. doi: 10.3945/ajcn.2010.29955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watkins BA, Li Y, Lippman HE, Fengs S. Modulatory effect of omega-3 polyunsaturated fatty acids on osteoblast function and bone metabolism. Prostaglandins Leukot Essenti Fatty Acids. 2003;68:387–398. doi: 10.1016/s0952-3278(03)00063-2. [DOI] [PubMed] [Google Scholar]
- 23.El-Sayed E, Ibrahim K. Effect of the types of dietary fats and non-dietary oils on bone metabolism. Crit Rev Food Sci Nutr. 2015;16 doi: 10.1080/10408398.2014.914889. [DOI] [PubMed] [Google Scholar]
- 24.Devlin MJ, Grasemann C, Cloutier AM, Louis L, Palmert M, Bouxsein ML. Maternal perinatal diet induces developmental programming of bone architecture. J Endocrinol. 2013;217:69–81. doi: 10.1530/JOE-12-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horowitz MC, Lorenzo JA. The origins of osteoclasts. Curr Opin Rheumatol. 2004;16:464–468. doi: 10.1097/01.bor.0000127825.05580.eb. [DOI] [PubMed] [Google Scholar]
- 26.Gimble JM, Robinson CE, Wu X, Kelly KA. The function of adipocytes in the bone marrow stroma: an update. Bone. 1996;19:421–428. doi: 10.1016/s8756-3282(96)00258-x. [DOI] [PubMed] [Google Scholar]
- 27.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki Sh, et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;39:1329–1337. doi: 10.1210/endo.139.3.5837. [DOI] [PubMed] [Google Scholar]
- 28.Fuller K, Wong B, Fox S, Choi Y, Chambers T. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med. 1998;188:997–1001. doi: 10.1084/jem.188.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Interleukin-1β and tumor necrosis factor-α, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone. 1999;25:255–259. doi: 10.1016/s8756-3282(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Lazarenko O, Wu X. Obesity reduces bone density associated with activation of PPARγand suppression of Wnt/β-catenin in rapidly growing male rats. PLoS One. 2010;5:13704. doi: 10.1371/journal.pone.0013704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saika M, Inoue D, Kido S, Matsumoto T. 17‚-Estradiol stimulates expression of osteoprotegerin by a mouse stromal cell line, ST-2, via estrogen receptor. Endocrinology. 2001;142:2205–2212. doi: 10.1210/endo.142.6.8220. [DOI] [PubMed] [Google Scholar]
- 32.Chen XW, Garner SC, Anderson JJ. Isoflavones regulate interleukin-6 and osteoprotegerin synthesis during osteoblast cell differentiation via an estrogen-receptordependent pathway. Biochem Biophys Res Commun. 2002;295:417–422. doi: 10.1016/s0006-291x(02)00667-8. [DOI] [PubMed] [Google Scholar]
- 33.Shevde NK, Bendixen AC, Dienger KM, Pike JW. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci USA. 2000;97:7829–7834. doi: 10.1073/pnas.130200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srivastava S, Toraldo G, Weitzmann MN, Cenci S, Ross FP, Pacifici R. Estrogen decreases osteoclast formation by down-regulating receptor activator of NF-ÎB ligand (RANKL)-induced JNK activation. J Biol Chem. 2001;276:8836–8840. doi: 10.1074/jbc.M010764200. [DOI] [PubMed] [Google Scholar]
- 35.Hughes DE, Dai A, Tiffee JC, Hiu Li HH, Mundy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-β. Nat Med. 1996;2:1132–1136. doi: 10.1038/nm1096-1132. [DOI] [PubMed] [Google Scholar]


